Abstract
In this paper we report the construction of a Schizosaccharomyces pombe strain that facilitates analysis of replicating DNA. The strain co-expresses the Herpes simplex virus thymidine kinase gene (hsv-tk) and a human equilibrative nucleoside transporter (hENT1). The double integrant efficiently incorporates 3H-thymidine into nuclear DNA as monitored by scintillation counting. These strains also incorporate the thymidine analog Bromodeoxy uridine (BUdR) into newly replicated DNA, which can be detected by immunofluorescence and flow cytometry. This strain provides a valuable tool for direct study of DNA replication in S.pombe.
INTRODUCTION
Fission yeast Schizosaccharomyces pombe offers numerous advantages to studies of DNA replication and offers an excellent model system between budding yeast and metazoans (reviewed in 1). Fission yeast is easily manipulated physically and genetically and many mutants defective for DNA replication, as well as the corresponding proteins, have been well characterized. While the budding yeast origin of replication is a 100 bp element with a short, highly conserved consensus sequence, the fission yeast origin is 10 times larger (reviewed in 2–4). There is no consensus sequence in the fission yeast origin, and no single mutation abolishes function (3,5,6). Significantly, a large, diffuse structure appears to be characteristic of metazoan replicators as well (reviewed in 7), suggesting the fission yeast origin is particularly appropriate as a model for function of higher eukaryotic origins. This difference in origin structure may have consequences for the activity and organization of replication initiation proteins, which in many cases behave differently in Saccharomyces cerevisiae than in S.pombe and other cells (see for example 8–13). There is ample precedent for meaningful differences in the two yeast species in the regulation of S phase progression, origin structure and behavior of conserved replication proteins. However, most methods used to assay actual DNA synthesis are indirect, and measure bulk DNA synthesis in a population of cells (e.g. by flow cytometry). It is therefore difficult to ascertain unequivocally whether replication has actually taken place in a single cell.
Mammalian cells efficiently incorporate labeled nucleoside analogs via a thymidine uptake pathway, which acts by importing the compound and phosphorylating it with thymidine kinase (tk). This has permitted single-cell analysis of replication patterns and timing. Yeast cells, however, lack a thymidine kinase gene and rely instead on a significantly different pathway to generate thymidine (14); thus yeast cannot normally incorporate labeled nucleoside analogs. Saccharomyces cerevisiae strains have been engineered to express hsv-tk, allowing conversion of exogenous thymidine to thymidine monophosphate (TMP), which then enters the pathway of thymidine triphosphate formation (15). This pathway can be utilized to incorporate radiolabeled thymidine or halogenated analogs such as 5-bromo-2′-deoxyuridine (BUdR) (16). However, the efficiency of incorporation of these nucleoside analogs is poor. Investigators have compensated for this by eliminating the endogenous pathway, generating mutants that are auxotrophic for TMP (15,17), or using folate agonist drugs such as sulfanilamide, methotrexate and amethopterin (18,19). Alternatively, investigators have integrated multiple copies of the hsv-tk gene (20) or employed methods to enrich for the labeled DNA such as double immunoprecipitations (21). Thymidine uptake (tut) mutants that improve uptake have also been employed (15).
Recently, it was shown that expression of human transporter proteins increases the efficiency of thymidine import into budding yeast cells, making integrated hsv-tk genes more effective (19,22). The human equilibrative nucleoside transporter (hENT1) is an integral membrane protein that facilitates the diffusion of purine and pyrimidine nucleosides down their concentration gradients (19,23). Use of this transporter in S.cerevisiae has enabled phenotypic complementation studies to take place using small concentrations of thymidine (19,23).
This report describes the construction and characterization of a S.pombe strain with integrated hsv-tk and hENT1 transgenes that are constitutively expressed. Together, these allow efficient uptake of exogenous nucleosides such as thymidine and halogenated analogs such as BUdR that can be measured easily to identify the extent of replication in fission yeast cells. This provides a powerful new tool for more detailed study of DNA replication in S.pombe.
MATERIALS AND METHODS
Plasmid construction
To generate plasmid pJAH29, a KpnI linker was inserted into plasmid pYhENT1 (19) by digesting with SphI and filling in the overhang using DNA polymerase I (Klenow). Digesting with KpnI then isolated the hENT1 gene. The hENT1 gene was inserted into a modified version of integration vector pJK148 (24) with the constitutive adh promoter from pEVP11 (25) inserted into its SalI site.
Plasmid pJAH31 was generated by digesting pJL261 (26) with HindIII, filling in with Klenow and subsequently digesting with EcoRI to liberate the adh-tk fragment. This was cloned into the SmaI and EcoRI sites of integration vector pJK210 (24). The intermediate was digested with SpeI, filled in and then digested with KpnI, which was then cloned into the KpnI and SmaI sites of pEA2 (27).
Yeast strains and transformation
All strains (Table 1) were maintained on yeast extract plus supplement (YES) agar plates or under selection on Edinburgh minimal media (EMM) with appropriate supplements using standard techniques. Yeast cells were transformed by electroporation (28).
Table 1. Yeast strains used in this study.
| Strain | Genotype: description |
|---|---|
| T6 | h– leu1-32::[hsv-tk leu1+] ura4-D18 (pf-1 integrant [SV40 hsv-tk leu1+]) from F.Antequera |
| FY6 | h– leu1-32 ura4-294 ade6-704 |
| FY435 | h+ leu1-32 his7-366 ura4-D18 ade6-M210 |
| FY2315 | h+ leu1-32::[hENT1 leu1+] his7-366 ura4-D18 ade6-M210 (pJAH29 integrant) |
| FY2316 | h+ leu1-32 his7-366::[hsv-tk his7+] ura4-D18 ade6-M210 (pJAH31 integrant) |
| FY2317 | h+ leu1-32::[hENT1 leu1+] his7-366::[hsv-tk his7+] ura4-D18 ade6-M210 (pJAH29 and pJAH31 integrant) |
| FY2319 | h– leu1-32::[hENT1 leu1+] ura4-294::[hsv-tk ura4+] ade6-704 (pJAH31 and pJK210-tk+ integrant) |
To generate hENT1 and hsv-tk integrants, the strain FY6 was cotransformed with plasmid pJAH29, linearized in the leu1 gene by NdeI digestion, and another plasmid with adh-tk in pJK210 (24), linearized in the ura4 gene and plated on EMM selecting for leucine and uracil. Transformants were streaked to YES and then back to EMM to confirm stable integration. Expression of hsv-tk was confirmed by streaking to rich media containing 20 mg/l FUdR (Sigma F-0503). FUdR-sensitive transformants were screened for integration at the proper locus and expression of hENT1 and hsv-tk by Southern and northern blot. The integrant FY2319 was mated with strain FY435, sporulated and clones that were leu+ and his– selected for, creating strain FY2315. The strains FY2315 and FY435 were then transformed with pJAH31, which was linearized by digesting in the his7 gene with StuI. The resulting transformants were streaked to rich media and back to minimal to confirm stable integration. Three FUdR-sensitive transformants were selected for both hsv-tk and hENT1/hsv-tk integrants to create strains FY2316 (isolate 7) and FY2317 (isolate 4). Strains FY2316 and FY2317 thus contain integrated hENT1 and hsv-tk at linked loci (leu1 and his7) to facilitate further genetic crosses.
The fission yeast strain T6, which expresses hsv-tk under control of the SV40 promoter was obtained from F. Antequera (21). This strain contains three to four copies of hsv-tk integrated in tandem into the leu1+ gene (F.Antequera, personal communication).
3H-thymidine incorporation assay
Incorporation of 3H-thymidine was performed by growing wild type (FY435) and hENT1- and hsv-tk-containing strains to mid-log phase. Equivalent cell numbers (2.25 × 107 cells) were collected, volumes were normalized and 3H-thymidine (ICN24041.2) was added to a final concentration of 1 µCi/ml. Cells were subsequently labeled for 30 min at 32°C and transferred to 2 ml screwcap tubes and pelleted, then washed in 1 ml cold H2O and pelleted. After removing all but 250 µl of supernatant, cells were resuspended and transferred to a 2 ml screwcap tube containing 300 µl phenol:chloroform and an equal volume of glass beads (Sigma G-8772). Cells were lysed by mechanical shearing in a FastPrep (Bio101, FP120), six pulses of 30 s each at setting 5.5. After spinning for 5 min to separate phases, the aqueous phase was removed to a new tube and ethanol precipitated. The precipitate was aspirated, dried, resuspended in 100 µl TE and transferred to liquid scintillation vials. After adding 4 ml of EcoLume (ICN 882470) scintillation fluid the samples were counted using the 3H channel of a Beckman LS 6000 SC scintillation counter.
Nitrogen starvation and release
Cells were grown to late log growth phase in EMM, harvested and washed twice in EMM lacking nitrogen (EMM –N). Starvation and G1 arrest was achieved by resuspending cells in EMM –N + 7 µg/ml adenine to a volume equal to the original culture and grown overnight at 25°C. Half of the culture was released by transferring to a fresh flask and refeeding with an equal volume of EMM plus appropriate supplements. Cultures were harvested for 3H-thymidine incorporation as described above, except that cells were labeled for 30 min at 25°C after 4 h release at 25°C.
Southern and northern blot analysis
DNA for Southern blotting was prepared as described in (28). For Southern blots, 10 µg of DNA was digested with 20 U of StuI or 40 U of NdeI for 3.5 h. Southern blotting and upward capillary transfer were performed as described in (29), using a 0.6% TAE gel and alkaline transfer to a positively charged nylon membrane (Genescreen Plus, NEN NEF976).
RNA for northern blotting was prepared from 3 × 107 logarithmically growing cells using the RNEasy Mini Kit from Qiagen (cat. 74104). Northern blotting was performed using 10 µg of RNA and formaldehyde gels as described in (29) with an alkaline transfer to a positively charged nylon membrane (Genescreen Plus, NEN NEF976).
Probes for both Southern and northern blots were prepared by restriction digests of plasmids pJAH29 and pJAH31. Southern probes to the leu1+ and his7+ fragments were isolated by digesting with EcoRV or ClaI/XbaI, respectively. Northern probes for hsv-tk and hENT1 were isolated by digesting with BamHI or BbsI/XmnI, respectively.
Both Southern and northern hybridizations used 10 ml Ekono hybridization solution (Gene Technologies, Inc., 786-100) and 95 µg/ml salmon testes DNA (Sigma, D-7656) as prehybridization solution. Twenty-five nanograms of probe were labeled with 50 µCi [α-32P]dCTP (PerkinElmer NEG013H) using the Prime-It II kit (Stratagene 300385), hybridized overnight at 65°C in 10 ml Ekono hybridization solution and washed with SSC/SDS buffer as described (29). Washed blots were exposed for 2 and 3 days, respectively and visualized on a Phosphorimager™ with ImageQuant™ v1.2 software (Molecular Dynamics, Sunnyvale, CA).
Immunofluorescence
Logarithmically growing cells (10 ml) were labeled for 4 h at 25°C in media containing 200 µg/ml BUdR (Sigma B-9285). Flasks were wrapped in aluminum foil to protect cells from light. Whenever possible, cells were kept in the dark while being prepared to protect from DNA damage; all incubations were carried out at room temperature unless otherwise noted. Cells were fixed in 70% ethanol for 10 min, washed in PBS and then treated with 0.5 mg/ml Zymolyase 20T (Seikagaku 120491) in PBS for 10 min. After washing in PBS, cells were resuspended in 1 ml of 4 M HCl and incubated for 10 min to denature the DNA. Cells were washed extensively in PBS then blocked in PBS with 10% fetal calf serum (Invitrogen 16000036) for 1 h. Cells were incubated overnight in α-BUdR antibody (Becton-Dickinson 347580) at 1:50 in PBS with 10% fetal calf serum and 0.05% Tween-20. Cells were then washed in PBS and incubated with α-mouse-AlexaFluor 488 (Molecular Probes 11029) at 1:250 in PBS with 10% fetal calf serum and 0.05% Tween-20 for 2 h. Cells were washed and resuspended in PBS then put on cover slips previously treated with poly-l-lysine (Sigma). DNA was detected with 4-6′ diamidino-2-phenylindole (DAPI). Cells were visualized using a Leica DMR microscope and OpenLab software (Improvision).
Chromosome spreads
Schizosaccharomyces pombe chromosome spreads were prepared as described (30), except that spheroplasted cells washed in MES/sorbitol were resuspended in 100 ml MES/sorbitol plus 360 ml 4% (w/v) paraformaldehyde and spread onto glass slides. After 30 min, excess cells were rinsed off the slides with 0.2% Photoflo (Kodak), air-dried and heat fixed before processing for anti-BUdR immunostaining as described above.
Flow cytometry
Cells grown to early log phase were labeled with BUdR for 4 h as described above, then collected and fixed in 70% ethanol. Cells were washed in 50 mM sodium citrate then treated with 100 µg/ml RNAse in 50 mM sodium citrate for 2 h at 37°C. Four samples containing ∼5 × 106 cells were differentially processed to detect the DNA. One sample was stained with 4 µg/ml propidium iodide (PI). The second sample was processed for immunofluorescence to detect BUdR as described above, except that anti-rabbit fluorescein (FITC) was used as a secondary antibody (Jackson ImmunoResearch, 115-096-062). The third sample was stained for both anti-BUdR followed by FITC and PI. The fourth sample was left unstained. DNA content was monitored using a two-color acquisition on a FACScan (Becton Dickinson) as previously described (31). Data analysis was carried out with Cell Quest software. Nitrogen starved and released cells were stained with Sytox Green as described (32).
RESULTS
Strain construction
To develop a fission yeast strain that could be efficiently labeled with BUdR, we first integrated the herpes virus thymidine kinase hsv-tk into the chromosome at the leu1 locus (26). We observed that expression of hsv-tk in fission yeast alone leads to very inefficient thymidine uptake; moreover, deletion of the fission yeast tmp1+ gene leads to a lethal phenotype that cannot be rescued by exogenous thymidine even in the presence of hsv-tk (J.A.Hodson, unpublished data). Other groups that have expressed a single copy of hsv-tk in fission yeast also observe inefficient nucleoside uptake (N.Rhind, personal communication). Therefore, we tested whether co-expression of the human endonucleoside transporter hENT1 gene could promote thymidine uptake in our strains to increase labeling efficiency. We determined that constitutive expression of hENT1 and hsv-tk together creates an efficient labeling system.
We constructed two plasmids to integrate the hENT1 and the hsv-tk genes under control of the constitutive alcohol dehydrogenase promoter adh1 (Fig. 1A; for details, see Materials and Methods). These plasmids were integrated into the leu1-32 and his7-366 loci, respectively, to generate leu1+::pJAH29 and his7+::pJAH31 (Fig. 1B). The leu1-32 and his7-366 markers were chosen due to their proximity to each other on chromosome II [∼8 cM (33)]. This will facilitate further strain construction by reducing the frequency of recombination between the two integrations, so they will tend to segregate together.
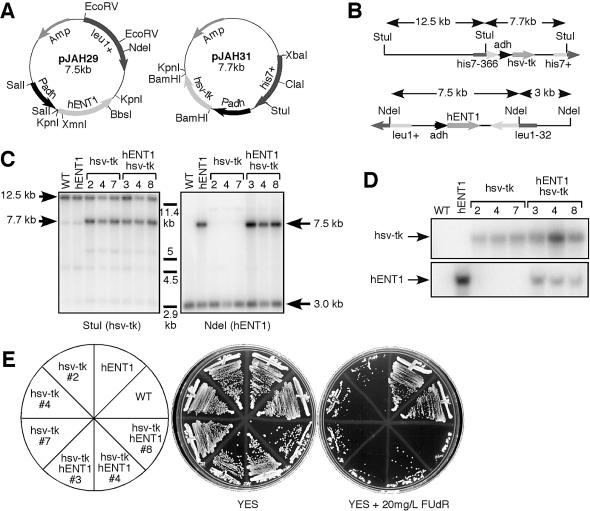
Figure 1.
Construction and confirmation of hENT1/hsv-tk integrants. (A) Diagram of plasmids constructed for integration of hENT1 and hsv-tk. (B) Schematic of integration of hENT1 and hsv-tk at leu1 and his7 loci, respectively. Not drawn to scale. (C) Southern blot confirming integration of hENT1 and hsv-tk at proper loci. (D) Northern blot confirming mRNA expression of hENT1 and hsv-tk. (E) Confirmation of FUdR sensitivity of hENT1/hsv-tk integrants. Cells were streaked onto YES plates and YES plates containing 20 mg/l FUdR and grown for 3 days at 32°C.
DNA and RNA from three his7+ leu1+ isolates were analyzed by Southern and northern hybridization. Figure 1c shows that hsv-tk is integrated properly at the his7+ locus, and hENT1 is integrated at the leu1+ locus (Fig. 1C). Both genes are expressed under control of the constitutive adh promoter as assayed by mRNA levels on a northern blot (Fig. 1D).
Analysis of uptake
Despite inefficient uptake of thymidine analogs, strains expressing hsv-tk are sensitive to the halogenated thymidine derivative FUdR (26). Fluorodeoxyuridine is converted by thymidine kinase to fluorodeoxyuridine monophosphate (FdUMP), which is a potent inhibitor of yeast thymidylate synthesis, preventing DNA replication and leading to cell death (15,34). FUdR sensitivity provides a convenient phenotypic marker for fission yeast strains containing hsv-tk. Our strains FY2316 (hsv-tk) and FY2317 (hsv-tk hENT1) were unable to grow on plates containing FUdR, although some background colonies were able to form, presumably due to selection for rearrangement or recombination of hsv-tk alleles. However, wild type strains, as well as a strain expressing hENT1 alone (FY2315) were both proficient at colony formation (Fig. 1e). Thus, expression of hsv-tk specifically confers sensitivity to FUdR.
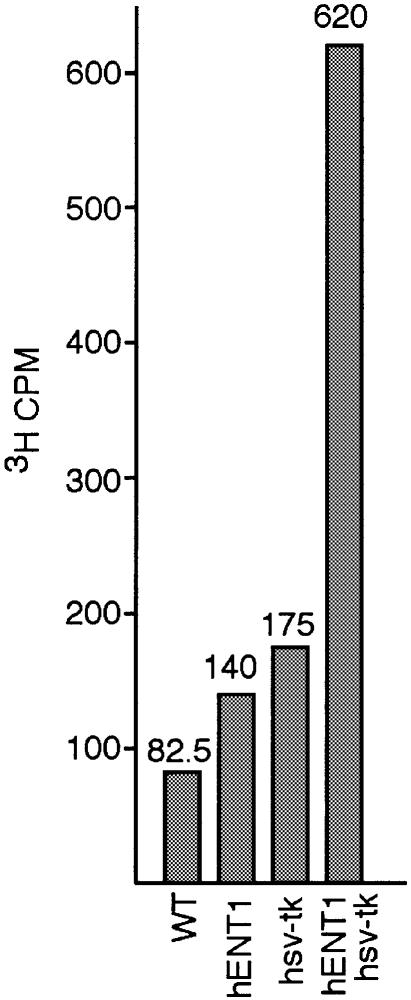
Cells that contain hsv-tk should be able to incorporate 3H-labeled thymidine during DNA replication (16). We tested wild type, hsv-tk, hENT1 and hsv-tk hENT1 strains for thymidine incorporation. When grown in the presence of 3H-thymidine for 30 min, only those cells expressing both transgenes showed incorporation of radiolabeled thymidine (Fig. 2). It should be noted that there is some degree of background associated with this assay, most likely due to the sensitivity of scintillation counting and lack of extensive purification of unincorporated nucleosides in the DNA extraction method.
Figure 2.
Incorporation of 3H-thymidine. Logarithmically growing cells were labeled with 1 µCi/ml 3H-thymidine for 30 min. DNA was extracted and counted on a scintillation counter using the 3H channel.
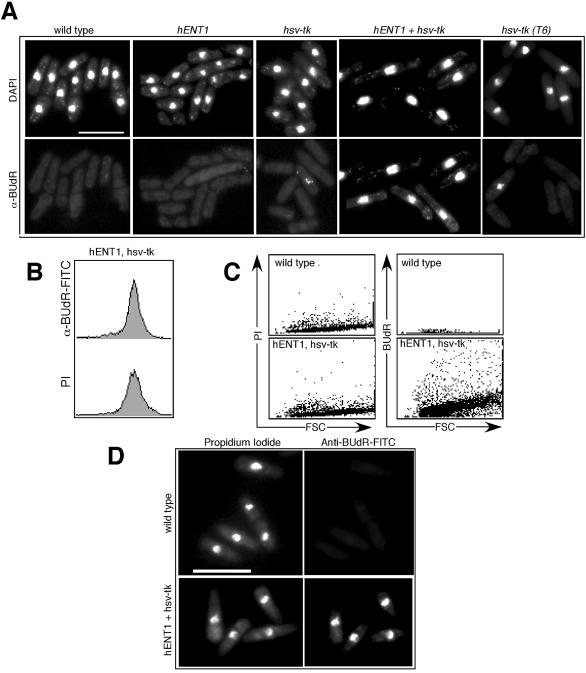
Hsv-tk-expressing cells can also incorporate other derivatives of thymidine such as BUdR (16), which has been used widely in mammalian cells, and more recently in S.cerevisiae, as a marker for replicating DNA (35,36). There are several commercially available α-BUdR antibodies, which can be used to detect incorporation by indirect immunofluorescence. We grew cells in the presence of BUdR for one cell cycle (4 h) and were able to detect nuclear BUdR staining in cells coexpressing hENT1 and hsv-tk when assayed by indirect immunofluorescence (Fig. 3A). More limited BUdR incorporation was detected when cells were BUdR labeled for shorter time periods (J.M.Bailis, unpublished observations). We observed that the timing of cell cycle progression and cell morphology in cells labeled for one cell cycle or less with BUdR appeared similar to wild type, whereas cells became elongated upon extended exposure to BUdR (e.g., more than one cell cycle; J.M.Bailis, unpublished data), presumably due to DNA damage caused by BUdR incorporation. Only limited incorporation of BUdR into the nuclear chromatin was detected in cells expressing hsv-tk alone, in either our parent strain (FY2316) or in the T6 strain, which expresses multiple copies of hsv-tk under control of the SV40 promoter (21). This is consistent with our observations that expression of hsv-tk alone permits only limited thymidine analog uptake.
Figure 3.
Incorporation of BUdR into replicating cells. Logarithmically growing cells were labeled with BUdR for one cell cycle (4 h) and then processed for indirect immunofluorescence and flow cytometry. (A) Cells were stained with anti-BUdR antibody and DAPI. Although the strains containing hsv-tk demonstrate limited uptake of BUdR, only the strain that coexpresses hsv-tk with hENT1 efficiently incorporates BUdR into most of the cells. Scale bar, 10 µm. (B) BUdR labeling can be used to monitor DNA content by flow cytometry. A G2 DNA content, typical of asynchronous S.pombe cells, is observed both when hENT1 hsv-tk cells are stained for PI (propidium iodine, measuring total DNA content) and when cells labeled with BUdR for one cell cycle are detected using anti-BUdR antibody. (C) Comparison of staining by flow cytometry of wild type cells and hENT1 hsv-tk cells following BUdR labeling for one cell cycle. Cells were simultaneously labeled for total DNA with PI and for BUdR with anti-BUdR and FITC. FSC, forward scatter, is a measure of cell size distribution. (D) Cells shown in (C). Bar, 10 µm.
Incorporation of BUdR into replicating DNA can also be monitored by flow cytometry. We labeled cells containing hsv-tk and hENT1 with BUdR for one cell cycle then stained the cells to detect the DNA (using propidium iodide) and to detect BUdR incorporated into the DNA (using anti-BUdR antibodies). Single labeling of the cells with either PI or anti-BUdR revealed a 2C DNA content of the cells, as expected because the cells were labeled with BUdR for an entire cell cycle (Fig. 3B). As shown in Figure 3C, wild type cells showed no significant labeling with BUdR as detected by flow cytometry. Cells could be labeled simultaneously for BUdR incorporation and for total DNA content, which could be distinguished by microscopy (Fig. 3D). The ability to use flow cytometry to monitor newly replicated DNA labeled with BUdR should prove useful in determining the amount of DNA replication that can occur in S phase mutants.
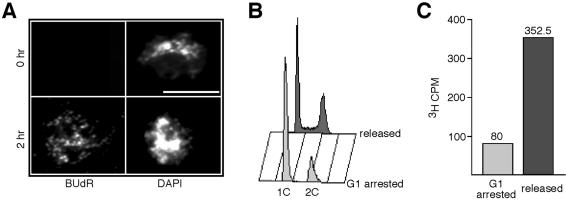
To ensure that only cells actively replicating DNA were able to incorporate BUdR and radiolabeled thymidine, we blocked cells in G1 by nitrogen starvation then released cells into a synchronized S phase. Cells arrested in G1 failed to show BUdR incorporation into chromosomes when assayed by chromosome spreads, whereas synchronously replicating cells did show incorporation (Fig. 4A). Figure 4B confirms that nitrogen starved cells were indeed arrested in G1 and released cells were undergoing S phase as assayed by flow cytometry (Fig. 4B). Arrested cells also failed to incorporate 3H-thymidine as opposed to replicating cells, which showed significant incorporation (Fig. 4C).
Figure 4.
Incorporation of nucleoside analogs in G1-arrested and synchronous cells. (A) Cells from wild type (FY435) and hENT1 hsv-tk (FY2317) were starved in EMM-N plus supplements for 15.5 h, then BUdR was added to 200 µg/ml. Cells were starved for another 30 min before refeeding. Chromosome spreads were prepared at 0, 2 and 4 h after release from nitrogen starvation. Shown are examples of chromosome spreads at the 0 and 2 h time points stained for BUdR and DAPI. Scale bar, 10 µm. (B) DNA content of G1 arrested and released cells. Cells from hENT1 hsv-tk (FY2317) were nitrogen starved or released for 4 h and sampled and stained for flow cytometry. (C) 3H-thymidine incorporation of G1 arrested and released cells. G1 arrested and released FY2317 cells were labeled with 1 µCi/ml 3H-thymidine for 30 min. DNA was extracted and counted on a scintillation counter using the 3H channel.
DISCUSSION
In this report we describe the construction of a S.pombe strain that expresses both the herpes virus thymidine kinase gene hsv-tk and the human endonucleoside transporter 1 hENT1 under the control of separate alcohol dehydrogenase promoters. These genes are integrated into the genome at two loci, which are genetically linked on chromosome II. Location of the integrations was confirmed by Southern hybridization and expression confirmed via northern blot.
Strains expressing hsv-tk alone were unable to grow on media containing FUdR (26), but were not efficiently labeled with 3H-thymidine or BUdR. The strains that express both hENT1 and hsv-tk incorporated 3H-labeled thymidine. Other thymidine analogs like BUdR are also incorporated by strains expressing both transgenes and could be detected by immunofluorescence as well as by flow cytometry. Cells arrested in G1 by nitrogen starvation and not actively replicating DNA did not incorporate significant levels of BUdR or 3H-thymidine in comparison to similar strains released out of nitrogen starvation.
The disparity in background levels of nucleoside analog uptake in the 3H-thymidine incorporation assay versus anti-BUdR immunofluorescence may be due to the varying levels of sensitivity between the two techniques. Scintillation counting is extremely sensitive, and our DNA preparation method is relatively crude, so it is likely this assay would have a high level of background. In contrast, indirect immunofluorescence requires binding of multiple ligands and is likely to have a threshold limit of detection below which any signal is unable to be visualized. Furthermore, the different nucleoside analogs may be taken up with varying efficiencies, which are not directly comparable when different concentrations of the analogs are used.
This strain can be a powerful tool for study of DNA replication. The tight linkage of the two integrations should facilitate cloning of replication mutants since most (92%) offspring of a cross should contain both the hsv-tk and hENT1 genes. Additionally, the ease of screening for presence of the hsv-tk gene by FUdR sensitivity greatly simplifies confirmation of products.
Previous studies have been unable to obtain significant levels of thymidine kinase activity unless folate agonist drugs such as sulfanilamide and methotrexate were used to induce thymidine starvation (18) or mutants in thymidine uptake are included (17). The inclusion of hENT1 eliminates the need for folate agonists or uptake mutants by increasing the intake of exogenous thymidine to detectable levels. A previous study relied on expression of multiple copies of hsv-tk alone under control of the SV40 promoter in strain T6 (21). Although that study showed significant incorporation of BudR when multiple immunoprecipitations were carried out, we found that we could not observe significant BudR staining by indirect immunofluorescence in either our strain (FY2316) or the previously published strain (T6), both of which express hsv-tk alone. In contrast, we easily detected BudR by this method in our strain FY2317 coexpressing hENT1. There may be a higher threshold level of BudR incorporation needed for cytological visualization. Thus the S.pombe strain that we describe here allows for robust, reproducible detection of BudR incorporation by indirect immunofluorescence, providing an important cytological tool.
Another manner in which this strain will facilitate the study of replication is by providing a direct method by which DNA synthesis can be assayed and quantified. Radiolabeled thymidine can be easily monitored by scintillation counting, and incorporated BUdR or other analogs can be visualized by indirect immunofluorescence or flow cytometry. This will enable researchers to visualize and quantify the amount of synthesis taking place, even in single cells. Analysis of various replication mutants in the hsv-tk/hENT1 strain background can be used to confirm whether or not such alleles are essential for DNA synthesis, and whether the mutants undergo partial DNA replication or complete S phase. Furthermore, it is now possible to carry out pulse–chase experiments with different nucleoside analogs, such as BUdR followed by chlorodeoxyuridine (CldU) to compare early and late replication patterns as has been demonstrated in mammalian cells (37). The combination of this strain with molecular combing techniques, which has proved a powerful approach in both S.cerevisiae and mammalian cells, could allow the visualization of replicated stretches of chromosomes by microscopy, shedding light on such topics as origin usage and polymerase processivity (20). This knowledge would be especially useful since these features in S.pombe are much more similar to those of higher eukaryotes than in S.cerevisiae (1).
In summary, we describe a system in S.pombe that allows cells to uptake labeled nucleoside analogs, by co-expressing thymidine kinase with a nucleoside transporter. This opens a wide variety of applications to the field of S.pombe DNA replication which have not been available to researchers before now.
Acknowledgments
ACKNOWLEDGEMENTS
We thank: Carol Cass, Mark Vickers and other members of the Cass laboratory for their helpful advice and generous contribution of the YhENT1 plasmid; Nick Rhind for tips on 3H-thymidine incorporation assays and communication of unpublished work; Francisco Antequera for providing the T6 strain and additional information; and Joel Huberman for helpful suggestions for improving this manuscript. This study was supported by NIH GM59321 to S.L.F. J.M.B. was supported by the Damon Runyon Cancer Research Foundation (DRG-1634).
REFERENCES
- 1.Forsburg S.L. (1999) The best yeast. Trends Genet., 15, 340–344. [DOI] [PubMed] [Google Scholar]
- 2.Newlon C.S. and Theis,J.F. (1993) The structure and function of yeast ARS elements. Curr. Opin. Gen. Dev., 3, 752–758. [DOI] [PubMed] [Google Scholar]
- 3.Clyne R.K. and Kelly,T.J. (1995) Genetic analysis of an ARS element from the fission yeast Schizosaccharomyces pombe. EMBO J., 14, 6348–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J., Carlson,D.L., Dubey,D.D., Sharma,K. and Huberman,J.A. (1994) Comparison of two major ARS elements of the ura4 replication origin region with other ARS elements in the fission yeast Schizosaccharomyces pombe. Chromosoma, 103, 414–422. [DOI] [PubMed] [Google Scholar]
- 5.Kim S.M. and Huberman,J.A. (1998) Multiple orientation-dependent, synergistically interacting, similar domains in the ribosomal DNA replication origin of the fission yeast, Schizosaccharomyces pombe. Mol. Cell Biol., 18, 7294–7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubey D.D., Kim,S.-M., Todorov,I.T. and Huberman,J.A. (1996) Large, complex modular structure of a fission yeast DNA replication origin. Curr. Biol., 6, 467–473. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert D.M. (2001) Making sense of eukaryotic DNA replication origins. Science, 294, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen V.Q., Co,C., Irie,K. and Li,J.J. (2000) Clb/Cdc28 kinases promote nuclear export of the replication initiator proteins Mcm2-7. Curr. Biol., 10, 195–205. [DOI] [PubMed] [Google Scholar]
- 9.Labib K., Kearsey,S.E. and Diffley,J.F. (2001) MCM2-7 proteins are essential components of prereplicative complexes that accumulate cooperatively in the nucleus during G1-phase and are required to establish, but not maintain, the S-phase checkpoint. Mol. Biol. Cell, 12, 3658–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasion S.G. and Forsburg,S.L. (1999) Nuclear localization of fission yeast Mcm2/Cdc19p requires MCM complex formation. Mol. Biol. Cell, 10, 4043–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman D.A. and Forsburg,S.L. (1998) Schizosaccharomyces pombe Mcm3p, an essential nuclear protein, associates tightly with Nda4p (Mcm5p). Nucleic Acids Res., 26, 3955–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okishio N., Adachi,Y. and Yanagida,M. (1996) Fission yeast nda1 and nda4, MCM homologs required for DNA replication, are constitutive nuclear proteins. J. Cell Sci., 109, 319–326. [DOI] [PubMed] [Google Scholar]
- 13.Maiorano D., Blom van Assendelft,G. and Kearsey,S.E. (1996) Fission yeast cdc21, a member of the MCM protein family, is required for onset of S phase and located in the nucleus throughout the cell cycle. EMBO J., 15, 861–872. [PMC free article] [PubMed] [Google Scholar]
- 14.Grivell A.R. and Jackson,J.F. (1968) Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms and the relevance of this to the specific labelling of deoxyribonucleic acid. J. Gen. Microbiol., 54, 307–317. [DOI] [PubMed] [Google Scholar]
- 15.Sclafani R. and Fangman,W.L. (1986) Thymidine utilization by TUT mutants and facile cloning of mutant alleles by plasmid conversion in S. cerevisiae. Genetics, 114, 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNeil J.B. and Friesen,J.D. (1981) Expression of the Herpes simplex virus thymidine kinase gene in Saccharomyces cerevisiae. Mol. Gen. Genet., 184, 386–393. [DOI] [PubMed] [Google Scholar]
- 17.Leff J. and Lam,K.B. (1976) Bromodeoxyuridine 5′-monophosphate incorporation into yeast nuclear and mitochondrial deoxyribonucleic acid. J. Bacteriol., 127, 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little J.G. and Haynes,R.H. (1979) Isolation and characterization of yeast mutants auxotrophic for 2′-deoxythymidine 5′-monophosphate. Mol. Gen. Genet., 168, 141–151. [DOI] [PubMed] [Google Scholar]
- 19.Vickers M.F., Mani,R.S., Sundaram,M., Hogue,D.L., Young,J.D., Baldwin,S.A. and Cass,C.E. (1999) Functional production and reconstitution of the human equilibrative nucleoside transporter (hENT1) in Saccharomyces cerevisiae. Biochem. J., 339, 21–32. [PMC free article] [PubMed] [Google Scholar]
- 20.Lengronne A., Pasero,P., Bensimon,A. and Schwob,E. (2001) Monitoring S phase progression globally and locally using BrdU incorporation in TK+ yeast strains. Nucleic Acids Res., 29, 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez M. and Antequera,F. (1999) Organization of DNA replication origins in the fission yeast genome. EMBO J., 18, 5683–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths M., Beaumont,N., Yao,S.Y., Sundaram,M., Boumah,C.E., Davies,A., Kwong,F.Y., Coe,I., Cass,C.E., Young,J.D. and Baldwin,S.A. (1997) Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nature Med., 3, 89–93. [DOI] [PubMed] [Google Scholar]
- 23.Vickers M.F., Young,J.D., Baldwin,S.A., Ellison,M.J. and Cass,C.E. (2001) Functional production of mammalian concentrative nucleoside transporters in Saccharomyces cerevisiae. Mol. Memb. Biol., 18, 73–79. [PubMed] [Google Scholar]
- 24.Keeney J.B. and Boeke,J.D. (1994) Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics, 136, 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell P. (1989) Gene cloning and expression in fission yeast. In Nasim,A., Young,P. and Johnson,B.F. (eds), Molecular Biology of the Fission Yeast. Academic Press, San Diego, CA, pp. 244–272. [Google Scholar]
- 26.Kiely J., Haase,S.B., Russell,P. and Leatherwood,J. (2000) Functions of fission yeast Orp2 in DNA replication and checkpoint control. Genetics, 154, 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apolinario E., Nocero,M., Jin,M. and Hoffman,C.S. (1993) Cloning and manipulation of the Schizosaccharomyces pombe his7+ gene as a new selectable marker for molecular genetic studies. Curr. Genet., 24, 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Methods Enzymol., 194, 795–823. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J. and Russell,D. (2001) Molecular Cloning: A Laboratory Manual, 3rd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, NY. [Google Scholar]
- 30.Ogawa Y., Takahashi,T. and Masukata,H. (1999) Association of fission yeast Orp1 and Mcm6 proteins with chromosomal replication origins. Mol. Cell. Biol., 19, 7228–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sazer S. and Sherwood,S.W. (1990) Mitochondrial growth and DNA synthesis occur in the absence of nuclear DNA replication in fission yeast. J. Cell Sci., 97, 509–516. [DOI] [PubMed] [Google Scholar]
- 32.Liang D.T., Hodson,J.A. and Forsburg,S.L. (1999) Reduced dosage of a single fission yeast MCM protein causes genetic instability and S phase delay. J. Cell Sci., 112, 559–567. [DOI] [PubMed] [Google Scholar]
- 33.Kohli J., Hottinger,H., Munz,P., Strauss,A. and Thuriaux,P. (1977) Genetic mapping in Schizosaccharomyces pombe by mitotic and meiotic analysis and induced haploidization. Genetics, 87, 471–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bisson L. and Thorner,J. (1977) Thymidine 5′-monophosphate-requiring mutants of Saccharomyces cerevisiae are deficient in thymidylate synthetase. J. Bacteriol., 132, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan B. and Karpen,G. (2001) Centromere identity in Drosophila is not determined in vivo by replication timing. J. Cell Biol., 154, 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Versini G., Comet,I., Wu,M., Hoopes,L., Schwob,E. and Pasero,P. (2003) The yeast Sgs1 helicase is differentially required for genomic and ribosomal DNA replication. EMBO J., 22, 1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blower M.D., Sullivan,B.A. and Karpen,G.H. (2002) Conserved organization of centromeric chromatin in flies and humans. Dev. Cell, 2, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]