Abstract
Mutations in the Berardinelli-Seip congenital lipodystrophy 2 gene (BSCL2) are the underlying defect in patients with congenital generalized lipodystrophy type 2. BSCL2 encodes a protein called seipin, whose function is largely unknown. In this study, we investigated the role of Bscl2 in the regulation of adipocyte differentiation. Bscl2 mRNA is highly up-regulated during standard hormone-induced adipogenesis in 3T3-L1 cells in vitro. However, this up-regulation does not occur during mesenchymal stem cell (C3H10T1/2 cells) commitment to the preadipocyte lineage. Knockdown of Bscl2 by short hairpin RNA in C3H10T1/2 cells has no effect on bone morphogenetic protein-4-induced preadipocyte commitment. However, knockdown in 3T3-L1 cells prevents adipogenesis induced by a standard hormone cocktail, but adipogenesis can be rescued by the addition of peroxisome proliferator-activated receptor-γ agonist pioglitazone at an early stage of differentiation. Interestingly, pioglitazone-induced differentiation in the absence of standard hormone is not associated with up-regulated Bscl2 expression. On the other hand, short hairpin RNA-knockdown of Bscl2 largely blocks pioglitazone-induced adipose differentiation. These experiments suggest that Bscl2 may be essential for normal adipogenesis; it works upstream or at the level of peroxisome proliferator-activated receptor-γ, enabling the latter to exert its full activity during adipogenesis. Loss of Bscl2 function thus interferes with the normal transcriptional cascade of adipogenesis during fat cell differentiation, resulting in near total loss of fat or lipodystrophy.
Mutations in Seipin underlie Berardinelli-Seip congenital generalized lipodystrophy-2; Seipin is required for adipocyte differentiation, working upstream or at the level of PPARγ during adipogenesis.
Berardinelli-Seip syndrome (Bscl) is an autosomal recessive disease characterized by a near total absence of adipose tissue from birth or early infancy (1,2). Like other patients with congenital generalized lipodystrophy (CGL), affected individuals often develop severe insulin resistance, diabetes, hypertriglyceridemia, and fatty liver. To date, mutations involving three different genes, BSCL1, BSCL2, and CAVEOLIN1, have been identified to underlie this rare genetic disorder with the first two accounting for about 95% of reported cases (3,4,5,6). Most of the disease-associated alterations in these three genes involve nonsense, splice, or frame shift mutations, which likely result in complete loss of function (3,4,5,6). BSCL1 encodes acylglycerol phosphate acyltransferase (AGPAT)-2 that catalyzes the formation of phosphatidic acid, a crucial intermediate step in the biosynthesis of triglycerides (6,7). Mutations at BSCL2 cause a more severe form of CGL with almost complete absence of adipose tissue and high incidence of mental retardation (4,8). BSCL2 encodes a protein called seipin, of which the function is largely unknown.
Human BSCL2 protein is predicted from its primary sequence to traverse the plasma membrane twice with both termini facing the cytoplasm and a glycosylation site in the luminal segment (9). BSCL2-enhanced green fluorescent protein (eGFP) was found to be localized to the endoplasmic reticulum (ER) in human umbilical vein endothelial cells (EA.hy926) (10). Interestingly, heterozygous missense mutations in the glycosylation sites of BSCL2 are associated with distal hereditary motor neuropathy and silver syndrome, a dominant heritable disease (10). Recent studies in yeast have shown that the lack of a yeast Bscl2 ortholog, fewer lipid drops (FLD), causes abnormal lipid droplet assembly and maintenance possibly due to aberrant phospholipid metabolism in the absence of the protein (11,12). The role of BSCL2 in triglyceride metabolism and adipocyte differentiation in mammals is largely unknown. Lack of BSCL2 has been postulated to directly disrupt adipocyte differentiation/function or interfere with mesenchymal stem cell commitment into preadipocytes, leading to near total absence of adipose tissue, although, to date, there is little experimental evidence supporting these hypotheses (13,14). It has been shown recently, however, that BSCL2 knockdown inhibits the differentiation of C3H10T1/2 cells, a mesenchymal stem cell-line, into adipocytes (37).
Here we present evidence that Bscl2 may play a direct role in adipocyte differentiation. We found that Bscl2 is markedly up-regulated during standardized hormone [mixture of insulin, isobutylmethylxanthine (IBMX), and dexamethasone, heretofore referred to as DMI]-induced, but not peroxisome proliferator-activated receptor (PPAR)-γ selective agonist pioglitazone (pio)-induced, adipogenesis in 3T3-L1 preadipocytes. Knockdown of Bscl2 has no major effect on mesenchymal stem cell commitment but blocks DMI-induced adipocyte differentiation, and this inhibition can be fully rescued by addition of pio. However, pio alone in the absence of DMI does not restore the differentiation of Bscl2-knockdown preadipocytes. We conclude that the presence of Bscl2 is required for PPARγ’s full activation to initiate the adipogenesis transcription program. Taken together, these observations underscore a critical role for Bscl2 in normal adipocyte differentiation, which, when disrupted, leads to impaired adipogenesis and lipodystrophy.
Materials and Methods
Cell culture
The 3T3-L1 cell line (American Type Culture Collection, Manassas, VA) was propagated in DMEM containing 10% fetal bovine serum (FBS). Adipocyte differentiation was initiated after 2 d at confluence in DMEM (25 mm glucose) containing 10% FBS, supplemented with 1 μm insulin, 0.5 mm IBMX, and 1 μm DMI for 2 d and then supplemented with 1 μm insulin alone for 2 d. After 4 d, cells were cultured in DMEM 10% FBS medium. Murine primary preadipocytes from the sc fat stromal vascular fraction were prepared as described (15). Briefly, sc fat from 6- to 7-wk-old C57BL/6J mice were isolated, minced, and digested in 2 mg/ml collagenase IV (Sigma, St. Louis, MO) with 20 mg/ml BSA at 37 C for 40 min. The digested mixtures were then filtered through a 100-μm cell strainer, spun at 250 × g for 8 min. The pellet containing the stromal vascular fraction was then resuspended in preadipocyte growth media (Cell Applications, Inc., San Diego, CA) and cultured for induction of differentiation by using the standard 3T3-L1 differentiation protocol. Human adipose stem cells were cultured in EGM-2MV (Cambrex, East Rutherford, NJ) plus penicillin/streptomycin and differentiated in adipocyte differentiation medium (ADM; Cell Applications) with three cycles of exchange from ADM to DMEM plus 1 μm insulin. C3H10T1/2 cells were maintained in DMEM high glucose plus 10% FBS and penicillin/streptomycin.
Bone morphogenetic protein (BMP)-4-induced commitment of C3H10T1/2 cells to the adipocyte lineage
The commitment of C3H10T1/2 cells to adipocyte lineage was performed as described previously (16). Briefly, C3H10T1/2 cells were split at low density and cultured in DMEM containing 10% FBS in the presence of 50 ng/ml purified recombinant BMP4 (R&D Systems, Minneapolis, MN). After cells reached 2-day postconfluence, they were induced to differentiate using the standard (DMI) adipogenic induction media.
RT-PCR and RNA quantitation
Total RNA was isolated from tissues or cultured cells with TRIzol (Invitrogen, Carlsbad, CA) and reverse transcribed using SuperScript II reverse transcriptase using random primers (Invitrogen). Real-time quantitative RT-PCR was performed on the MX3000 real time detection system (Stratagene, La Jolla, CA) using iQ SYBR Green PCR reagent kit (Bio-Rad Laboratories, Hercules, CA).
Generation of retroviral constructs and retroviral infections
Murine Bscl2 cDNA (GenBank accession no. AF180471) was subcloned into pBPCW-green fluorescent protein (GFP) retroviral expression vectors (CLONTECH, Palo Alto, CA) and pMSCVpuro-NHM with puromycin selectable marker. Four small interfering RNA sequences targeting mouse Bscl2 (13a, 14a, 15a, 16a) were obtained from Dharmacon, Inc. (Lafayette, CO) and synthesized as short hairpin RNA (shRNA) using TTCAAGAGA as linker. shRNA against luciferase was used as control. The shRNA constructs were then subcloned into the RNAi-Ready pSiren-RetroQ vector (CLONTECH) using the EcoRI and BamHI restriction sites. An effect had to be seen with at least two hairpins. The best targeted sequences that delivered the most efficient knockdown were shBs13a and shBs14a. Retroviral packaging Bosc-23 (American Type Culture Collection) cells were cotransfected with the targeting plasmids and packaging vector pCL-eco (Imgenex, Sorrento Valley, CA). Forty-eight hours after transfection, the culture media containing the virus particles were collected and mixed with DMEM 10% FBS at 1:2 to infect cells in the presence of 8 μg/ml polybrene. Cells were infected and selected as described (15). Experiments were repeated at least three times.
Immunofluorescence microscopy
Cells were cultured on collagen-coated glass coverslips, fixed with 4% paraformaldehyde, permeabilized with 0.2% Nonidet P-40, blocked with 5% goat serum, and then probed with antibody against calreticulin (Cell Signaling, Beverly, MA) at 1:25 and then incubated with Alexa-fluor 555-conjugated goat antirabbit IgG (H+L; Molecular Probes, Eugene, OR). The slides were mounted with Vectorshield mounting medium with 4′6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Images were taken with a Axiovert inverted microscope (Zeiss, New York, NY).
Triglyceride content measurement and oil-red O staining
At indicated days, the intracellular triglyceride in the adipocytes was extracted by chloroform and methanol. The amount of triglyceride was measured with an Infinity triglyceride assay kit as described (17). The concentration of cellular protein was determined using a DC protein assay (Bio-Rad Laboratories). Intracellular triglyceride content was calculated as per milligram of protein. Oil-red O staining was performed as described (15) and photographed with camera or under microscopy.
Statistical analysis
Statistical analyses of the data were performed using a two-tailed unpaired t test with unequal variance. Data are presented as the means ± sd with statistical significance set at a P value of <0.05.
Results
Bscl2 is differentially regulated during adipogenesis
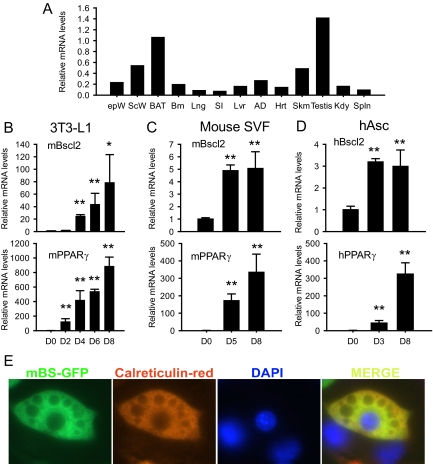
Previous studies using Northern blotting demonstrated that Bscl2 is highly expressed in human testis and brain (4). We used real-time PCR-based expression analysis to determine the expression of Bscl2 in various tissues isolated from 4-h-fasted 10-wk-old male C57BL/6J mice. The expression level is highest in testis followed by brown fat, sc fat, skeletal muscle, epididymal fat, and other tissues (Fig. 1A).
Figure 1.
Bscl2 is expressed in adipose tissue and highly up-regulated during adipocyte differentiation. A, Bscl2 mRNA expression in mouse tissues as determined by real-time RT-PCR in epididymal (epW) and sc (scW) white adipose tissue, brown adipose tissue (BAT), brain (Brn), lung (Lng), small intestine (SI), liver (Lvr), adrenal gland (AD), heart (Hrt), skeletal muscle (Skm), testis, kidney (Kdy), and spleen (Spln) isolated from 4-h-fasted, 10-wk-old male C57BL/J6 mice. Data are normalized to cyclophilin A mRNA. Time course of Bscl2 and PPARγ mRNA expression during induced differentiation in 3T3-L1 cells was by DMI (B), mouse stromal vascular cells (SVF) by DMI (C), and human adipocyte stem cells (hAsc) by ADM (D) as described in Materials and Methods. Data are means ± sd (n = 3) relative to d 0. *, P < 0.05; **, P <0.005 vs. d 0. E, 3T3-L1 cells stably infected with retroviruses expressing GFP-tagged Bscl2 were induced to differentiate for 8 d and stained with anticalreticulin antibody. Merged images show overlay of the two proteins in which yellow indicates colocalization (×630). DAPI, 4′6-Diamidino-2-phenylindole.
After demonstrating that adipose tissue is a major site for Bscl2 expression, we examined whether its expression level is regulated during adipocyte differentiation in 3T3-L1 cells induced by a standard hormone cocktail (DMI; see Materials and Methods) (Fig. 1B, upper panel). The time-dependent expression pattern of PPARγ (18) was also determined for these cells for comparison (Fig. 1B, lower panel). A 25-fold increase in Bscl2 mRNA was detected at d 4; it went up to about 40-fold at d 6 and further to about 70-fold when the cells were fully differentiated at d 8. Therefore, the increase in Bscl2 mRNA lagged behind that of PPARγ. Bscl2 mRNA was also up-regulated during hormonal induced adipocyte differentiation of primary mouse stromal vascular cells (Fig. 1C) and isolated human adipocyte stem cells (Fig. 1D; also see Materials and Methods).
A number of factors including cAMP response element-binding protein (19), Early frowth factor 2 (Egr2/Krox20) (20) and lipin 1β (21) are induced very early in the differentiation cascade within the first few hours after DMI treatment of 3T3-L1 cells. No significant increase in Bscl2 expression was detectable at 48 h (d 2) (Fig. 1B). To determine whether there was a Bscl2 peak before 48 h, we performed a detailed analysis of some earlier time points but found no evidence of any rise in Bscl2 expression between 2 and 34 h after DMI addition (supplemental Fig. S1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). In contrast, CCAAT enhancer-binding protein (C/EBP)-β, which is known to be transiently induced during the first day of differentiation, showed a significant increase in expression at as early as 2 h; the up-regulation persisted at least until 24 h before returning toward baseline later (supplemental Fig. S1B). As previously reported (18), PPARγ mRNA level rose mildly but significantly at 4 h and substantially at 34 h and afterward (Fig. 1B and supplemental Fig. S1C). Therefore, in DMI-induced 3T3-L1 differentiation, there was no evidence of biphasic stimulation of Bscl2 expression; marked up-regulation of Bscl2 was detectable at d 4 (Fig. 1B), much later than that for PPARγ.
Bscl2 localizes to the ER in adipocytes
Bscl2-GFP fusion protein was shown to localize in the ER membrane in EA.hy926 cells (10). To determine the subcellular compartment of Bscl2 expression in an adipocyte cell line, we created a retrovirus vector expressing a BSCL2-eGFP fusion protein and used it to stably transduce 3T3-L1 preadipocytes. We induced differentiation by DMI and stained the mature differentiated adipocytes with an antibody against calreticulin (a specific ER marker) and nuclear dye 4′6-diamidino-2-phenylindole. As shown in Fig. 1E, the Bscl2-eGFP fusion protein was localized mostly around the nuclei and colocalized with ER marker calreticulin. Biochemical fractionation further confirmed that overexpression of N-terminal c-Myc tagged murine Bscl2 in 3T3-L1 adipocytes led to aggregates that stayed most in the wells on sodium dodecyl sulfate gel electrophoresis (supplemental Fig. S2); the small amount that entered the gel was mainly localized to the microsome fraction, which contains ER and correlates with the location of the ER marker calreticulin (supplemental Fig. S2). These data indicate that, in adipocytes, Bscl2 is predominantly an ER resident protein.
Bscl2 expression is not altered and not essential during mesenchymal stem cell commitment to preadipocytes
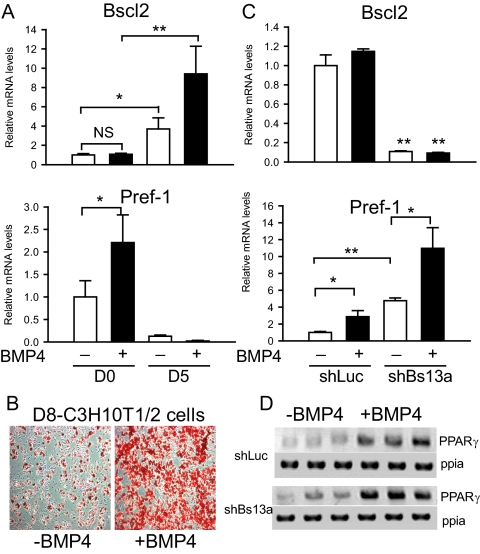
In addition to its late appearance during preadipocyte differentiation into mature adipocytes, we asked whether Bscl2 might also be involved in preadipocyte lineage commitment. To examine its possible role in the early pluripotent stem cell commitment to the preadipocyte lineage, we used a well-characterized murine mesenchymal stem cell line C3H10T1/2 as a model. BMP4, a member of the TGFβ superfamily, has been reported to induce commitment of the C3H10T1/2 cells to the preadipocyte lineage (16). We cultured these cells with and without BMP4 until 2 d after confluence (d 0). DMI was then added to induce differentiation. As shown in Fig. 2A, we observed no change in Bscl2 mRNA level in the 2-d postconfluent cells (d 0) with or without BMP4 treatment. The BMP4-induced commitment was corroborated by the stimulation of preadipocyte factor (Pref)-1 expression, a preadipocyte marker and a negative regulator of adipogenesis (22). As shown in Fig. 2A, Pref-1 mRNA level doubled in the BMP4-treated C3H10T1/2 cells at d 0. Further differentiation of these cells into mature adipocytes (d 5) after DMI addition achieved almost 100% differentiation and was marked by a 10-fold increase in Bscl2 mRNA in the BMP4-committed cells, whereas cells not treated with BMP4 showed a much smaller approximately 3-fold increase in expression (Fig. 2A). DMI addition caused only a small fraction (about 20%) of the cells to become adipocytes in non-BMP4-committed cells (Fig. 2B), resulting in a mild Pref-1 mRNA reduction (Fig. 2A, compare white bars). In contrast, in BMP4-committed cells, DMI treatment markedly lowered Pref-1 expression to essentially undetectable levels on d 5 (Fig. 2A, compare black bars) when about 100% of these cells became adipocytes (Fig. 2B). Therefore, BMP4-induced commitment of mesenchymal stem cells to the preadipocyte lineage did not affect Bscl2 mRNA expression until the terminal stages of adipocyte differentiation (d 5).
Figure 2.
Bscl2 is not induced and essential during the commitment of C3H10T1/2 mesenchymal stem cells to preadipocytes. A, Bscl2 and Pref-1 mRNA expression in d 0 preadipocytes and d 5 adipocytes with or without BMP4 treatment. Data are normalized to cyclophilin A and expressed as fold of expression compared with control at d 0 without BMP4 treatment. B, Oil-red O staining of d 8 adipocytes with or without BMP4 treatment. C, Bscl2 and Pref-1 mRNA expression were assayed by real-time RT-PCR in C3H10T1/2 cells stably infected with retroviruses expressing control shLuc or Bscl2 targeting shBs13a.in d 0 preadipocytes with or without BMP4 treatment. Data are normalized to cyclophilin A (ppia) and expressed as fold of expression compared with control at d 0 without BMP4 treatment. Data are means ± sd (n = 3). *, P < 0.05; **, P <0.005. D, Semiquantitative RT-PCR analyses of PPARγ expression in C3H10T1/2 cells stably infected with retroviruses expressing shLuc or shBs13a.in d 0 preadipocytes with or without BMP4 treatment. The amounts of cyclophilin A (ppia) are used as loading control. NS, Not significant.
We next performed loss-of-function experiments in C3H10T1/2 cells to examine whether basal expression of Bscl2 is required for BMP4- induced preadipocyte commitment. C3H10T1/2 cells were transduced with retroviruses expressing shRNA targeting murine Bscl2 (shBs13a); luciferase shRNA (shLuc) was used as control. Stably transduced cells were selected, expanded, and submitted to BMP4-induced commitment until 2 d after confluence. As shown in Fig. 2C (upper panel), basal Bscl2 mRNA expression was knocked down by about 90% in shBs13a cells compared with shLuc-transduced control cells with or without BMP4 treatment. Unexpectedly, in the absence of BMP4 treatment, we observed an approximately 4.7-fold up-regulation in Pref1 expression in shBs13a knockdown cells compared with control cells. BMP4 treatment increased Pref-1 expression to about the same degree, increasing its level in control cells by 2.8-fold and shBs13a knockdown cells by 2.3-fold (increased from 4.7 to 10.9, Fig. 2C, lower panel). Thus, judging by the similar relative change in the expression of preadipocyte marker Pref-1, Bscl2 knockdown did not appear to impact BMP4-induced commitment of mesenchymal stem cells. Meanwhile, the expression of the master transcription factor PPARγ, normally not expressed in mesenchymal stem cells, was increased to similar levels after BMP4 treatment in both control and Bscl2 knockdown cells (Fig. 2D), further implicating that Bscl2 knockdown in C3H10T1/2 cells has no effect in PPARγ induction during mesenchymal stem cell commitment to the preadipocyte lineage. We note that the elevated Pref-1 expression in knockdown cells in the absence of BMP4 was not observed in two other Bscl2 shRNA constructs (shBs15a and shBs16a), although a similar fold of induction of Pref-1 and PPARγ expression by BMP4 treatment was also obtained in the presence of these latter shRNAs (data not shown).
Knockdown of Bscl2 impairs standard hormone-induced adipogenesis in vitro
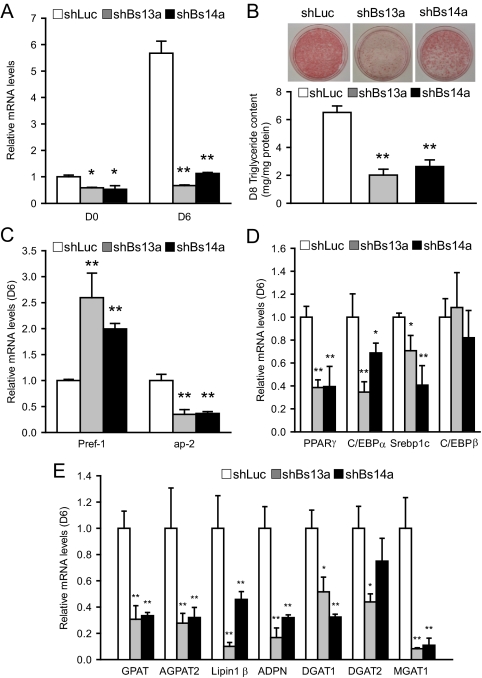
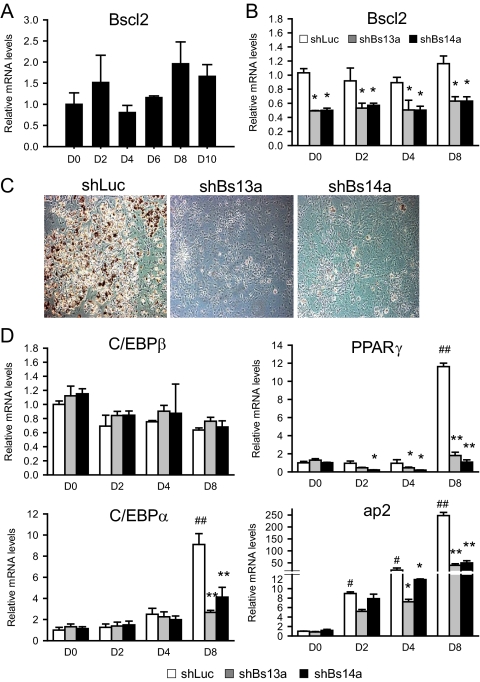
We next examined the effect of Bscl2 deficiency on adipocyte maturation in 3T3-L1 cells, a standard preadipocyte cell line. We transduced 3T3-L1 preadipocytes with retroviruses expressing shRNA targeting murine Bscl2 (shBs13a and shBs14a), using shLuc as control. Stably transduced cells were selected, expanded, and induced to differentiation by DMI. Baseline Bscl2 mRNA at d 0 in control 3T3-L1 cells remained relatively low until d 4 of DMI-induced adipogenesis (Fig. 1B). Basal Bscl2 mRNA was down-regulated by 42% (shBs13a) and 47% (shBs14a), respectively, on d 0. On d 6 after DMI treatment, the much higher level of Bscl2 mRNA was knocked down to a much greater extent, by 89% (shBs13a) and 80% (shBs14a), respectively (Fig. 3A). shLuc-treated control cells underwent DMI-induced adipose differentiation and triglyceride accumulation normally. In contrast, knockdown of Bscl2 expression led to marked inhibition of triglyceride accumulation, at about 30–40% of shLuc-treated control, as revealed both by oil-red O staining and direct measurement of triglyceride content on d 8 (Fig. 3B). The inhibition of triglyceride accumulation is also associated with a substantially higher expression of Pref-1, a preadipocyte marker and inhibitor of differentiation, and a significantly reduced expression of a mature adipocyte marker adipocyte protein 2 (ap2), both indications of markedly impaired differentiation in Bscl2-knockdown cells (Fig. 3C). The expression of three major adipogenic transcription factors PPARγ, C/EBPα, and adipocyte determination differentiation factor 1 (ADD1/sterol regulatory element-binding protein (Srebp)-1c) that are known to be important in 3T3-L1 differentiation was significantly suppressed at d 6, but no effect on C/EBPβ expression was detected (Fig. 3D). Knockdown of Bscl2 mRNA also significantly down-regulated the expression of genes involved in triglyceride synthesis, including glycerol phosphate acyltransferase, l-acylglycerol-3-phosphate-O-acyltransferase 2, lipin 1β, adiponutrin, diacylglycerol acyltransferase 1, and diacylglycerol acyltransferase 2 as well as monoacyglycerol acyltransferase 1 (Fig. 3E). Furthermore, Western blot analysis confirmed that, compared with control cells at d 8, Bscl2 knockdown cells led to down-regulation at the protein level of PPARγ as well as ap2, a direct target gene of PPARγ (supplemental Fig. S3). Together these data indicate that normal levels of Bscl2 are important for normal adipogenesis.
Figure 3.
Bscl2 knockdown reduces adipogenesis in 3T3-L1 cells. 3T3-L1 cells were stably infected with retroviruses expressing control shLuc or Bscl2 targeting shBs13a and shBs14a. A, Bscl2 mRNA was assayed by real-time RT-PCR, and values were expressed as fold of expression by normalizing to control cells at d 0 relative to cyclophilin A expression. B, Cells expressing shLuc, shBs13a, or shBs14a were differentiated and lipid accumulation assessed by oil-red O staining (upper panel) and quantitated by triglyceride analysis kit after lipid extraction (lower panel) on d 8. C–E, Expression of mRNA encoding mature adipocyte marker proteins, adipocyte transcription factors, and major triglyceride synthesis enzymes analyzed by real-time PCR in d 6 differentiated shLuc-, shBs13a-, and shBs14a-transduced cells. Data are means ± sd (n = 3). *, P < 0.05; **, P <0.005 vs. control cells at d 6. GPAT, Glycerol phosphate acyltransferase; AGPAT2, l-acylglycerol-3-phosphate-O-acyltransferase 2; ADPN, adiponutrin; DGAT1, diacylglycerol acyltransferase 1; DGAT2, diacylglycerol acyltransferase 2; MGAT1, monoacyglycerol acyltransferase 1.
PPARγ agonist rescues DMI-induced adipocyte differentiation in Bscl2 knockdown 3T3-L1 cells
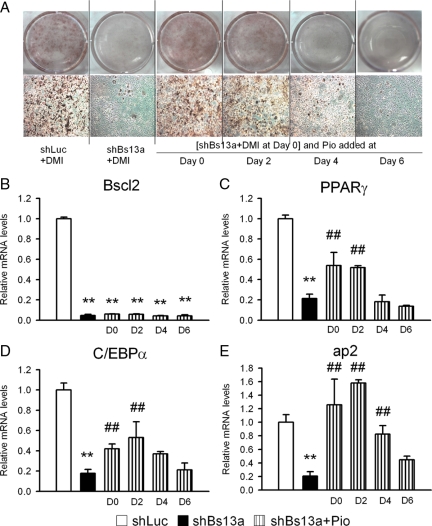
The expression of PPARγ, a master regulator of adipogenesis, was significantly inhibited in Bscl2-knockdown cells (Fig. 3D). Therefore, we examined whether activation of PPARγ by a PPARγ agonist, pio, would reverse the adipocyte differentiation blockade by Bscl2 knockdown. We added pio at different times after DMI treatment in 3T3-L1 cells stably expressing shBs13a. As in the last experiment, Bscl2 knockdown blocked DMI- induced adipocyte differentiation of 3T3-L1 preadipocytes as compared with control shLuc cells (Fig. 4A). Addition of pio at d 0 and 2 allowed the Bscl2-knocked-down 3T3-L1 cells to differentiate into triglyceride containing adipocytes on d 8 to a similar extent as shLuc cells (Fig. 4A). The timing of PPARγ activation was important because addition of pio at d 4 and 6 failed to rescue the differentiation as observed on d 8. Real-time PCR analysis of mRNA isolated from d 8 3T3-L1 cells showed that Bscl2 mRNA was effectively knocked down in all shBs13a-treated cells with or without pio treatment (Fig. 4B). Addition of pio at d 0 and 2 largely rescued the expression of PPARγ (Fig. 4C) and C/EBPα (Fig. 4D) and completely restored ap2 expression (Fig. 4E) to that of shLuc cells. Therefore, the expression of Bscl2 appears essential to the activation of PPARγ, but activation of the latter by an exogenous agonist can obviate the need for Bscl2 expression for DMI-induced differentiation of 3T3-L1 preadipocytes into adipocytes.
Figure 4.
Rescue of differentiation blockade of Bscl2 knockdown 3T3-L1 cells in the presence of insulin, dexamethasone, and IBMX (DMI) by exogenous PPARγ ligand pio. At d 0 (2 d of confluence), 3T3-L1 cells stably infected with shLuc and shBs13a retroviruses were treated with DMI. One micromole pio was added at d 0, 2, 4, or 6 of differentiation and kept at that level until d 8. A, At d 8, cells were stained with oil-Red-O and photographed under digital camera and microscopy. RNA were extracted at d 8 for real-time RT-PCR analysis of Bscl2 (B), PPARγ (C), C/EBPα (D), and ap2 (E). Data are means ± sd (n = 3). **, P < 0.005 vs. control cells at d 8; ##, P < 0.005 vs. shBs13a cells at d 8.
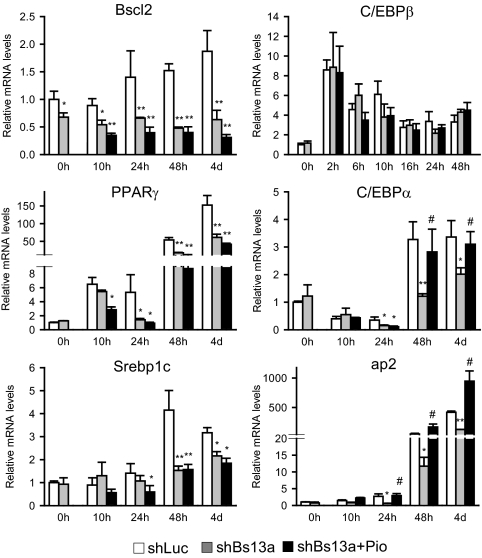
To gain additional insight on how Bscl2 knockdown affects normal adipogenesis and the role of PPARγ activation in restoring 3T3-L1 differentiation, we performed a detailed analysis of the time-dependent effect of Bscl2 knockdown on adipocyte gene expression profile during DMI induction. As shown in Fig. 5, Bscl2 expression was inhibited throughout the entire differentiation period in shBs13a-transduced cells whether the cells were treated with pio. Knockdown of Bscl2 had no effect at d 0 on the expression of any of genes examined. Induction of C/EBPβ was known to be an early event preceding and critical to the subsequent induction of the key adipocyte transcription regulators, PPARγ and C/EBPα (23). When Bscl2 knockdown cells were induced to differentiate for different times up to d 4, the early induction of C/EBPβ (0–48 h) was not different from shLuc control cells, and pio treatment had no significant effect on C/EBPβ expression at any time point. In contrast, shBs13a inhibition of PPARγ and C/EBPα as well as ap2 expression was evident as early as 24 h (d 1) after induction. By 48 h (d 2), pio rescued the mRNA expression of PPARγ direct target genes C/EBPα and ap2 to a level at or above that of control cells without restoring PPARγ’s own mRNA expression level through the positive feedback loop. In addition, the induction of Srebp1c mRNA was significantly inhibited from d 2 to d 4 in Bscl2 knockdown cells, and pio treatment failed to reverse the inhibition. Therefore, normal Bscl2 expression appears to be required for the optimal activation of the major transcription factor PPARγ that initiates the adipocyte differentiation transcription program. Despite the down-regulation of PPARγ expression in Bscl2 knockdown cells, its activation by an exogenously added ligand pio in the presence of DMI successfully drove the expression of its downstream target adipogenic genes, restoring normal adipogenesis in 3T3-L1 cells.
Figure 5.
Inhibition of PPARγ, C/EBPα, Srebp1c, and ap2 but not C/EBPβ induction by Bscl2 knockdown during early adipogenesis and pio rescue of PPARγ target gene expression in 3T3-L1 cells. Control cells or cells expressing Bscl2 targeting shBs13a without or with pio added at d 0 were induced to differentiate by DMI for different periods. The expression of mRNAs was determined by quantitative PCR for Bscl2, C/EBPβ, PPARγ, C/EBPα, Srebp1c, and ap2. Data were normalized to cyclophilin A mRNA expression and presented as fold of expression relative to d 0 control cells. Data are means ± sd (n = 3). *, P < 0.05; **, P < 0.005 vs. control cells at the same time point; #, P < 0.05 vs. control cells at d 0.
Normal Bscl2 expression is essential for DMI-independent PPARγ agonist-induced adipocyte differentiation
PPARγ activation alone in the absence of other standard hormones such as DMI has been shown to be sufficient to induce adipocyte differentiation (18,24,25). The capability of pio in rescuing adipocyte differentiation in Bscl2 down-regulated 3T3-L1 cells prompted us to examine whether PPARγ agonist-induced adipocyte differentiation is associated with changes in Bscl2 mRNA expression. Treatment with pio alone markedly up-regulated PPARγ mRNA expression (supplemental Fig. S4A) as well as that of its downstream target C/EBPα (supplemental Fig. S4B). It also led to a marked up-regulation of ap2 mRNA, a mature adipocyte marker (supplemental Fig. S4C). Throughout this differentiation process, there were only minor and inconsistent fluctuations in Bscl2 expression (Fig. 6A). These data indicate that PPARγ does not regulate Bscl2 expression. Therefore, up-regulation of Bscl2 expression appears not essential for the pio-induced differentiation of preadipocytes into mature adipocytes.
Figure 6.
Basal Bscl2 expression not increased but required during pio-induced adipogenesis. A, Bscl2 mRNA levels during differentiation in 3T3-L1 cells in the constant presence of exogenous pio (1 μm) in the absence of DMI. B, 3T3-L1 cells stably infected with retroviruses expressing shLuc, shBs13a, and shBs14a in the presence of pio (1 μm) and no DMI. RNAs were extracted at d 0, 2, 4, and 8 for real-time RT-PCR analysis of Bscl2. C, At d 8, cells were stained with oil-Red-O and photographed under microscopy (×100). D, Relative mRNA levels of C/EBPβ, PPARγ, and C/EBPα and mature adipocyte marker ap2 quantified by real-time RT-PCR, normalized to cyclophilin A mRNA expression and relative to d 0 control cells. Data are means ± sd (n = 3). *, P < 0.05; **, P <0.005 vs. shLuc control cells at each time point; #, P < 0.05; ##, P <0.005 vs. control cells at d 0.
We showed in Fig. 5 that knockdown of Bscl2 in 3T3-L1 preadipocytes attenuates the DMI-induced differentiation and the impaired differentiation could be rescued by addition of the PPARγ agonist pio. We next asked whether Bscl2 knockdown also blocks pio-induced differentiation in the absence of DMI. As shown in Fig. 6B, shBs13a and shBs14a each individually knocked down Bscl2 expression by about 50% during the entire period of pio-induced differentiation (from d 0 to d 8). Unexpectedly, we observed an essentially complete inhibition of pio-induced adipocyte differentiation in knockdown cells as compared with control shLuc cells (Fig. 6C). The mRNA expression of C/EBPβ was not changed at any of the each time points comparing Bscl2 knockdown cells with control cells (Fig. 6D). Although the basal mRNA level of PPARγ and its direct target genes C/EBPα and ap2 were not changed at d 0, expression of PPARγ and ap2 were mildly but significantly down-regulated at d 2 and d 4, and the expression of these two genes as well as of C/EBPα was markedly reduced in Bscl2-knockdown cells on d 8 (Fig. 6D). Therefore, even in the presence of a PPARγ agonist, pio, normal levels of basal Bscl2 expression appear to be essential for PPARγ activation and normal adipocyte differentiation in the absence of DMI.
Discussion
In this study, we demonstrate that Bscl2 is markedly induced during adipogenesis and appears to play a key role in the differentiation of 3T3-L1 cells into adipocytes. Knockdown of Bscl2 expression leads to down-regulation of the major transcription factors PPARγ and C/EBPα along with many other adipogenic genes including lipin 1 and AGPAT2 as well as other enzymes in triglyceride biosynthesis (21,26,27). Mutations in AGPAT2 cause BSCL type 1 lipodystrophy in humans (7), whereas lipin 1-deficient (fld) mice also develop lipodystrophy (28). Mutations at BSCL2 are known to be associated with a severe from of lipodystrophy; we hypothesized that lack of Bscl2 expression may interfere with the expression of key transcription factors, including PPARγ, and their downstream target enzymes that are involved in normal adipogenesis, producing a phenotype more severe than that seen with loss of AGPAT2 alone.
Recent studies in yeast suggest that Bscl2 may play a role in lipid droplet formation and maintenance (11,12). Whereas we cannot exclude such a possibility in mammalian cells, the data obtained from retrovirus transduced shRNA Bscl2 knockdown C3H10T1/2 cells and 3T3-L1 preadipocytes suggest that Bscl2 may not be involved in early mesenchymal stem cell commitment to preadipocytes; instead, Bscl2 appears crucial in the process of preadipocyte differentiation to mature adipocytes. In Bscl2 knockdown 3T3-L1 cells, the failure of PPARγ induction was evident as early as 24 h of adipocyte differentiation before lipid droplets made their appearance in these cells. Therefore, deficiency of Bscl2 leads to a defect in the adipogenic transcription program early during the differentiation of 3T3-L1 cells.
Based on a proposed model for key roles of several major transcription factors in adipogenesis, C/EBPβ and -δ are among the earliest factors to respond to adipogenic stimuli in a committed precursor cell; they in turn induce the expression of PPARγ and C/EBPα. The latter two sustain each other’s expression through a positive feedback loop and promote the establishment of the ultimate terminally differentiated phenotype (29,30,31). Our experiments demonstrate that a knockdown of Bscl2 in 3T3-L1 preadipocytes renders them deficient in expression of PPARγ, C/EBPα, Srebp1c, and ap2, key markers of adipocyte differentiation (Fig. 3), suggesting that Bscl2 plays an important role early in the adipogenesis differentiation program. Pio, a PPARγ agonist, can fully rescue DMI-induced adipogenesis in Bscl2 knockdown cells (Fig. 4), consistent with Bscl2 function being upstream or at the level of PPARγ activation. Bscl2 deficiency blunts the up-regulation of ADD1/Srebp1c (Fig. 5), which may compound or cause changes in adipogenic gene expression, ADD1/Srebp1c being implicated in the production of an endogenous ligand for PPARγ (32). However, it appears paradoxical that a PPARγ ligand can rescue DMI-induced differentiation but cannot by itself (in the absence of DMI) induce adipocyte differentiation in Bscl2 knockdown preadipocytes. Because the basal PPARγ level is not detectably changed in Bscl2 knockdown cells (Fig. 5), this defect is likely not the result of reduced PPARγ expression. We speculate that Bscl2 may act on some unidentified factor(s) that is required for ligand-dependent or independent PPARγ activation. When the hormonal inducers (DMI) are present, they may be able to act on the same factor(s) to compensate for the loss of Bscl2. Whether Bscl2 works mainly through ADD1/Srebp1c or other pathways in regulating PPARγ activation has yet to be determined.
Many factors required for PPARγ activation tend to go up within the first few hours after induction of differentiation, i.e. before PPARγ up-regulation (20,33,34,35,36). We observed an increase in Bscl2 only on d 4 of DMI induction, significantly lagging behind the increase in PPARγ expression (Fig. 1B). However, in Bscl2 knockdown cells, the activation of PPARγ by hormone cocktail DMI or PPARγ agonist pio alone are all blocked at as early as within 24 h (Figs. 5 and 6). This time line suggests that the normal basal expression of Bscl2 is required for endogenous or exogenous ligand-dependent or independent PPARγ activation. The increased Bscl2 expression that occurs at a later time during differentiation may not be essential for this process, although it may help reinforce or maintain the full activation of PPARγ at the later stages. Additional experiments are required to substantiate such an interpretation.
Our preliminary data suggest that Bscl2 by itself is not proadipogenic because ectopic expression of Bscl2 in 3T3-L1 cells in the presence of hormonal inducers does not further stimulate adipocyte marker expression or lipid accumulation compared with control cells (data not shown). However, exogenously expressed Bscl2 has been shown to readily form aggregates (10) (supplemental Fig. S2) possibly due to its high hydrophobicity, which may make the interpretation of overexpression experiments difficult.
We showed that PPARγ activation does not stimulate Bscl2 expression in the later time points of adipogenesis because PPARγ agonist-induced 3T3-L1 differentiation does not detectably alter Bscl2 expression (Fig. 6). Furthermore, ectopic expression of nuclear form ADD1/Srebp1c, C/EBPβ, and C/EBPα in mouse primary stromal vascular cells and NIH-3T3 cells also fails to stimulate Bscl2 expression (data not shown), suggesting that other additional factors are involved in up-regulating Bscl2 expression during adipogenesis. Thus, Bscl2 may function in a separate pathway regulated by as-yet-unidentified factors induced by hormonal inducers that may be necessary for the full activation of the master differentiation transcription factor PPARγ.
In this investigation, we have performed many different experiments to decipher the role of Bscl2 in adipocyte differentiation. While we were preparing this manuscript for publication, Payne et al. (37) reported a study in C3H10T1/2 mesenchymal stem cells that partially overlapped one of our experiments (presented in Fig. 2). Whereas their observations largely corroborate our findings, there are some major differences. First, we performed a much more extensive series of experiments in 3T3-L1 cells, a well-characterized preadipocyte cell line. We did include experiments in C3H10T1/2 cell to show that Bscl2 mRNA expression is not up-regulated during BMP4-induced mesenchymal stem cell commitment to the preadipocyte lineage (Fig. 2) and that up-regulation of Bscl2 expression happens only at the late stage of preadipocyte differentiation into adipocyte. Meanwhile, knockdown of Bscl2 in C3H10T1/2 cells has no major effect on lineage commitment. All these data pinpoints the role of Bscl2 in adipocyte maturation instead of lineage commitment. Second, we observed unique patterns of expression of key adipogenic genes in Bscl2 knockdown cells. Notably, basal expression of Srebp1c in Bscl2 knockdown 3T3-L1 cells was not suppressed until 48 h (Fig. 5), and activation and up-regulation of PPARγ was inhibited at both the early and late stages of adipocyte differentiation instead of only at the late stage of adipocyte differentiation, suggesting different mechanisms in different cell lines. Finally, we demonstrated that the differentiation blockade in Bscl2 knockdown 3T3-L1 cells could be overcome by addition of a PPARγ agonist. The consistent requirement for normal expression of Bscl2 in DMI-induced as well as PPARγ agonist-induced but DMI-independent adipocyte differentiation strongly suggests a key role of Bscl2 in the PPARγ activation. In a nutshell, this absolute requirement of Bscl2 for full activation of PPARγ supports a crucial role of Bscl2 in adipocyte development, providing a plausible explanation for the failure in adipose tissue formation in patients with CGL2.
Supplementary Material
Acknowledgments
We thank Dr. Minako Imamura for discussions and suggestions.
Footnotes
This work was supported by Grant HL-51586 from the National Institutes of Health (to L.C.), Grant P30DK079638 from the Diabetes and Endocrinology Research Center, the Betty Rutherford Chair in Diabetes Research from St. Luke’s Episcopal Hospital, and the T.T. and W.F. Chao Foundation. W.C. was supported, in part, by Mentor-based Postdoctoral Fellowship from American Diabetes Association No. 7-06-MN-10 and Postdoctoral Fellowship from American Heart Association No. 0825134 F.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 2, 2009
Abbreviations: ADD1, Adipocyte determination differentiation factor 1; ADM, adipocyte differentiation medium; AGPAT, acylglycerol phosphate acyltransferase; ap2, adipocyte protein 2; BMP, bone morphogenetic protein; Bscl, Berardinelli-Seip syndrome; C/EBP, CCAAT enhancer-binding protein; CGL, congenital generalized lipodystrophy; DMI, dexamethasone; eGFP, enhanced GFP; ER, endoplasmic reticulum; FBS, fetal bovine serum; GFP, green fluorescent protein; IBMX, isobutylmethylxanthine; pio, pioglitazone; PPAR, peroxisome proliferator-activated receptor; Pref, preadipocyte factor; shLuc, luciferase shRNA; shRNA, short hairpin RNA; Srebp, sterol regulatory element-binding protein.
References
- Berardinelli W 1954 An undiagnosed endocrinometabolic syndrome: report of 2 cases. J Clin Endocrinol Metab 14:193–204 [DOI] [PubMed] [Google Scholar]
- Seip M, Trygstad O 1996 Generalized lipodystrophy, congenital and acquired (lipoatrophy). Acta Paediatr Suppl 413:2–28 [DOI] [PubMed] [Google Scholar]
- Garg A, Wilson R, Barnes R, Arioglu E, Zaidi Z, Gurakan F, Kocak N, O'Rahilly S, Taylor SI, Patel SB, Bowcock AM 1999 A gene for congenital generalized lipodystrophy maps to human chromosome 9q34. J Clin Endocrinol Metab 84:3390–3394 [DOI] [PubMed] [Google Scholar]
- Magré J, Delépine M, Khallouf E, Gedde-Dahl Jr T, Van Maldergem L, Sobel E, Papp J, Meier M, Mégarbané A, Bachy A, Verloes A, d'Abronzo FH, Seemanova E, Assan R, Baudic N, Bourut C, Czernichow P, Huet F, Grigorescu F, de Kerdanet M, Lacombe D, Labrune P, Lanza M, Loret H, Matsuda F, et al. 2001 Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28:365–370 [DOI] [PubMed] [Google Scholar]
- Kim CA, Delépine M, Boutet E, El Mourabit H, Le Lay S, Meier M, Nemani M, Bridel E, Leite CC, Bertola DR, Semple RK, O'Rahilly S, Dugail I, Capeau J, Lathrop M, Magré J 2008 Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab 93:1129–1134 [DOI] [PubMed] [Google Scholar]
- Magré J, Delépine M, Van Maldergem L, Robert JJ, Maassen JA, Meier M, Panz VR, Kim CA, Tubiana-Rufi N, Czernichow P, Seemanova E, Buchanan CR, Lacombe D, Vigouroux C, Lascols O, Kahn CR, Capeau J, Lathrop M 2003 Prevalence of mutations in AGPAT2 among human lipodystrophies. Diabetes 52:1573–1578 [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A 2002 AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 31:21–23 [DOI] [PubMed] [Google Scholar]
- Simha V, Garg A 2003 Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes. J Clin Endocrinol Metab 88:5433–5437 [DOI] [PubMed] [Google Scholar]
- Lundin C, Nordström R, Wagner K, Windpassinger C, Andersson H, von Heijne G, Nilsson I 2006 Membrane topology of the human seipin protein. FEBS Lett 580:2281–2284 [DOI] [PubMed] [Google Scholar]
- Windpassinger C, Auer-Grumbach M, Irobi J, Patel H, Petek E, Hörl G, Malli R, Reed JA, Dierick I, Verpoorten N, Warner TT, Proukakis C, Van den Bergh P, Verellen C, Van Maldergem L, Merlini L, De Jonghe P, Timmerman V, Crosby AH, Wagner K 2004 Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat Genet 36:271–276 [DOI] [PubMed] [Google Scholar]
- Szymanski KM, Binns D, Bartz R, Grishin NV, Li WP, Agarwal AK, Garg A, Anderson RG, Goodman JM 2007 The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci USA 104:20890–20895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei W, Shui G, Gaeta B, Du X, Kuerschner L, Li P, Brown AJ, Wenk MR, Parton RG, Yang H 2008 Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol 180:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal AK, Garg A 2006 Genetic disorders of adipose tissue development, differentiation, and death. Annu Rev Genomics Hum Genet 7:175–199 [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Garg A 2004 Seipin: a mysterious protein. Trends Mol Med 10:440–444 [DOI] [PubMed] [Google Scholar]
- Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED 2007 Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol Cell Biol 27:743–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD 2004 Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA 101:9607–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Erickson RL, Gerin I, DeRose PM, Bajnok L, Longo KA, Misek DE, Kuick R, Hanash SM, Atkins KB, Andresen SM, Nebb HI, Madsen L, Kristiansen K, MacDougald OA 2002 Microarray analyses during adipogenesis: understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor α in adipocyte metabolism. Mol Cell Biol 22:5989–5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM 1994 mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8:1224–1234 [DOI] [PubMed] [Google Scholar]
- Reusch JE, Colton LA, Klemm DJ 2000 CREB activation induces adipogenesis in 3T3-L1 cells. Mol Cell Biol 20:1008–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Torrens JI, Anand A, Spiegelman BM, Friedman JM 2005 Krox20 stimulates adipogenesis via C/EBPβ-dependent and -independent mechanisms. Cell Metab 1:93–106 [DOI] [PubMed] [Google Scholar]
- Phan J, Péterfy M, Reue K 2004 Lipin expression preceding peroxisome proliferator-activated receptor-γ is critical for adipogenesis in vivo and in vitro. J Biol Chem 279:29558–29564 [DOI] [PubMed] [Google Scholar]
- Smas CM, Sul HS 1993 Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 73:725–734 [DOI] [PubMed] [Google Scholar]
- Darlington GJ, Ross SE, MacDougald OA 1998 The role of C/EBP genes in adipocyte differentiation. J Biol Chem 273:30057–30060 [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM 1994 Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79:1147–1156 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM 1995 A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 83:813–819 [DOI] [PubMed] [Google Scholar]
- Péterfy M, Phan J, Reue K 2005 Alternatively spliced lipin isoforms exhibit distinct expression pattern, subcellular localization, and role in adipogenesis. J Biol Chem 280:32883–32889 [DOI] [PubMed] [Google Scholar]
- Gale SE, Frolov A, Han X, Bickel PE, Cao L, Bowcock A, Schaffer JE, Ory DS 2006 A regulatory role for 1-acylglycerol-3-phosphate-O-acyltransferase 2 in adipocyte differentiation. J Biol Chem 281:11082–11089 [DOI] [PubMed] [Google Scholar]
- Péterfy M, Phan J, Xu P, Reue K 2001 Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet 27:121–124 [DOI] [PubMed] [Google Scholar]
- Elberg G, Gimble JM, Tsai SY 2000 Modulation of the murine peroxisome proliferator-activated receptor γ2 promoter activity by CCAAT/enhancer-binding proteins. J Biol Chem 275:27815–27822 [DOI] [PubMed] [Google Scholar]
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM 1999 Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell 3:151–158 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM 2000 Transcriptional regulation of adipogenesis. Genes Dev 14:1293–1307 [PubMed] [Google Scholar]
- Kim JB, Spiegelman BM 1996 ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev 10:1096–1107 [DOI] [PubMed] [Google Scholar]
- Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, Nakamura K, Nakamura T, Yamauchi T, Kubota N, Kadowaki T, Matsuki Y, Ogawa W, Hiramatsu R, Kasuga M 2005 Role of Kruppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem 280:12867–12875 [DOI] [PubMed] [Google Scholar]
- Hamm JK, Park BH, Farmer SR 2001 A role for C/EBPβ in regulating peroxisome proliferator-activated receptor γ activity during adipogenesis in 3T3-L1 preadipocytes. J Biol Chem 276:18464–18471 [DOI] [PubMed] [Google Scholar]
- Akerblad P, Lind U, Liberg D, Bamberg K, Sigvardsson M 2002 Early B-cell factor (O/E-1) is a promoter of adipogenesis and involved in control of genes important for terminal adipocyte differentiation. Mol Cell Biol 22:8015–8025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, Suzuki R, Kitamura T, Akira S, Kadowaki T, Nagai R 2005 Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab 1:27–39 [DOI] [PubMed] [Google Scholar]
- Payne VA, Grimsey N, Tuthill A, Virtue S, Gray SL, Dalla Nora E, Semple RK, O'Rahilly S, Rochford JJ 2008 The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes 57:2055–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.