In July 1998 a six year old boy with insulin dependent diabetes presented one year after diagnosis with an urticarial rash involving all insulin injection sites. The pruritic rash started 10 minutes after injection of human Mixtard 30 (Novo Nordisk) in the arm. He was otherwise well. The rash occurred at injection sites on the arms, thighs, and buttocks (not used for two months), which disappeared spontaneously within 12 hours. Subsequently in September 1998 (figure), December 1998, and February 1999 he had milder episodes soon after insulin injection. When the insulin was changed to Humulin M3 (Lilly) he had three urticarial reactions in the first two weeks and further reactions in September 1999 and April 2000. The mean insulin dose was 0.61 units/kg/day. There was no history of atopy.
His mean haemoglobin A1c was 8.0%, and the blood count was normal. Tests for insulin specific IgE gave negative results but there was a high titre for insulin specific IgG. Tests for reactions to the constituents of Humulin M3 and Mixtard 30 were not performed.
The Committee on Safety of Medicines has received one report of rapid onset itching and erythema at all previous injection sites. This concerned a 68 year old woman treated with Protophane (now called Insulatard; Novo Nordisk), Velosulin (Novo Nordisk), and enalapril.
To my knowledge this is the first published report in a child of a human insulin preparation causing intermittent urticaria simultaneously affecting only previous injection sites. The boy had never received animal insulin and there had been no interruption in his treatment. Mixtard 30 and Humulin M3 are identical in composition (human insulin, m-cresol, zinc oxide, sodium hydroxide, hydrochloric acid, sodium phosphate, phenol, and protamine) except for glycerol in Humulin M3. It seems likely that this reaction was mediated by insulin specific IgE fixed in mast cells located at insulin injection sites. Zinc allergy is unlikely as it is associated with delayed reactions.1 Reaction to protamine is another possible explanation, with intermittent rash occurring as a result of variation in protamine concentration owing to incomplete mixing in the injection device.2,3
This rare insulin reaction initially caused major concern because of the uncertain prognosis. Two years after onset any urticarial rashes are treated by the boy's parents with antihistamine (chlorpheniramine).
Figure.
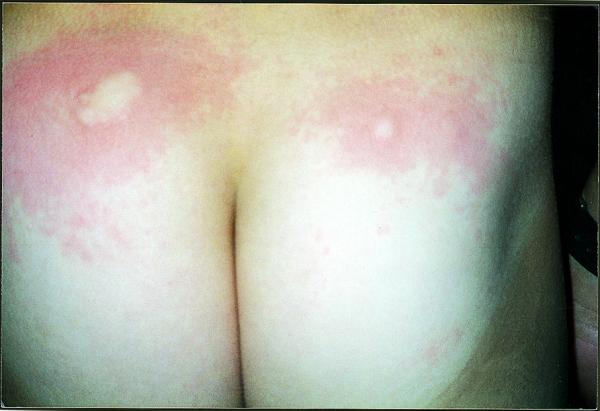
Urticarial rash on buttocks. Reproduced with parents' permission
Footnotes
Competing interests: None declared.
References
- 1.Feinglos MN, Jegasothy BV. “Insulin” allergy due to zinc. Lancet. 1979;1:122. doi: 10.1016/s0140-6736(79)90517-8. [DOI] [PubMed] [Google Scholar]
- 2.Bollinger ME, Hamilton RG, Wood RA. Protamine allergy as a complication of insulin hypersensitivity: a case report. J Allergy Clin Immunol. 1999;104:462–465. doi: 10.1016/s0091-6749(99)70394-5. [DOI] [PubMed] [Google Scholar]
- 3.Jehle PM, Micheler C, Jehle DR, Breiteg D, Boehm B. Inadequate suspension of neutral protamine Hagendorn (NPH) insulin in pens. Lancet. 1999;354:1604–1607. doi: 10.1016/S0140-6736(98)12459-5. [DOI] [PubMed] [Google Scholar]


