Abstract
Exposure to endocrine-disrupting chemicals during development could alter the epigenetic programming of the genome and result in adult-onset disease. Methoxychlor (MXC) and its metabolites possess estrogenic, antiestrogenic, and antiandrogenic activities. Previous studies showed that fetal/neonatal exposure to MXC caused adult ovarian dysfunction due to altered expression of key ovarian genes including estrogen receptor (ER)-β, which was down-regulated, whereas ERα was unaffected. The objective of the current study was to evaluate changes in global and gene-specific methylation patterns in adult ovaries associated with the observed defects. Rats were exposed to MXC (20 μg/kg·d or 100 mg/kg·d) between embryonic d 19 and postnatal d 7. We performed DNA methylation analysis of the known promoters of ERα and ERβ genes in postnatal d 50–60 ovaries using bisulfite sequencing and methylation-specific PCRs. Developmental exposure to MXC led to significant hypermethylation in the ERβ promoter regions (P < 0.05), whereas the ERα promoter was unaffected. We assessed global DNA methylation changes using methylation-sensitive arbitrarily primed PCR and identified 10 genes that were hypermethylated in ovaries from exposed rats. To determine whether the MXC-induced methylation changes were associated with increased DNA methyltransferase (DNMT) levels, we measured the expression levels of Dnmt3a, Dnmt3b, and Dnmt3l using semiquantitative RT-PCR. Whereas Dnmt3a and Dnmt3l were unchanged, Dnmt3b expression was stimulated in ovaries of the 100 mg/kg MXC group (P < 0.05), suggesting that increased DNMT3B may cause DNA hypermethylation in the ovary. Overall, these data suggest that transient exposure to MXC during fetal and neonatal development affects adult ovarian function via altered methylation patterns.
Developmental exposure to the pesticide methoxychlor leads to DNA methylation changes in the ovary.
Recent reports describing the role of epigenetic mechanisms in the fetal and neonatal basis of adult disease have highlighted the concerns of the scientific community. The emerging threat of adverse environmental conditions such as improper nutrition, stressors, and endocrine-disrupting chemicals (EDCs) and the consequent damage to the epigenome resulting from such exposures has serious implications on human health. EDCs are of specific concern among the detrimental environmental factors because they are widespread in the environment (1,2,3,4,5,6). A sex- and stage-specific exposure to EDCs during early development could alter the epigenetic programming of the genome and result in adult-onset disease (4,7). Importantly, these effects can be epigenetically transmitted to the next generation (1,5,7). Furthermore, the observations that endocrine disruptors alter reproductive behaviors in generations that are not directly exposed to these compounds raise the possibility that EDCs have evolutionary and trans-population health implications (8).
Epigenetic mechanisms such as DNA methylation, histone modifications, and noncoding RNAs are heritable elements of DNA that influence gene expression without changing the gene sequence (9). DNA methylation is one of the most commonly studied epigenetic mechanisms involved in the regulation of gene expression and chromosomal stability (10,11). Alterations in DNA methylation patterns are implicated in several complex diseases including cancer (12,13,14). However, the full impact of epigenetic mechanisms in disease etiology is not completely understood.
Among the functions of the adult ovary are the interdependent processes of folliculogenesis and steroidogenesis, which are required for ovulation and corpus luteum formation. These processes occur through a dynamic bidirectional communication between the germ line and the supporting somatic cells (granulosa cells and theca cells). Many critical events in early follicular development such as oocyte nest breakdown, primordial follicle assembly, and the initial primordial to primary follicle transition occur between embryonic day (E) 19 and postnatal day (PND) 7 in female rats. When exposed to EDCs during their development, other organ systems including the mammary gland, prostate, uterus, and testis develop adult dysfunction associated with alterations in DNA methylation patterns (1,4,7,15). It is likely that similar defects could occur in the ovary (16). Importantly, female germ cell epigenetic reprogramming (i.e. remethylation of DNA) occurs during early ovarian development and could be perturbed by exposure to EDCs. Therefore, it is imperative to explore the possibility that EDCs lead to such epigenetic alterations in the ovary.
Two stages of early development, embryogenesis and gametogenesis, have heightened DNA methylation activity wherein DNA methylation patterns are actively erased and reestablished (epigenetic reprogramming) (17). Normal mammalian development requires the action of DNA methyltransferases (DNMTs) for the de novo establishment (DNMT3A and B) and maintenance (DNMT1) of DNA methylation within the genome. In addition, DNMT3L (DNMT3-like) that has similar sequence as DNMT3A and DNMT3B but lacking in enzymatic activity functions as a regulator of DNMT3A and DNMT3B (18,19). The expression level of these enzymes is highly regulated and peaks during specific stages of postnatal ovary development (20). Therefore, measurement of the levels of DNMTs can be used to assess the epigenetic status of the ovary.
Methoxychlor (MXC) was used in the United States as a replacement for dichlorodiphenyltrichloroethane for more than 50 yr and is a well-studied EDC (21). Whereas the use of MXC is currently restricted within the United States, its use in the rest of the world is unknown. The levels of MXC in the environment range from 160 mg/liter, or 160 ppm (in waters downstream from MXC sprayed areas), to 0.1 ng/kg·d, the latter being the Food and Drug Administration’s calculated average daily intake of MXC in adults (22). Studies of MXC’s effects in the adult ovary have shown that antral follicles are particular targets for its adverse actions (23,24), which are likely to be through an estrogen receptor (ER)-mediated pathway (25). MXC along with its metabolites possesses estrogenic, antiestrogenic, and antiandrogenic activities. EDCs with similar activities are common in the environment; therefore, MXC represents a model EDC (21). The MXC metabolite 2,2-bis-(p-hydroxyphenyl)-l,l,l-trichloroethane (HPTE) possesses the ability to bind to both ER subtypes and acts as an estrogen agonist when bound to ERα or an antagonist when bound to ERβ (26,27,28).
ERα and ERβ are nuclear receptors as well as steroid receptors that act as ligand-regulated transcription factors. Models of cooperation as well as competition between the two ERs have been proposed (29,30,31,32). The N-terminal A/B domain is the most variable region of ERα and ERβ sharing less than 20% amino acid identity in this region, indicating that this domain may contribute to ER subtype-specific actions on target genes. Both nuclear ER subtypes are expressed in the ovary during development as well as adulthood. Gene deletion studies in mice show that ERβ, expressed in the granulosa cells, plays a more significant role locally in the ovary. ERβ is known to be essential for FSH-directed granulosa cell differentiation as well as for LH responsiveness (33,34). ERα, expressed in the theca cells, is necessary for steroidogenesis and estrogen-mediated feedback in the hypothalamus and pituitary (35).
A recent study investigated the effects of transient exposure to environmentally relevant low and high doses of MXC during the periods of fetal and neonatal ovarian development (36). This report investigated several reproductive parameters including morphology and levels of key regulatory markers in the ovary. The high dose of MXC caused accelerated entry into puberty and the first estrus, increased irregular cyclicity, reduced litter sizes, and caused premature reproductive aging. In addition, the high dose of MXC collectively reduced the superovulatory response and serum progesterone and increased serum LH levels. We also found altered follicular composition, specifically, an increase in the number of preantral and early antral follicles and a reduced number of corpora lutea. In addition, this ovarian dysfunction was associated with a reduction in levels of ovarian ERβ expression along with reduced LH receptor and cytochrome P450 side-chain cleavage (36).
The objective of the current study is to evaluate changes in global and gene-specific methylation patterns that are associated with dysfunction in adult ovaries after a transient fetal and neonatal exposure to MXC.
Materials and Methods
Animals
Fischer (CDF) inbred rats were obtained from Charles River Laboratories (Wilmington, MA) to generate timed-pregnant females. The inbred strain was used because it has minimal polymorphisms, which facilitates the detection of treatment effects. The animals were maintained in a room with controlled illumination (lights on 0700–2100 h), temperature (26–28 C), and humidity (30–70%). Rats were given a soy-free diet rat chow (5V01, Lab Diet; PMI Nutrition International LLC, Brentwood, MO) and tap water ad libitum. The soy-free diet was given to reduce the quantity of phytoestrogens in the feed and to minimize background-level exposure to estrogenic compounds (37). All procedures were carried out in accordance with the guidelines of the Rutgers University Animal Care and Facilities Committee.
Treatments
Timed-pregnant females received two different treatment dosages of MXC (Sigma, St. Louis, MO): 20 μg/kg·d (low dose MXC) and 100 mg/kg·d (high dose MXC) in 1 ml/kg vehicle. Control animals received only vehicle (dimethyl sulfoxide-sesame oil; 1:2; control). The rats were treated for 12 d between E19 and PND7. On E19, three to five dams were randomly assigned to each treatment group. To precisely control the dosage, we used parenteral routes. The treatment was administered to the dams via an ip injection. The day of birth was designated as PND0. The litter size was culled to eight to ten offspring/dam. The female offspring were treated via sc injection daily from PND0 to PND7. The first injection was within the first 24 h after birth. No significant differences were observed in the weight of the animals at collection, suggesting no overt toxicity.
Tissue collection
On the proestrus day of the third regular cycle, PND50–60 animals were killed by decapitation and ovaries were collected. One ovary from each animal was snap frozen and stored at −80 C until further use, whereas the other ovary was fixed in Bouin’s solution. The frozen ovary was bisected and one half used for DNA and RNA extractions, respectively. Ovaries from five to six animals per treatment group were collected. At least three female offspring belonging to different litters were used in the following experiments/assays, except in control and 20 μg/kg·d MXC groups (see Fig. 4), in which two ovaries for each were used.
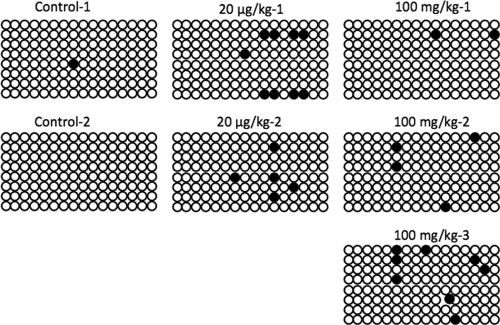
Figure 4.
Effect of MXC on CpG sites in PAPP-A gene in adult ovary. Bisulfite sequencing was conducted to examine the regions −566 to −329 and +8 to +300 upstream and downstream, respectively, from the transcription start site of the PAPP-A gene (shown above are data from the +8 to +300 group). Eight separate clones were analyzed from two or three animals from each treatment group. All predicted CpGs that were converted to thymine are shown as open circles and methylated cytosines that remain as cytosines are shown as filled circles. Although no changes were observed in the upstream region of the start site, numerous alterations were observed in the 16 CpGs in the +8 to +300 region. Only one predicted CpG site was methylated in control ovaries. However, numerous CpGs were methylated in ovaries exposed to 20 μg/kg·d and 100 mg/kg·d MXC.
Histology and immunohistochemistry
Histology and immunohistochemistry was performed as previously described (36). Briefly, sections were dewaxed in Citrisolv (d-limonene; Fisher catalog no. 04-355-121) and rehydrated in PBS for 10 min. After the antigen retrieval and blocking procedures, primary antibodies, anti-ERα or anti-ERβ (rabbit polyclonal antibodies, catalog no. sc-542 or PA1 310B; Santa Cruz Biotechnology, Santa Cruz, CA, or Affinity Bioreagents, Golden, CO, respectively) were diluted to 1:100 or 1:50 in 1% normal serum and applied to the sections for overnight incubation at room temperature. Biotinylated antirabbit secondary antibodies (Santa Cruz Biotechnology) were then added at a 1:200 dilution and incubated for 60 min at room temperature. Subsequently streptavidin-fluorescein isothiocyanate (catalog no. SA-5001; Vector Labs, Burlingame, CA) was added at 1:50 dilution.
All sections were also stained with ethidium homodimer (catalog no. E3599; Invitrogen, Carlsbad, CA) at 2 μg/ml as a nuclear counterstain (not shown) to identify the follicular cell type. Slides were mounted in Prolong Gold anti-fade reagent (catalog no. P36934; Invitrogen). Negative control sections were treated identically, except that the primary antibody was replaced with PBS (data not shown). Sections were observed under an Eclipse E800 microscope with epifluorescence attachments (Nikon, Tokyo, Japan). Images were acquired with a Nikon DXM1200F camera with ACT1 software (version 2), and minimal and equal adjustments for brightness on all images were made with Photoshop CS (Adobe, San Jose, CA). As previously described by Armenti et al. (36), mean staining intensity per unit area of selected structures for each marker was determined using Image J software (National Institutes of Health, http://rsb.info.nih.gov/ij/). The polygonal selection tool of the software was used to select the specific structures. The mean staining intensity was determined for granulosa cells (ERβ) or theca cells (ERα) of each follicle, excluding oocyte and antral space, in randomly selected preantral/early antral stage follicles for ERβ and large antral follicles for ERα. At least five follicles per animal (n = 3, total of 15 follicles per treatment group) were quantified in this manner.
Methylation-sensitive arbitrarily primed PCR (AP-PCR)
Global changes in the DNA methylation pattern of the genome of MXC-treated rats were identified by AP-PCR as previously described (1). Briefly, 4 μg of genomic DNA were digested with 10 U/μl each of RsaI alone (R), RsaI and methylation-sensitive HpaII (H), or RsaI and methylation-insensitive MspI (M) overnight at 37 C. This was followed by PCR using 10 primer sets designed to amplify methylation sites, as previously described (1,38). PCR products were separated using PAGE and visualized by SYBR green staining (Invitrogen). Hypo- or hypermethylation was determined by the relative band intensity using Kodak 1D Image Analysis software (Eastman Kodak Digital Sciences, New Haven, CT). The H-digested PCR products that were differentially amplified between control and MXC groups were isolated, reamplified, cloned (pGEM-T Easy Vector system; Promega, Madison, WI), and sequenced. The identity and chromosomal location was determined by using the basic local alignment sequence tool (BLAST; National Center for Biotechnology Information, United States National Library of Medicine, Bethesda, MD) in the rat Ensembl database.
Bisulfite treatments
Genomic DNA (5 μg) was digested with RsaI (10 U/μl) at 37 C overnight and then treated with bisulfite solution using a commercially available kit (EZ-DNA methylation kit; Zymo Research, Orange, CA) according to the manufacturer’s instructions. After purification, the bisulfite-converted DNA was used as a template for bisulfite-sequencing PCR (BSPCR) and methylation-specific PCR (MSPCR).
BSPCR
Sequence-specific primers to amplify the CpG-rich regions of interest were designed using the MethPrimer program (www. urogene.org/methprimer). PCR products were amplified and cloned (pGEM-T Easy; Promega). Clones were selected through blue-white screening. Five to eight clones were sequenced per animal per treatment (Genewiz, South Plainfield, NJ) to characterize the methylation status of the CpG sites in ERα and pregnancy-associated plasma protein-A (PAPP-A). Direct sequencing was conducted for ERβ with the same primers as used for PCR amplification. The peak heights were used to calculate the levels of methylation (C/C+T*100) at each predicted CpG.
MSPCR
Due to lack of robust bisulfite sequencing primers in the region of interest of rat ERβ exon 0N, methylation levels were measured using MSPCR. Primers were designed to amplify unmethylated alleles (UM) and methylated alleles (M) from both ERβ and Hprt (housekeeping gene) promoters. Levels of methylation were calculated based on the ratio of intensity of amplicons from PCRs amplifying methylated to unmethylated alleles as normalized to the Hprt amplicons. Primer sequences and conditions are presented in supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Semiquantitative RT-PCR for analysis of mRNA levels of Dnmt3a, Dnmt3b, and Dnmt3l
Two micrograms of RNA (extracted by the Trizol method; Invitrogen) were reverse transcribed using the ABI high-capacity-cDNA synthesis system (Applied Biosystems Inc., Foster City, CA). Samples were diluted to 20 ng and used for PCR. Primers to amplify Dnmt3a, Dnmt3b, Dnmt3l, and Hprt have been previously described, and all primer sets were adapted to rat Dnmts (details are included in Table 1). NIH Image J was used to quantitate the gel band intensities, and all values were normalized to the Hprt values. Samples were collected from three to four animals per treatment group.
Table 1.
Genes hypermethylated in adult ovary after MXC exposure between E19 and PND7
| Number | Ensembl gene Id | Potential candidate (abbreviation) | Chr | Function | References |
|---|---|---|---|---|---|
| 1 | ENSRNOG00000000142 | Plexin domain containing 2 (Plxdc2) | 17 | Integral membrane protein | (90,91) |
| 2 | ENSRNOG00000006711 | Zinc finger protein 212 (Zfp212) | 4 | Transcription factor | |
| 3 | ENSRNOG00000032822 | 60S Ribosomal subunit L29 (Rpl29) | 9 | Ribosomal protein | (92,93,94) |
| 4 | ENSRNOG00000033527 | Pregnancy associated plasma protein-A (PAPP-A) | 5 | Endopeptidase | (40,42,95,96) |
| 5 | ENSRNOG00000012167 | POU domain, class 4, transcription factor 2 (Pou4f2) | 19 | Transcription factor | (97) |
| 6 | ENSRNOG00000022804 | Forkhead box P4 (FoxP4) | 9 | Transcription factor | (98,99) |
| 7 | ENSRNOG00000006641 | Dopamine β-hydroxylase precursor (Dbh) | 3 | Dopamine biosynthesis | (44,86,87) |
| 8 | ENSRNOG00000000640 | Early growth response protein 2 (Egr2) | 20 | Transcription factor | (100) |
| 9 | ENSRNOG00000017177 | similar to Acidic ribosomal phosphoprotein P1 | 18 | Ribosomal protein | (101) |
| 10 | ENSRNOG00000032137 | Forkhead box B2 (Foxb2) | 1 | Transcription factor | (102,103) |
Chr, Chromosome.
Data analysis
The data were analyzed using one-way ANOVA for all experiments except for percent methylation of ERβ promoter region −625 to −392, in which two-way ANOVA was used. Statistically significant differences were determined using the Dunnett’s test for comparing to the vehicle-treated control or the Bonferroni test for multiple comparisons. GraphPad Prism graphing and analysis software (version 4a; GraphPad Software, Inc., San Diego, CA) was used for all statistical analyses. A statistically significant difference was confirmed at P < 0.05.
Results
ERα expression is unaffected by MXC exposure, whereas ERβ expression is down-regulated
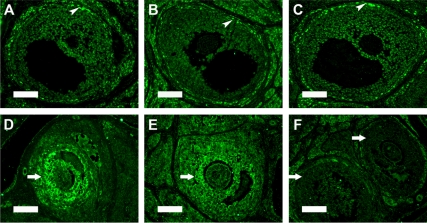
Previous studies from our laboratory demonstrated that MXC exposure during fetal and neonatal periods inhibits the levels of ERβ but has no effect on ERα in adult females. To confirm our previous observations (36) and also assess the status of estrogen receptors in adult ovarian follicles, we conducted immunohistochemical evaluation of ERα and ERβ expression. ERα was found predominantly in theca and interstitial cells surrounding all stages of follicles with intense expression in the corpora lutea of control ovaries (shown in large antral follicles, Fig. 1A). Mean staining intensity in theca cells was quantified using Image J software. Control ovaries were found to have 24.13 ± 13.34 (arbitrary intensity value; mean ± sd), and MXC treatment did not significantly alter the expression of ERα, as shown in Fig. 1, B and C (23.87 ± 7.39 in 20 μg/kg treated ovaries and 19.33 ± 6.96 in 100 mg/kg treated ovaries). On the other hand, ERβ expression was predominantly in the granulosa cells of multiple stages of follicles in the control ovaries, with a more intense staining in the preantral and early antral follicles (Fig. 1D, 36.71 ± 5.47). The 100 mg/kg MXC treatment caused a significant reduction in the levels of ERβ staining intensity in these follicles (Fig. 1F, 19.9 ± 1.06, P < 0.05), whereas the staining intensity in preantral and early antral follicles of 20 μg/kg-treated ovaries (Fig. 1E, 31.78 ± 5.22) had a trend to be reduced.
Figure 1.
Effects of MXC exposure on ERα and ERβ localization in adult ovaries. Shown are PND 50–60 ovarian sections with immunohistochemical staining for ERα and ERβ from rats treated with vehicle (control; A and D), 20 μg/kg MXC (B and E), or 100 mg/kg MXC (C and F) daily between E19 and PND7 (as described in Materials and Methods and also in Ref. 36). Staining with streptavidin-fluorescein isothiocyanate label (green) indicates the immunoreactivity for ERα and ERβ. ERα expression is unaffected (shown in theca cells in large antral follicles, arrowheads, A–C), whereas early antral follicles (shown in granulosa cells, arrows) have reduced ERβ expression in 100 mg/kg dose MXC-treated ovaries (F). Scale bar, 100 μm (A–C) and 50 μm (D-F); (n = 3–4 animals/treatment).
ERα and ERβ promoter regions are differentially methylated by transient MXC exposure
To determine the role of epigenetic regulation in ER expression in MXC-treated ovaries, we performed methylation profiling of the known promoters of ERα and ERβ genes.
We performed BSPCR and cloned a 140-bp PCR product from the ERα promoter spanning −186 to −45 and found no significant changes in the methylation patterns of the six CpG dinucleotides in this region (supplemental Fig. 1). This finding correlates with the lack of significant effects on the levels of ovarian ERα protein (Fig. 1, 36).
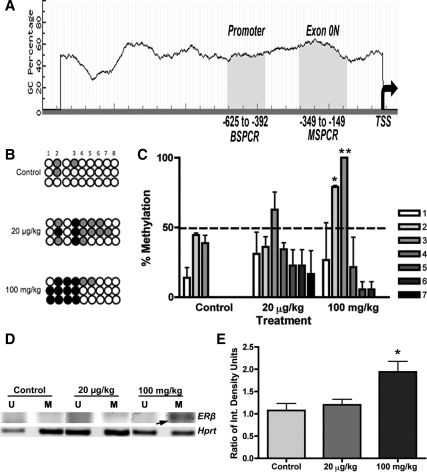
As shown in Fig. 2A, the promoter region of rat ERβ has two CpG islands that include important regulatory elements (39). Whereas one of these regions spans the canonical proximal promoter of ERβ (−625 to −392, CpG island 1), the other spans an untranslated exon region (−349 to −149, CpG island 2, exon 0N). In an effort to evaluate both CpG islands, two different but complementary approaches were taken: BSPCR and MSPCR as depicted in Fig. 2A.
Figure 2.
Effects of MXC on methylation of CpG sites in ERβ promoter region in adult ovaries. A, Bisulfite sequencing PCR (BSPCR) for region-625 to -392 (B, C) or methylation-specific PCR (MSPCR) for section -349 to -149, exon ON, (D, E) of ERβ promoter were performed as described in Materials and Methods. TSS, Transcription start site. CpG islands are shown in shaded area. B, PCR products were amplified with primers described in supplemental Table 1 and then directly sequenced. Peak heights were obtained from raw chromatograms, and percent cytosine (C) to thymine (T) conversions were calculated using the formula C/C+T*100 for each predicted CpG. All unmethylated cytosines that were converted to thymine are shown as open circles. Methylated cytosines are shown as filled circles (0–50%, gray; 50–100%, black circles). Whereas all predicted CpGs were methylated less than 50%, sites 2 and 3 were significantly hypermethylated in 100 mg/kg·d MXC-treated ovaries (C). Note that the non-CpG after site 2 consistently remained as cytosine. D, Levels of methylation were also measured using MSPCR for region −349 to −149, exon 0N. Primers were designed to amplify unmethylated alleles (U) and methylated alleles (M). Representative picture of PCR products is shown. E, One hundred milligrams per kilogram per day MXC (100 mg/kg) caused a significant increase in the levels of methylated (M) alleles in the ovary (arrow). *, P < 0.05; **, P < 0.01 (n = 3 animals/treatment). All experiments were repeated twice.
A 190-bp product spanning eight CpG dinucleotides was amplified with primers described in supplemental Table 1. PCR products were gel purified and directly sequenced. Bisulfite-treated DNA from three different animals from each treatment group was sequenced twice from separate bisulfite treatments. As shown in Fig. 2B (black filled circles) there were more hypermethylation events in the 100 mg/kg group. These methylation events were then quantified using the peak heights in the chromatograms. Significantly increased methylation was found in the CpG dinucleotides at sites 2 and 3 (P < 0.05 and 0.01, respectively) in the 100 mg/kg group (Fig. 2C). Interestingly, the cytosine preceding the CpG at site 3 was consistently unconverted to thymine suggesting steric hindrance.
To evaluate the levels of methylation in CpG island 2, MSPCR was conducted with primers described in supplemental Table 1. After normalization to the levels of unmethylated and methylated alleles in the Hprt gene (Fig. 2, D and E), we found significantly increased levels of methylation in the 100 mg/kg group (P < 0.05).
Global DNA methylation is altered by developmental MXC exposure in adult ovaries
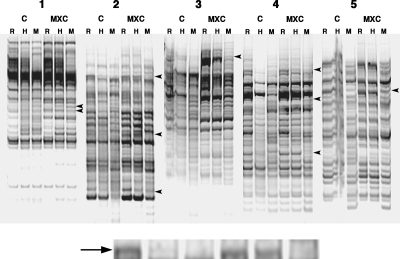
To further explore the role of epigenetic alterations on ovarian dysfunction, we assessed global DNA methylation changes using methylation-sensitive AP-PCR. To identify the maximum number of potential methylation events, we conducted AP-PCR with ovarian DNA obtained from control and MXC-treated (100 mg/kg) rats. Preliminary screening of H-digested PCR products revealed more than 25 alterations in methylation patterns. These PCR products were cut out, reamplified, and cloned. At least five to six clones were sequenced, and the sequences were matched to genes with 95–100% homology. After elimination of repeats and nonhomology matches, 10 genes were identified to be hypermethylated in ovaries from MXC-exposed rats (Fig. 3 and Table 1). Most of these proteins serve critical functions in the ovary (40,41,42,43,44,45). Among the candidate genes, PAPP-A is particularly relevant to the MXC-induced ovarian phenotype, i.e. a reduction in both follicular maturation and ovulation. Therefore, we analyzed PAPP-A using bisulfite sequencing (Fig. 4). We examined both the promoter and exon/intron regions for changes in methylation patterns. Our results demonstrated that whereas the promoter region (−566 to −329) was unchanged, the exon region (+8 to +300) of the PAPP-A gene was hypermethylated in ovaries from rats exposed to not only 100 mg/kg MXC but also 20 μg/kg MXC.
Figure 3.
Effects of MXC on the global DNA methylation in ovaries at PND 50–60. Methylation-sensitive AP-PCR was used. Four micrograms of ovarian genomic DNA were digested with RsaI (R) or RsaI with methylation-sensitive HpaII (H) or -insensitive MspI (M) restriction enzymes, followed by PCR with 10 degenerate primer sets designed to amplify methylation sites (five representative PCR product sets are shown, 1–5; each panel shows one set). C, Control; MXC, 100 mg/kg·d MXC. See Materials and Methods for details. The H-digested PCR products that were differentially amplified between control and MXC (arrowheads; enlarged inset, and arrow) were identified and listed as potential candidates for further investigation (Table 1); (n = 3 animals per treatment). All experiments were repeated three times.
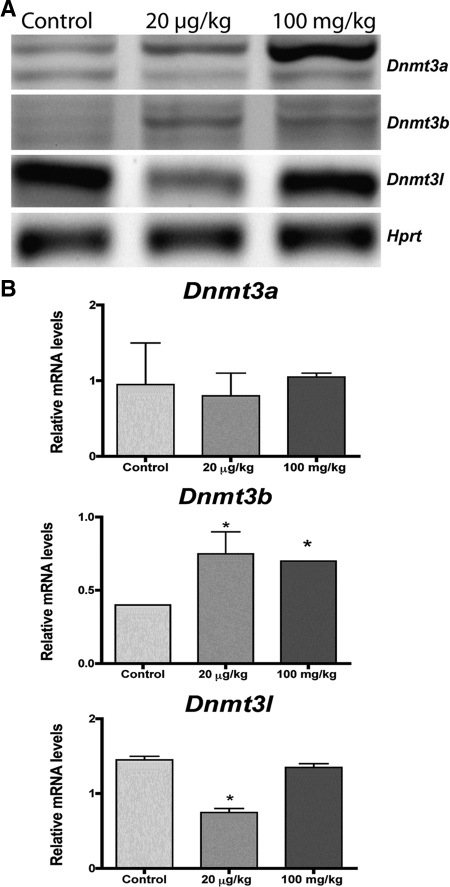
Developmental MXC exposure up-regulates expression of DNMTs in the adult ovary
De novo DNMTs are responsible for the addition of new methyl groups on DNA. To determine whether the MXC-induced gene-specific and global DNA hypermethylation is associated with increased DNMT levels, we measured the mRNA expression levels of Dnmt3a, Dnmt3b, and Dnmt3l in ovaries from control and MXC-treated (20 μg/kg and 100 mg/kg) rats between E19 and PND7 (Fig. 5A). Whereas Dnmt3a was unchanged, Dnmt3b expression was stimulated, nearly 2-fold, in ovaries of the 20 μg/kg and 100 mg/kg MXC groups (P < 0.05, Fig. 5B). Interestingly, the levels of Dnmt3l were reduced in the 20 μg/kg-treated ovaries and unchanged in 100 mg/kg dose ovaries.
Figure 5.
Effects of MXC exposure on the Dnmt expression pattern in adult ovaries. Ovaries from females that were treated with vehicle (control), 20 μg/kg·d MXC, or 100 mg/kg·d MXC between E19 and PND7 were collected on PND 50–60. RNA isolation and semiquantitative RT-PCR were performed as described in Materials and Methods. Housekeeping gene Hprt was used as an internal control. A representative gel is shown (A). Quantification (B) showed a significant stimulation of Dnmt3b by MXC treatment. *, P < 0.05 (n = 3–4 ovaries/treatment), and all PCRs were repeated three times. The error bars are not apparent in two groups in Dnmt3b graph due to small variation. The multiple bands in the Dnmt3a and 3b represent the different isoforms of each Dnmt.
Discussion
We previously demonstrated that a transient exposure to MXC during early development causes reduced fertility due to dysregulation of numerous ovarian genes, including ERβ (36). In addition, superovulation studies using exogenous gonadotropins in prepubertal females showed that 100 mg/kg MXC treatment reduced the number of eggs ovulated, suggesting a direct effect on the ovary. In the current study, we have provided evidence that there is a direct correlation between such an exposure and global and gene-specific hypermethylation in the adult ovary. This study is the first to show that DNA hypermethylation events, possibly via increased Dnmt expression, are associated with the changes in gene expression and dysfunction in the adult ovary.
Human epidemiological studies have shown that women exposed to EDCs such as agricultural pesticides (organochlorines) have prolonged/irregular estrous cycles and difficulty in achieving pregnancy, failed assisted reproductive technology attempts, and loss of pregnancies (46,47). Studies of female reproductive function after exposure to EDCs have documented defects such as aneuploidy in oocytes, formation of multioocyte follicles, and disruption of estrus cyclicity in rodent and other species, which collectively support the data obtained from the human epidemiological studies (16,48,49,50,51,52,53).
Pathologies of several organs (e.g. uterus, prostate, mammary gland, and cardiovascular tissues) are associated with alterations in DNA methylation patterns and the resulting changes in gene expression (13,54,55,56). For example, increased ERβ gene expression and decreased DNA methylation were observed in endometriotic tissues compared with normal endometrial tissues (57). Hypermethylation and reduced gene expression of ERβ were also observed in atherosclerosis and during vascular aging (58). Similar observations were made in breast and prostate cancers (59,60,61,62,63). The current study has confirmed that exposure to high dose MXC during fetal and neonatal periods dramatically down-regulates ERβ expression in the adult ovary due to pronounced hypermethylation in the promoter regions of ERβ. Our results indicating that ERα was unaffected suggest that MXC primarily acts via ERβ.
ERs are expressed in the neonatal stages during which MXC exposure occurred in the current study. Our unpublished observations have suggested that ERα is primarily expressed in the germinal epithelium, stromal cells, and theca cells in the rat ovary starting at PND 1. This pattern was disrupted by MXC treatment, and granulosa cells from MXC-treated ovaries had strong expression of ERα. In addition, intense immunoreactivity was observed in the oocytes in MXC-treated ovaries. These data are similar to previous studies using prepubertal mouse ovaries wherein there was ectopic expression of ERα on treatment with genistein, an estrogenic EDC (48). Furthermore, ERβ expression was intensely up-regulated in primary follicles by PND 7 in MXC-treated ovaries (Zama, A. M., J.E. Marano, and M. Uzumcu, unpublished data). The ligand-binding cavity of ERβ is significantly smaller (<20%) than that of ERα, and this may have implications for the selective affinity and pharmacology of ligands (64,65). Complicating this scenario is the existence of multiple isoforms of ERβ; ERβ1 is the most active and necessary isoform found in most ERβ-expressing tissues. ERβ 2-ERβ 5 have been found in multiple tissue and cell types (66,67). These isoforms have been found to heterodimerize with ERβ 1 for full activation. In addition, ERβ 2 has been found to be a dominant-negative isoform of ERβ that attenuates the activity of ERβ 1 (67,68,69,70,71). Our antibody against ERβ possibly detects all isoforms of ERβ. It would be valuable to identify the specific ERβ isoform(s) produced in MXC-treated ovaries due to the hypermethylation in the promoter region. It is quite possible that due to the misexpression of ERs, heterodimerization of hitherto noncoexpressing isoforms of ER can occur (72), resulting in the transactivation of noncanonical targets.
Another interesting possibility is that alterations in ER methylation patterns due to stress, such as the early exposure to MXC, potentiate the ovarian epigenome as a whole to further epigenetic changes (73,74) that are then either erased or fixed in the epigenome through later hormonal interplay (75,76,77). The 100 mg/kg MXC-treated ovaries had high levels of Dnmt3b, whereas Dnmt3l was unchanged. On the other hand, the 20 μg/kg-treated ovaries had high levels of Dnmt3b with low levels of Dnmt3l. Because DNMT3L modulates DNMT3B activity, these data potentially correlate with an overall increase in DNMT activity in the 100 mg/kg MXC-treated ovaries. It has been documented in different types of cancers that differential expression of the Dnmt genes, as seen in the current study, might contribute to the CpG island methylator phenotype wherein hypermethylation patterns are observed in clusters of genes related to the particular cancer (60,78,79,80,81,82). Detailed analysis of follicle stage-specific DNMT protein expression patterns, using immunohistochemistry, is necessary to further examine possible effects of MXC on DNMTs.
We have now identified candidate genes using AP-PCR. The majority of these genes were either transcription factors or ribosomal proteins; some of these have documented roles in ovarian function. One of the most promising results was that MXC caused hypermethylation of exon regions of the PAPP-A gene. PAPP-A is an IGF binding protein (IGFBP) endopeptidase that maintains IGFBP/IGF balance and is thus necessary for normal folliculogenesis (45). IGFs stimulate processes of FSH-directed follicle selection and final follicular maturation (41,83). Reduced PAPP-A expression due to increased methylation could limit its availability in follicles and thus increase IGFBP content and sequester IGF-I. This could lead to the observed defect in follicle selection and maturation (36). PAPP-A expression pattern is well documented in late stage antral follicles and mid to late corpora lutea in both humans and rodents (84,85). Our studies detected reduced luteal expression of PAPP-A in MXC-treated ovaries (not shown); however, studies to confirm the expression patterns after hormonal stimulation are pending. Another interesting candidate is the enzyme involved in dopamine biosynthesis, dopamine β-hydroxylase (Dbh). Catecholamines have been found in the oocyte (44) and norepinephrine has been found in the ovarian sympathetic nerve fibers. The function of catecholamines is essential for follicular maturation in the ovary (86,87). Potential alteration in the Dbh methylation profile could cause reduced dopamine production in the ovary, which also could lead to reduced follicular maturation. Whereas the candidate search using AP-PCR is not exhaustive, it provides a good starting point for revealing the effects of MXC on DNA methylation patterns. In the future, oocyte- vs. somatic cell-specific DNA methylation arrays can be conducted.
The physiological levels/ratio of its metabolites could define the overall activity of MXC as being estrogenic vs. antiestrogenic (88). Although the low dose (20 μg/kg·d) is higher than the estimated average daily intake (0.1 ng/kg·d) for adults (22), it is considered an environmentally relevant dose. We observed that the low dose leads to significant alterations in some ovarian parameters (36) and DNA methylation changes in the PAPP-A gene and a hypermethylation trend in the ERβ promoter (current study). Developmental exposure to this low level of MXC can lead to alterations in reproductive behavior and increase uterine growth (88,89). These described effects were observed after a short-term exposure to MXC done under controlled experimental conditions. Most humans are exposed to multiple EDCs during their lifetime. Therefore, the results of the low-dose exposure studies are worth pursuing with longer-term exposure protocols in conjunction with exposures involving combinations of EDCs.
Additional work is needed to fully understand the developmental epigenetic role of EDCs on adult ovarian dysfunction. Other studies should also investigate the possibility that these effects are transmitted to subsequent generations via epigenetic alterations in female germ cells because the exposure to MXC is occurring during the period of female germ cell epigenetic reprogramming. This understanding is likely to provide us with better prevention and treatment options against the abnormalities induced by EDCs, thus improving human health and reducing future health costs.
Supplementary Material
Acknowledgments
We thank Dr. Kathy Manger for her assistance in the preparation of this manuscript, Dr. Rob Zachow for his critical reading of the manuscript, and Justin George and Alison Caruana for technical assistance.
Footnotes
This work was supported by Grant ES013854 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 9, 2009
Abbreviations: AP-PCR, Arbitrarily primed PCR; BSPCR, bisulfite-sequencing PCR; DNMT, DNA methyltransferase; E, embryonic day; EDC, endocrine-disrupting chemical; ER, estrogen receptor; IGFBP, IGF binding protein; MSPCR, methylation-specific PCR; MXC, methoxychlor; PAPP-A, pregnancy-associated plasma protein-A; PND, postnatal day.
References
- Anway MD, Cupp AS, Uzumcu M, Skinner MK 2005 Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308:1466–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL 2007 Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA 104:13056–13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Bosagna CM, Sabat P, Valdovinos FS, Valladares LE, Clark SJ 2008 Epigenetic and phenotypic changes result from a continuous pre- and postnatal dietary exposure to phytoestrogens in an experimental population of mice. BMC Physiol 8:17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS 2006 Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66:5624–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN 2006 Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 147:S11–S17 [DOI] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M 2006 Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA 103:3480–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR 2004 Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol 199:142–150 [DOI] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK 2007 Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA 104:5942–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK 2007 Environmental epigenomics and disease susceptibility. Nat Rev Genet 8:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E 2002 Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 3:662–673 [DOI] [PubMed] [Google Scholar]
- Slotkin RK, Martienssen R 2007 Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet 8:272–285 [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Ohlsson R, Henikoff S 2006 The epigenetic progenitor origin of human cancer. Nat Rev Genet 7:21–33 [DOI] [PubMed] [Google Scholar]
- Esteller M 2007 Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet 8:286–298 [DOI] [PubMed] [Google Scholar]
- Egger G, Liang G, Aparicio A, Jones PA 2004 Epigenetics in human disease and prospects for epigenetic therapy. Nature 429:457–463 [DOI] [PubMed] [Google Scholar]
- Soto AM, Vandenberg LN, Maffini MV, Sonnenschein C 2008 Does breast cancer start in the womb? Basic Clin Pharmacol Toxicol 102:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette Jr LJ 2008 Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril 90:911–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J 2001 Epigenetic reprogramming in mammalian development. Science 293:1089–1093 [DOI] [PubMed] [Google Scholar]
- Lucifero D, Chaillet JR, Trasler JM 2004 Potential significance of genomic imprinting defects for reproduction and assisted reproductive technology. Hum Reprod Update 10:3–18 [DOI] [PubMed] [Google Scholar]
- La Salle S, Mertineit C, Taketo T, Moens PB, Bestor TH, Trasler JM 2004 Windows for sex-specific methylation marked by DNA methyltransferase expression profiles in mouse germ cells. Dev Biol 268:403–415 [DOI] [PubMed] [Google Scholar]
- Schaefer CB, Ooi SK, Bestor TH, Bourc'his D 2007 Epigenetic decisions in mammalian germ cells. Science 316:398–399 [DOI] [PubMed] [Google Scholar]
- Cummings AM 1997 Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol 27:367–379 [DOI] [PubMed] [Google Scholar]
- ATSDR 2002 Toxicological profile for methoxychlor. Atlanta: U.S. Department of Health and Human Services, Public Health Service [Google Scholar]
- Borgeest C, Miller KP, Gupta R, Greenfeld C, Hruska KS, Hoyer P, Flaws JA 2004 Methoxychlor-induced atresia in the mouse involves Bcl-2 family members, but not gonadotropins or estradiol. Biol Reprod 70:1828–1835 [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA 2005 Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci 88:213–221 [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA 2006 Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci 93:180–188 [DOI] [PubMed] [Google Scholar]
- Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, Safe S 1999 Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors α and β. Endocrinology 140:5746–5753 [DOI] [PubMed] [Google Scholar]
- Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S 2000 Interaction of methoxychlor and related compounds with estrogen receptor α and β, and androgen receptor: structure-activity studies. Mol Pharmacol 58:852–858 [PubMed] [Google Scholar]
- Waters KM, Safe S, Gaido KW 2001 Differential gene expression in response to methoxychlor and estradiol through ERα, ERβ, and AR in reproductive tissues of female mice. Toxicol Sci 63:47–56 [DOI] [PubMed] [Google Scholar]
- Zhao C, Matthews J, Tujague M, Wan J, Ström A, Toresson G, Lam EW, Cheng G, Gustafsson JA, Dahlman-Wright K 2007 Estrogen receptor β2 negatively regulates the transactivation of estrogen receptor α in human breast cancer cells. Cancer Res 67:3955–3962 [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA 2003 Estrogen signaling: a subtle balance between ER α and ERβ. Mol Interv 3:281–292 [DOI] [PubMed] [Google Scholar]
- Matthews J, Wihlén B, Tujague M, Wan J, Ström A, Gustafsson JA 2006 Estrogen receptor (ER) β modulates ERα-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol 20:534–543 [DOI] [PubMed] [Google Scholar]
- Papoutsi Z, Zhao C, Putnik M, Gustafsson JA, Dahlman-Wright K, 2009 Binding of estrogen receptor α/β heterodimers to chromatin in MCF-7 cells. J Mol Endocrinol 43:65–72 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS 2005 Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 146:3247–3262 [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Mayo KE 2005 To β or not to β: estrogen receptors and ovarian function. Endocrinology 146:3244–3246 [DOI] [PubMed] [Google Scholar]
- Armenti AE, Zama AM, Passantino L, Uzumcu M 2008 Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol 233:286–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger-Tong H, Murthy L, Chiappetta C, Kirkland JL, Goodwin B, Adlercreutz H, Stancel GM, Makela S 1998 A case of a laboratory animal feed with high estrogenic activity and its impact on in vivo responses to exogenously administered estrogens. Environ Health Perspect 106:369–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong CX, Mass MJ 2001 Both hypomethylation and hypermethylation of DNA associated with arsenite exposure in cultures of human cells identified by methylation-sensitive arbitrarily primed PCR. Toxicol Lett 122:223–234 [DOI] [PubMed] [Google Scholar]
- Zhang X, Leung YK, Ho SM 2007 AP-2 regulates the transcription of estrogen receptor (ER)-β by acting through a methylation hotspot of the 0N promoter in prostate cancer cells. Oncogene 26:7346–7354 [DOI] [PubMed] [Google Scholar]
- Kalli KR, Chen BK, Bale LK, Gernand E, Overgaard MT, Oxvig C, Cliby WA, Conover CA 2004 Pregnancy-associated plasma protein-A (PAPP-A) expression and insulin-like growth factor binding protein-4 protease activity in normal and malignant ovarian surface epithelial cells. Int J Cancer 110:633–640 [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Bondy CA, Zhou J, Monget P 2003 The insulin-like growth factor system: a key determinant role in the growth and selection of ovarian follicles? A comparative species study. Reprod Domest Anim 38:247–258 [DOI] [PubMed] [Google Scholar]
- Spicer LJ 2004 Proteolytic degradation of insulin-like growth factor binding proteins by ovarian follicles: a control mechanism for selection of dominant follicles. Biol Reprod 70:1223–1230 [DOI] [PubMed] [Google Scholar]
- Regev A, Goldman S, Shalev E 2005 Expression of plexin-B1 in the mouse ovary and its possible role in follicular development. Fertil Steril 84(Suppl 2):1210–1219 [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Smith GD, Danilchik M, Levine JE, Wolf DP, Dissen GA, Ojeda SR 1998 Oocytes are a source of catecholamines in the primate ovary: evidence for a cell-cell regulatory loop. Proc Natl Acad Sci USA 95:10990–10995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune JE, Rivera GM, Yang MY 2004 Follicular development: the role of the follicular microenvironment in selection of the dominant follicle. Anim Reprod Sci 82–83:109–126 [DOI] [PubMed] [Google Scholar]
- Fuortes L, Clark MK, Kirchner HL, Smith EM 1997 Association between female infertility and agricultural work history. Am J Ind Med 31:445–451 [DOI] [PubMed] [Google Scholar]
- Younglai EV, Holloway AC, Foster WG 2005 Environmental and occupational factors affecting fertility and IVF success. Hum Reprod Update 11:43–57 [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR 2002 Neonatal exposure to genistein induces estrogen receptor (ER)α expression and multioocyte follicles in the maturing mouse ovary: evidence for ERβ-mediated and nonestrogenic actions. Biol Reprod 67:1285–1296 [DOI] [PubMed] [Google Scholar]
- Jefferson W, Newbold R, Padilla-Banks E, Pepling M 2006 Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod 74:161–168 [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME 2007 Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 148:3580–3590 [DOI] [PubMed] [Google Scholar]
- Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ 2003 Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol 13:546–553 [DOI] [PubMed] [Google Scholar]
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA 2007 Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet 3:e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain DA, Guillette Jr LJ 1998 Reptiles as models of contaminant-induced endocrine disruption. Anim Reprod Sci 53:77–86 [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Cui H, Ohlsson R 2002 DNA methylation and genomic imprinting: insights from cancer into epigenetic mechanisms. Semin Cancer Biol 12:389–398 [DOI] [PubMed] [Google Scholar]
- Baylin SB, Esteller M, Rountree MR, Bachman KE, Schuebel K, Herman JG 2001 Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet 10:687–692 [DOI] [PubMed] [Google Scholar]
- Esteller M, Corn PG, Baylin SB, Herman JG 2001 A gene hypermethylation profile of human cancer. Cancer Res 61:3225–3229 [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE 2007 Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod 77:681–687 [DOI] [PubMed] [Google Scholar]
- Kim J, Kim JY, Song KS, Lee YH, Seo JS, Jelinek J, Goldschmidt-Clermont PJ, Issa JP 2007 Epigenetic changes in estrogen receptor β gene in atherosclerotic cardiovascular tissues and in vitro vascular senescence. Biochim Biophys Acta 1772:72–80 [DOI] [PubMed] [Google Scholar]
- Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC, Gustafsson JA, Dahlman-Wright K 2003 Expression of estrogen receptor β isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene 22:7600–7606 [DOI] [PubMed] [Google Scholar]
- Roll JD, Rivenbark AG, Jones WD, Coleman WB 2008 DNMT3b overexpression contributes to a hypermethylator phenotype in human breast cancer cell lines. Mol Cancer 7:15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F, Akahira J, Miura I, Suzuki T, Ito K, Hayashi S, Sasano H, Yaegashi N 2008 Loss of estrogen receptor β isoform expression and its correlation with aberrant DNA methylation of the 5′-untranslated region in human epithelial ovarian carcinoma. Cancer Sci 99:2365–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima D, Li LC, Dharia A, Perinchery G, Ribeiro-Filho L, Yen TS, Dahiya R 2001 CpG hypermethylation of the promoter region inactivates the estrogen receptor-β gene in patients with prostate carcinoma. Cancer 92:2076–2083 [DOI] [PubMed] [Google Scholar]
- Sasaki M, Tanaka Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto S, Dahiya R 2002 Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J Natl Cancer Inst 94:384–390 [DOI] [PubMed] [Google Scholar]
- Pike AC, Brzozowski AM, Walton J, Hubbard RE, Bonn T, Gustafsson JA, Carlquist M 2000 Structural aspects of agonism and antagonism in the oestrogen receptor. Biochem Soc Trans 28:396–400 [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engström O, Ohman L, Greene GL, Gustafsson JA, Carlquist M 1997 Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758 [DOI] [PubMed] [Google Scholar]
- Petersen DN, Tkalcevic GT, Koza-Taylor PH, Turi TG, Brown TA 1998 Identification of estrogen receptor β2, a functional variant of estrogen receptor β expressed in normal rat tissues. Endocrinology 139:1082–1092 [DOI] [PubMed] [Google Scholar]
- Okada A, Ohta Y, Buchanan DL, Sato T, Inoue S, Hiroi H, Muramatsu M, Iguchi T 2002 Changes in ontogenetic expression of estrogen receptor α and not of estrogen receptor β in the female rat reproductive tract. J Mol Endocrinol 28:87–97 [DOI] [PubMed] [Google Scholar]
- Ho SM, Leung YK, Chung I 2006 Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer. Ann NY Acad Sci 1089:177–193 [DOI] [PubMed] [Google Scholar]
- Leung YK, Mak P, Hassan S, Ho SM 2006 Estrogen receptor (ER)-β isoforms: a key to understanding ER-β signaling. Proc Natl Acad Sci USA 103:13162–13167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak P, Leung YK, Tang WY, Harwood C, Ho SM 2006 Apigenin suppresses cancer cell growth through ERβ. Neoplasia 8:896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda K, Miyaura C, Okada T, Shizuta Y 2002 Dietary bisphenol A prevents ovarian degeneration and bone loss in female mice lacking the aromatase gene (Cyp19). Eur J Biochem 269:2214–2222 [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- Fliss AE, Benzeno S, Rao J, Caplan AJ 2000 Control of estrogen receptor ligand binding by Hsp90. J Steroid Biochem Mol Biol 72:223–230 [DOI] [PubMed] [Google Scholar]
- Ruden DM, Xiao L, Garfinkel MD, Lu X 2005 Hsp90 and environmental impacts on epigenetic states: a model for the trans-generational effects of diethylstibesterol on uterine development and cancer. Hum Mol Genet 14(Spec No 1):R149–R155 [DOI] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM 2008 Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology 149:5922–5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Otsu E, Negishi H, Utsunomiya T, Arima T 2007 Aberrant DNA methylation of imprinted loci in superovulated oocytes. Hum Reprod 22:26–35 [DOI] [PubMed] [Google Scholar]
- Fortier AL, Lopes FL, Darricarrère N, Martel J, Trasler JM 2008 Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet 17:1653–1665 [DOI] [PubMed] [Google Scholar]
- Ahluwalia A, Hurteau JA, Bigsby RM, Nephew KP 2001 DNA methylation in ovarian cancer. II. Expression of DNA methyltransferases in ovarian cancer cell lines and normal ovarian epithelial cells. Gynecol Oncol 82:299–304 [DOI] [PubMed] [Google Scholar]
- Ahluwalia A, Yan P, Hurteau JA, Bigsby RM, Jung SH, Huang TH, Nephew KP 2001 DNA methylation and ovarian cancer. I. Analysis of CpG island hypermethylation in human ovarian cancer using differential methylation hybridization. Gynecol Oncol 82:261–268 [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterland RA, Dill AL, Webber MM, Waalkes MP 2007 Tumor suppressor gene inactivation during cadmium-induced malignant transformation of human prostate cells correlates with overexpression of de novo DNA methyltransferase. Environ Health Perspect 115:1454–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Shigematsu H, Shames DS, Sunaga N, Takahashi T, Shivapurkar N, Iizasa T, Minna JD, Fujisawa T, Gazdar AF 2007 Methylation and gene silencing of the Ras-related GTPase gene in lung and breast cancers. Ann Surg Oncol 14:1397–1404 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Dowdy SC, Xue A, Shujuan J, Eberhardt NL, Podratz KC, Jiang SW 2005 Opposite alterations of DNA methyltransferase gene expression in endometrioid and serous endometrial cancers. Gynecol Oncol 96:601–609 [DOI] [PubMed] [Google Scholar]
- deMoura MD, Choi D, Adashi EY, Payne DW 1997 Insulin-like growth factor-I-mediated amplification of follicle-stimulating hormone-supported progesterone accumulation by cultured rat granulosa cells: enhancement of steroidogenic enzyme activity and expression. Biol Reprod 56:946–953 [DOI] [PubMed] [Google Scholar]
- Rhoton-Vlasak A, Gleich GJ, Bischof P, Chegini N 2003 Localization and cellular distribution of pregnancy-associated plasma protein-a and major basic protein in human ovary and corpora lutea throughout the menstrual cycle. Fertil Steril 79:1149–1153 [DOI] [PubMed] [Google Scholar]
- Hourvitz A, Kuwahara A, Hennebold JD, Tavares AB, Negishi H, Lee TH, Erickson GF, Adashi EY 2002 The regulated expression of the pregnancy-associated plasma protein-A in the rodent ovary: a proposed role in the development of dominant follicles and of corpora lutea. Endocrinology 143:1833–1844 [DOI] [PubMed] [Google Scholar]
- Sotomayor-Zarate R, Dorfman M, Paredes A, Lara HE 2008 Neonatal exposure to estradiol valerate programs ovarian sympathetic innervation and follicular development in the adult rat. Biol Reprod 78:673–680 [DOI] [PubMed] [Google Scholar]
- Greiner M, Paredes A, Rey-Ares V, Saller S, Mayerhofer A, Lara HE 2008 Catecholamine uptake, storage, and regulated release by ovarian granulosa cells. Endocrinology 149:4988–4996 [DOI] [PubMed] [Google Scholar]
- Alworth LC, Howdeshell KL, Ruhlen RL, Day JK, Lubahn DB, Huang TH, Besch-Williford CL, vom Saal FS 2002 Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses. Toxicol Appl Pharmacol 183:10–22 [DOI] [PubMed] [Google Scholar]
- Palanza P, Morellini F, Parmigiani S, vom Saal FS 2002 Ethological methods to study the effects of maternal exposure to estrogenic endocrine disrupters: a study with methoxychlor. Neurotoxicol Teratol 24:55–69 [DOI] [PubMed] [Google Scholar]
- Miller SF, Summerhurst K, Rünker AE, Kerjan G, Friedel RH, Chédotal A, Murphy P, Mitchell KJ 2007 Expression of Plxdc2/TEM7R in the developing nervous system of the mouse. Gene Expr Patterns 7:635–644 [DOI] [PubMed] [Google Scholar]
- Mintz PJ, Cardó-Vila M, Ozawa MG, Hajitou A, Rangel R, Guzman-Rojas L, Christianson DR, Arap MA, Giordano RJ, Souza GR, Easley J, Salameh A, Oliviero S, Brentani RR, Koivunen E, Arap W, Pasqualini R 2009 An unrecognized extracellular function for an intracellular adapter protein released from the cytoplasm into the tumor microenvironment. Proc Natl Acad Sci USA 106:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn-Safran CB, Julian J, Fongemie JE, Hoke DE, Czymmek KJ, Carson DD 2002 Changes in the cytologic distribution of heparin/heparan sulfate interacting protein/ribosomal protein L29 (HIP/RPL29) during in vivo and in vitro mouse mammary epithelial cell expression and differentiation. Dev Dyn 223:70–84 [DOI] [PubMed] [Google Scholar]
- Julian J, Das SK, Dey SK, Baraniak D, Ta VT, Carson DD 2001 Expression of heparin/heparan sulfate interacting protein/ribosomal protein l29 during the estrous cycle and early pregnancy in the mouse. Biol Reprod 64:1165–1175 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheong D, Chan S, Hooi SC 1999 Heparin/heparan sulfate interacting protein gene expression is up-regulated in human colorectal carcinoma and correlated with differentiation status and metastasis. Cancer Res 59:2989–2994 [PubMed] [Google Scholar]
- Matsui M, Sonntag BB, Hwang SS, Byerly T, Hourvitz A, Adashi EY, Shimasaki S, Erickson GF 2004 Pregnancy-associated plasma protein-a production in rat granulosa cells: stimulation by follicle-stimulating hormone and inhibition by the oocyte-derived bone morphogenetic protein-15. Endocrinology 145:3686–3695 [DOI] [PubMed] [Google Scholar]
- Rivera GM, Fortune JE 2003 Selection of the dominant follicle and insulin-like growth factor (IGF)-binding proteins: evidence that pregnancy-associated plasma protein A contributes to proteolysis of IGF-binding protein 5 in bovine follicular fluid. Endocrinology 144:437–446 [DOI] [PubMed] [Google Scholar]
- Budhram-Mahadeo V, Parker M, Latchman DS 1998 POU transcription factors Brn-3a and Brn-3b interact with the estrogen receptor and differentially regulate transcriptional activity via an estrogen response element. Mol Cell Biol 18:1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel A, Wong EA, Mukhopadhyay M, Malik N, Westphal H 2003 FoxP4, a novel forkhead transcription factor. Biochim Biophys Acta 1627:147–152 [DOI] [PubMed] [Google Scholar]
- Li S, Weidenfeld J, Morrisey EE 2004 Transcriptional and DNA binding activity of the Foxp1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol Cell Biol 24:809–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano RL, Wilson AC 2003 HCF-1 functions as a coactivator for the zinc finger protein Krox20. J Biol Chem 278:51116–51124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artero-Castro A, Kondoh H, Fernández-Marcos PJ, Serrano M, Ramón y Cajal S, Lleonart ME 2009 Rplp1 bypasses replicative senescence and contributes to transformation. Exp Cell Res 315:1372–1383 [DOI] [PubMed] [Google Scholar]
- Hannenhalli S, Kaestner KH 2009 The evolution of Fox genes and their role in development and disease. Nat Rev Genet 10:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Schütz G, Monaghan AP 1996 Expression of the winged helix genes fkh-4 and fkh-5 defines domains in the central nervous system. Mech Dev 55:221–230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.