Abstract
The PTH type 1 receptor (PTH1R) and PTHrP are expressed in vessels, where they contribute to regulating vascular smooth muscle cell (VSMC) function. Elevated PTHrP levels in VSMC are often associated with hyperplasia. In contrast, exogenous PTHrP, acting through the PTH1R, inhibits VSMC proliferation. In this study, we investigated the regulation of PTH1R expression by endogenous PTHrP and the associated effects on VSMC proliferation. Blocking binding of secreted PTHrP fragments to the PTH1R by treatment with either an antagonist or an antibody against PTHrP, and inhibition of PTHrP expression by small interfering RNA significantly increased PTH1R expression. Interestingly, treatment of the cells with a PTHrP analog (Bpa1-PTHrP) that activates the PTH1R without inducing its internalization had the same effect on receptor expression. To examine the association between receptor expression and the antiproliferative effect of N-terminal fragments of PTHrP, VSMC were treated with exogenous PTHrP (1–36) acutely and chronically to induce receptor down-regulation. Stimulation of VSMC with exogenous PTHrP (1–36) significantly reduced cell proliferation during the first 18 h of treatment but was no longer effective after 3 d, a time when PTH1R was down-regulated. In contrast, treatment with the noninternalizing agonist Bpa1-PTHrP strongly inhibited cell proliferation at all time points. In conclusion, our study show that PTHrP, after its intracellular processing and secretion, promotes down-regulation of the PTH1R in VSMC, thereby regulating cell proliferation in an auto/paracrine fashion. This regulatory mechanism may have important implication during vascular remodeling, in particular in the development of neointima after arterial injury, where PTHrP overexpression occurs.
Endogenously secreted N-terminal fragments of parathyroid hormone-related protein regulate PTH1R levels and proliferation of vascular smooth muscle cells in an auto/paracrine fashion.
PTHrP was first detected in neoplastic tissues associated with humoral hypercalcemia of malignancy (1). Although PTHrP is undetectable in the circulation of healthy individuals, it is expressed in a variety of tissues where it exerts both intracrine and auto/paracrine actions (2). PTHrP is expressed in the vasculature, both in vascular smooth muscle cells (VSMC) and in the endothelium (2,3). In addition, VSMC also express the PTH/PTHrP type 1 receptor (PTH1R), a class B (or class 2) G protein-coupled receptor (4), that recognizes the N-terminal regions of PTH and PTHrP with equal affinity and in most cell types is coupled to multiple G proteins. The initial finding of the hypotensive action of parathyroid extract (5) clearly indicated that the vasculature is one of the target tissues for PTH. Similarly, PTHrP, and in particular its N-terminal region, PTHrP (1–36), exerts vasodilatory actions (6,7). More recently, PTHrP has been identified as a key molecule involved in vascular remodeling. PTHrP is up-regulated in human coronary atherosclerotic lesions and in experimentally induced restenosis (8,9,10).
One prominent effect of PTHrP in VSMC is the control of proliferation. Thus, overexpression of intact full-length PTHrP in VSMC increases cell proliferation both in vitro and in animal models of neointima formation after angioplasty (11,12,13). In addition, the proliferation rate of VSMC from PTHrP knockout mice is significantly reduced compared with wild-type mice (11), suggesting that PTHrP may be a physiological regulator of vascular proliferation. The proliferative effect of PTHrP has been attributed to the presence of a nuclear localization signal within the C terminus of the molecule (14). On the other hand, PTHrP is translated with a signal sequence and is processed in VSMC leading to the secretion of various fragments, including PTHrP (1–36) (15). These fragments specifically bind the PTH1R. In many cells, stimulation of the PTH1R by its cognate ligands activates at least two distinct intracellular signaling cascades: the Gs/adenylyl cyclase/cAMP and the Gq/inositol triphosphate/intracellular calcium pathways (16). In VSMC, the PTH1R couples mostly, if not exclusively, to Gs (4,17,18). These findings are consistent with the notion that N-terminal fragments of PTH and PTHrP, acting via the PTH1R, inhibit VSMC proliferation in a cAMP/protein kinase A-dependent fashion (18,19,20). However, high levels of PTHrP expression were found in neointima where VSMC hyperplasia occurs, suggesting that other regulatory mechanisms are involved in the overall action of PTHrP in the vasculature.
To begin addressing the mechanisms at the basis of the regulatory role of PTHrP on VSMC proliferation, we hypothesized that endogenously secreted N-terminal fragments of PTHrP down-regulate the PTH1R in VSMC, thereby reducing its antiproliferative effects. Herein, we present a series of experiments supporting this hypothesis. These studies provide a mechanistic basis to understand some of the complex effects of PTHrP in VSMC.
Results
Characterization of endogenously expressed PTH1R and PTHrP in A10 cells
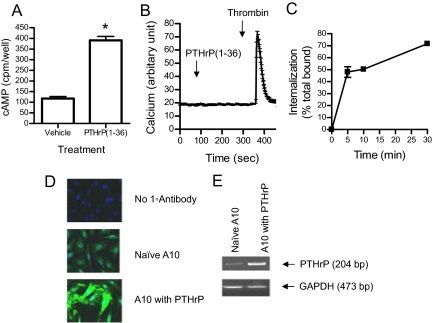
To study the role of endogenous PTHrP on PTH receptor expression and function, we used A10 cells (a clonal cell line originated from embryonic rat aortic smooth muscle cells) as a model system. Consistent with previous reports, detectable PTH1R expression, measured by [125I]PTH binding, was observed in A10 cells (KD = 30 nm, approximately 5000 receptors per cell). Significant increases in intracellular cAMP (maximum 3.5-fold) were observed upon stimulation with PTHrP (1–36) (Fig. 1A). Intracellular calcium release, however, was not observed in A10 cells stimulated with up to 1 μm PTHrP (1–36) (Fig. 1B). PTHrP (1–36) also did not stimulate inositol phosphates production even after incubation for up to 2 h (data not shown). Receptor endocytosis was assessed using radioligand internalization assay in A10 cells transiently expressing human PTH1R. As shown in Fig. 1C, receptor-associated [125I]PTH was rapidly and extensively internalized.
Figure 1.
Characterization of the PTH1R and PTHrP expression in VSMC. A, cAMP production in response to PTHrP (1–36). A10 cells were plated on 24-well plates and incubated with or without 100 nm PTHrP (1–36) at 37 C for 30 min. Results are presented as means and se in one of three experiments performed in triplicate. *, P < 0.05. B, Real-time recordings of intracellular [Ca2+]. A10 cells were plated on glass-bottom dishes, loaded with Fluo4, and treated with 1 μm PTHrP (1–36) and thrombin (10 U/ml) at the times indicated. Fluorescence intensity was measured every 3 sec for 500 sec. Graph shows the average and se of fluorescence intensity of 40 cells. Similar results were obtained in five independent experiments. C, Internalization of radiolabeled PTH (1–34) in A10 cells. The percentage of specific radioligand internalization was evaluated as described in Materials and Methods. Results are presented as means ± se in one of two independent experiments performed in triplicate. D, Immunostaining of PTHrP in naive A10 cells and in cells transiently transfected with PTHrP. Cells were fixed and stained with anti-PTHrP antibody followed by Alexa488-conjugated secondary antibody and DAPI. All images were taken with identical microscope settings. E, RT-PCR of PTHrP mRNA. Total RNA was extracted from either naive A10 cells or after transfection with PTHrP. PTHrP and GAPDH genes were amplified from cDNA using the specific primer sets described in Materials and Methods.
Because we found the antibody against the N-terminal region of PTHrP unsuitable for immunoblot analysis, PTHrP expression in A10 cells was detected by immunostaining and RT-PCR (Fig. 1, D and E). PTHrP was localized in the cytoplasm in naive A10 cells, and cells overexpressing PTHrP showed an increased intensity of protein expression (Fig. 1D). A 204-bp band was detected in RNA extracts of naive A10 cells, which increased in intensity in cells transfected with PTHrP (Fig. 1E). No band was detected in the A10 cells when PCR was carried out in the absence of reverse transcriptase (data not shown). Collectively, these data confirm the presence of endogenous PTHrP and PTH1R in A10 cells, and the exclusive activation of the Gs/cAMP signaling pathway. These data also establish the rapid and extensive internalization of the PTH1R in response to agonists.
PTH1R is down-regulated by endogenously secreted PTHrP
VSMC express PTHrP endogenously, raising the possibility that secreted N-terminal fragments of PTHrP may exert auto/paracrine effects on receptor activation and regulation. Because of the ability of PTH1R agonists to efficiently internalize PTH1R in A10 cells, we hypothesized that secreted PTHrP may down-regulate PTH1R. To test this hypothesis, we used three independent ways of blocking the action of secreted PTHrP.
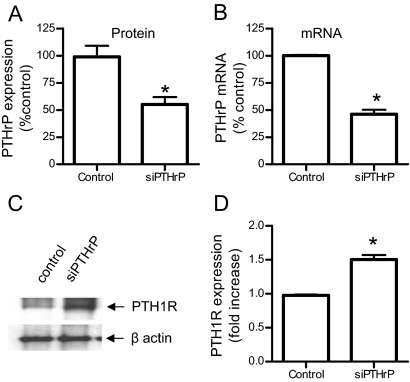
In the first approach, we used small interfering RNA (siRNA) oligonucleotides designed to target the region 404-422 of rat PTHrP (GenBank accession no. NM012636) to decrease the expression of PTHrP in A10 cells. After optimizing conditions for duplex siRNA transfection using siGLO (control siRNA with green fluorescence), we tested whether PTHrP expression was efficiently inhibited in A10 cells. In our experimental conditions, PTHrP siRNA decreased PTHrP mRNA and protein levels of PTHrP by about 50% (Fig. 2, A and B). We then examined whether PTH1R levels were affected by inhibition of PTHrP expression. A10 cells were transfected with heme agglutinin (HA)-tagged PTH1R and PTHrP siRNA (or control siRNA) and cultured in DMEM containing 1% fetal bovine serum (FBS) for 3 d. Cell lysates were separated in SDS-PAGE and blotted with anti-HA and anti-β-actin antibodies. As shown in Fig. 2C, PTHrP siRNA increased total receptor expression in A10 cells. Cell surface expression of HA-PTH1R determined by ELISA in fixed and nonpermeabilized cells was also significantly increased (50%) in cells transfected with PTHrP siRNA (Fig. 2D). These experiments suggest that endogenous PTHrP expressed in A10 cells is able to down-regulate the PTH1R.
Figure 2.
Inhibition of PTHrP expression by siRNA increases PTH1R expression. A, Expression of PTHrP in A10 cells transfected without or with PTHrP siRNA was determined by immunostaining after 72 h of transfection, as described in Materials and Methods. Graph shows average intensity ± se of PTHrP expression in five random fields. B, Quantitative real-time PCR of PTHrP. Total RNA was extracted from A10 cells 48 h after transfection with PTHrP siRNA. PTHrP mRNA levels were determined by real-time RT-PCR, normalized against GAPDH using the comparative Ct (ΔΔCt) method. Values for PTHrP mRNA are the average (± range) percentage of control from two independent experiments. C, Western blot analysis for the expression of HA-PTH1R. After transfection with HA-PTH1R and either control or small interfering PTHrP (siPTHrP), cells were cultured in 1% FBS medium for 3 d Cell lysates were separated on 12% SDS-PAGE and immunoblotted with anti-HA (upper panel) or anti-β-actin (lower panel) antibodies. D, ELISA for the surface expression of PTH1R. After transfection with HA-PTH1R and either control or siPTHrP, cells were fixed but not permeabilized and HA-PTH1R expression quantified by ELISA. Results are presented as means and se in one of three independent experiments performed in triplicate. *, P < 0.05. Some error bars are smaller than the line thickness.
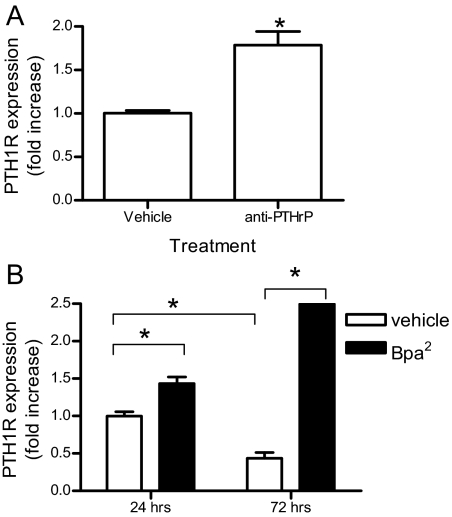
Next, we examined whether blocking the action of secreted N-terminal forms of PTHrP by incubation with anti-PTHrP (1–36) antibody regulates PTH receptor expression. A10 cells expressing HA-tagged PTH1R were cultured in DMEM with 1% serum for 3 d in the presence or absence of anti-PTHrP (1–36) antibody and receptor expression was determined by ELISA. In cells treated with anti-PTHrP antibody, PTH1R expression was significantly higher (approximately 75%) than in untreated cells (Fig. 3A).
Figure 3.
Blockade of secreted N-terminal forms of PTHrP increases PTH1R expression. A, A10 cells transfected with HA-PTH1R were cultured in 1% FBS medium in the absence (vehicle) or presence of anti-PTHrP (1–34) antibody (1 mg/ml) for 3 d. Surface receptor expression was measured by ELISA. B, A10 cells expressing HA-PTH1R treated in the absence (vehicle) or presence of 1 μm Bpa2-PTHrP for the indicated times. Surface receptor expression was measured by ELISA in nonpermeabilized cells using anti-HA antibody. The graph represents the mean fold increases ± se from vehicle-treated cells in one of three independent experiments performed in triplicate. *, P < 0.05.
In the third approach, binding of secreted PTHrP fragments to the PTH1R was inhibited using the antagonist Bpa2-PTHrP (21). A10 cells expressing HA-PTH1R were incubated without or with 1 μm Bpa2-PTHrP for up to 3 d. Determination of the expression of PTH1R by ELISA after 24 and 72 h culture in the presence or absence of 1 μm Bpa2-PTHrP revealed that surface PTH1R was decreased by 50% in untreated cells after 3 d, whereas treatment with the antagonist Bpa2-PTHrP caused a significant increase in receptor expression at both times (Fig. 3B). Importantly, Bpa2-PTHrP exerted no effect on the expression of HA-tagged TRH receptor, a distinct member of the G protein-coupled receptor superfamily, supporting the specific effect of Bpa2-PTHrP on PTH1R (data not shown). Total receptor expression, determined by Western blot, was markedly increased in cells treated with Bpa2-PTHrP as compared with untreated cells (Fig. 4D). Therefore, endogenous secreted PTHrP causes a time-dependent down-regulation of PTH1R that can be prevented by treatment with a competitive antagonist.
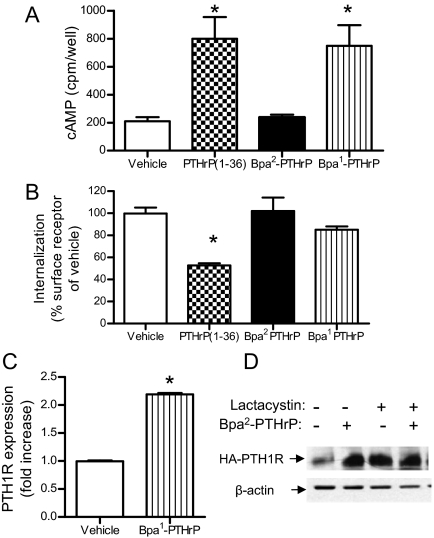
Figure 4.
A noninternalizing PTH1R agonist increases PTH1R expression. A, cAMP production in A10 cells. A10 cells expressing HA-PTH1R were treated with 100 nm PTHrP (1–36), the antagonist Bpa2-PTHrP, or the agonist Bpa1-PTHrP, as indicated. cAMP accumulation was measured as described in Materials and Methods. B, Loss of surface PTH1R. A10 cells expressing HA-PTH1R were treated with 100 nm PTHrP (1–36), the antagonist Bpa2-PTHrP, or the agonist Bpa1-PTHrP at 37 C for 15 min to induce receptor internalization. Surface PTH1R expression was measured by ELISA using anti-HA antibody. C, PTH1R expression. A10 cells were transfected with HA-PTH1R, cultured in 1% FBS DMEM for 3 d in the absence (white bars) or presence (gray bars) of 1 μm Bpa1-PTHrP. Total receptor expression was measured by ELISA using anti-HA antibody. Graph shows mean ± se performed in one of three independent experiments performed in triplicate. D, Total PTH1R expression measured by Western blot with anti-HA antibody from lysates of A10 cells transfected with HA-PTH1R and treated in the absence or presence of 10 μm lactacystin and with or without the antagonist Bpa2-PTHrP (1 μm) for 24 h. *, P < 0.05 vs. vehicle.
To examine whether inhibition of PTH1R internalization was sufficient to block down-regulation, we took advantage of a well characterized PTHrP agonist, Bpa1-PTHrP (21). This analog binds to the receptor and activates the cAMP/adenylyl cyclase pathway without inducing receptor internalization (Fig. 4, A and B). HA-PTH1R expression in cells treated with and without Bpa1-PTHrP was measured by ELISA. Similar to the effect of the antagonist Bpa2-PTHrP, the noninternalizing agonist Bpa1-PTHrP increased PTH1R expression in A10 cells (Fig. 4C). These experiments suggest that posttranslational regulation and/or protein degradation might be involved in the receptor down-regulation. Indeed, in A10 cells treated with 10 μm lactacystin (a proteasome inhibitor), total PTH1R receptor greatly increased compared with untreated cells (Fig. 4D). The receptor levels in these conditions were equivalent to those measured in A10 cells treated with the antagonist Bpa2-PTHrP (Fig. 4D).
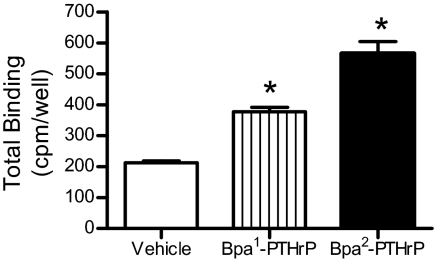
The effects of Bpa1- and Bpa2-PTHrP on endogenous PTH1R expression were determined by [125I]PTH binding. Confluent A10 cells were treated with vehicle or Bpa1- or Bpa2-PTHrP for 3 d and washed with 50 mm glycine buffer (pH 3.0) to remove receptor-bound ligands. Cells were then incubated with radiolabeled PTH on ice for 2 h. Consistent with the previous experiments, both Bpa1- and Bpa2-PTHrP significantly increased endogenous PTH receptor expression (Fig. 5).
Figure 5.
Regulation of endogenous PTH1R expression by PTHrP analogs. Naive A10 cells in 24-well plates were incubated with DMEM/1% FBS containing 100 nm of the agonist Bpa1-PTHrP or the antagonist Bpa2-PTHrP, as indicated, for 3 d. Receptor expression was measured by radioligand binding assay as described in Materials and Methods. Each point represents the mean percentage ± se from triplicate determinations. Similar results were obtained in an additional experiment. *, P < 0.05 vs. vehicle.
Collectively, these experiments indicate that PTHrP secreted by VSMC acts in an auto/paracrine fashion to down-regulate PTH1R, an effect that can be reversed by blocking either endogenous PTHrP with appropriate antibodies or by antagonizing PTHrP binding to the PTH1R. These results also show that PTH1R internalization by endogenously secreted PTHrP is accompanied by a reduction of total and surface receptor expression in VSMC.
PTH1R levels determine the effect of endogenous PTHrP on cell proliferation
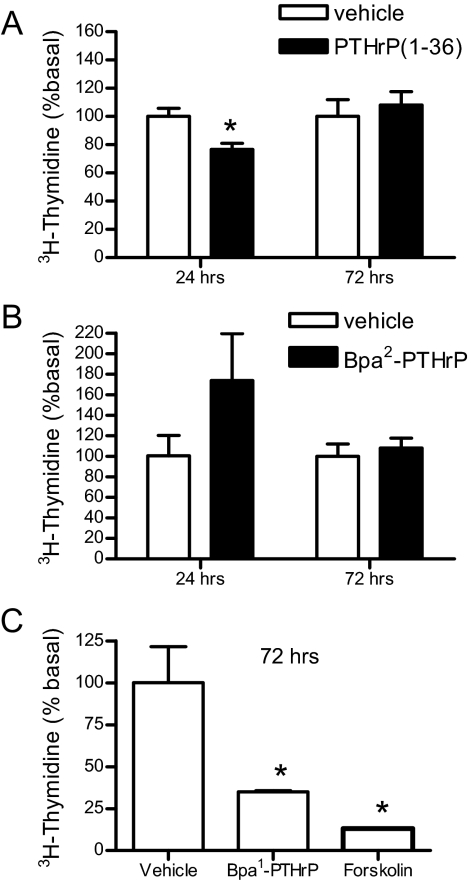
One of the most prominent effects of PTH1R agonists in VSMC is the regulation of cell proliferation. Indeed, PTHrP (1–36) (100 nm for 24 h) significantly decreased proliferation of A10 cells to approximately 75% of control vehicle-treated cells (Fig. 6A). However, when A10 cell proliferation was assayed after 3 d incubation with PTHrP (1–36), no inhibition was observed (Fig. 6A). In contrast, similar experiments performed with the antagonist Bpa2-PTHrP showed an increase in cell proliferation during the first 24 h of treatment (due to the inhibition of endogenous PTHrP), but no increase in thymidine incorporation was observed after 72 h (Fig. 6B). These data suggest that although PTHrP acutely inhibits VSMC proliferation, chronic exposure to the ligand (at endogenous levels) results in receptor down-regulation (Fig. 3B) and consequent inability to decrease cell growth. The lack of modulation of cell proliferation by PTHrP and Bpa2-PTHrP upon longer incubation prompted us to determine the specificity of this effect. Consistent with previous reports (18), agents that increase cAMP in VSMC (such as forskolin, an activator of adenylyl cyclase) significantly decreased cell proliferation (Fig. 6C). Interestingly, the non-desensitizing analog Bpa1-PTHrP inhibited cell proliferation by 65% (Fig. 6C) after 72 h, an effect that was not observed in response to either PTHrP or the antagonist Bpa2-PTHrP (Fig. 6, A and B).
Figure 6.
Cell proliferation in A10 cells treated with PTHrP analogs. A10 cells were incubated in DMEM/1% FBS containing 100 nm PTHrP (A), μm Bpa2-PTHrP (B), 1 μm Bpa1-PTHrP and 10 μm forskolin (C) for indicated time. Cells were incubated with [3H]thymidine during the last 18 h. [3H]Thymidine incorporation was measured with a β-counter. Each point represents the mean percentage ± se from triplicate determinations. Similar results were obtained in one additional experiment. *, P < 0.05.
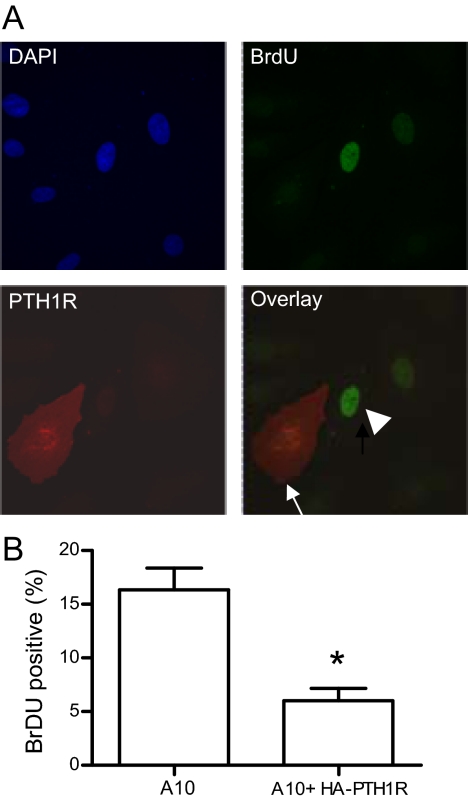
To establish whether PTH1R levels control the antiproliferative action of secreted PTHrP, proliferation of A10 cells transiently transfected with HA-tagged PTH1R was determined by bromodeoxyuridine (BrDU) staining. BrDU-positive nuclei (proliferating cells) were observed in 16% of nontransfected cells, whereas only 6% of cells expressing HA-PTH1R were BrDU positive (Fig. 7), suggesting that increased receptor density in A10 cells is sufficient to inhibit cell proliferation in response to endogenous PTHrP.
Figure 7.
Increasing PTH1R number inhibits A10 cell proliferation. A, A10 cells were transiently transfected with HA-PTH1R and incubated with BrDU for 18 h. Cell nuclei were stained with DAPI (top left) followed by immunostaining with anti-BrDU (top right) and anti-HA (bottom left) antibodies as detailed in Materials and Methods. The overlay picture (bottom right) shows a cell expressing HA-PTH1R, which is negative for BrDU (arrow), and a nontransfected cell positive for BrDU (arrowhead). B, Quantitation of cell proliferation in naive and PTH1R-expressing cells. The number of BrDU-positive cells was counted in five different fields. The white bar shows the percentage of BrDU-positive, untransfected cells ± se; the gray bar shows the percentage of BrDU-positive, HA-PTH1R-expressing cells ± se. *, P < 0.05.
PTHrP regulates cell proliferation of primary VSMC
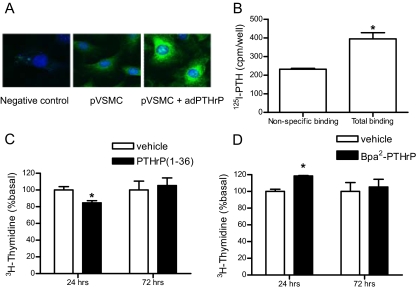
The preceding studies were performed in clonal A10 cells. It was therefore essential to establish whether endogenous, secreted PTHrP regulates cell proliferation in primary VSMC. Primary VSMC were isolated from the aorta of C57BL6 mice and cultured using standard techniques described in Materials and Methods. These cell preparations expressed α-smooth muscle actin (a specific marker for VSMC), which formed well organized fibers (data not shown). Similar to A10 cells, VSMC expressed PTHrP (determined by immunostaining, Fig. 8A) and PTH1R (determined by binding of radioiodinated PTH, Fig. 8B). In these cells, treatment with PTHrP for 24 h inhibited thymidine incorporation by 15%, but no inhibition was observed after 72 h treatment (Fig. 8C). In contrast, treatment with the antagonist Bpa2-PTHrP increased VSMC proliferation during the first 24 h of incubation but not after 72 h (Fig. 8D). Collectively, these observations confirm that the regulation of cell proliferation by endogenous PTHrP observed in clonal cell lines is fully operational in primary VSMC.
Figure 8.
Primary VSMC (pVSMC). A, Immunostaining of PTHrP in primary VSMC and in cells infected with adenovirus encoding human PTHrP. Cells were fixed and stained with anti-PTHrP antibody (1:1000) or anti-HA (1:2000, as a negative control) followed by Alexa488-conjugated secondary antibody and DAPI. All images were taken with identical microscope settings. B, PTH1R expression. Receptor expression was measured by radioligand binding assay as described in Materials and Methods, either in the absence (total binding) or in the presence (nonspecific binding) of 1 μm PTHrP. C and D, Primary VSMC were incubated in DMEM/1% FBS containing 100 nm PTHrP (C) or 1 μm Bpa2-PTHrP (D) for 24 or 72 h. Cells were incubated with [3H]thymidine during the last 18 h. [3H]Thymidine incorporation was measured with β-counter. Each point represents the mean percentage ± se from triplicate determinations. Similar results were obtained in an additional experiment. *, P < 0.05.
Discussion
The effects of PTHrP and its N-terminal peptides on VSMC proliferation are complex. Elevated PTHrP levels are often associated with VSMC proliferation. In particular, after angioplasty, PTHrP is up-regulated in the proliferating VSMC, and the development of restenosis is exacerbated by the local application of adenovirus expressing PTHrP (12). Studies in vitro have shown that overexpression of PTHrP in A10 cells increases proliferation, an effect that is dependent on the presence of a nuclear localization signal within the C-terminal region of the protein (11). However, overexpression of PTHrP in A10 cells also results in higher secretion of biologically active N-terminal fragments of PTHrP [such as PTHrP (1–36)] (15). This raises a paradoxical situation, because activation of the PTH1R by exogenously administered PTHrP (1–36) exerts antiproliferative effects on VSMC, both in vivo and in vitro (8,11,19). Therefore, both proliferative (by the intracrine action of full-length PTHrP) and antimitogenic effects (by the autocrine action of secreted N-terminal fragments of PTHrP) originate from the same molecule, albeit via completely distinct mechanisms. In this study, we examined the possibility that prolonged exposure of VSMC to endogenously produced and secreted PTHrP (1–36) causes down-regulation of PTH1R with consequent attenuation of the antiproliferative signal. Down-regulation of PTH1R by exogenous PTH or PTHrP have been reported in osteoblastic and kidney cell lines (22,23,24). However, similar studies in HEK293 cells did not reveal PTH1R down-regulation (25). These studies have been performed with relatively high concentrations of agonist (10–100 nm) in cells that may express PTHrP at different levels and, in the case of HEK293 cells, in a system that does not express PTH1R endogenously and may lack key regulatory components. Therefore the ability of endogenous PTHrP to regulate PTH1R levels in VSMC remained unknown.
We addressed these issues using three different ways of blocking either expression or function of endogenous PTHrP. First, inhibition of PTHrP expression in A10 cells by siRNA resulted in a significant increase in PTH1R levels, indicating that receptor down-regulation is dependent on PTHrP expression. Second, treatment of A10 cells with an anti-PTHrP (1–36) antibody also increased PTH1R expression, indicating that secreted N-terminal fragments of PTHrP are responsible for the down-regulation. Third, by using the potent antagonist Bpa2-PTHrP, we show that secreted N-terminal forms of PTHrP must bind the PTH1R for down-regulation to occur.
Modification of residue 1 (Ala) in PTHrP (1–36) by Bpa does not significantly alter the affinity for the PTH1R and the efficacy in acutely stimulating the Gs/adenylyl cyclase signaling (21,26). However, in contrast to PTHrP (1–36), Bpa1-PTHrP does not induce mobilization of β-arrestin2, a required event for PTH1R desensitization and endocytosis (21,27). In this study, we observed that both the antagonist Bpa2-PTHrP and the agonist Bpa1-PTHrP efficiently inhibit receptor down-regulation by preventing secreted PTHrP (1–36) from internalizing the PTH1R. Further support for this interpretation comes from the observation that treatment of A10 cells with the proteasome inhibitor lactacystin increased the total amount of PTH1R, suggesting that receptor degradation occurs constitutively in these cells.
Collectively, these observations direct us to propose that endogenous PTHrP, after its intracellular processing and secretion, promotes down-regulation of the PTH1R in VSMC by continuously inducing its internalization. It is noteworthy that these effects are significant even at levels of secreted N-terminal fragments of PTHrP that are hardly detectable by RIA (14). Yet, the regulation of PTH1R expression in these conditions is a slow process, because it requires several days to become apparent. It is therefore possible that such an autocrine regulation is active only in cells and tissues that express PTHrP endogenously and for prolonged periods. This is certainly the case in the vasculature, especially in the pathological setting of high remodeling such as after angioplasty and in atherosclerotic lesions, where PTHrP expression increases considerably (10,28).
Variations in PTH1R levels in VSMC have relevant effects on their proliferation. Our study shows that increasing receptor number in A10 cells is sufficient to inhibit proliferation independently from the addition of agonists. This effect is likely due to the endogenous production and secretion of N-terminal fragments of PTHrP that act in an auto/paracrine fashion to inhibit proliferation. Indeed, blocking the PTH1R with the antagonist Bpa2-PTHrP resulted in an increase of cell proliferation both in A10 and in primary VSMC. Consistent with these observations, treatment with the PTH1R antagonist PTHrP (7–34) enhanced cuff-induced intimal thickness in rats (8). On the other hand, exogenous PTHrP (1–36) is able to inhibit VSMC proliferation during the first 24 h but is ineffective upon prolonged incubation, reflecting the profound receptor down-regulation and consequent loss of function. In contrast, Bpa1-PTHrP, which does not induce PTH1R desensitization, internalization, and down-regulation, was still effective after 3 d, further supporting the proposed mechanism of regulation of PTH1R levels and VSMC proliferation. It is noteworthy that essentially identical results were obtained in clonal A10 cells and in primary VSMC, indicating that these effects are mediated by endogenous PTHrP and PTH1R and that the mechanisms of regulation of receptor levels and proliferation are physiologically relevant. Also, this mechanism are consistent with the observation that proliferation of VSMC after angioplasty in β-arrestin2 knockout mice was significantly lower than in wild-type mice (29). Our findings would suggest that reduced PTH1R endocytosis in β-arrestin2-null cells may restrain receptor down-regulation, thereby enhancing PTHrP (1–36)-dependent inhibition of VSMC proliferation.
In summary, we have demonstrated that endogenous PTHrP regulates PTH1R expression and cell proliferation in VSMC. The auto/paracrine actions of PTHrP in these cells may have important implications during vascular remodeling, in particular in the development of neointima after arterial injury, where PTHrP overexpression occurs.
Materials and Methods
Peptide synthesis and radioligand preparation
The synthesis, purification, and characterization of PTHrP (1–36)-NH2 [PTHrP (1–36)], [Bpa1, Ile5, Arg11,13, Tyr36]PTHrP (1–36)-NH2 (Bpa1-PTHrP), [Bpa2, Ile5, Arg11, 13, Tyr36]PTHrP (1–36)-NH2 (Bpa2-PTHrP) were carried out as previously described (26). The pure products were characterized by analytical HPLC and electron spray mass spectrometry. Radioiodination and HPLC purification of PTH (1–34) was carried out as reported (30).
Cell culture and transfection
Monolayer cultures of A10 cells (American Type Culture Collection, Manassas, VA) were grown in DMEM (Invitrogen Life Technologies, Grand Island, NY) supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA) at 37 C in a humidified 95% room air, 5% CO2 incubator. Cells were transfected with plasmids encoding HA-PTH1R (1 μg/ml) (31) using Fugene 6 (Roche, Indianapolis, IN) according to the manufacturer’s instructions. Cells were used for experiments 1–3 d after transfection.
Primary VSMC culture
VSMC cultures were prepared from mouse aorta. Briefly, the aorta was isolated surgically, the endothelial layer scraped, and the tissue minced into small pieces. The pieces were plated onto 12-well plates and cultured in DMEM with 10% FBS and 100 U/ml penicillin and streptomycin in a humidified atmosphere of 5% CO2 and 95% air. More than 95% of cells were VSMC as characterized by immunohistochemical staining for smooth muscle-specific α-actin (Sigma Chemical Co., St. Louis, MO). Cells from passages 7–15 were used in the experiments. Adenovirus encoding wild-type human PTHrP was used for the overexpression of PTHrP in primary VSMC according to a previous report (12). The experimental protocol was approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
PTH binding and internalization
Binding and internalization of radiolabeled PTH (1–34) was performed in confluent cells as described (21). Briefly, cells prepared as described above in 24-well plates were incubated on ice for 2 h with 100,000 cpm [125I]PTH-(1–34)NH2 in 250 μl DMEM containing 10% FBS. Cells were washed twice with ice-cold PBS and collected in 0.5 ml 0.1 N NaOH, and bound [125I]PTH-(1–34)NH2 was assessed by γ-spectrometry. To determine ligand internalization, cells were washed twice with ice-cold PBS and incubated at room temperature in DMEM/10% FBS. At the indicated time points, surface-bound ligand was extracted by two 5-min incubations on ice with 50 mm glycine buffer (pH 3.0) containing 0.1 m NaCl, and the remaining cell-associated (internalized) radioligand was collected in 0.5 ml 0.1 N NaOH. The amount of radioligand in each fraction was assessed by γ-spectrometry. Radioligand internalization is expressed as the ratio (percentage) of internalized fraction over the total cell-associated ligand (surface plus internalized). Nonspecific binding and internalization were measured in parallel experiments carried out in the presence of 1 μm unlabeled PTH (1–34).
Adenylyl cyclase activity and intracellular calcium determinations
cAMP accumulation was determined as previously described (30). Briefly, ligand-stimulated cAMP accumulation was measured in cells preincubated for 15 min with 3-isobutyl-1-methylxanthine (1 mm) and subsequently exposed to the indicated concentrations of the appropriate ligand for 30 min at 37 C. Reactions were stopped with 1.2 m trichloroacetic acid, and cAMP was isolated by the two-column chromatographic method (32). For the determination of intracellular calcium levels, cells plated on glass-bottom dishes were loaded with the calcium dye Fluo4 (4 μm) in Hanks’ balanced salt solution containing 1.8 mm calcium and 20 mm HEPES (pH 7.4) for 30 min. Cells were washed with Hanks’ balanced salt solution twice. The fluorescent intensity of cells were acquired every 3 sec on a Zeiss LSM 5 Pascal confocal microscope (Carl Zeiss, Thornwood, NY) with a ×25 oil immersion objective.
siRNA preparation and transfection
PTHrP siRNA was generated by Dhamarcon (Thermo Scientific) as follows: 5′-CUCUUUGUACGUCUCCACCdTdT-3′ (sense) and 5′-GGUGGAGACGUACAAAGAGdTdT-3′ (antisense). As a control siRNA, double-stranded, chemically synthesized RNA with green fluorescence (siGLO, Dharmacon, Thermo Scientific) was used. A10 cells were plated onto coverslips or 12-well plates and transfected with siPTHrP (200 nm), siGLO (200 nm) and/or HA-PTH1R (1 μg/ml) using DharmaFECT Duo (3 μl/ml) transfection reagent (Dharmacon, Thermo Scientific) in DMEM with 1% FBS in the absence of antibiotic. Cells were used for experiments after 48 h (for RNA) or 72 h (for protein detection) of transfection. Based on the control siGLO, the transfection efficiency in A10 cells was over 90%.
Real time RT-PCR
Total RNA from A10 cells was obtained by using the RNA extraction kit (QIAGEN, Valencia, CA) following the instructions of the manufacturer. One microgram of total RNA was reverse transcribed in the following reaction buffer (Promega, Madison, WI): ImProm-II reverse transcriptase (1 μl), 1× reaction buffer, ribonuclease inhibitor (20 U), dNTP (10 mm each), and oligo(dT)15 primer (1 μm) at 42 C for 1 h. As a negative control, water was used instead of reverse transcriptase. cDNA was used to amplify PTHrP and GAPDH using specific primer sets (PTHrP, 204 bp, forward 5′-ATGACAAGGGCAAGTCCAT-3′ and reverse 5′-TCTCCACCTTGTTGGTTTCC-3′; GAPDH, 474 bp, 5′-CTCATGACCACAGTCCATGC-3′and 5′-ATGTAGGCCATGAGGTCCAC-3′). PCR was performed in 20 μl reaction condition containing 2 μl cDNA, 1 μl dNTP (10 mm), 1 μl each primer (10 mm), 1 U GoTaq polymerase (Promega), and 15 mm MgCl2 in 20 cycles for the GAPDH and 30 cycles for PTHrP. PCR products were separated on 1.5% agarose gel containing 0.5 μg/ml ethidium bromide.
Quantitative gene expression analysis was performed on an ABI PRISM 7700 using SYBR Green technology. In 48-well optical plates, 10 μl SYBR Green master mix (Applied Biosystems, Warrington, UK) was added to 2 μl cDNA (corresponding to 50 ng total RNA input) and 300 nm forward and reverse primers in water. After 10 min at 95 C, 40 cycles of 15 sec at 95 C, 30 sec at 60 C, and 60 sec at 72 C were applied. At the end of the run, samples were heated to 95 C to check melting curve. The absence of genomic DNA contamination in the RNA preparations was confirmed by using total RNA samples that had not been subjected to reverse transcription. GAPDH was used as the standard housekeeping gene. Relative PTHrP gene expression was normalized by GAPDH expression.
Fluorescence microscopy
To visualize PTHrP expression, cells grown on 25-mm coverslips were fixed for 20 min at room temperature with 4% paraformaldehyde in PBS. Cells were incubated with blocking buffer containing 5% goat serum and 0.2% Nonidet P-40 (NP-40) in PBS. A rabbit anti-PTHrP antibody (Peninsula Laboratories, San Carlos, CA) was applied at a dilution of 1:1000 in the same buffer overnight at 4 C. Coverslips were washed with PBS, incubated with Alexa488-conjugated antirabbit secondary antibody (1:500; Molecular Probes, Eugene, OR) and 4′,6-diamidino-2-phenylindole (DAPI; 0.1 μg/ml; Sigma) for 3 h and washed again. Coverslips were mounted for immunofluorescence microscopy and analyzed with an Olympus Fluoview confocal laser-scanning microscope with a ×63 oil immersion objective. To quantify PTHrP expression, images were acquired in five regions of the coverslip where cells were confluent using an identical microscope setting. The focus was adjusted to obtain maximal intensity of the DAPI signal. The fluorescence intensity of each image was determined with Image J software (National Institutes of Health, Bethesda, MD).
Western blot analysis
Cells were solubilized by incubation for 10 min in ice-cold lysis buffer [150 mm NaCl, 50 mm Tris, 1% Triton X-100, 1 mm EDTA (pH 8.0) containing a protease inhibitor cocktail]. The cell lysates were resolved on 12% SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane, which was then subjected to two sequential 2-h incubations with appropriate antibodies (anti-HA or anti-β-actin) at 1:2000 and horseradish peroxidase-conjugated antimouse IgG antibody at 1:2000. Immunoreactivity was detected by chemiluminescence.
PTH1R expression by ELISA
Cells transiently expressing HA-PTH1R were grown in 12-well plates and treated with various peptides or reagents at 37 C, as indicated. Cells were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 20 min. For determination of total receptor, cells were blocked and permeabilized with 5% milk and 0.2% NP-40 for 20 min at room temperature. For cell surface receptor determinations, NP-40 was omitted. Cells were incubated with anti-HA antibody (Covance Research Products Inc., Berkeley, CA) at 1:2000 after blocking with 5% milk in PBS. Cells were washed three times in PBS and incubated with horseradish peroxidase-conjugated antimouse secondary antibody (1:3000; Cell Signaling Technology, Beverly, MA). Antibody binding was visualized by adding 0.2 ml substrate solution for horseradish peroxidase (BM-blue-POD solution; Roche). After incubation for 20 min at room temperature, the reaction was terminated by adding 0.2 ml 10% sulfuric acid, and the plate was read at 450 nm in a microplate reader.
Proliferation assays
Cells were plated on 24-well plates and cultured until 70–80% confluent. Cells were incubated with 1 μCi/ml [3H]thymidine in the culture media for 18 h at 37 C, rinsed with PBS, and exposed to 10% trichloroacetic acid for 10 min. Trichloroacetic acid was removed and the cell monolayers dissolved in 1 N NaOH for the determination of radioactivity. For BrDU staining, A10 cells were seeded on coverslips and then cultured after transfection. Cells were treated with 100 μm BrDU in DMEM containing 1% FBS for 18 h. Cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.5% of Triton X-100 (Sigma) for 30 min, and incubated with blocking solution (1% BSA and 1% FBS) for 20 min at room temperature. Cells were incubated with anti-BrDU primary antibody (1:100; Biodesign International, Saco, ME) solution containing 1% BSA, RQ1 deoxyribonuclease I (1:10; Promega), and anti-HA antibody (1:500) for 1 h at 37 C. Alexa 488-conjugated donkey antisheep secondary antibody (1:750; Molecular Probes) and Alexa 546-conjugated antimouse secondary antibody (1:500) were then added for 1 h. DAPI was used to stain the nuclei. Images were recorded with an Olympus Fluoview confocal laser-scanning microscope. The number of BrDU-positive cells was counted in five different fields. A BrDU-labeling index was calculated by dividing the number of BrDU-positive cells by the total number of cells expressing HA-tagged receptors in each of the five fields.
Statistical analysis
Results from each experiment were averaged and expressed as mean ± se. Results were analyzed by ANOVA with Tukey’s test or Student’s t test. P values were considered statistically significant when lower than 0.05.
Acknowledgments
We thank Dr. Peter Friedman (University of Pittsburgh, Pittsburgh, PA) for the HA-tagged PTH1R and Dr. Patricia Hinkle (University of Rochester Medical Center, Rochester, NY) for the HA-tagged TRHR. Particular thanks go to Stacey Barrick for her outstanding technical support.
Footnotes
This work was supported by National Institutes of Health Grant DK071158 (to A. B.).
Disclosure Summary: N.F.T. is coinventor on U.S. patent application no. 20050261183. N.F.T. has a financial interest in Vasculostatin LLC. G.S. and A.B. have nothing to disclose.
First Published Online July 2, 2009
Abbreviations: BrDU, Bromodeoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; FBS, fetal bovine serum; HA, heme agglutinin; NP-40, Nonidet P-40; PTH1R, PTH/PTHrP receptor; siRNA, small interfering RNA; VSMC, vascular smooth muscle cell.
References
- Fiaschi-Taesch NM, Stewart AF 2003 Parathyroid hormone-related protein as an intracrine factor: trafficking mechanisms and functional consequences. Endocrinology 144:407–411 [DOI] [PubMed] [Google Scholar]
- Clemens TL, Cormier S, Eichinger A, Endlich K, Fiaschi-Taesch N, Fischer E, Friedman PA, Karaplis AC, Massfelder T, Rossert J, Schlüter KD, Silve C, Stewart AF, Takane K, Helwig JJ 2001 Parathyroid hormone-related protein and its receptors: nuclear functions and roles in the renal and cardiovascular systems, the placental trophoblasts and the pancreatic islets. Br J Pharmacol 134:1113–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Kupfer J, Enomoto H, Sharifi B, Giannella-Neto D, Forrester JS, Singer FR, Goltzman D, Hendy GN, Pirola C 1991 Abundant expression of parathyroid hormone-related protein in primary rat aortic smooth muscle cells accompanies serum-induced proliferation. J Clin Invest 88:1841–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano K, Wu S, Huang X, Pirola CJ, Juppner H, Abou-Samra AB, Segre GV, Iwasaki K, Fagin JA, Clemens TL 1994 Parathyroid hormone (PTH)/PTH-related protein (PTHrP) receptor and its messenger ribonucleic acid in rat aortic vascular smooth muscle cells and UMR osteoblast-like cells: cell-specific regulation by angiotensin-II and PTHrP. Endocrinology 135:1093–1099 [DOI] [PubMed] [Google Scholar]
- Collip JB 1925 The internal secretion of the parathyroid glands. Proc Natl Acad Sci USA 11:484–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter KD, Piper HM 1998 Cardiovascular actions of parathyroid hormone and parathyroid hormone-related peptide. Cardiovasc Res 37:34–41 [DOI] [PubMed] [Google Scholar]
- Mok LL, Nickols GA, Thompson JC, Cooper CW 1989 Parathyroid hormone as a smooth muscle relaxant. Endocr Rev 10:420–436 [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Akishita M, Kozaki K, Toba K, Namiki A, Yamaguchi T, Orimo H, Ouchi Y 2000 Expression of parathyroid hormone-related protein in human and experimental atherosclerotic lesions: functional role in arterial intimal thickening. Atherosclerosis 152:97–105 [DOI] [PubMed] [Google Scholar]
- Pirola CJ, Wang HM, Strgacich MI, Kamyar A, Cercek B, Forrester JS, Clemens TL, Fagin JA 1994 Mechanical stimuli induce vascular parathyroid hormone-related protein gene expression in vivo and in vitro. Endocrinology 134:2230–2236 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Ohtsuru A, Enomoto H, Namba H, Ozeki S, Shibata Y, Yokota T, Nobuyoshi M, Ito M, Sekine I, Yamashita S 1994 Coronary atherosclerotic smooth muscle cells overexpress human parathyroid hormone-related peptides. Biochem Biophys Res Commun 200:1028–1035 [DOI] [PubMed] [Google Scholar]
- Massfelder T, Dann P, Wu TL, Vasavada R, Helwig JJ, Stewart AF 1997 Opposing mitogenic and anti-mitogenic actions of parathyroid hormone-related protein in vascular smooth muscle cells: a critical role for nuclear targeting. Proc Natl Acad Sci USA 94:13630–13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi-Taesch N, Takane KK, Masters S, Lopez-Talavera JC, Stewart AF 2004 Parathyroid-hormone-related protein as a regulator of pRb and the cell cycle in arterial smooth muscle. Circulation 110:177–185 [DOI] [PubMed] [Google Scholar]
- Fiaschi-Taesch N, Sicari BM, Ubriani K, Bigatel T, Takane KK, Cozar-Castellano I, Bisello A, Law B, Stewart AF 2006 Cellular mechanism through which parathyroid hormone-related protein induces proliferation in arterial smooth muscle cells: definition of an arterial smooth muscle PTHrP/p27kip1 pathway. Circ Res 99:933–942 [DOI] [PubMed] [Google Scholar]
- de Miguel F, Fiaschi-Taesch N, López-Talavera JC, Takane KK, Massfelder T, Helwig JJ, Stewart AF 2001 The C-terminal region of PTHrP, in addition to the nuclear localization signal, is essential for the intracrine stimulation of proliferation in vascular smooth muscle cells. Endocrinology 142:4096–4105 [DOI] [PubMed] [Google Scholar]
- Yang KH, Stewart AF 1996 Parathyroid hormone-related protein: the gene, its mRNA species, and protein products. In: Bilezikian JP, Raisz L, Rodan G, eds. Principles of bone biology. San Diego: Academic Press; 347–376 [Google Scholar]
- Abou-Samra AB, Jüppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts Jr JT 1992 Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 89:2732–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Wu S, Jüppner H, Green J, Aragay AM, Fagin JA, Clemens TL 1996 Cell-specific signal transduction of parathyroid hormone (PTH)-related protein through stably expressed recombinant PTH/PTHrP receptors in vascular smooth muscle cells. Endocrinology 137:3154–3162 [DOI] [PubMed] [Google Scholar]
- Wu S, Pirola CJ, Green J, Yamaguchi DT, Okano K, Jueppner H, Forrester JS, Fagin JA, Clemens TL 1993 Effects of N-terminal, midregion, and C-terminal parathyroid hormone-related peptides on adenosine 3′,5′-monophosphate and cytoplasmic free calcium in rat aortic smooth muscle cells and UMR-106 osteoblast-like cells. Endocrinology 133:2437–2444 [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Akishita M, Kozaki K, Toba K, Namiki A, Yamaguchi T, Orimo H, Ouchi Y 1998 Amino-terminal fragment (1–34) of parathyroid hormone-related protein inhibits migration and proliferation of cultured vascular smooth muscle cells. Atherosclerosis 136:59–66 [DOI] [PubMed] [Google Scholar]
- Pirola CJ, Wang HM, Kamyar A, Wu S, Enomoto H, Sharifi B, Forrester JS, Clemens TL, Fagin JA 1993 Angiotensin II regulates parathyroid hormone-related protein expression in cultured rat aortic smooth muscle cells through transcriptional and post-transcriptional mechanisms. J Biol Chem 268:1987–1994 [PubMed] [Google Scholar]
- Bisello A, Chorev M, Rosenblatt M, Monticelli L, Mierke DF, Ferrari SL 2002 Selective ligand-induced stabilization of active and desensitized parathyroid hormone type 1 receptor conformations. J Biol Chem 277:38524–38530 [DOI] [PubMed] [Google Scholar]
- Ureña P, Iida-Klein A, Kong XF, Jüppner H, Kronenberg HM, Abou-Samra AB, Segre GV 1994 Regulation of parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acid by glucocorticoids and PTH in ROS 17/2.8 and OK cells. Endocrinology 134:451–456 [DOI] [PubMed] [Google Scholar]
- Abou-Samra AB, Jueppner H, Potts Jr JT, Segre GV 1989 Inactivation of pertussis toxin-sensitive guanyl nucleotide-binding proteins increase parathyroid hormone receptors and reverse agonist-induced receptor down-regulation in ROS 17/2.8 cells. Endocrinology 125:2594–2599 [DOI] [PubMed] [Google Scholar]
- Yamamoto I, Shigeno C, Potts Jr JT, Segre GV 1988 Characterization and agonist-induced down-regulation of parathyroid hormone receptors in clonal rat osteosarcoma cells. Endocrinology 122:1208–1217 [DOI] [PubMed] [Google Scholar]
- Chauvin S, Bencsik M, Bambino T, Nissenson RA 2002 Parathyroid hormone receptor recycling: role of receptor dephosphorylation and β-arrestin. Mol Endocrinol 16:2720–2732 [DOI] [PubMed] [Google Scholar]
- Behar V, Bisello A, Bitan G, Rosenblatt M, Chorev M 2000 Photoaffinity cross-linking identifies differences in the interactions of an agonist and an antagonist with the parathyroid hormone/parathyroid hormone-related protein receptor. J Biol Chem 275:9–17 [DOI] [PubMed] [Google Scholar]
- Syme CA, Friedman PA, Bisello A 2005 Parathyroid hormone receptor trafficking contributes to the activation of extracellular signal-regulated kinases but is not required for regulation of cAMP signaling. J Biol Chem 280:11281–11288 [DOI] [PubMed] [Google Scholar]
- Ozeki S, Ohtsuru A, Seto S, Takeshita S, Yano H, Nakayama T, Ito M, Yokota T, Nobuyoshi M, Segre GV, Yamashita S, Yano K 1996 Evidence that implicates the parathyroid hormone-related peptide in vascular stenosis. Increased gene expression in the intima of injured carotid arteries and human restenotic coronary lesions. Arterioscler Thromb Vasc Biol 16:565–575 [DOI] [PubMed] [Google Scholar]
- Kim J, Zhang L, Peppel K, Wu JH, Zidar DA, Brian L, DeWire SM, Exum ST, Lefkowitz RJ, Freedman NJ 2008 β-Arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res 103:70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari SL, Behar V, Chorev M, Rosenblatt M, Bisello A 1999 Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves β-arrestin2. Real-time monitoring by fluorescence microscopy. J Biol Chem 274:29968–29975 [DOI] [PubMed] [Google Scholar]
- Wang B, Bisello A, Yang Y, Romero GG, Friedman PA 2007 NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J Biol Chem 282:36214–36222 [DOI] [PubMed] [Google Scholar]
- Salomon Y, Londos C, Rodbell M 1974 A highly sensitive adenylate cyclase assay. Anal Biochem 58:541–548 [DOI] [PubMed] [Google Scholar]