Abstract
Introduction
Dysregulation of neuronal networks has been suggested to underlie the cognitive and perceptual abnormalities observed schizophrenia.
Discussions
An in vitro model of psychosis is proposed based on the two different approaches to cause aberrant network activity in layer V pyramidal cells of prefrontal brain slices: (1) psychedelic hallucinogens such as lysergic acid diethylamide and (2) minimal GABAA receptor antagonism, modeling the GABA interneuron deficit in schizophrenia. A test of this model would be to determine if drugs that normalize aberrant networks in brain slices have efficacy in the treatment of schizophrenia. Selective agonists of glutamate mGlu2/3 metabotropic receptors, which are highly effective in suppressing aberrant network activity in slices, are the most advanced toward reaching that clinical endpoint. In accord with the model, a recent phase II clinical trial shows that an mGlu2/3 receptor agonist is equivalent in efficacy to a standard antipsychotic drug for both negative and positive symptoms in schizophrenic patients, but without the usual side effects. D1/5 dopamine receptor agonists are also effective in normalizing aberrant network activity induced by both hallucinogens and minimal GABAA antagonism; clinical efficacy remains to be determined. A general model of network regulation is presented, involving astrocytes, GABA interneurons, and glutamatergic pyramidal cells, revealing a wide range of potential sites hitherto not considered as therapeutic targets.
Keywords: Antipsychotic, Astrocyte, GABA, Glutamate, Hallucinogens, Network, Prefrontal cortex, Pyramidal cell, Schizophrenia, Serotonin
Introduction
The hallmark of cortical circuits both in vivo and in vitro is the prominence of periodic states of depolarization (UP states) that interact with internal and external inputs (McCormick et al. 2003; Petersen et al. 2003). Riding upon these UP states is a nearly balanced mixture of excitatory and inhibitory synaptic potentials both in vivo (Haider et al. 2006) and in vitro (Shu et al. 2003), indicative of recurrent network activation. The association with recurrent network activation distinguishes this type of UP state from non-network related UP states that are generated intrinsically within a neuron. Network-generated UP states have been suggested to provide a neuronal “context” within which information is interpreted and decisions are made (McCormick 2005). It has been hypothesized that aberrations in the modulation of neuronal networks may account for perceptual and cognitive abnormalities in schizophrenia (Winterer and Weinberger 2004; Lewis et al. 2005). In schizophrenic patients, and to a lesser degree their siblings, there is an elevated level of background electrophysiological noise, especially in prefrontal regions, leading to a decrease in the signal to noise ratio (Winterer and Weinberger 2004). Within this framework, schizophrenia can be seen as a disease of disordered network regulation in which high fluctuating background noise interferes with the stability of cortical representations of external and internal stimuli.
Modeling “psychosis” in prefrontal cortical slices
The elements of recurrent network activity are preserved in brain slices of prefrontal cortex. UP states and associated network activity may occur spontaneously or be evoked with an electrical stimulus applied to mid-layers of ferret cortical slice (McCormick et al. 2003; Shu et al. 2003) or applied to thalamus in mouse thalamocortical slice (Beierlein et al. 2002; Rigas and Castro-Alamancos 2007). Of particular interest for this review is that stimulus-induced UP states can be enhanced markedly in layer V pyramidal cells of prefrontal cortex by psychedelic hallucinogens acting via serotonin 5-HT2A receptors (Aghajanian and Marek 1999). Originally, this type of UP state was termed “asynchronous” late excitatory postsynaptic currents (EPSCs). However, as the hallucinogen-enhanced “late EPSCs” in layer V pyramidal cells in rat brain slice are comprised of mixed inhibitory and excitatory components rather than simply being EPSCs (Lambe and Aghajanian 2007), they clearly represent the persistent activity of an activated network. On that basis, it is preferable to use the term “recurrent network activity” instead of “UP state”, as the latter term has also been used to denote non-network generated plateau potentials. The rapid alteration between EPSCs and IPSCs results in recurrent network activity that is prominently in the gamma frequency range both under basal conditions (McCormick et al. 2003; Shu et al. 2003) and when enhanced by hallucinogens (Lambe and Aghajanian 2007). Interestingly, the elevated electrophysiological “noise” that occurs in schizophrenic patients also has a prominent gamma frequency component (Winterer and Weinberger 2004), but it not known directly if this is generated by intrinsic recurrent network activity.
The intensity of stimuli evoking recurrent activity must be kept within a narrow window: higher intensities recruit negative feedback inhibition sufficient to suppress the recurrent activity, indicative of the importance by inhibitory circuits in its regulation (Lambe and Aghajanian 2006; Rigas and Castro-Alamancos 2007). In this regard, it is interesting that reducing tonic inhibition with a low concentration of the GABAA antagonist bicuculline enhances stimulus-induced network activity in a manner that resembles in many respects the effect of hallucinogens (Aghajanian and Marek 1999; Lambe and Aghajanian 2007).
The enhancement of network activity by hallucinogens or minimal GABA antagonism is characterized by an increase in duration and a decrease in refractory period, which increases the probability a response will occur during repeated low frequency stimulation (Aghajanian and Marek 1999). The effects of hallucinogens are prevented by interfering with the diffusion of synaptic glutamate, indicating a dependence upon spillover of synaptic glutamate into the extrasynaptic space (Lambe and Aghajanian 2006). These electrophysiological indicators of glutamate spillover are paralleled in vivo by microdialysis studies showing that LSD (d-lysergic acid diethylamide) and mescaline-like hallucinogens induce an increase in extracellular glutamate levels in prefrontal cortex (Scruggs et al. 2003; Muschamp et al. 2004). Consistent with a glutamate spillover mechanism, the hallucinogen-induced recurrent activity is blocked by selective antagonists of the NR2B subtype of NMDA receptor (Lambe and Aghajanian 2006), which is mainly located extrasynaptically in mature brain (Charton et al. 1999). Interestingly—perhaps because of a primarily extrasynaptic location—selective NR2B antagonists do not produce psychotomimetic effects in human subjects (Merchant et al. 1999) and cognitive deficits in rodents (Higgins et al. 2005) that are typically seen with non-selective NMDA antagonists such as ketamine or phencylidine (Krystal et al. 1994). As yet, no clinical trials with selective NR2B antagonists in schizophrenic patients have been reported.
The ability of psychedelic hallucinogens to increase glutamate spillover, taken together with its dependence on extrasynaptic NR2B receptors, forms the basis for the spillover model of recurrent network activity. As depicted in Fig. 1, the poststimulus response consists of two components: (1) recurrent network activity and (2) a slow inward current (SIC). LSD as well as other psychedelic hallucinogens cause the wave of recurrent activity to spread to cells that otherwise would not be activated by a given external or internal stimulus (Lambe and Aghajanian 2006). A spreading of network activity between different neuronal ensembles would have the effect of breaking down the barriers between set of neurons in otherwise distinct neuronal ensembles. However, there is no experimental evidence that such a mechanism is responsible for the blurring of perceptual and cognitive boundaries reported by people who have ingested psychedelic hallucinogens. Furthermore, it is not known whether similar mechanisms underlie the network abnormalities that have been proposed to occur in psychoses such as schizophrenia (Winterer and Weinberger 2004; Lewis et al. 2005). Nevertheless, the induction of aberrant network activity in prefrontal brain slice represents a potentially useful in vitro model for discovery of novel sites involved in the regulation of intrinsic cortical networks.
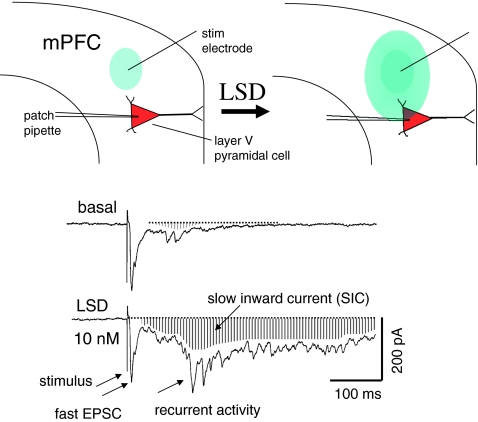
Fig. 1.
LSD enhances spread of recurrent network activity in medial prefrontal cortex (mPFC) induced by focal electrical stimulation (stim electrode) (top diagram). Recurrent activity, recorded in mPFC slice by whole cell patch pipette, is depicted as spreading from initial zone (blue oval) to impinge upon a more distant layer V pyramidal cell. Traces below show example of LSD-induced increase in recurrent activity over basal condition; recurrent activity consists of a mix of EPSCs and IPSCs. Downward deflection of baseline reveals a slow inward current or SIC (shaded area) accompanying the recurrent activity. Also note that the fast EPSC is not altered appreciably (provided by G. Aghajanian)
There has been considerable debate over whether the psychedelic hallucinogens or non-competitive NMDA antagonists or NMDA antagonists such as ketamine more faithfully model naturally occurring psychoses such as schizophrenia. A recent double-blind crossover study in healthy volunteers has addressed this issue directly in by comparing the psychological effects of the psychedelic hallucinogen N,N-dimethyltrytamine (DMT) with sub-anesthetic doses of the non-competitive NMDA receptor antagonist (S)-ketamine (Gouzoulis-Mayfrank et al. 2005). The authors conclude that the two classes of drugs model different aspects of schizophrenia: positive symptoms being more prominent with DMT while negative symptoms are more pronounced with ketamine. Interestingly, on a mechanistic level, both the psychedelic hallucinogens (Scruggs et al. 2003; Muschamp et al. 2004) and NMDA antagonists (Moghaddam et al. 1997) produce an increase in extracellular glutamate levels in prefrontal cortex. Based on that shared ability to increase glutamate release, it has been suggested that hallucinogens and NMDA receptors antagonists share certain components of a final glutamatergic pathway (Aghajanian and Marek 2000). However, it is not surprising that the clinical pattern of response to the two types of agents differ since the initial receptor sites at which NMDA antagonists and hallucinogens produce this effect is quite different. In vivo studies indicate that sub-anesthetic doses of NMDA agonists in prefrontal cortex acts predominantly to decrease the firing of putative GABA interneurons, leading to a disinhibition of glutamatergic pyramidal cells (Homayoun and Moghaddam 2007). Such a disinhibitory effect of NMDA antagonists is not seen in prefrontal slice, presumably because interneurons have little spontaneous activity in this preparation. Nevertheless, a disinhibitory effect can be approximated in the slice by minimal GABAA receptor antagonism, an effect resembling that of hallucinogens in promoting strong aberrant network activity (Aghajanian and Marek 1999; Lambe and Aghajanian 2007). Each of these two approaches for inducing aberrant network activity is of interest in its own way: (1) the effect of hallucinogens is suggestive of possible mechanisms by which disordered cortical networks may affect cognition and perception and (2) the minimal GABAA antagonist paradigm represents a way of modeling in vitro the GABA deficit in schizophrenia (Ford et al. 2007).
The usefulness of the in vitro network model ultimately will depend on whether novel treatments specifically designed to normalize aberrant network activity in brain slice predict efficacy in ameliorating psychosis in patients. As recurrent network activity involves a complex interaction between glutamatergic pyramidal cells, GABAergic interneurons, and glial cells. This complexity offers the opportunity for intervening at diverse sites within the network. A few of these that are most advanced toward clinical testing are discussed in the following sections.
Suppression of aberrant network activity by mGluR 2/3 agonists
One way to limit the effects of glutamate spillover upon network activity would be to restrict glutamate release. The mGluR2 and mGluR3 metabotropic glutamate autoreceptors are known to serve as negative feedback regulators of glutamate release (Conn and Pin 1997). On that basis, it was hypothesized that mGluR2/3 agonist would reduce hallucinogen-induced glutamate overflow and associated aberrant network activity. Consistent with that idea, early studies in prefrontal slices showed that fast synaptic EPSCs induced by serotonin via 5-HT2A receptors were suppressed by treatment with a preferential mGluR2/3 agonist (Aghajanian and Marek 1997). Subsequently, it was found that a more selective mGluR2/3 agonist LY354740 was highly efficacious in suppressing the enhancement recurrent network activity induced by the mescaline-like hallucinogen DOI (Marek et al. 2000; Fig. 2, lower panel).
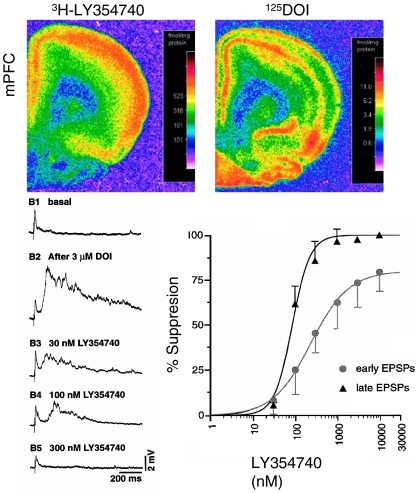
Fig. 2.
Autoradiograpy showing overlap between binding at mGlu2/3 receptors (labeled by 3H-SCH354740) and 5-HT2A/C receptors of the mescaline-like hallucinogen 125DOI (top) in mPFC. Traces (lower left) show marked enhancement of basal late evoked slow depolarization by the mescaline-like hallucinogen DOI in a layer V mPFC cell (B2); note synaptic potentials riding upon the wave of depolarization. Subsequent traces show a dose-dependent suppression of the DOI effect by the mGlu2/3 agonist LY354740. Plot (lower right) shows summary dose–response data. Note the late “EPSP” is more sensitive to LY354740 than the early EPSP. Also note that the recordings were in current clamp rather than voltage clamp mode; thus, unlike Figs. 1, 3, and 4, the responses are given in terms of potential rather current—its voltage clamp counterpart. Montage adapted from Marek et al. 2000
In view of the striking laminar overlap between 5-HT2 receptors labeled by the hallucinogen DOI and mGluR2/3 receptors labeled by LY354740 in layer V of medial prefrontal cortex (Fig. 2, upper panel), it was originally assumed that the interaction between 5-HT2A and mGluR2/3 receptors simply represented a physiological antagonism at separate molecular or cellular sites (Marek et al. 2000). However, a recent study reported that 5-HT2A and mGluR2 receptors form a macromolecular complex, allowing allosteric interactions to occur between the two receptors in the same cells (Gonzalez-Maeso et al. 2008). Particularly intriguing is the possibility that activation of 5-HT2A receptors by the hallucinogen DOI may lower the affinity of mGlu2 receptors for mGlulR2/3 agonists. A reduced affinity of mGluR2 receptors for glutamate would impair their negative feedback function in opposing an excessive increase in recurrent network activity in response to elevated extracellular glutamate. In contrast, when extracellular glutamate is raised through inhibition of glutamate uptake, there is strong activation of mGluR2/3 receptors and suppression of recurrent activity (Lambe and Aghajanian 2006).
Based on the above in vitro electrophysiological studies as well as in vivo microdialysis studies using the phencylidine/ketamine model (Moghaddam and Adams 1998), it has been suggested that mGlu2/3 agonists have potential as therapeutic agents in schizophrenia or other psychoses. However, early clinical trials employing LY354740, a first generation drug, proved to be disappointing. Subsequently, LY2140023—a prodrug of the mGlu2/3 agonist LY404039 with improved bioavailability—was tested in a randomized, double-blind phase 2 clinical trial (Patil et al. 2007). Over a 4-week period, LY2140023 was found to be equivalent in efficacy to the commonly used “atypical” antipsychotic drug olanzapine in ameliorating both negative and positive symptoms. However, in contrast to traditional antipsychotic drugs, LY2140023 did not lead to any increase in body weight, prolactin, or extrapramidal symptoms. The absence of such side effects can be explained by the fact that LY404039, the active metabolite of LY2140023, is a glutamate analog that does not act upon dopamine, norepinephrine, serotonin, and many other common neurotransmitter receptors that have been implicated in producing these effects (Rorick-Kehn et al. 2007).
The positive clinical results with LY2140023, if confirmed, lend credence to the idea that normalization of aberrant network activity in the prefrontal slice may be a useful model for discovering novel agents in the treatment of schizophrenia. It should be noted, however, that the beneficial effects of LY2140023 emerged no faster than with a standard antipsychotic drug, developing slowly over 4 weeks (Patil et al. 2007). This contrasts with the rapid suppression of recurrent network activity in vitro, which occurs within minutes after application of mGluR2/3 agonists (Marek et al. 2000). The delayed clinical response suggests that there may be molecular or structural deficits in schizophrenia that are slow to reverse (see below).
D1/5 agonists and recurrent network activity
As hallucinogen-enhanced network activity depends on glutamate spillover (Lambe and Aghajanian 2006) and associated increase in extracellular glutamate (Scruggs et al. 2003; Muschamp et al. 2004), it was hypothesized treatments that decrease extracellular glutamate in prefrontal cortex would have the opposite effect. This property is displayed by D1/5 dopamine receptor agonists, which have been found to cause a decrease in extracellular glutamate in vivo in prefrontal cortex (Abekawa et al. 2000; Harte and O’Connor 2004). As illustrated in Fig. 3 (upper traces), D1/5 receptor agonists such as SKF38393 are extremely potent suppressants of both basal and hallucinogen-induced network activity in prefrontal slices (Lambe and Aghajanian 2007). Unlike mGluR2/3 agonists, D1/5 agonists do not cause any reduction of the fast EPSC. The suppression of recurrent network activity by SKF 38393 is mimicked by forskolin, a direct activator of adenylyl cyclase, and by 8-Br-cAMP, a phosphodiesterase-resistant analog of cAMP, consistent with the known coupling of D1/5 receptors through the Gs/cAMP pathway. These results are paralleled by recent optical imaging studies in prefrontal slices showing that D1/D5 agonists decrease the amplitude, duration, and lateral spread of activation in local cortical networks (Bandyopadhyay and Hablitz 2007).
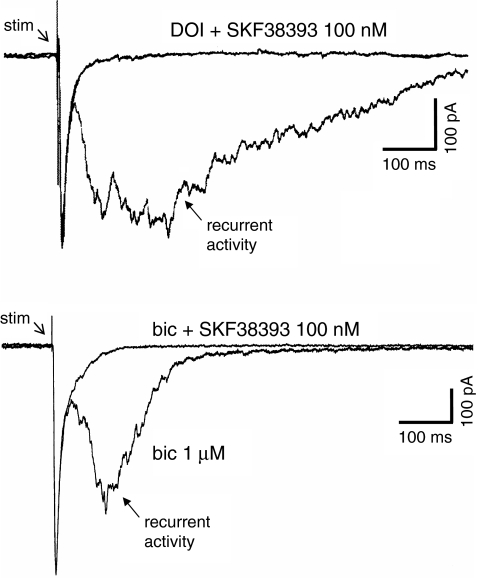
Fig. 3.
Recurrent activity (and associated SIC) induced by either DOI (upper traces) or the GABAA antagonist bicuculline (bic) (lower traces) is suppressed by a nanomolar concentration of the D1 dopamine receptor antagonist SKF383393. Note in the superimposed traces that there is no reduction in the fast EPSC. Modified from Lambe and Aghajanian 2007
In behaving animals, D1 receptor activation dose-dependently suppresses the sustained neuronal firing that takes place during the “delay” period in prefrontal cortex engaged in a working memory task (Vijayraghavan et al. 2007). This suppression has an inverted-U relation to working memory: moderate levels of D1 receptor stimulation partially reduces firing rate but leads to an enhancement in spatial tuning, whereas at higher levels of D1 stimulation, the suppression of firing becomes more pronounced, leading to losses in spatial information capacity and detuning of spatial memory-related information. As these in vivo studies did not examine the relationship of delay firing to recurrent network activity, a direct comparison cannot be made to the progressive suppressant effect of D1 agonists on recurrent activity that we find in vitro in the prefrontal slice (Lambe and Aghajanian 2007). Nevertheless, as delay firing is most likely sustained by recurrent networks (McCormick et al. 2003), there may be a common mechanism underlying the two phenomena.
There are at least two possible mechanisms by which D1/D5 receptors could limit excitation and promote inhibition in cortical networks. First, through a presynaptic effect, D1/D5 receptor stimulation directly attenuates recurrent excitation in layer V pyramidal neurons (Gao et al. 2001). Second, D1/D5 receptors can enhance inhibition within the network by directly exciting GABAergic neurons (Seamans et al. 2001; Gorelova et al. 2002; Bandyopadhyay and Hablitz 2007). Thus, multiple mechanisms are likely to contribute to a D1/D5 receptor-mediated suppression of hallucinogen-induced recurrent activity. Interestingly, D1/5 agonists are also able to suppress the increase in network activity produced by low concentrations of the GABAA antagonist bicuculline (Fig. 3, lower traces), demonstrating the ability of D1/5 agonists to restore inhibitory balance in networks in a variety of situations.
On the basis of various clinical and preclinical studies it has been proposed that D1/D5 agonists may be useful in treatment of schizophrenia (Abi-Dargham and Moore 2003; Marcus et al. 2005). Clinical trials show that D1/D5 antagonists, in contrast to D2 receptor antagonists, exacerbate psychosis in schizophrenic subjects (de Beaurepaire et al. 1995). In addition, in vivo imaging studies show an upregulation of D1 receptors in prefrontal cortex of schizophrenic patients as compared to healthy controls (see Abi-Dargham and Moore 2003). The D1 upregulation is believed to be due to chronic hypostimulation of the D1 receptors in that patient population and was predictive of poor performance during a working memory task. As yet there has been only one clinical trial testing the effects of a D1/D5 agonist in schizophrenic patients (George et al. 2007). That study employed a single, slow subcutaneous infusion of dihydrexidine, which is a short-acting full D1/D5 agonist with ~10-fold selectivity of D1/D5 over D2 receptors. The drug was relatively well tolerated, but no short-term improvement was seen either in clinical ratings or neuropsychological tests. The authors regarded this result as not surprising in view of the fact that dihydrexidine was given as a single dose and has only has 30-min half life. More extended clinical studies were thought to be needed with longer-acting D1/D5 agonists to fully evaluate potential therapeutic usefulness. In that regard, a partial rather than full agonist may be preferable given the possible detrimental effects of supranormal stimulation of D1/5 receptors (Zahrt et al. 1997; Vijayraghavan et al. 2007).
Relative merits of proposed treatments
Two examples have been given as to how modulators of recurrent network activity could useful in the treatment of schizophrenia, one already tested in Phase II trials and the other at a much earlier stage. The Phase II clinical trials with the mGluR2/3 agonist prodrug LY2140023 show antipsychotic efficacy equivalent in efficacy to a standard antipsychotic comparison drug, with the important advantage of avoiding many undesirable side effects such as weight gain and extrapyramidal side effects (Patil et al. 2007). However, a potential drawback of mGluR2/3 agonists is their tendency to produce some reduction in the fast EPSC, albeit to a lesser degree than their effect on persistent recurrent network activity (Marek et al. 2000). Interestingly, selective genetic deletions show that mGlu2, but not mGlu3 receptor, are most predictive of the antipsychotic-like activity of mGlu2/3 agonists in preclinical behavioral studies (Fell et al. 2008; Woolley et al. 2008). Similarly, pharmacological studies show that a positive allosteric modulator selective for mGlu2 receptors mimics the ability of a combined mGlu2/3 agonist to suppress behavioral effects hallucinogenic drugs (Benneyworth et al. 2007). It remains to be seen whether mGlu2-selective agonists (or positive modulators) would have less tendency than mixed mGlu2/3 agonists to reduce the fast EPSC relative to network activity. As mentioned earlier, D1/D5 agonists can reduce late network activity in the absence of any suppression of the fast EPSC (Lambe and Aghajanian 2007), possibly conferring a therapeutic advantage. However, as D1/D5 receptors are located at diverse sites in the brain and periphery, it is difficult to predict whether actions of systemically administered D1/D5 agonists at extraneous sites would limit their potential therapeutic usefulness in treating schizophrenia (Marcus et al. 2005).
The crucial role of GABA interneurons in network regulation
The clinical response to mGlu2/3 agonist treatment is no more rapid than with a standard antipsychotic drug (Patil et al. 2007), suggesting the existence of underlying structural changes in schizophrenia that are inherently slow to reverse. For example, there is growing evidence for a net reduction in GABA-mediated inhibitory modulation in schizophrenia due to decline in GABA synthesizing enzymes and certain interneuron populations in cortex (Akbarian et al. 1995; Benes and Berretta 2001; Lewis et al. 2001). Ford and colleagues have suggested that the search for new treatments would be facilitated by in vitro experiments by which “…GABA antagonists introduced into the Petri dish produce the schizophrenia pattern…” of electrophysiological oscillations (Ford et al. 2007). Predating this suggestion, it was earlier shown that a subconvulsive concentration of the GABAA antagonist bicuculline promotes aberrant network activity in brain slices (Aghajanian and Marek 1999). Moreover, D1/D5 receptor agonists are as effective in suppressing bicuculline-induced effects on network activity as they are in suppressing the effects of hallucinogens (Lambe and Aghajanian 2006). The presumed mechanism for this suppression is that D1/D5 agonists counteract the GABAergic deficit produced by bicuculline by activating GABAergic interneurons. It is significant that a low, preconvulsant concentration of bicuculline has relatively little effect on fast synaptic GABA transmission (Luhmann and Prince 1990; Aghajanian and Marek 1999). This selectivity implies that bicuculline’s effect is mainly upon extrasynaptic GABAA channels, which are activated tonically by low concentrations of GABA rather than high synaptic concentrations (Yeung et al. 2003). It has been proposed that non-sedating, subtype-selective positive allosteric modulators of GABAA receptors potentially may have a more rapid therapeutic effect than existing treatments since they address more directly the issue of underlying pathophysiology (Guidotti et al. 2005; Rudolph and Mohler 2006; Lewis et al. 2008). To date, only one such drug—MK-0777—has been tested clinically in schizophrenic patients. This drug, which has high α2/α3 selectivity, was found to improve working memory and other measures of prefrontal function in a 4-week trial in chronic schizophrenic patients (Lewis et al. 2008). Although there was no overall improvement in the Brief Psychiatry Rating Scale, it was felt the improvement in cognitive function had sufficient promise to proceed with further clinical trials with MK-0777 or other related compounds.
Role of astrocytes in the network model
The role of astrocytes in the regulation of recurrent network activity has been largely neglected. Yet in postmortem brain tissue from schizophrenic subjects, a significant reduction has been reported in layer V glial cells of dorsolateral (Rajkowska et al. 2002) and anterior cingulate prefrontal cortex (Stark et al. 2004), a region homologous to the area in rat medial prefrontal where most of the slice recordings are performed. There are a large variety of receptors that can promote release of glutamate from astrocytes. These include the metabotropic glutamate mGlu1/5 receptors, located on both astrocytes and neurons and the protease-activated receptor 1 (PAR1), located primarily on astrocytes (see Haydon and Carmignoto 2006); the latter are activated through the tPA/plasminogen/plasmin serine protease pathway. The co-activation of PAR1 and mGlu1/5 receptors is particularly effective in inducing a slow and prolonged release of glutamate from astrocytes. The slowly released glutamate can then act upon extrasynaptic neuronal NR2B receptors to produce slow inward currents or SICs (Fellin et al. 2004). The astrocytic dependence of NR2B-mediated SICs would explain how a transient spillover of synaptic glutamate is able to trigger sustained recurrent network activity (Fig. 4, top panel). According to this model, two waves of glutamate are involved the production of SICs as well as concurrent recurrent activity: an initial wave of synaptic glutamate spillover onto astrocytes and a secondary astrocytic amplification of the neuronal glutamate signal. Consistent with this idea are preliminary experiments showing that highly selective PAR1 antagonists are potent blockers of both basal and hallucinogen-induced SICs and as well as recurrent network activity (Fig. 4, bottom panel).
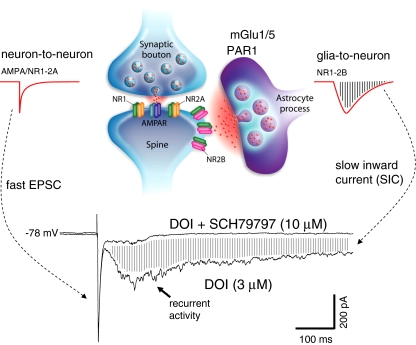
Fig. 4.
Proposed role of astrocytes in the generation of recurrent activity and slow inward currents (SICs). Drawing in upper panel depicts a synapse in which an adjacent astrocyte process slowly releases glutamate (in response to glutamate spillover) onto extrasynaptic NR2B receptors to give rise to glia-to-neuron SICs (adapted from Haydon and Carmignoto 2006). In contrast, the fast neuron-to-neuron response is of much shorter in duration. Traces below illustrate blockade of DOI-induced SICs by SCH79797, a selective antagonist of the astrocytic PAR1 receptor. Note the concomitant suppression of associated recurrent activity. Also note that the fast EPSC is unchanged as indicated by superimposition of the two traces (provided by G. Aghajanian)
Limitations of the in vitro network model
The main strength of the prefrontal brain slice preparation is that it allows for a dissection of mechanisms underlying intrinsic recurrent network activity. However, the prefrontal slice preparation has the inherent limitation of being disconnected from subcortical efferents and afferents, including major reciprocal connections with monoaminergic, mesolimbic, and thalamic systems (Groenewegen and Uylings 2000). Among these, the midline/intralaminar thalamic inputs are of particular interest since they comprise the final link in the ascending arousal pathway to prefrontal regions. The midline/intralaminar projections are distinctive in terminating upon apical dendrites of layer V pyramidal cells of medial prefrontal cortex—categorized as agranular as it lacks a layer IV, the normal target for thalamic inputs (see Lambe and Aghajanian 2003). This arrangement creates the unusual situation in which layer V pyramidal cells serve both as the main receptive cells for thalamic input and the main output cells to subcortical regions. Another distinctive feature of cells in midline/intralaminar versus other thalamic nuclei is that they are selectively excited by the wake-promoting peptides hypocretin 1 and 2 (orexin A and B) as well as nicotine via α4β2 receptors; this excitation occurs at the level axon terminals as well as the relay cell bodies (Lambe and Aghajanian 2003).
In behavioral studies, hypocretin or nicotine infused into medial prefrontal cortex of awake animals improves performance in a complex cognitive task requiring divided attention (Lambe et al. 2005). Postmortem studies have found diminished connectivity between anterior thalamic nuclei and prefrontal cortical areas, which may contribute to cognitive deficits that are detectable even at early stages of schizophrenia (Andreasen et al. 1996; Danos et al. 1998; Portas et al. 1998; Lewis et al. 2001). These findings are supported by MRI scans in patients with first episode schizophrenia showing that fiber pathways in the anterior limb of the internal capsule, which connect midline/anterior thalamic nuclei to prefrontal cortex, are reduced in volume (Lang et al. 2006). The evidence for an underlying loss of thalamocortical connectivity suggests that there may a deficit in cortical processing of incoming information from the ascending arousal system in schizophrenia.
In vivo electrophyiological studies give important insights on how sensory activation of thalamic inputs interacts with cortical recurrent network activity. The interaction of sensory responses with spontaneous depolarizations (“UP states”) has been studied with whole cell in vivo recordings in pyramidal cells of rodent somatosensory cortex (Petersen et al. 2003). During the UP state, but not quiescent or DOWN states, there appears to be a high level of intrinsic synaptic noise indicative of recurrent network activity. Surprisingly, despite cell depolarization, a sensory stimulus produces fewer action potentials during UP states than DOWN states. Thus, the UP state does not seem to prime for a greater reception of sensory information—instead there seems to be a competition or interference between sensory-evoked responses and ongoing spontaneous activity. If this pattern holds for prefrontal cortex, then excessive intrinsic network activity within the cortex might be expected to interfere with sensory processing. Although not directly demonstrated, this attenuation of sensory responses by high levels of intrinsic activity may be responsible for the decrease in signal to noise ratio that has been found in schizophrenic patients (Winterer and Weinberger 2004). Unfortunately, it not feasible to model this relationship directly in vitro since anatomical thalamic connections are not readily retained in the medial prefrontal slice preparation, limiting its ability to model interactions with afferent inputs that may influence the effects of drugs acting upon the candidate sites described below.
Targeting novel therapeutic targets
The successful phase II trials with an mGluR2/3 agonist provides strong incentive for testing agents directed at alternative sites within the network model for their clinically usefulness. Table 1 lists a wide array of cellular and receptor sites located at critical nodal points involved in the modulation of intrinsic network activity in prefrontal cortex that are potential therapeutic targets. These should be regarded only as illustrative of the many factors, known and unknown, that are involved in regulation of a highly complex network. As many atypical antipsychotics already include potent antagonist activity at 5-HT2A receptors as part of their profile, this receptor has not been listed. The most selective and potent of these is risperidone, which has ~10- to 20-fold selectivity for the 5-HT2A over the D2 receptor (Schotte et al. 1996). However, it has been reported that at typical clinical doses, risperdone has as high a level of D2 receptor occupancy as low dose typical antipsychotics, making it difficult to isolate the role of 5-HT2A blockade (Kapur et al. 1999). Although phase III trials with a selective 5-HT2A agonist (M100907) have been conducted in schizophrenic patients, they were said to be discontinued because of less than optimal efficacy (de Paulis 2001). However, the detailed results of these trials have not been made available in the open literature, leaving open the question of whether activation of 5-HT2A receptors contribute significantly to disordered networks in schizophrenia.
Table 1.
Candidate sites for modulation of aberrant networks
| Cellular target | Candidate site | Clinical efficacy | Status |
|---|---|---|---|
| Pyramidal cell | mGluR2/3 ( +) | high | Phase IIb clinical |
| NR2B (−) | – | Early clinical | |
| α2 GABAA (+) | – | Early clinical | |
| α5 GABAA (+) | Preclinical | ||
| Interneuron | D1 (+) | – | Early clinical |
| Astrocyte | PAR1 (−) | – | Preclinical |
| mGluR1/5 (−) | – | Preclinical |
Negative sign (−) antagonist, positive sign (+) agonist/positive modulator
Undoubtedly, some of the novel antagonists or agonists that show efficacy in vitro will have serious side effects in vivo—including unexpected adverse interactions with efferents and afferents—greatly outweighing their possible therapeutic benefit. Ideally, selection of the most appropriate therapeutic target will be informed by the underlying deficit in a given patient. Two examples of suggested preexisting structural or molecular changes in schizophrenia have been mentioned previously: deficits GABA interneurons and anterior thalamocortical connections. In addition, it is likely that many of the susceptibility gene polymorphisms identified in schizophrenia, such as those that modulate glutamate or GABA transmission, contribute to network dysregulation. Ultimately, as the affected site(s) can vary across patient subtypes, knowledge about underlying genetic makeup and developmental pathophysiology will provide the best guidance for selecting optimal targets within the network.
Acknowledgements
This study was funded by NIMH and the State of Connecticut.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abekawa T, Ohmori T, Ito K, Koyama T (2000) D1 dopamine receptor activation reduces extracellular glutamate and GABA concentrations in the medial prefrontal cortex. Brain Res 867:250–4 [DOI] [PubMed]
- Abi-Dargham A, Moore H (2003) Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist 9:404–16 [DOI] [PubMed]
- Aghajanian GK, Marek GJ (1997) Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36:589–599 [DOI] [PubMed]
- Aghajanian GK, Marek GJ (1999) Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res 825:161–171 [DOI] [PubMed]
- Aghajanian GK, Marek GJ (2000) Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Rev 31:302–312 [DOI] [PubMed]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE Jr, Jones EG (1995) Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 52:258–266 [DOI] [PubMed]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD (1996) Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal–thalamic–cerebellar circuitry. Proc Natl Acad Sci U S A 93:9985–9990 [DOI] [PMC free article] [PubMed]
- Bandyopadhyay S, Hablitz JJ (2007) Dopaminergic modulation of local network activity in rat prefrontal cortex. J Neurophysiol 97:4120–4128 [DOI] [PubMed]
- Beierlein M, Fall CP, Rinzel J, Yuste R (2002) Thalamocortical bursts trigger recurrent activity in neocortical networks: layer 4 as a frequency-dependent gate. J Neurosci 22:9885–9894 [DOI] [PMC free article] [PubMed]
- Benes FM, Berretta S (2001) GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25:1–27 [DOI] [PubMed]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E (2007) A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol 72:477–484 [DOI] [PubMed]
- Charton JP, Herkert M, Becker CM, Schroder H (1999) Cellular and subcellular localization of the 2B-subunit of the NMDA receptor in the adult rat telencephalon. Brain Res 816:609–617 [DOI] [PubMed]
- Conn PJ, Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237 [DOI] [PubMed]
- Danos P, Baumann B, Bernstein HG, Franz M, Stauch R, Northoff G, Krell D, Falkai P, Bogerts B (1998) Schizophrenia and anteroventral thalamic nucleus: selective decrease of parvalbumin-immunoreactive thalamocortical projection neurons. Psychiatry Res 82:1–10 [DOI] [PubMed]
- de Beaurepaire R, Labelle A, Naber D, Jones BD, Barnes TR (1995) An open trial of the D1 antagonist SCH 39166 in six cases of acute psychotic states. Psychopharmacology (Berl) 121:323–327 [DOI] [PubMed]
- de Paulis T (2001) M-100907 (Aventis). Curr Opin Investig Drugs 2:123–32 [PubMed]
- Fell MJ, Svensson KA, Johnson BG, Schoepp DD (2008) Evidence for the Role of metabotropic glutamate (mGlu) 2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (–)-(1R, 4S, 5S, 6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4, 6-dicarboxylic acid (LY404039). J Pharmacol Ex Ther 326:209–217 [DOI] [PubMed]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G (2004) Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43:729–743 [DOI] [PubMed]
- Ford JM, Krystal JH, Mathalon DH (2007) Neural synchrony in schizophrenia: from networks to new treatments. Schizophrenia Bulletin Schizophr Bull 33:848–852 [DOI] [PMC free article] [PubMed]
- Gao WJ, Krimer LS, Goldman-Rakic PS (2001) Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci U S A 98:295–300 [DOI] [PMC free article] [PubMed]
- George MS, Molnar CE, Grenesko EL, Anderson B, Mu Q, Johnson K, Nahas Z, Knable Z, Fernandes P, Juncos J, Huang X, Nichols D, Mailman RB (2007) A single 20 mg dose of dihydrexidine (DAR-0100), a full dopamine D1 agonist, is safe and tolerated in patients with schizophrenia. Schizophrenia Research 93:42–50 [DOI] [PubMed]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452:93–97 [DOI] [PMC free article] [PubMed]
- Gorelova N, Seamans JK, Yang CR (2002) Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol 88:3150–66 [DOI] [PubMed]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar K-A (2005) Psychological effects of (S)-ketamine and N, N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry 38:301–311 [DOI] [PubMed]
- Groenewegen HJ, Uylings HB (2000) The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res 126:3–28 [DOI] [PubMed]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E (2005) GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 180:191–205 [DOI] [PubMed]
- Haider B, Duque A, Hasenstaub AR, McCormick DA (2006) Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci 26:4535–4545 [DOI] [PMC free article] [PubMed]
- Harte M, O’Connor WT (2004) Evidence for a differential medial prefrontal dopamine D1 and D2 receptor regulation of local and ventral tegmental glutamate and GABA release: a dual probe microdialysis study in the awake rat. Brain Res 1017:120–129 [DOI] [PubMed]
- Higgins GA, Ballard TM, Enderlin M, Haman M, Kemp JA (2005) Evidence for improved performance in cognitive tasks following selective NR2B NMDA receptor antagonist pre-treatment in the rat. Psychopharmacology (Berl) 179:85–98 [DOI] [PubMed]
- Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500 [DOI] [PMC free article] [PubMed]
- Haydon PG, Carmignoto G (2006) Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86:1009–1031 [DOI] [PubMed]
- Kapur S, Zipursky RB, Remington G (1999) Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 156:286–293 [DOI] [PubMed]
- Krystal JH, Karper LP, Seibyl JP et al (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuro-endocrine responses. Arch Gen Psychiatry 51:199–214 [DOI] [PubMed]
- Lambe E, Aghajanian G (2003) Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron 40:139–150 [DOI] [PubMed]
- Lambe EK, Aghajanian GK (2006) Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology 31:1682–1689 [DOI] [PubMed]
- Lambe EK, Aghajanian GK (2007) Prefrontal cortical network activity: opposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation. Neuroscience 145:900–910 [DOI] [PMC free article] [PubMed]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK (2005) Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J Neurosci 25:5225–5229 [DOI] [PMC free article] [PubMed]
- Lang DJ, Khorram B, Goghari VM, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Honer WG (2006) Reduced anterior internal capsule and thalamic volumes in first-episode psychosis. Schizophr Res 87:89–99 [DOI] [PubMed]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN (2001) Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry 158:1411–1422 [DOI] [PubMed]
- Lewis DA, Hashimoto T, Volk DW (2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–24 [DOI] [PubMed]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D (2008) Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry 165:1585–1593 [DOI] [PMC free article] [PubMed]
- Luhmann HJ, Prince DA (1990) Transient expression of polysynaptic NMDA receptor-mediated activity during neocortical development. Neurosci Lett 111:109–115 [DOI] [PubMed]
- Marcus MM, Jardemark KE, Wadenberg ML, Langlois X, Hertel P, Svensson TH (2005) Combined alpha2 and D2/3 receptor blockade enhances cortical glutamatergic transmission and reverses cognitive impairment in the rat. Int J Neuropsychopharmacol 8:315–327 [DOI] [PubMed]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (2000) Physiological antagonism between 5-hydroxytryptamine2A and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292:76–8 [PubMed]
- McCormick DA (2005) Neuronal networks: flip-flops in the brain. Curr Biol 15:R294–R296 [DOI] [PubMed]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T (2003) Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex 13:1219–31 [DOI] [PubMed]
- Merchant RE, Bullock MR, Carmack CA, Shah AK, Wilner KD, Ko G et al (1999) A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101, 606 in patients with a mild or moderate traumatic brain injury. Ann NY Acad Sci 890:42–50 [DOI] [PubMed]
- Moghaddam B, Adams BW (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352 [DOI] [PubMed]
- Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927 [DOI] [PMC free article] [PubMed]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA (2004) Lysergic acid diethylamide and [ ]-2, 5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res 1023:134–140 [DOI] [PubMed]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a andomized Phase 2 clinical trial. Nat Med 13:1102–1107 [DOI] [PubMed]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B (2003) Interaction of sensory responses with spontaneous depolarization in layer 2 ⁄ 3 barrel cortex. Proc Natl Acad Sci U S A 100:13638–13643 [DOI] [PMC free article] [PubMed]
- Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG, Fischer I, Kikinis R, Donnino R, Jolesz FA, McCarley RW (1998) Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry 43:649–659 [DOI] [PubMed]
- Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C (2002) Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res 57:127–138 [DOI] [PubMed]
- Rigas P, Castro-Alamancos MA (2007) Thalamocortical UP states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci 27:4261–4272 [DOI] [PMC free article] [PubMed]
- Rorick-Kehn LM, Johnson BG, Knitowski KM, Salhoff CR, Witkin JM, Perry KW, Griffey KI, Tizzano JP, Monn JA, McKinzie DL, Schoepp DD (2007) In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology (Berl) 193:121–136 [DOI] [PubMed]
- Rudolph U, Mohler H (2006) GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol 6:18–23 [DOI] [PubMed]
- Schotte A, Janssen PFM, Gommeren W, Luyten WHML, VanGompel P, Lesage AS, DeLoore K, Leysen JE (1996) Risperiidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 124:57–73 [DOI] [PubMed]
- Scruggs JL, Schmidt D, Deutch AY (2003) The hallucinogen 1-[2, 5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett 346:137–140 [DOI] [PubMed]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ (2001) Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A 98:301–6 [DOI] [PMC free article] [PubMed]
- Shu Y, Hasenstaub A, McCormick DA (2003) Turning on and off recurrent balanced cortical activity. Nature 423:288–93 [DOI] [PubMed]
- Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B (2004) Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry 161:882–888 [DOI] [PubMed]
- Vijayraghavan S, Wang M, Birnbaum SG, Bruce CJ, Williams GV, Arnsten AFT (2007) Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10:376–384 [DOI] [PubMed]
- Winterer G, Weinberger DR (2004) Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci 27:683–690 [DOI] [PubMed]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DNC (2008) The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 196:431–440 [DOI] [PubMed]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA (2003) Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol 63:2–8 [DOI] [PubMed]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF (1997) Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17:8528–35 [DOI] [PMC free article] [PubMed]