Gold catalysis has recently generated a variety of valuable methods for the synthesis of complex structures from simple starting materials.1 While the majority of efforts have focused on intramolecular rearrangement and addition reactions, a number transformations taking advantage of intermolecular reaction of the a gold-stabilized cationic intermediate generated from the 1,2-rearrangement of propargyl esters have been described.2 In these reactions, the cationic intermediate shows reactivity analogous to that reported for electrophilic metal-stabilized vinylcarbenoids.3-5 For example, we have shown that sulfoxides react with intermediate A to form carbonyl compounds (eq 1).5 On the basis of this reactivity, we postulated that allylgold intermediate B, generated by reaction of A with a nucleophile, could be induced to react with electrophiles. Herein, we report the realization of this goal leading to a convenient method for the construction of azepines.
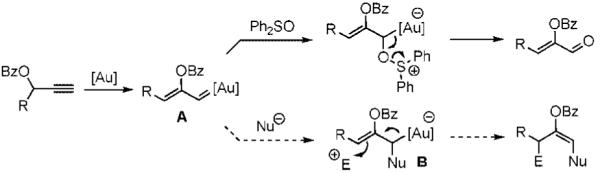 |
(1) |
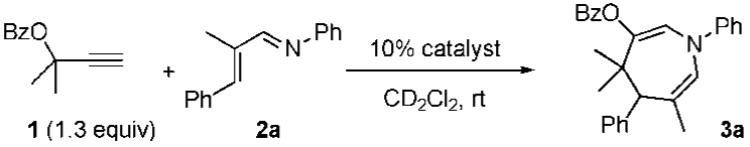
In analogy to related reactions of rhodium-stabilized vinylcarbenoids,6 we reasoned that generation of allylgold intermediate B and a proximate electrophile could be accomplished by reaction of A with a nucleophilic diene, such as an α,β-unsaturated imine. On the basis of this hypothesis, we were pleased to find that subjecting propargyl ester 1 and N-phenyl imine 2 to our typical conditions for cationic triphenylphosphinegold(I)-catalyzed reactions afforded a trace amount of azepine 3 (Table 1, entry 1). While changing the ligand from triphenylphosphine to an N-heterocyclic carbene only slightly improved the yield (entry 2), the use of 5 mol % of AuCl allowed for the formation of azepine 3 in 44% yield (Table 1, entry 3). On the basis of reports that suggest AuCl may form Au(III) species in situ,7 we subsequently examined Au(III) sources and were pleased to find that picolinic acid derived catalyst 4 catalyzed formation of the desired product with increased efficiency (65% yield, entry 5).8
Table 1.
Optimization of the Au-Catalyzed [4 + 3]-Cycloaddition
 | ||||
|---|---|---|---|---|
| entry | catalyst | time (h) | yield (%)a | |
| 1 | Ph3PAuCl + AgsbF6 | 24 | 6 |  |
| 2 | IMesAuCl + AgSbF6 | 24 | 17 | |
| 3 | AuCl | 4 | 44 | |
| 4 | AuCl3 | 4 | 33 | |
| 5 | PicAuCl2 (5%) | 2 | 65 | |
By 1H NMR versus an internal standard.
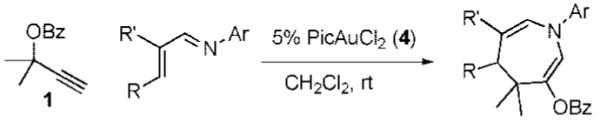
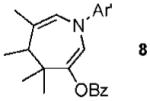
With conditions in hand, we examined the scope of the gold-catalyzed [4 + 3]-cycloaddition (Table 2). In general, the highest yields were obtained with substrates containing electron-rich N-aryl groups on the imine nitrogen (entries 1-5). On the other hand, the reaction proved highly tolerant of variation at the other positions of the unsaturated imine component. For example, having the olefin conjugated with electron-rich and electron-deficient aryl groups had little impact on the yield of the cycloaddition (entries 6 and 7). The olefin substituents can also be aliphatic. For example, imine 9 underwent chemoselective [4 + 3]-cycloaddition to afford 10 in 60% yield without cyclopropanation of the isolated alkene (entry 9). Additionally, gold-catalyzed cycloaddition of vinyl bromide 11 produced a 63% yield of bromoazepine 12, a potential cross-coupling partner (entry 10).
Table 2.
[4 + 3]-Cycloaddition of α,β-Unsaturated Imines
 | |||
|---|---|---|---|
| entry | imine | azepine | Yield |
| 1 | 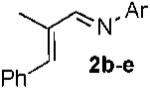 |
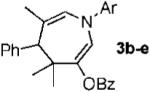 |
b Ar = 4-HO-2,6-Me2-C6H2 87% |
| 2 | c Ar = 2,6-Me2-C6H3 80% | ||
| 3 | d Ar = 2,3-Me2-C6H3 88% | ||
| 4 | e Ar = 4-MeO-C6H4 65% | ||
| 5 | f Ar = 4-F-C6H4 55% | ||
| 6 | 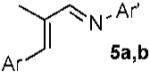 |
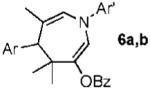 |
a Ar = 4-NO2C6H4 70% |
| 7 | b Ar = 4-MeOC6H4 80% | ||
| 8 | 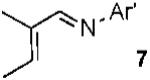 |
 |
65% |
| 9 | 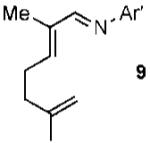 |
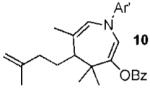 |
62% |
| 10 | 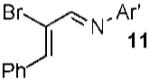 |
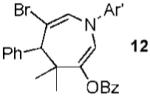 |
63% |
Conditions: 1.3 equiv of 1, 5% 4, CH2 Cl2, rt. Ar’ = 4-HO-2,6-Me2-C6H2.
We next turned to examine the scope of the propargyl ester component of the cycloaddition (Table 3). With secondary benzylic propargyl esters 13 and 15, the reactions provided azepine products 14 and 16 in good yields and as single diastereomers (entries 1 and 2).9 Tertiary propargyl esters also participated in the cycloaddition addition, smoothly affording all-carbon quaternary centers in azepines 18 and 20, albeit with diminished diastereocontrol (entries 3 and 4). Similarly, tert-butylcyclohexanone derived ester 21 underwent the gold-catalyzed cycloaddition to generate 22 with 2.5:1 dr with respect to the axial stereocenter (entry 5).
Table 3.
Diastereoselective Transformations of Propargyl Esters
 | ||||
|---|---|---|---|---|
| entry | propargyl ester | imine | azepine | yield (dr)a |
| 1 | 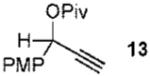 |
5a | 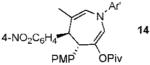 |
99% (> 20:1) |
| 2 | 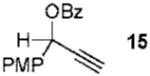 |
11 | 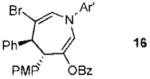 |
73% (> 20:1) |
| 3 | 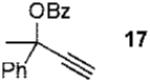 |
2b | 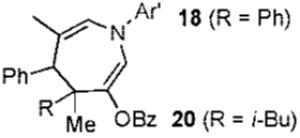 |
73% (3.3:1) |
| 4 | 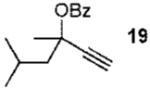 |
2b | 83% (1.4:1) | |
| 5 | 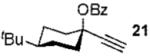 |
2b | 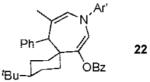 |
58%b (2.5:1) |
Conditions: 1.3 equiv of propargyl ester, 5% 4, CH2Cl2, rt.
Conditions: 2 equiv of propargyl ester, 10% 4, dichloromethane, 60 °C. Ar’ = 4-HO-2,6-Me2-C6H2.
A proposed mechanism that accounts for this diastereoselectivity is detailed in Scheme 1. Gold-promoted isomerization of the propargyl ester leads to gold-carbenoid intermediate A.10,11 Subsequent nucleophilic addition of the imine nitrogen generates allylgold intermediate 23 that undergoes intramolecular nucleophilic addition onto the pendant iminium electrophile via transition state 24.
Scheme 1.
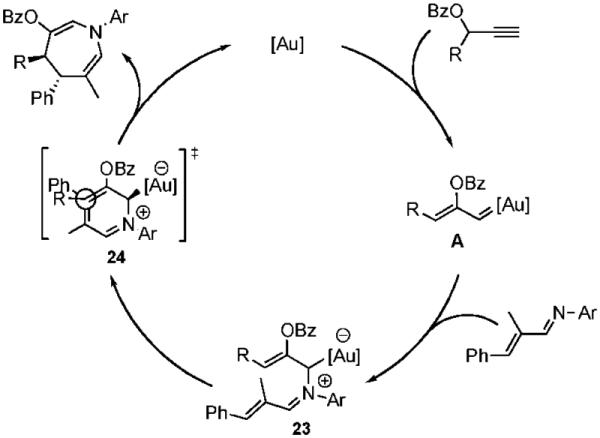
Mechanistic Hypothesis
Additional studies revealed that electron-donating substituents on the N-aryl and β-aryl groups enhance the rate of the gold-catalyzed cycloaddition, supporting a stepwise mechanism in which formation of iminium 23 is rate-determining.12 On the basis of this observation, we envisioned that heteroaryl imines might also serve as heterodienes in the gold-catalyzed [4 + 3]-cycloaddition. We were pleased to find that indole azepine 26 was formed from the gold-catalyzed cycloaddition of 1 with imine 25, albeit at slightly elevated temperatures and increased catalyst loading (eq 2). On the other hand, quinoline imine 27 underwent gold-catalyzed coupling with propargyl ester 1 to furnish tricyclic azepine 28 in 93% yield at room temperature (eq 3).
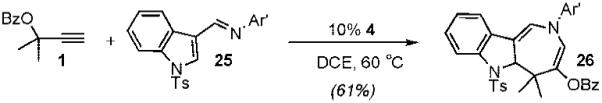 |
(2) |
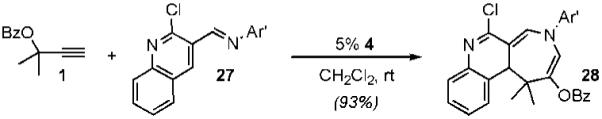 |
(3) |
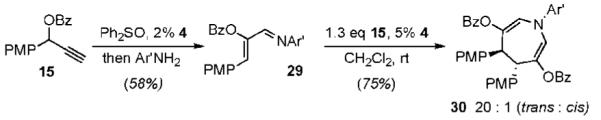 |
(4) |
In conclusion, we have developed a Au(III)-catalyzed synthesis of azepines via the annulation of simple, readily available starting materials. This is exemplified by the fact that both components employed in the cycloaddition reaction to form azepine 30 can be generated from gold-catalyzed rearrangements of propargyl ester 15 (eq 4). In addition to representing a rare example of a Au-catalyzed intermolecular annulation reaction,13 the [4 + 3]-cycloaddition highlights the generation and subsequent electrophilic trapping of an allyl-gold intermediate from gold-stablized vinylcarbenoid A. The development of reactions that take advantage of this mechanistic paradigm is ongoing in our laboratories and will be reported in due course.
Supplementary Material
Acknowledgment
We gratefully acknowledge NIHGMS (RO1 GM073932), Merck Research Laboratories, Bristol-Myers Squibb, Amgen Inc., and Novartis for funding. N.D.S. thanks Eli Lilly for a graduate fellowship.
References
- (1).For recent reviews of gold-catalyzed reactions, see:Jiménez-Nunez E, Echavarren AM. Chem. Commun. 2007:333. doi: 10.1039/b612008c.Gorin DJ, Toste FD. Nature. 2007;446:395. doi: 10.1038/nature05592.Furstner A, Davies PW. Angew. Chem., Int. Ed. 2007;46:3410. doi: 10.1002/anie.200604335.Hashmi ASK. Chem. Rev. 2007;107:3180. doi: 10.1021/cr000436x.Shen HC. Tetrahedron. 2008;64:3885.
- (2).(a) Marion N, Nolan SP. Angew. Chem., Int. Ed. 2007;46:2750. doi: 10.1002/anie.200604773. [DOI] [PubMed] [Google Scholar]; (b) Correa A, Marion N, Fensterbank L, Malacria M, Nolan SP, Cavallo L. Angew. Chem., Int. Ed. 2008;47:718. doi: 10.1002/anie.200703769. [DOI] [PubMed] [Google Scholar]
- (3).(a) Miki K, Ohe K, Uemura S. J. Org. Chem. 2003;68:8505. doi: 10.1021/jo034841a. [DOI] [PubMed] [Google Scholar]; (b) Johansson MJ, Gorin DJ, Staben ST, Toste FD. J. Am. Chem. Soc. 2005;127:18002. doi: 10.1021/ja0552500. [DOI] [PubMed] [Google Scholar]; (c) Gorin DJ, Dube P, Toste FD. J. Am. Chem. Soc. 2006;128:14480. doi: 10.1021/ja066694e. [DOI] [PubMed] [Google Scholar]; (d) Gorin DJ, Watson IDG, Toste FD. J. Am. Chem. Soc. 2008;130:3736. doi: 10.1021/ja710990d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Amijs CHM, López-Carrillo V, Echavarren AM. Org. Lett. 2007;9:4021. doi: 10.1021/ol701706d. [DOI] [PubMed] [Google Scholar]; (b) Davies PW, Albrecht SJ-C. Chem. Commun. 2008:238. doi: 10.1039/b714813e. [DOI] [PubMed] [Google Scholar]
- (5).Witham CA, Mauleón P, Shapiro ND, Sherry BD, Toste FD. J. Am. Chem. Soc. 2007;129:5838. doi: 10.1021/ja071231+. [DOI] [PubMed] [Google Scholar]
- (6).Doyle MP, Hu W, Timmons DJ. Org. Lett. 2001;3:3741. doi: 10.1021/ol016703i.Doyle MP, Yan M, Hu W, Gronenberg LS. J. Am. Chem. Soc. 2003;125:4692. doi: 10.1021/ja029745q.Davies HML, Hu B, Saikali E, Bruzinski PR. J. Org. Chem. 1994;59:4535.For a related reaction of Fischer carbenes, see:Barluenga J, Toḿas M, Ballesteros A, Santamaria J, Carbajo RJ, López-Ortiz F, Garcia-Granda S, Pertierra P. Chem.—Eur. J. 1996;2:88.Barluenga J, Tomás M, Rubio E, Lopez-Pelegrin JA, Garcia-Granda S, Priede MP. J. Am. Chem. Soc. 1999;121:3065.Barluenga J, Tomás M, Rubio E, Lopez-Pelegrin JA, Garcia-Granda S, Priede MP. J. Am. Chem. Soc. 1999;121:3065.
- (7).Lemiere G, Gandon V, Agenet N, Goddard JP, de Kozak A, Aubert C, Fensterbank L, Malacria M. Angew. Chem., Int. Ed. 2006;45:7596. doi: 10.1002/anie.200602189. [DOI] [PubMed] [Google Scholar]
- (8).(a) Hashmi ASK, Weyrauch JP, Rudolph M, Kurpejovic E. Angew. Chem., Int. Ed. 2004;43:6545. doi: 10.1002/anie.200460232. [DOI] [PubMed] [Google Scholar]; (b) Hashmi ASK, Kurpejovic E, Wölfle M, Frey W, Bats JW. Adv. Synth. Catal. 2007;349:1743. [Google Scholar]
- (9).The trans-diaryl stereochemistry, which is opposite to that produced in related rhodium-catalyzed cycloadditions,6a was established by an X-ray crystal structure (see Supporting Information) of 13.
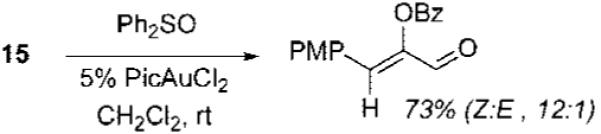
- (10).As in the intermolecular cyclopropanation of these intermediates,3b chirality was not transferred in the cycloaddition of enantioenriched propargyl ester 15 with imine 2b (see Supporting Information).
-
(11).The observation that the E/Z-selectivities obtained by trapping with sulfoxides are not identical to the diastereoselectivity observed in the cycloaddition suggests that A is formed reversibly2b and reacts with nucleophile dependent selectively (see Supporting Information).
- (12).See Supporting Information for details.
- (13).For additional examples of intermolecular gold-catalyzed annulations, see:Melhado AD, Luparia M, Toste FD. J. Am. Chem. Soc. 2007;129:12638. doi: 10.1021/ja074824t.Hsu Y-C, Datta S, Ting C-M, Liu R-S. Org. Lett. 2008;10:521. doi: 10.1021/ol7030334.Zhang G, Huang X, Li G, Zhang L. J. Am. Chem. Soc. 2008;130:1814. doi: 10.1021/ja077948e.Barluenga J, Fernández-Rodríguez MA, García-García P, Aguilar E. J. Am. Chem. Soc. 2008;130:2764. doi: 10.1021/ja7112917.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


