Abstract
Heat shock protein 90 is a molecular chaperone that maintains function of numerous intracellular signaling nodes utilized by cancer cells for proliferation and survival. Hsp90 is also detected on the plasma membrane of tumor cells and its expression has been suggested to correlate with metastatic potential. Given the abundance and diverse functions of the intracellular pool of this protein, the precise contribution of cell surface Hsp90 to cell motility and tumor metastasis remains to be determined. In this study we utilized the small molecule DMAG-N-oxide, a novel cell-impermeable Hsp90 inhibitor, to specifically examine the role of cell surface Hsp90 in cell motility. We observed that, while not affecting intracellular Hsp90 function, DMAG-N-oxide significantly retarded tumor cell migration and integrin/extracellular matrix-dependent cytoskeletal reorganization. Concomitant with these findings, targeting cell surface Hsp90 significantly inhibited tumor cell motility and invasion in vitro, and had a dramatic impact on melanoma cell lung colonization in vivo. These data indicate that cell surface Hsp90 plays an important role in modulating cancer cell migration that is independent of the function of the intracellular Hsp90 pool, and that small molecule inhibitors of surface Hsp90 may provide a new approach to targeting the metastatic phenotype.
Keywords: heat shock protein 90, cell motility, cancer metastasis, molecularly targeted small molecules
Introduction
The majority of cancer deaths are caused by formation of secondary metastases rather than by the primary cancer (Fidler, 1999). Although cancer metastasis is a complex process, de-regulated cell migration and eventual colonization of distant tissue sites represent key components of the metastatic process (Entschladen et al., 2004; Yamaguchi et al., 2005). Cell migration is stimulated by environmental signals such as extracellular matrix [ECM] molecules [e.g., fibronectin] and growth factors. These environmental signals induce re-organization of the actin cytoskeleton and stimulate formation of cell protrusions, termed lamellipodia, at the leading edge of migrating cells. Localized actin polymerization is a driving force of cell migration (Wehrle-Haller & Imhof, 2003; Yamaguchi et al., 2005). Dynamic assembly and disassembly of focal adhesions [clusters of integrins and associated proteins, such as focal adhesion kinase and c-Src] is also integral to this process (Schlaepfer et al., 2004).
Heat shock protein [Hsp] 90 is a molecular chaperone that is important for maintaining stability and function of numerous client proteins (Neckers & Neckers, 2005). In many cases, Hsp90 client proteins are mutated or activated in cancer cells, and small molecule Hsp90 inhibitors, such as geldanamycin [GA] and its derivatives, 17-allylamino-17-demethoxygeldanamycin [17AAG] and 17-dimethylaminoethylamino-17-demethoxygeldanamycin [17DMAG], inhibit cancer cell proliferation in vitro and tumor growth in vivo concomitant with destabilization and degradation of these client proteins (Banerji et al., 2005; Munster et al., 2001; Nguyen et al., 2000; Solit et al., 2002). 17AAG, 17DMAG, and other Hsp90 inhibitors are currently being evaluated for anti-cancer activity in more than 20 phase II clinical trials (Heath et al., 2005; Solit et al., 2002).
Hsp90 is found not only intracellularly but also on the cell surface (Eustace et al., 2004). Cell surface expression of Hsp90 has been observed on melanoma cells, fibrosarcoma cells and on neuronal cells (Becker et al., 2004; Erkeller-Yuksel et al., 1992; Eustace et al., 2004; Sidera et al., 2004). In melanoma cells, cell surface Hsp90 expression correlates positively with metastatic potential (Becker et al., 2004), and inhibition of cell surface Hsp90 with antibody (Sidera et al., 2004) or GA coupled to cell-impermeable agarose beads (Eustace et al., 2004) has been reported to reduce cell migration in vitro. Thus, cell surface Hsp90 may play a role in cancer cell motility and metastasis distinct from but perhaps overlapping with its intracellular chaperone function.
Because these studies indicate that surface Hsp90 may be a target for development of novel metastasis inhibitors, we screened a panel of Hsp90 antagonists to identify small molecules that were both cell-impermeant, and thus specific for surface Hsp90, and that inhibited in vitro tumor cell motility and invasion. We found that the cell-impermeable Hsp90 inhibitor DMAG-N-oxide lacked the well-recognized characteristics of cell-permeable Hsp90 inhibitors yet profoundly affected cell motility. Both DMAG-N-oxide and an equivalently active Hsp90 antibody inhibited serum-dependent cell migration and actin re-organization, and fibronectin-dependent focal adhesion formation. Using a murine melanoma experimental metastasis model, we found that in vivo administration of cell-impermeable Hsp90 inhibitor reduced the frequency of lung colonization and significantly improved survival.
Results
Identification and characterization of DMAG-N-oxide as a cell-impermeable Hsp90 inhibitor
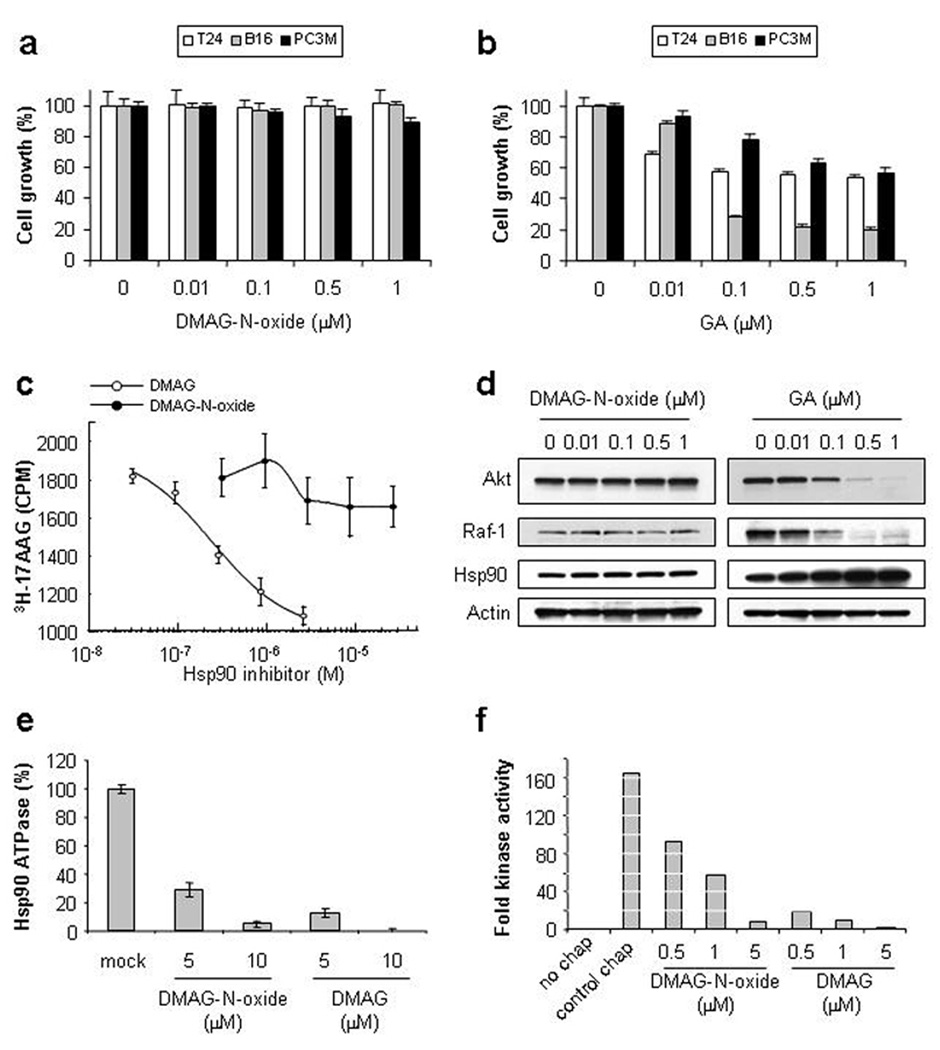
In analyzing a number of GA-derived Hsp90 inhibitors for their membrane permeability and affinity for Hsp90, we identified DMAG-N-oxide as a cell-impermeable Hsp90 inhibitor. Hsp90 affinity was determined using purified protein, while cell growth inhibition served as an initial screen for intracellular activity (or lack thereof) in intact cells. Although the Kd of DMAG-N-oxide for Hsp90 was determined to be 0.6 µM [similar to the Kds of the cell-permeable inhibitors 17AAG and 17DMAG (Tian et al., 2004)], the compound failed to demonstrate growth inhibitory activity when tested against 3 cancer cell lines [see Fig. 1a & b; GA is used as a positive control]. We confirmed the cell impermeability of DMAG-N-oxide by its inability to compete with tritium labeled 17AAG [3H-17AAG] for binding to intracellular Hsp90 in intact SkBr3 cells. Cells were pre-incubated with indicated concentrations of non-radioactive 17DMAG or DMAG-N-oxide, and then were further incubated with 3H-17AAG. Protein-bound 3H-17AAG [reflecting drug bound to intracellular Hsp90] was detected by liquid scintillation spectrometry. As shown in Fig. 1c, while 17DMAG dose-dependently inhibited 3H-17AAG incorporation into cells, DMAG-N-oxide failed to do so at concentrations up to 20 µM [more than 30 times its Kd for Hsp90], confirming that DMAG-N-oxide is poorly cell-penetrant.
Figure 1. DMAG-N-oxide is a cell-impermeable Hsp90 inhibitor.
a, b. DMAG-N-oxide does not affect cell growth. T24, B16-luc and PC3M cells were treated with indicated concentrations of DMAG-N-oxide or GA. Relative cell growth was determined 48 h after drug addition by the MTT assay. Values represent mean ± SD [a, n=4; b, n=3]. c. DMAG-N-oxide does not compete with 3H-17AAG for binding to intracellular Hsp90. SkBr3 cells were pre-treated with indicated concentrations of DMAG or DMAG-N-oxide and then exposed to 0.5 µM 3H-17AAG. Cells were lysed and proteins were precipitated with TCA. The amount of incorporated radioactivity was determined by liquid scintillation spectrometry. d. DMAG-N-oxide fails to destabilize intracellular Hsp90 client proteins. T24 cells were treated with the indicated concentrations of DMAG-N-oxide or GA. Whole cell extracts were prepared and analyzed by immunoblotting with antibodies recognizing Akt, Raf-1, Hsp90 or actin. e. DMAG-N-oxide inhibits the ATPase activity of purified Hsp90. Hsp90 ATPase activity was assayed as described in Methods in the presence of increasing concentrations of DMAG-N-oxide or DMAG. Values are expressed as a percentage of the untreated sample; means +/− SD are shown. f. DMAG-N-oxide inhibits Hsp90 chaperone activity. The ability of purified Hsp90 and accessory proteins to reconstitute the kinase activity of bacterially expressed GST-Chk1 was assessed, as described in Methods, in the presence of increasing concentrations of either DMAG-N-oxide or DMAG. Values are expressed as fold kinase activity above the ‘no chaperone’ control. The experiment was performed twice with similar results.
To date more than 100 intracellular Hsp90 client proteins have been identified. Cell-permeable Hsp90 inhibitors induce the degradation of these proteins because their stability depends on intracellular Hsp90 function (Neckers, 2002). Since our data suggested DMAG-N-oxide to be cell-impermeable, we examined the ability of DMAG-N-oxide to impact the stability of 2 well-characterized Hsp90 client proteins, Akt and Raf-1. T24 bladder carcinoma cells were incubated with the indicated concentrations of either DMAG-N-oxide or GA [as a positive control], and Akt and Raf-1 levels were monitored by western blotting. As shown in Fig. 1d, DMAG-N-oxide did not affect the steady-state level of either Akt or Raf-1, while GA significantly and dose-dependently decreased the expression of both proteins.
Heat shock factor [Hsf] is a transcription factor whose activity is negatively regulated by Hsp90 (Whitesell et al., 2003). By dissociating Hsf1 from Hsp90, cell-permeable Hsp90 inhibitors significantly increase Hsf activity, resulting in elevated expression of heat shock proteins, including Hsp90 itself (Whitesell et al., 2003). Consistent with its cell impermeance, DMAG-N-oxide failed to alter Hsp90 expression [Fig. 1d].
Although the Hsp90 binding affinity of DMAG-N-oxide is similar to that of 17DMAG itself, we wished to confirm that DMAG-N-oxide can inhibit Hsp90 function in cell free assays. Thus, we tested its activity in two in vitro assays that utilize purified protein – Hsp90 ATPase activity and Chk-1 reconstitution [Fig. 1e & f]. The N-terminal Hsp90 inhibitors identified to date, including the benzoquinone ansamycins such as 17AAG and 17DMAG, all bind in the ATP-binding cleft of the chaperone and inhibit its ATPase activity. Indeed, DMAG-N-oxide exhibited dose-dependent inhibition of the ATPase activity of purified Hsp90; inhibition with 17DMAG is shown for comparison. Likewise, DMAG-N-oxide displayed dose-dependent inhibition in the defined six-protein Chk-1 kinase reconstitution assay recently developed by Arlander et al., that is fully dependent on the presence of active Hsp90 (Arlander et al., 2006). The activity of 17DMAG is shown for comparison. Thus, in two distinct cell-free systems in which cell penetration is not required DMAG-N-oxide inhibited Hsp90 activity to a degree commensurate with its binding affinity to the purified protein. The inhibitory activity of DMAG-N-oxide did not involve down-regulation or internalization of extracellular Hsp90 since incubation with DMAG-N-oxide for up to 24 h did not affect the intensity of cell surface Hsp90 expression [see Supplemental Fig. 1].
Effect of cell-impermeable Hsp90 inhibitors on cancer cell invasion in vitro
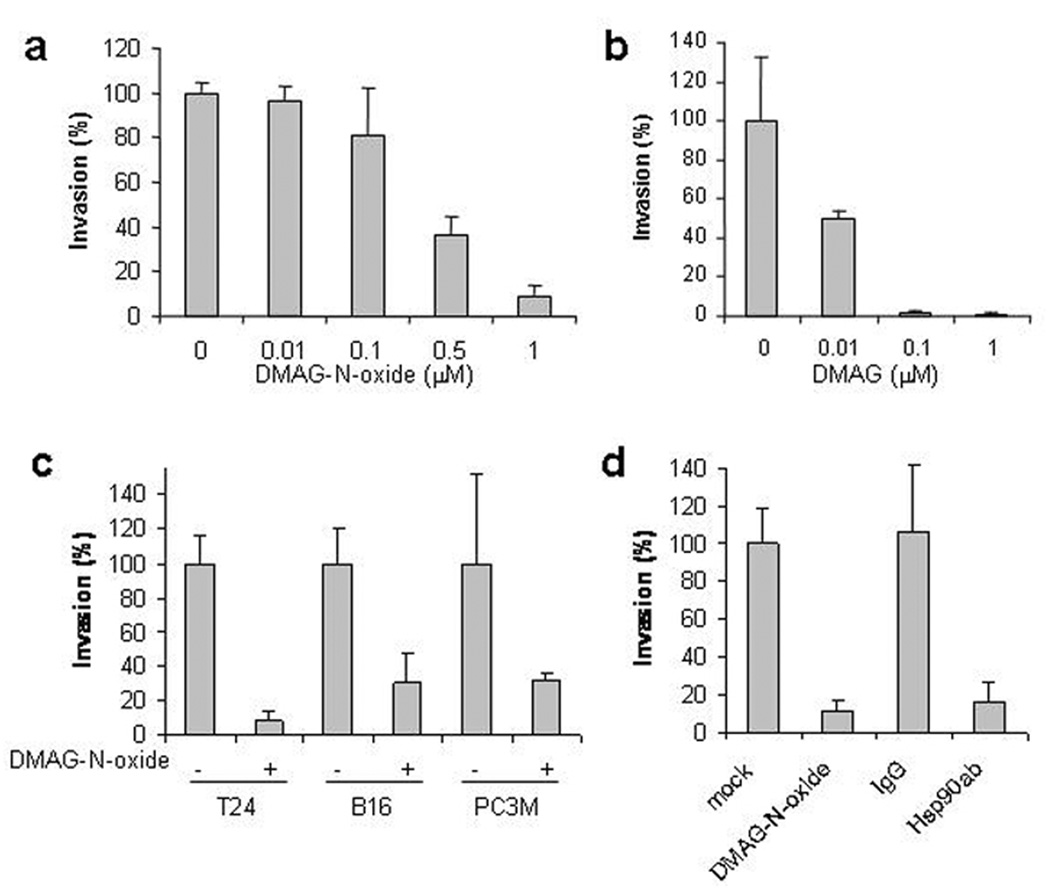
To examine whether DMAG-N-oxide might possess anti-metastatic activity independent of any growth inhibitory properties, we investigated its activity in a Matrigel invasion assay, a technique widely used as an in vitro model of the initial stages of cancer metastasis. As shown in Fig. 2a, DMAG-N-oxide significantly and dose-dependently inhibited T24 cell invasion at concentrations of 0.1–1.0 µM. The anti-invasion activity of DMAG-N-oxide was thus comparable to its affinity for Hsp90 [Kd = 0.6 µM]. For comparison, the activity of 17DMAG is shown in Fig. 2b. Its enhanced activity, compared to DMAG-N-oxide, suggests that intracellular Hsp90 client proteins likely also participate in aspects of cell invasion.
Figure 2. Cell-impermeable Hsp90 antagonists inhibit cell invasion.
a, b. T24 cells were treated with the indicated concentration of either DMAG-N-oxide or DMAG and analyzed in a Matrigel invasion assay. c. T24, B16-luc and PC3M cells were treated with DMAG-N-oxide [1 µM] and analyzed as above. d. T24 cells were treated with either normal mouse IgG [20 µg/ml], Hsp90 antibody [20 µg/ml], or DMAG-N-oxide [1 µM] and examined as above. Values represent mean ± SD [n=3].
We next examined the anti-invasion activity of DMAG-N-oxide towards other cell lines. As shown in Fig. 2c, invasion of B16 [melanoma] and PC3M [prostate cancer] cells was comparably inhibited by DMAG-N-oxide [1 µM], indicating that the anti-invasive activity of this cell-impermeable Hsp90 inhibitor is not restricted to a particular tumor type. All 3 cell lines examined [T24, B16, and PC3M] expressed detectable Hsp90 protein on the cell surface, and exposure to DMAG-N-oxide did not affect this expression [Supplemental Fig. 1 and data not shown].
Because Hsp90 antibody, after brief incubation, binds only to cell surface Hsp90 on living cells [see Supplemental Fig. 2 and (Sidera et al., 2004)], we examined the effect of a C-terminal domain-specific Hsp90 antibody [SPA-830] on cell invasion, and compared its activity with that of DMAG-N-oxide. As shown in Fig. 2d, Hsp90 antibody [20 µg/ml] significantly inhibited T24 cell invasion in the Matrigel assay, while an IgG control antibody was without effect. DMAG-N-oxide-treated and -untreated samples are shown for comparison.
Effect of cell-impermeable Hsp90 inhibitors on cell migration in vitro
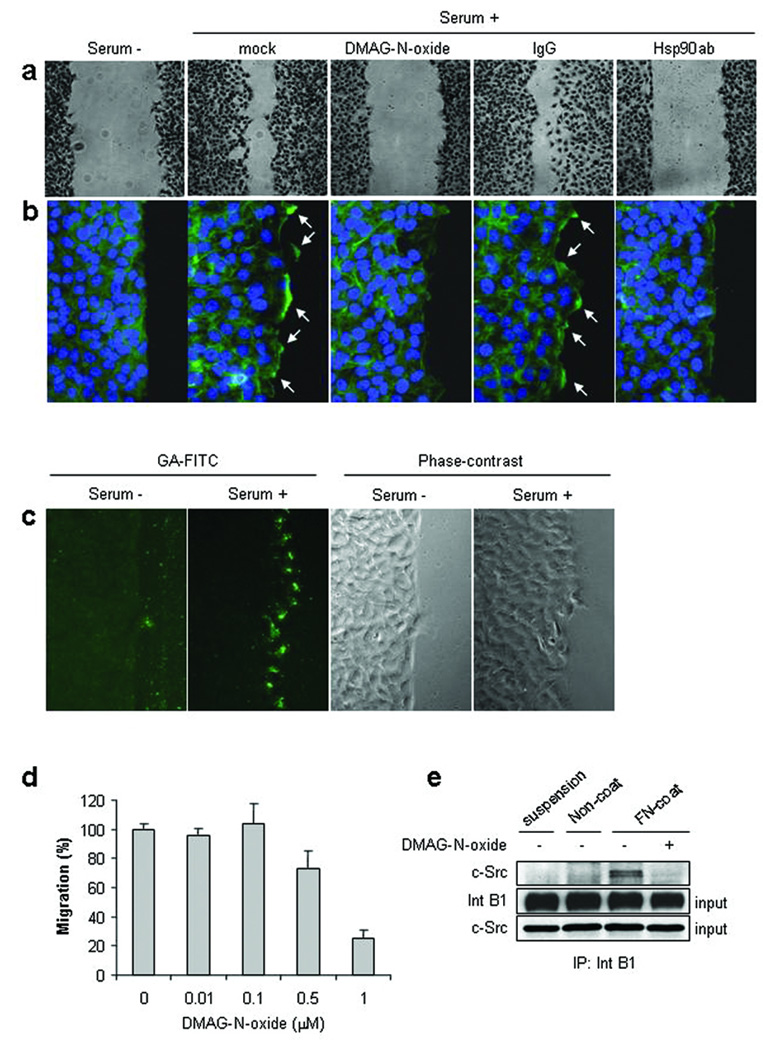
Cell migration is an initial step in cell invasion (Yamaguchi et al., 2005). To analyze further the cellular basis for the anti-invasive activity of cell-impermeable Hsp90 inhibitors, we used a wound-healing assay to examine whether these agents inhibit cell migration. T24 cells were pre-treated with DMAG-N-oxide [1 µM] or Hsp90 antibody [20 µg/ml] in the absence of serum, a wound was made by scraping the culture dish with a disposable pipette tip, and incubation was continued in the presence of serum. As shown in Fig. 3a, significant serum-dependent wound-healing was observed in cells treated with either vehicle or normal IgG, while both DMAG-N-oxide and Hsp90 antibody significantly inhibited this process. We further examined the anti-migration activity of cell-impermeable Hsp90 inhibitors using a Boyden chamber assay. DMAG-N-oxide significantly inhibited serum-stimulated cell migration [Fig. 3d]. The concentration-dependence of DMAG-N-oxide’s anti-migration activity paralleled the concentration-dependence of its anti-invasive activity [Fig. 2a].
Figure 3. Cell-impermeable Hsp90 antagonists inhibit cell migration.
a. T24 cells were grown to confluence, treated with DMAG-N-oxide [1 µM], normal IgG [20 µg/ml] or Hsp90 antibody [20 µg/ml] in the absence of serum, and then wounds were made by scraping with a disposable pipette tip; further incubation was carried out in the presence or absence of serum with additions as shown. Cells were visualized as described in Methods. b. Cells were fixed, permeabilized and stained with Alexa 488-conjugated phalloidin and DAPI [to detect cell nuclei]. F-actin polymers [green] and nuclei [blue] were observed with a fluorescence microscope. Arrows indicate F-actin polymers at the wound leading edge. c. Cells, exposed to serum or not, were fixed but not permeabilized and reacted with FITC-GA. FITC-GA labels cell surface Hsp90 at the ‘wound’ leading edge. d. T24 cells were treated with the indicated concentration of DMAG-N-oxide and migration was assessed using the Boyden chamber assay. Values represent mean ± SD [n=3]. e. T24 cells were maintained in suspension, replated in the absence of fibronectin [FN], or replated in the presence of FN with or without DMAG-N-oxide [1 µM] pre-treatment. After cell lysis, integrin β1 proteins were immunoprecipitated and examined for associated c-Src by immunoblot.
An initial step in cell migration is reorganization of the actin cytoskeleton (Yamazaki et al., 2005). We examined whether cell-impermeable Hsp90 inhibitors affected actin reorganization by staining cells in the wound-healing assay with fluorescently-labeled phalloidin, a toxin that interacts specifically with polymerized F-actin bundles, including the polymerized actin induced to form at the leading edge of migrating cells (Wehrle-Haller & Imhof, 2003). As shown in Fig. 3b, significant serum-dependent phalloidin reactivity was observed in untreated cells adjacent to the wound and in cells treated with vehicle or normal IgG. However, both DMAG-N-oxide and Hsp90 antibody markedly reduced phalloidin staining, especially at the leading edge of migrating cells [arrows]. Using FITC-GA to identify cell surface Hsp90 [see Supplemental Fig. 1 & 3], we observed strong reactivity at the leading edge of migrating cells [Fig. 3c], overlapping phalloidin reactivity. As was the case with phalloidin staining, leading edge Hsp90 positivity was strongly serum-dependent [and thus a property of migrating cells].
ECM-dependent dynamic assembly of focal adhesion molecules, including fibronectin-induced association of integrin β1 with c-Src at points where actin stress fibers are anchored to focal adhesions, is a key component of cell migration (Wozniak et al., 2004). Because of the close topological association between phalloidin staining and FITC-GA positivity in the wound-healing model, we examined whether cell impermeable Hsp90 inhibitor might interfere with the ECM-stimulated interaction of Src and integrin β1. As shown in Fig. 3e, while cells in suspension or cells plated on uncoated culture dishes for 1 h failed to demonstrate integrin β1/Src association, significant formation of this complex was observed 1 h after re-plating cells on fibronectin [FN]. However, these complexes failed to form in cells treated with DMAG-N-oxide. Further, DMAG-N-oxide disrupted FN-dependent actin polymerization and redistribution of focal adhesion kinase to sites of focal adhesion [see Supplemental Fig. 4].
Ex vivo treatment with cell-impermeable Hsp90 inhibitors reduces lung colonization by intravenously administered B16 melanoma
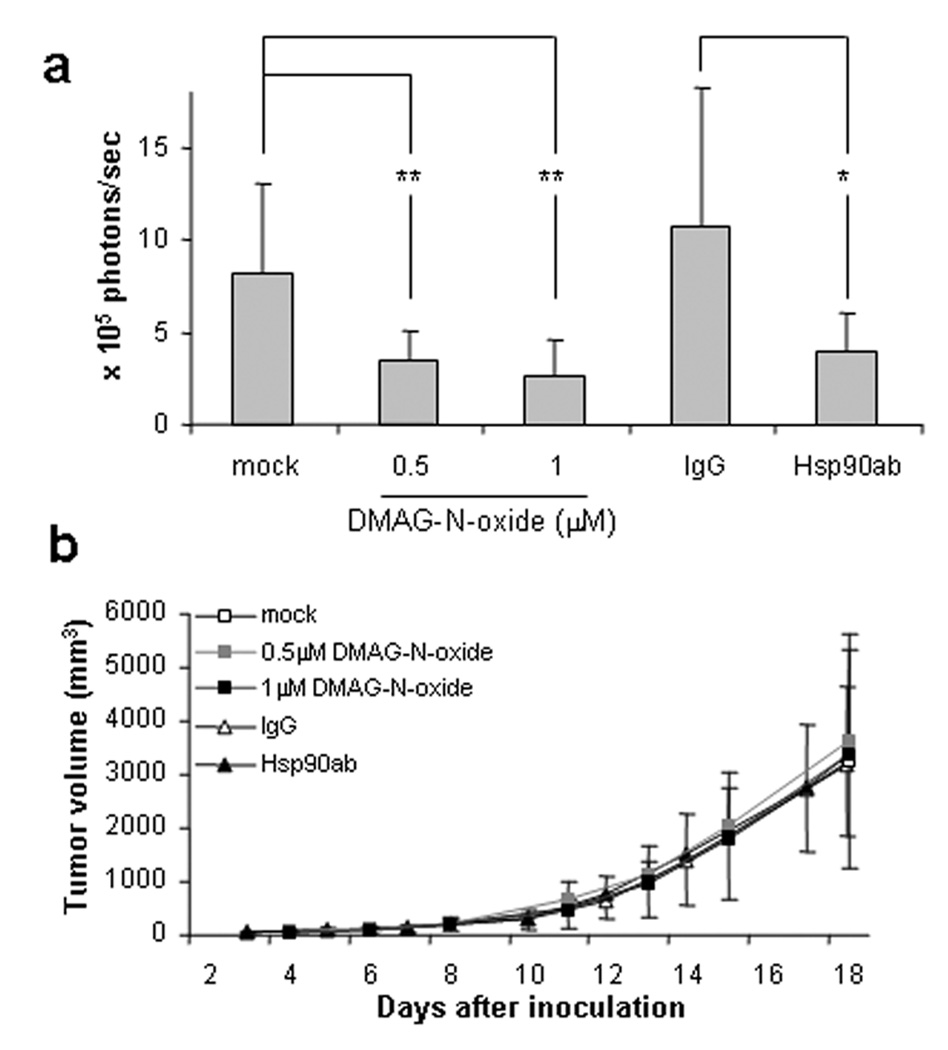
To examine whether cell-impermeable Hsp90 inhibitors affect aspects of cancer metastasis in vivo, we used the B16 murine melanoma lung colonization model (Brunson et al., 1978; Cranmer et al., 2005; Fidler, 1973). Because DMAG-N-oxide is structurally unstable in vivo, B16 cells stably expressing the luciferase gene were exposed to either DMAG-N-oxide [1 µM] or Hsp90 antibody [20 µg/ml] ex vivo. After the cells were intravenously injected into athymic mice, development of lung tumors was monitored non-invasively using an IVIS imaging system [see Methods]. As shown in Fig. 4b, significant lung colonization was detected in mice injected with B16 cells treated ex vivo with either DMSO or normal IgG, but colonization was significantly reduced in mice administered tumor cells previously exposed to either DMAG-N-oxide or Hsp90 antibody. Consistent with the data shown in Fig. 1a, neither DMAG-N-oxide nor Hsp90 antibody pre-treatment affected local growth of subcutaneously injected B16 cells [Fig. 4b].
Figure 4. Ex vivo treatment with cell-impermeable Hsp90 antagonists inhibits lung colonization by intravenously administered B16 melanoma.
B16-luc cells were treated with the indicated concentration of DMAG-N-oxide, or with 20 µg/ml normal IgG or Hsp90 antibody. Cells were intravenously or subcutaneously injected into Nu/Nu mice. Bioluminescence was detected following intravenous injection of luciferin using an IVIS Imaging System and was quantified as photons/second using Living Image software [a]. Tumor size following subcutaneous injection was measured three times per week with an electronic caliper [b]. Values represent mean ± SD [a: n=7; b: n=10]. For panel a, p values are as follows: * p< 0.05; ** p< 0.01.
Systemic administration of Hsp90 antibody inhibits lung colonization by B16 melanoma cells injected intravenously
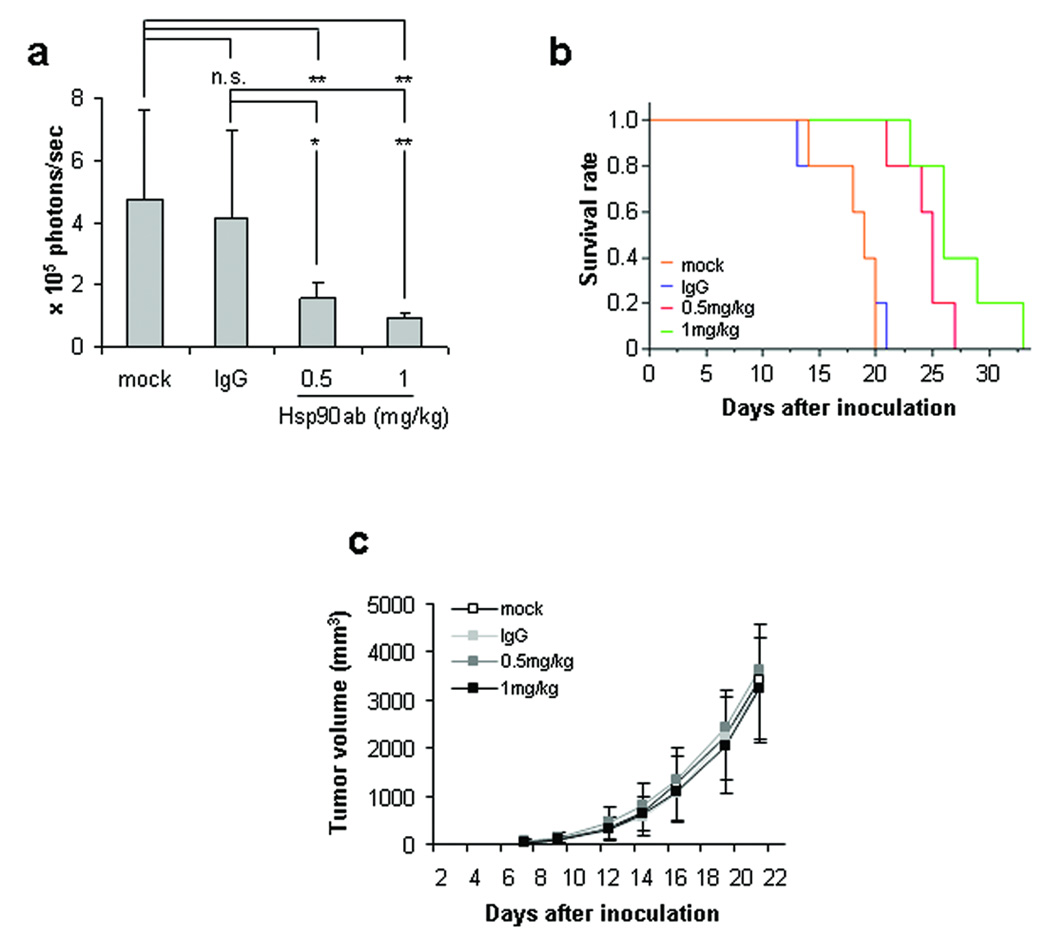
To assess the potential of cell-impermeable Hsp90 inhibitors as a possible anti-metastasis therapeutic strategy, we next examined whether twice weekly systemic administration of Hsp90 antibody could prevent or delay development of B16 lung colonization. Luciferase-expressing B16 cells were injected intravenously into athymic mice, and Hsp90 antibody or IgG control antibody [0.5 or 1 mg/kg] was administered twice weekly by the intraperitoneal route. As shown in Fig. 5a, significant lung seeding of melanoma cells was detected 10 days post tumor cell inoculation in mice receiving no treatment or twice weekly injections of normal IgG, but the incidence of lung seeding was markedly diminished in mice administered Hsp90 antibody.
Figure 5. Systemic administration of cell-impermeable Hsp90 antagonist inhibits lung colonization by intravenously injected B16 melanoma and improves survival.
B16-luc cells were intravenously [a – b] or subcutaneously [c] injected into Nu/Nu mice. a. Vehicle, normal IgG or Hsp90 antibody was intraperitoneally injected twice weekly at the indicated concentrations and luciferase activity was detected and quantified as in Fig. 4a. b. Kaplan-Meier plot depicting animal survival following intravenous injection of tumor cells. A log-rank test was used to assess the statistical significance of differences in survival time. Mock-injected animals: orange; normal IgG-injected animals: blue; 0.5 mg/kg Hsp90 antibody-injected animals: red; 1 mg/kg Hsp90 antibody-injected animals: green. c. Subcutaneous tumor masses were measured three times per week with an electronic caliper. Values represent mean ± SD [a: n=5; c: n=10]. For panel b, p values are as follows: * p< 0.05; ** p< 0.01.
Twice weekly administration of Hsp90 antibody was assessed for its ability to enhance survival. As shown in Fig. 5b, 50 percent of untreated or normal IgG-treated mice died within 19 days of intravenous tumor inoculation. In contrast, twice weekly administration of Hsp90 antibody significantly prolonged survival. Consistent with the findings of our ex vivo experiment, repeated systemic administration of Hsp90 antibody had no effect on local growth of subcutaneously injected B16 cells [Fig. 5c].
Discussion
In this study we identified a prototype cell-impermeable small molecule Hsp90 inhibitor, DMAG-N-oxide, and showed it to be an antagonist of cancer cell motility. DMAG-N-oxide inhibited cell migration and invasion in vitro and brief ex vivo exposure of tumor cells to the drug was sufficient to significantly reduce tumor colonization at distant sites in vivo. We observed similar activity with an Hsp90 antibody targeting the C-terminus of the chaperone, and we showed that these findings could be translated into enhanced survival in vivo following intravenous injection of metastatic melanoma cells [also see Supplemental Fig. 5].
Unlike the more commonly used cell-permeable Hsp90 targeting drugs, cell-impermeable inhibitors did not directly affect tumor growth either in vitro or in vivo, nor did they affect the stability of intracellular client proteins. Our findings suggest that effects of cell-impermeable Hsp90 inhibitors on cell motility and invasion are due, at least in part, to their ability to inhibit cell migration by interfering with leading edge actin polymerization and focal adhesion formation. ECM-stimulated, integrin-mediated focal adhesion kinase [FAK] localization to focal adhesions and FAK-mediated recruitment of c-Src to sites of integrin clustering are important components of FAK-mediated cell migration(Schlaepfer et al., 1999; Schlaepfer et al., 2004). Indeed, c-Src/FAK association at focal adhesions is thought to control the turnover of these structures during cell migration. Thus, our current data suggest that cell surface-localized Hsp90 plays a distinct and important role in this process. Although the precise molecular pathways requiring cell surface Hsp90 for motility remain to be fully elucidated, our data suggest that ECM-induced c-Src/integrin association, and re-organization of the actin cytoskeleton are important components.
Metastasis from a primary tumor requires progression through a series of highly complex and interrelated events, including [1] promotion of angiogenesis; [2] invasion into the circulation; [3] detachment and embolization of tumor aggregates; [4] transport through the circulation; [5] adhesion and arrest in capillary beds of specific organs; [6] extravasation; and [7] establishment at secondary sites (Cranmer et al., 2005). The mere presence of tumor cells in the blood does not necessarily indicate that metastatic disease will follow, as most blood-born malignant cells die rapidly in the circulation. The B16 melanoma model, with cells inoculated intravenously via the tail vein, has been used for more than 30 years to study mechanisms underlying tumor cell seeding and colonization of distant tissues once cells of the primary tumor have escaped from their original site and entered the bloodstream. Our findings, and the recent reports of others (Eustace et al., 2004; Sidera et al., 2004; Stellas et al., 2007), strongly implicate the participation of cell surface Hsp90 in this process. Our data further suggest that small molecule Hsp90 inhibitors, such as DMAG-N-oxide, can serve both as tools for dissecting the motility-related function(s) of intracellular and extracellular Hsp90 pools, and as proof of principle of the clinical usefulness of small molecule surface Hsp90 inhibitors.
Materials and Methods
Cell culture and reagents
The human bladder cancer cell line T24 and the human breast cancer cell line SkBr3 were cultured in Dulbecco’s modified Eagle’s minimal essential medium [DMEM; Invitrogen, Carlsbad, CA] supplemented with 10 % fetal bovine serum [FBS, Invitrogen], 100 U/ml penicillin [Invitrogen] and 100 µg/ml streptomycin [Invitrogen]. The murine melanoma cell line B16-luc [a kind gift of Dr. D. Bottaro, NCI] was cultured in DMEM supplemented with 10 % FBS and 3 mg/ml of puromycin [Invitrogen]. The human prostate cancer cell line PC3M was cultured in RPMI 1640 [Invitrogen] supplemented with 10 % FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. All cell lines were propagated at 37 °C in an atmosphere containing 5% CO2. GA, 17DMAG, and 17AAG [Kosan Biosciences, Hayward, CA] were dissolved in dimethylsulfoxide [DMSO] to a stock concentration of 10 mM, and then further diluted in aqueous medium. DMAG-N-oxide [Kosan Biosciences] was dissolved in distilled water. Chemical structures for GA, 17AAG, 17DMAG, DMAG-N-oxide, and FITC-GA are shown in Supplemental Figure 6.
Competition for [3H]17AAG uptake by SkBr3 cells
Competition for cellular uptake of [3H]17AAG was measured using a scintillating microplate method. SkBr3 cells in DMEM were grown in 96-well scintillating microplates [Cytostar T, GE Healthcare] to 90% confluency and overlayed with fresh medium containing 0.3 µM [3H]17AAG [30 Ci/mmol, Moravek Biochemicals] and 0–35 µM 17DMAG, DMAG-N-Oxide, or unlabeled 17AAG. Cultures were incubated for an additional 2h, then the scintillation proximity signal generated upon absorption of [3H]17AAG by adherent SkBr3 was measured using a Microbeta microplate scintillation counter.
Analysis of protein expression by immunoblotting
T24 cells were incubated with indicated concentration of drugs for 24 h. Whole-cell extracts were prepared as described previously (Xu et al., 2001). The protein concentration of samples was determined by BCA protein assay [Pierce, Rockford, IL]. Samples were applied to 4–20% polyacrylamide SDS gels [Bio-Rad, Hercules, CA], subjected to electrophoresis, and proteins were immunoblotted with antibodies against Akt, actin [Cell Signaling, Danvers, MA], Raf-1 [Santa Cruz Biotechnology, Santa Cruz, CA], or Hsp90 [Assay Designs, Ann Arbor, MI].
Analysis of cell growth
Cell growth was examined by MTT assay. 5 × 103 cells were incubated in a 96-well plate for 24 h. The indicated concentration of drugs was added, and incubations were continued for 48 h. At the conclusion of the experiment, MTT solution [Sigma-Aldrich, St. Louis, MO] was added to a final concentration of 1 mg/ml and plates were allowed to incubate at 37 °C for 3 h. At the end of the incubation period, DMSO was added to a final concentration of 50 %. Optical density at 570 nm was determined by spectrophotometer [Model ELx808IU, Bio-TEK instruments, Winooski, VT].
ATPase assay
Hsp90 ATPase activity was determined as described previously (Owen et al., 2002). Purified Hsp90 [5 µM] was incubated with 1µM [α-32P] ATP [2 mCi/ml] and indicated concentrations of Hsp90 inhibitors at 30°C in a buffer containing 40 mM HEPES-KOH, pH 7.4, 100 mM KCl, 2 mM MgCl2, and 2 mM DTT. Reactions were stopped by addition of an equal volume of a stop solution containing unlabeled AMP, ADP, ATP and EDTA [12 mM each]. Under the conditions used, the IC50 of GA for both human and yeast Hsp90 is 5 µM (Owen et al., 2002). Given these considerations, 5 and 10 µM DMAG and DMAG-N-oxide were used in these assays.
Chk1 kinase assay
Chk1 activity was determined as described previously (Arlander et al., 2006). Bead-bound Chk1 was incubated with [γ-32P] ATP, the Chk1 substrate GST-Cdc25C [residues 200–256], and indicated concentrations of Hsp90 inhibitors at 30°C for 10 min. Inhibitor concentrations were chosen based on previously published data (Arlander et al., 2006). Kinase reactions were terminated by addition of 4X SDS-PAGE sample buffer with heating at 100°C for 10 min. Reactions were separated by SDS-PAGE and transferred to Immobilon-P membrane [Millipore, Billerica, MA]. Membrane-bound radiolabeled proteins were detected and quantitated using a Storm 840 PhosphorImager [Amersham Biosciences, Piscataway, NJ].
Analysis of cell invasion/motility and F-actin polymerization
Anti-invasion activity was assessed by Matrigel invasion assay. Anti-motility activity was assessed by both wound-healing assay and Boyden chamber cell migration assay. Cells were detached from culture plates with PBS containing 2 mM EDTA, washed twice in culture media, and resuspended in serum-free culture medium. Cells were pre-treated with 20 µg/ml normal mouse IgG [Sigma-Aldrich, St. Louis, MO] or Hsp90 antibody [Assay Designs, SPA-830] for 2 h, and re-suspended in serum-free culture medium. Cells [2 × 104 T24, 3×105 B16-luc, or 1×105 PC3M cells] were added to the upper portion of a dual chamber separated by an 8 micron pore size filter; the upper chamber contained indicated concentrations of DMAG-N-oxide. For invasion assays, a Matrigel-coated transwell insert [BD Biosciences, Bedford, MA] was used, while for motility analysis, uncoated transwell inserts were used. Serum-containing culture medium and indicated concentrations of DMAG-N-oxide were added to the lower portion of the chamber and cells were allowed to migrate for 2 days at 37° C in 5 % CO2. Cells on the upper surface of the filter membrane were removed by cotton swab. Cells on the lower surface of the membrane were fixed and stained with Diff-Quik Stain kit [Dade Behring Inc., Newark, DE]. The membrane was mounted on a microscope slide and migrated cells were counted in 5 random high-power fields.
For wound-healing assays and staining for F-actin polymers, cells were cultured in LabTek II chamber slides [Nalge Nunc, Rochester, NY]. Confluent cultures were serum-starved for 24 h and incubated with either DMAG-N-oxide [1 µM], normal IgG or Hsp90 antibody [20 µg/ml] for 2 h, and then wounds were made by scraping with a pipette tip. After additional incubation in the presence of 10 % FBS for 6 h [wound-healing assay] or for 4 h [staining for F-actin polymers], cells were either fixed and stained with Diff-Quik Stain kit [wound-healing assay; Dade Behring], or were fixed in 10 % formaldehyde [Sigma-Aldrich] for 2 min, permeabilized with 0.1 % Triton X-100 [Sigma-Aldrich] for 3 min and stained with 1 unit of Alexa 488-conjugated phalloidin [Invitrogen] for 20 min in the presence of DAPI [Sigma-Aldrich]. Cells were observed in a fluorescence microscope [Model DMRXA, Leica, Allendale, NJ].
Detection of cell surface Hsp90
Cell surface Hsp90 was visualized using FITC-geldanamycin ([FITC-GA]. Cells were cultured in LabTekII chamber slides [Nalge Nunc] in the presence or absence of serum, and a wound was made by scraping with a pipette tip. Four hours after wounding, cells were washed, fixed in 10 % formaldehyde for 2 min, incubated with 2 µM FITC-GA [Invivogen, San Diego, CA] at 37°C for 1 h, and examined by fluorescence microscope.
Analysis of focal adhesion assembly by immunoprecipitation
Cells were serum-starved for 24 h and then detached from culture plates with PBS containing 2 mM EDTA, washed twice in culture medium, and resuspended in culture medium. While in suspension, cells were treated with Hsp90 inhibitor for 30 min, and then replated onto fibronectin-coated dishes [BD Biosciences], and further incubated for 1 h. For integrin β1 immunoprecipitation, 1 mg of total cell lysate proteins was incubated with integrin β1 antibody [BD Bioscience], followed by addition of protein G agarose beads [Invitrogen]. Beads were washed 4 times with lysis buffer, boiled in Laemmli buffer, and processed as described previously (Xu et al., 2001). As an indication of focal adhesion formation, resultant immunoblots were probed for fibronectin-dependent co-precipitation of c-Src and integrin β1.
Analysis of in vivo tumor colonization
Animal experiments and procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. For ex vivo DMAG-N-oxide treatment, B16-luc cells were incubated with the indicated concentration of DMAG-N-oxide for 16 h. Then cells were detached from culture plates with PBS containing 2 mM EDTA and prepared for intravenous injection as described below. For ex vivo Hsp90 antibody treatment, B16-luc cells were detached with PBS containing 2 mM EDTA, washed twice in DMEM, resuspended in DMEM, incubated for 90 min for recovery and further incubated with 20 µg/ml normal IgG or Hsp90 antibody for 2 h. After being washed twice and finally resuspended in DMEM, 5 × 105 cells were intravenously injected via tail vein into male Nu/Nu mice [6 weeks of age, TACONIC, Hudson, NY]. For systemic administration of Hsp90 antibody, B16-luc cells were detached with PBS containing 2 mM EDTA, washed twice and resuspended in DMEM, and 2 × 105 cells were intravenously injected via tail vein into Nu/Nu mice. Antibodies were dissolved in PBS to a final concentration of 0.2 mg/ml. A mock solution [PBS containing 10 % glycerol], normal IgG [1 mg/kg] or Hsp90 antibody [0.5 or 1 mg/kg] was injected intraperitoneally twice weekly thereafter [the first antibody injection was made 1 h before intravenous injection of tumor cells]. Bioluminescence emanating from tumor cells was detected either 10 days [ex vivo treatment with DMAG-N-oxide or systemic administration of Hsp90 antibody] or 11 days [ex vivo treatment with Hsp90 antibody] after intravenous injection of tumor cells as described previously (Wu et al., 2001). Five min prior to imaging, mice were intravenously injected with 150 mg/kg of D-luciferin [Xenogen] and bioluminescent imaging was performed using an IVIS Imaging System [Xenogen, Alameda, CA]. Bioluminescence was quantified as photons/second using Living Image software [Xenogen]. For examination of subcutaneous growth, drug-treated [for ex vivo assay] or untreated [for systemic drug evaluation assay] B16-luc cells [5 × 105 cells for ex vivo treatment assay and 2 × 105 cells for systemic evaluation assay] were subcutaneously injected into each Nu/Nu mouse in the hind footpad. Tumors were measured three times per week using an electronic caliper and their volumes were calculated using the following standard formula: width2 × length × 0.5. Because these tumors displayed no evidence of necrosis and were readily observable, Xenogen imaging was not employed to monitor their growth.
Statistical analysis
In the animal tumor studies [see Fig. 4 & Fig 5], statistically significant differences in tumor size were assessed by the unpaired Wilcoxon’s test. Kaplan-Meier plots and log-rank tests were used to assess statistically significant differences in animal survival. All statistical analyses were performed using JMP6 software [SAS, Cary, NC]. Differences were considered to be significant for values of p<0.05.
Supplementary Material
Footnotes
Supplementary information is available at Oncogene’s website
References
- Arlander SJ, Felts SJ, Wagner JM, Stensgard B, Toft DO, Karnitz LM. J Biol Chem. 2006;281:2989–2998. doi: 10.1074/jbc.M508687200. [DOI] [PubMed] [Google Scholar]
- Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, Judson I, Workman P. Clin Cancer Res. 2005;11:7023–7032. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- Becker B, Multhoff G, Farkas B, Wild PJ, Landthaler M, Stolz W, Vogt T. Exp Dermatol. 2004;13:27–32. doi: 10.1111/j.0906-6705.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- Brunson KW, Beattie G, Nicolsin GL. Nature. 1978;272:543–545. doi: 10.1038/272543a0. [DOI] [PubMed] [Google Scholar]
- Cranmer LD, Trevor KT, Bandlamuri S, Hersh EM. Melanoma Res. 2005;15:325–356. doi: 10.1097/00008390-200510000-00002. [DOI] [PubMed] [Google Scholar]
- Entschladen F, Drell TLt, Lang K, Joseph J, Zaenker KS. Lancet Oncol. 2004;5:254–258. doi: 10.1016/S1470-2045(04)01431-7. [DOI] [PubMed] [Google Scholar]
- Erkeller-Yuksel FM, Isenberg DA, Dhillon VB, Latchman DS, Lydyard PM. J Autoimmun. 1992;5:803–814. doi: 10.1016/0896-8411(92)90194-u. [DOI] [PubMed] [Google Scholar]
- Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, Scroggins BT, Neckers L, Ilag LL, Jay DG. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Nat New Biol. 1973;242:148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Cancer Chemother Pharmacol. 1999;43 Suppl:S3–S10. doi: 10.1007/s002800051091. [DOI] [PubMed] [Google Scholar]
- Heath EI, Gaskins M, Pitot HC, Pili R, Tan W, Marschke R, Liu G, Hillman D, Sarkar F, Sheng S, Erlichman C, Ivy P. Clin Prostate Cancer. 2005;4:138–141. doi: 10.3816/cgc.2005.n.024. [DOI] [PubMed] [Google Scholar]
- Munster PN, Srethapakdi M, Moasser MM, Rosen N. Cancer Res. 2001;61:2945–2952. [PubMed] [Google Scholar]
- Neckers L. Trends Mol Med. 2002;8:S55–S61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- Neckers L, Neckers K. Expert Opin Emerg Drugs. 2005;10:137–149. doi: 10.1517/14728214.10.1.137. [DOI] [PubMed] [Google Scholar]
- Nguyen DM, Desai S, Chen A, Weiser TS, Schrump DS. Ann Thorac Surg. 2000;70:1853–1860. doi: 10.1016/s0003-4975(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Owen BA, Sullivan WP, Felts SJ, Toft DO. J Biol Chem. 2002;277:7086–7091. doi: 10.1074/jbc.M111450200. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Sidera K, Samiotaki M, Yfanti E, Panayotou G, Patsavoudi E. J Biol Chem. 2004;279:45379–45388. doi: 10.1074/jbc.M405486200. [DOI] [PubMed] [Google Scholar]
- Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, Heller G, Tong W, Cordon-Cardo C, Agus DB, Scher HI, Rosen N. Clin Cancer Res. 2002;8:986–993. [PubMed] [Google Scholar]
- Stellas D, Karameris A, Patsavoudi E. Clin Cancer Res. 2007;13:1831–1838. doi: 10.1158/1078-0432.CCR-06-1585. [DOI] [PubMed] [Google Scholar]
- Tian ZQ, Liu Y, Zhang D, Wang Z, Dong SD, Carreras CW, Zhou Y, Rastelli G, Santi DV, Myles DC. Bioorg Med Chem. 2004;12:5317–5329. doi: 10.1016/j.bmc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Imhof BA. Int J Biochem Cell Biol. 2003;35:39–50. doi: 10.1016/s1357-2725(02)00071-7. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Bagatell R, Falsey R. Curr Cancer Drug Targets. 2003;3:349–358. doi: 10.2174/1568009033481787. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Wu JC, Sundaresan G, Iyer M, Gambhir SS. Mol Ther. 2001;4:297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, Neckers L. J Biol Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Wyckoff J, Condeelis J. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Yamazaki D, Kurisu S, Takenawa T. Cancer Sci. 2005;96:379–386. doi: 10.1111/j.1349-7006.2005.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.