Abstract
Nitrosyl hydride, HNO or nitroxyl, is the one-electron reduced and protonated form of nitric oxide. HNO is isoelectronic to singlet O2, and we have previously reported that deoxy myoglobin traps free HNO to form a stable adduct. In this report, we demonstrate that oxygen-binding hemoglobins from human, soy and clam also trap HNO to form adducts which are stable over a period of weeks. The same species can be formed in higher yield by careful reduction of the ferrous nitrosyl adducts of the proteins. Like the analogous O2-FeII adducts, the HNO adducts are diamagnetic, but with a characteristic HNO resonance in 1H NMR ca. 15 ppm that splits into doublets for H15NO adducts. The 1H and 15N NMR resonances, obtained by HSQC experiments, are shown to differentiate subunits and isoforms of proteins within mixtures. An apparent difference in reduction rates of the NO-adducts of the two subunits of human hemoglobin allows assignment of two distinct nitrosyl hydride peaks by a combination of UVvis, NMR and EPR analysis. The two peaks of HNO-hHb have a persistent 3:1 ratio during trapping reactions, demonstrating a kinetic difference between HNO binding at the two subunits. These results show NMR characterization of ferrous HNO adducts as a unique tool sensitive to structural changes within the oxygen-binding cavity, which may be of use in defining modes of oxygen binding in other heme proteins and enzymes.
Keywords: HNO, nitroxyl, nitrosyl hydride, dioxygen, globins, heme oxygenase
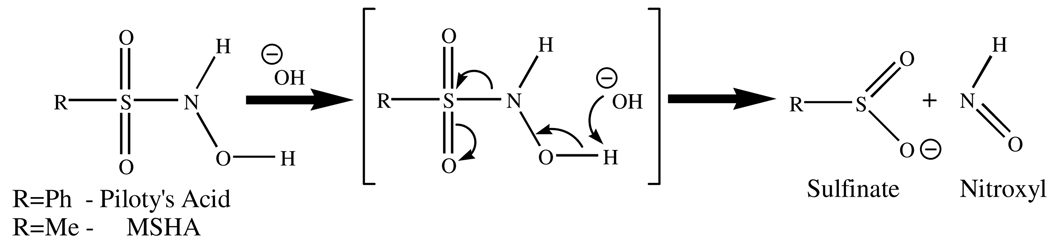
Nitrosyl hydride (HNO), the protonated form of nitroxyl anion (NO−), has distinct physicochemical properties from its congener nitric oxide (NO), much of which has been defined only recently.1,2 The anionic form is isoelectronic with dioxygen and exists as a triplet, 3NO− above pH 12; at lower pH the singlet 1HNO dominates, but is susceptible to rapid dimerization resulting in the formation of N2O, Eq 1.3,4 The rate of this dimerization has been reported as 8 × 106 M−1s−1,5 and thus severely limits the lifetime and concentration of HNO generated in solution.
| (1) |
HNO is the simplest analogue of alkylnitroso compounds, RNO, long known to bind to ferrous heme proteins.6 Mansuy and coworkers were the first to describe the binding of RNO compounds to ferrous globins myoglobin (Mb) and human hemoglobin (hHb),7 as well as to make the analogy of RNO binding to that of dioxygen.8 Although quite rare, a number of organometallic HNO complexes of Ir, Os, Re, and Ru have been identified,9–14 all of which are low spin d6 transition metal complexes and diamagnetic.15
| (2) |
| (3) |
In the last several years we have reported the preparation and characterization of the HNO adduct of myoglobin (HNO-Mb) by electrochemical16 or bulk reduction17 of the ferrous nitrosyl adduct (NO-Mb), Eq 2, and by trapping of free HNO by deoxy myoglobin (Mb-FeII), Eq 3.18 Like the isoelectronic oxyadduct of myoglobin (oxyMb), the ferrous nitrosyl hydride adduct (HNO-Mb) is diamagnetic and uniquely characterized by an 1H NMR signal of the N-bound hydride, which is well separated from other protein resonances at ca. 15 ppm. This unique resonance allowed more detailed structural characterization of the heme pocket of HNO-Mb by 2D NOE and COSY experiments.19
Several routes to HNO-metal complexes have been reported,15 but until this report only deoxymyoglobin (Mb-FeII) has been shown to directly trap free HNO in solution to form an identifiable HNO complex. Because of its limited lifetime, free HNO must be produced in situ from the decomposition of precursors like methylsulfonylhydroxylamic acid (MSHA) or Piloty’s acid (PS), Scheme 1.20 Using such reagents, the trapping of HNO by deoxyMb is observable in timecourse 1H NMR spectra by the formation of the diamagnetic HNO-Mb, or in UV-vis spectra by the loss of the deoxyMb Soret peak.18 The maximum HNO-Mb yield obtained by this method is ca. 80%, apparently due to competitive side reactions and further reactivity of Mb-HNO.21
Scheme 1.
In this report we demonstrate the ability of other oxygen-binding globins to trap free HNO to form long-lived HNO-adducts; examples include recombinant and native isoforms of leghemoglobin (lgHb),22 hemoglobin I isolated from the invertebrate clam Lucina pectinata (cHb),23 and human hemoglobin (hHb).24 These HNO-adducts can also be generated by careful reduction of the corresponding NO-adduct and are characterized by absorbance and 1H NMR spectra, as well as 1H-1H NOESY and 1H−15N HSQC methods. The unique 1H NMR hydride resonance serves as a sensitive indicator of structural changes within the heme pocket, distinguishing subunits and isoforms in protein mixtures.
MATERIALS AND METHODS
General
The HNO precursor phenylsulfonylhydroxamic acid (Piloty’s acid, PA) was purchase from Cayman Chemicals and recrystalized before use; the precursor methylsulfonylhydroxamic acid (MSHA) was synthesized and recrystallized by a literature procedure.25 15N-labeled sodium nitrite (15N 98.16 %) was purchased from Promy Chemicals. All other chemicals were purchased from Aldrich at 98% purity or better and used as received unless otherwise specified. Water was purified to a specified resistance of 18 MΩ/cm in a Barnstead nanopure water purification system before the addition of buffer salts.
Horse skeletal muscle myoglobin (95–100%) and lyophilized hemoglobin human were purchased from Sigma-Aldrich and were used as received. Leghemoglobin (lgHb) was expressed and purified as described previously.26 A mixture of leghemoglobin isoforms isolated from soybeans was obtained from Fraser Bergeson, and purified using size exclusion chromatography.27 Hemoglobin I (cHb) from Lucina pectinata was isolated and purified using size exclusion and ion exchange chromatography.23 All manipulations of ferrous proteins were inside an anaerobic glove box. Purification of proteins was carried out on pre-equilibrated Sephadex G-25 columns in 50 mM phosphate buffer either at pH 7 or 9.4.
Absorption spectra for kinetic experiments were recorded with a Hewlett Packard 8453 Diode Array spectrophotometer, or for reductive titrations on an Ocean Optics USB 2000 spectrometer in an anaerobic glovebox. X-band EPR spectra were recorded with a Bruker EMX spectrometer equipped with a standard TE102 (ER 4102ST) or a high sensitivity ER 4119HS resonator. Proton NMR experiments were recorded on Bruker Avance 600 and Varian 800 MHz spectrometers. The spectra were acquired by direct saturation of the residual water peak during the relaxation delay. Chemical shifts were referenced to the residual water peak at 4.8 ppm.
Preparation of ferrous heme proteins
The ferrous states of the heme proteins (lgHb, cHb, hHb) were prepared by the reduction of the ferric proteins (300 µL, 0.5–0.7 mM) with ∼ 30-fold excess of sodium dithionite in carbonate buffer at pH 9.4; the resulting ferrous forms were purified on Sephadex G-25 size exclusion column pre-equilibrated with carbonate buffer at pH 9.4 and then concentrated on a YM-10 membrane filter to give 0.5–0.7 mM solution.
Reactions of globins with HNO precursors, characterization of HNO adducts
An anaerobic stock solution of HNO precursor in pH 7 phosphate buffer was freshly prepared in deionized water before each set of experiments, all manipulations were carried out under N2 atmosphere in a glovebox. Samples of HNO adducts of the monomeric globins were prepared anaerobically by addition of 100 µL of an 8.9 mM solution of PA to a ca. 0.5 mM solution of ferrous heme proteins in 50 mM carbonate buffer at pH 9.4. The solution was allowed to stand for 45 min and then purified on a G-25 column using 50 mM phosphate buffer at pH 7. For 1H NMR experiments, the proteins were concentrated on a YM-10 membrane filter to concentrations of 0.5–0.7 mM; typically 50 µl of D2O was added to ca. 450 µL sample of the HNO-adduct before measurements.
Similarly, the reaction of 100 µL of 46 mM MSHA with ca. 0.5 mL of 1 mM hHb in 50 mM pH 9.4 carbonate buffer was followed by timecourse NMR spectra at hour intervals over a day. Afterwards, G-25 column filtration was used to remove impurities, and quantification of NO-Hb concentrations was obtained by comparison of doubly integrated EPR spectra of the products with that of an authentic NO-hHb sample. Determined yields of HNO-hHb over 6 trials ranged from 32% to 48%, per heme content.
Preparation of NO adducts of heme proteins
In a typical procedure, a 200 µL solution of ca. 10 µM ferric heme proteins (lgHb, cHb, hHb) in 50 mM phosphate buffer at pH 7 was prepared anaerobically, a 10-fold excess of sodium nitrite added and allowed to stand for 5 min. before the addition of a 30-fold excess of sodium dithionite. The resulting nitrosyl adduct solution was purified by size exclusion chromatography using a Sephadex G25 column and then concentrated on a YM-10 membrane. Samples of 15NO-ferrous adducts were prepared using 99% Na15NO2.
Reactions of nitrosyl globins with reduced DTDP to form HNO adducts
This procedure for chemical reduction follows that previously described for HNO-Mb.28 A sample of 4,4’-dimethyl-1,1’ – trimethylene-2,2’-dipyridinium (DTDP) (0.0024 g, 0.062 mmol) was dissolved in 200 µL anaerobic 50 mM carbonate buffer at pH 9.4, and reduced with Zn/Hg amalgam (2 mg). Small aliquots (10 µL) of the reduced solution of DTDP were added to NO-hHb (1 mL, 1.5 mM) in an anaerobic glove box, followed by in situ UVvis absorbance measurements. An increase and shift of the Soret band from 418 to 420 nm is characteristic of HNO adduct formation; a loss of Soret intensity indicates over-reduction, generating deoxy hHb. The resulting HNO-adduct solution was filtered through glass wool, run on a G-25 column using 50 mM phosphate buffer pH 7 and concentrated for NMR experiments using a YM-10 Centricon as above.
Quantification of impurities
As described for HNO-hHb, the percentage of ferrous NO adduct impurities in prepared HNO adduct samples were obtained by comparison of EPR spectra with authentic samples, as previously reported for HNO-Mb.18,28 For example, EPR spectra of NO-cHb and HNO-cHb samples were recorded at the same concentration (normalized by the protein absorbance at 280 nm) and the integrated signals were used to calculate the percentage of NO-cHb impurities in the HNO-cHb sample.
Results and Discussion
HNO adducts of monomeric globins
The globin protein family is widespread in nature with greater than 700 members,29 the best known are oxygen-binders, e.g., myoglobin and hemoglobin. Leghemoglobin (lgHb) is a small 16 kDa protein responsible for the binding and transport of O2 to bacteroids in root nodules of legumes; it has an oxygen affinity ca. 20 times higher than Mb.30,31 Another single heme globin, cHb, from the clam Lucina pectinata, binds H2S in its native role, but also forms a stable O2 adduct; cHb has been widely studied due to a very hydrophobic distal pocket and altered ligand binding affinities.32
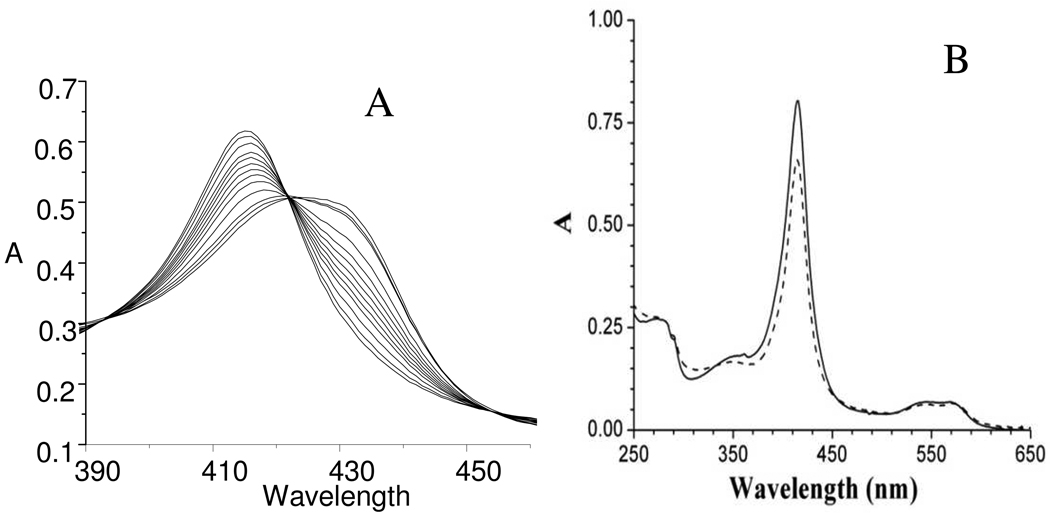
Stoichiometric reactions of the deoxy states of lgHb and cHb with HNO donor agent PA at pH 9.4, as illustrated for lgHb in Figure 1, results in absorbance changes similar to those seen during similar reaction with deoxyMb;18 the sequential loss of the deoxy state’s Soret absorbance gives a direct measure of the reaction with HNO. The reactions are stopped after 45 minutes by passing the reaction mixtures through a size-exclusion column, analogous to the general method developed for generating HNO-Mb.21 After purification, the solutions are stable over weeks when kept anaerobic and in the dark.28 These solutions are easily concentrated and the buffer exchanged from pH 7 to 9.5 without appreciable change in the UVvis or 1H NMR spectra, Table 1. As of yet there is no evidence for a stable, deprotonated nitroxyl adduct of any protein studied.
Figure 1.
(A) Sequential UV-vis spectra of formation of HNO trapping by deoxy lgHb, forming HNO-lgHb over thirty minutes during reaction with 4-fold excess PA in a 50 mM carbonate pH 9.4 buffer. (B) UV-vis spectra of HNO-lgHb (solid) and NO-lgHb (dotted) at equivalent concentration, ca. 50 uM in 50 mM pH 7 phosphate buffer.
Table 1.
UV-vis and 1H NMR data of ferrous globins adducts
| Protein | UVvis λmax (nm) | 1HNMR (ppm) | |
|---|---|---|---|
| FeII-NO | FeII-HNO | FeII-HNO | |
| lgHb | 414, 548, 571 | 415, 544, 569 | 15.0 |
| cHb | 420, 541, 568 | 421, 542, 565 | 15.53 |
| Mb | 421, 548, 580 | 423, 546, 578 | 14.80 |
| hHb | 418, 543, 580 | 420, 544 (br) | 14.63, 14.80 |
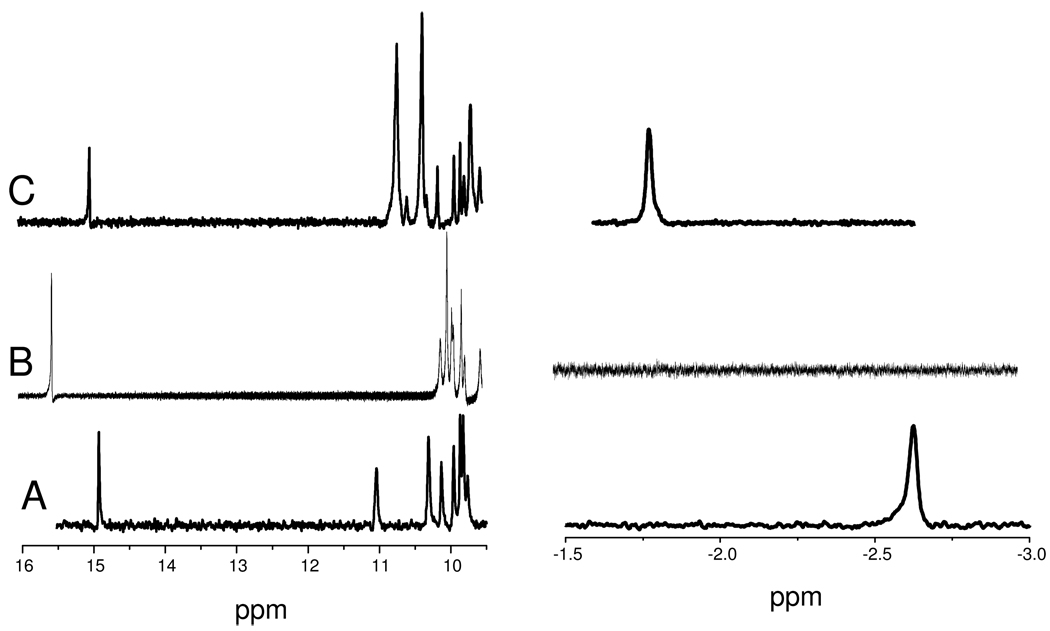
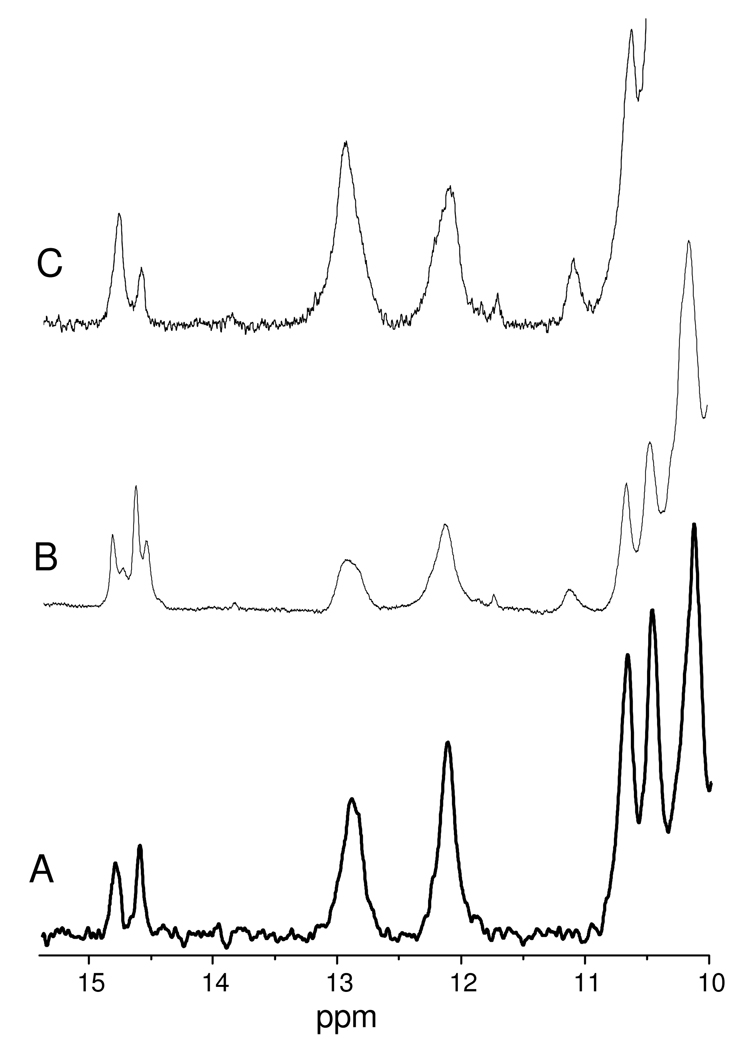
The formation of HNO adducts is confirmed by 1H NMR spectra of the product solutions which show a characteristic HNO peak between 13 and 16 ppm, Figure 2.15 Also characteristic are peaks below 0 ppm assignable to amino acid residues close to the heme plane; these resonances are shifted upfield due to the porphyrin ring current. The two valine methyl groups in HNO-Mb are seen as two distinct peaks at −0.88 and −2.63 ppm, the latter has strong NOE interactions with the nitrosyl hydride peak.19 A similar peak for a distal pocket isoleucine is seen at −1.80 ppm for HNO-lgHb, but cHb has no equivalent alkyl group above the heme, and no resonance is seen in this region. The hydrophobic distal pocket of this HNO-cHb also has the lowest field hydride absorbance at 15.53 ppm.
Figure 2.
1H NMR spectra of HNO adducts of (A) Mb, (B) cHb, (C) lgHb generated by reaction of deoxy proteins with PA in pH 9.4 carbonate buffers, then purified to ∼ 0.5 mM in 50 mM phosphate buffer at pH 7.0.
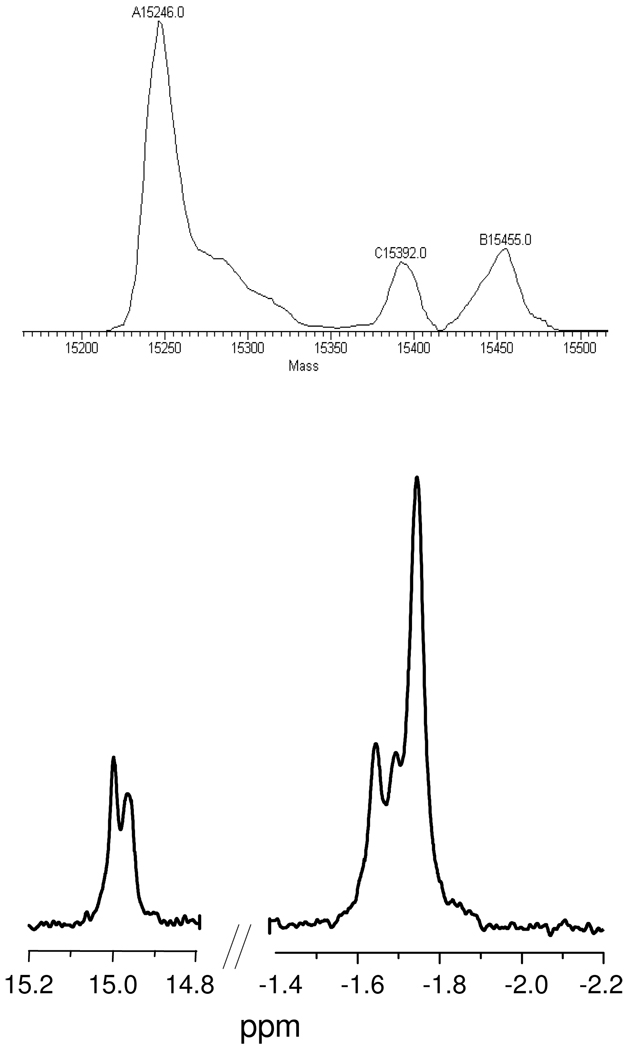
As isolated from soybeans, lgHb is obtained as mixtures of isoforms whose expression ratios change over the life of the plant, and which can be identified by HPLC-MS.33 A native lgHb mixture was reduced and reacted with PA as with the recombinant form, and the product solution analyzed by 1H NMR. As seen in Figure 3, the nitrosyl hydride region shows two overlapping peaks while the distal isoleucine resonances are split into three discernible peaks; Gaussian modeling of the isoleucine resonances give a ratio of 8:2:4 (supplemental); LC-MS of the native lgHb mixture distinguished three isoforms in a ratio of ca. 4:1:1, in rough agreement with the NMR analysis.
Figure 3.
Nanospray LC-MS of wildtype lgHb isoform mixture (top) and 1H NMR spectra (bottom) of HNO-lgHb adducts of this sample (500 µL, 0.7 mM) in 50 mM phosphate buffer at pH 7.0.
HNO adducts of human hemoglobin
Similar reactions of tetrameric human hemoglobin and PA yield solutions containing HNO-hHb species.1H NMR analysis of these solutions show two nitrosyl hydride peaks at 14.63 and 14.80 ppm, Figure 4, in a 3:1 ratio, attributable to HNO adducts of the α and β subunits (vide supra), with resonances assigned to distal pocket valine methyls at −2.18 and −2.23 ppm in a similar ratio. The characteristic doublet splitting of both hydride resonances is also seen for 15N-labeled samples prepared by reduction of 15NO-hHb.
Figure 4.
1H NMR spectra of HNO-hHb samples prepared by different methods: A) H14NO-hHb prepared by DTDP/Zn-Hg reduction of NO-hHb; B) H15NO-hHb prepared by reduction of 15NO-hHb; C) H14NO-hHb prepared from deoxy hHb by trapping of HNO produced by PA. All spectra of samples in pH 7 buffer at ca. 1 mM concentration.
Significantly, two broad resonances are observed at 12.3 and 13.0 ppm, Figure 4, analogous to peaks in 1H NMR spectra of oxy-hHb assigned to protons on Hisα122 and Hisα103 which take part in strong H-bonds between the subunits.34 Changes in these signals have been linked to transitions between tensed and relaxed allosteric states upon the binding of O2, and similar changes are observable in timecourse spectra obtained during HNO trapping using the donor MSHA, which releases HNO over hours under these conditions, Figure 5.18,20 The shifting of the peak at 12.3 ppm occurs during the initial stages of HNO-adduct formation, as might be expected for an allosteric response to ligand binding.
Figure 5.
1H NMR timecourse spectra of reaction of 40 mM MSHA with 2.0 mM deoxy hHb in 0.1 M phosphate buffer at pH 9.4 and 25°C, showing the growth of two nitroxyl peaks at 14.6 ppm and 14.8 ppm and changes in the Hisα122 peak at 12.3 ppm, at hour intervals over 16 hours.
The two nitrosyl hydride peaks have a persistent 3:1 ratio during the MSHA trapping reaction, as was also seen in product mixtures from reactions of deoxy hHb with PA, demonstrating a kinetic difference in free HNO binding at the two subunits. Similarly, a difference in reduction rates of the NO-adducts of the two subunits has allowed assignment of the nitrosyl hydride peaks by sequential reduction of NO-Hb and concurrent UVvis, NMR and EPR analysis, as follows.
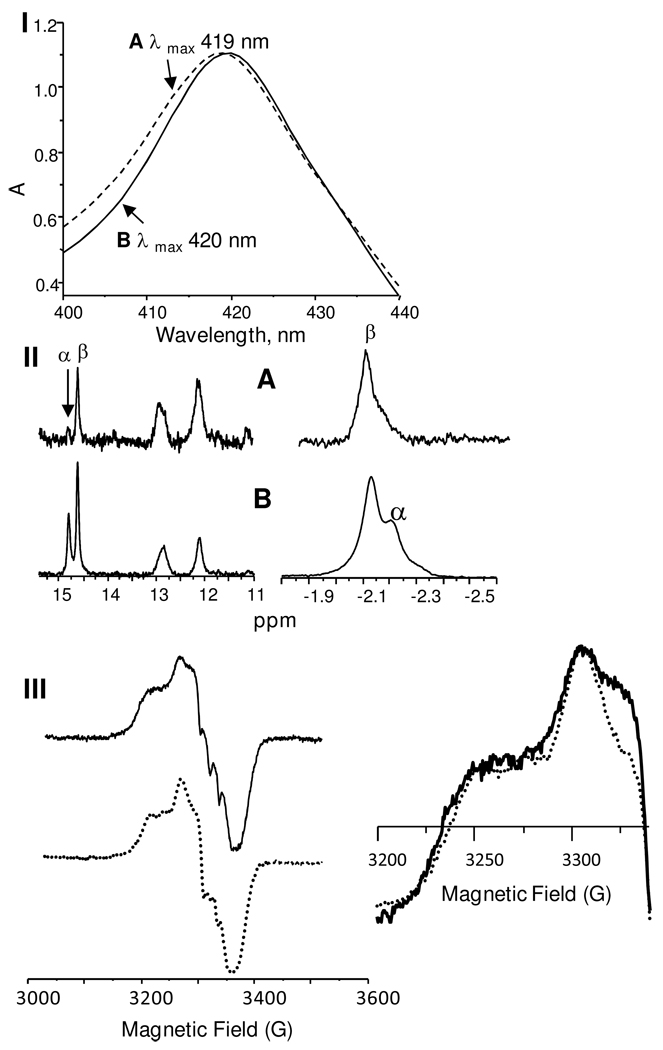
A solution of NO-hHb was titrated with reduced DTDP and the reaction monitored by UV-vis spectrophotometry. Sequential samples (A and B) were taken from the solution after titrations had shifted the Soret absorbance from 418 nm of NO-Hb to 419 and 420 nm, as shown in Figure 6. 1H NMR spectra of these samples after concentration displayed the two characteristic HNO-hHb peaks, but that of sample A had a significantly smaller peak at 14.8 ppm as compared to sample B, Figure 6. Comparison of the EPR spectra of these isolated samples revealed significant differences that can be attributed to different subunit content of ferrous nitrosyl adducts; the spectrum of sample A has an enhanced resonance ca. 3235 and 3330 G, which are characteristic of the ferrous nitrosyl of the α subunit.35,36 An excess of the nitrosyl adduct in the α subunit implies that of the β subunit has been lost by conversion to the HNO-FeII state. This logic assigns the 1H NMR peaks at 14.6 and 14.8 ppm to β and α subunits of HNO-Hb, respectively, and peaks at −2.13 and −2.20 ppm to E11 Val peaks of β and α subunits.
Figure 6.
I) Soret band absorbances of partially reduced NO-hHb samples A, with maxima at 419 (dashed line), and B, at 420 nm (solid line). II) 1H NMR of HNO-hHb samples A and B. III) EPR spectra of samples A (solid line) and B (dashed line).
2D NMR characterization
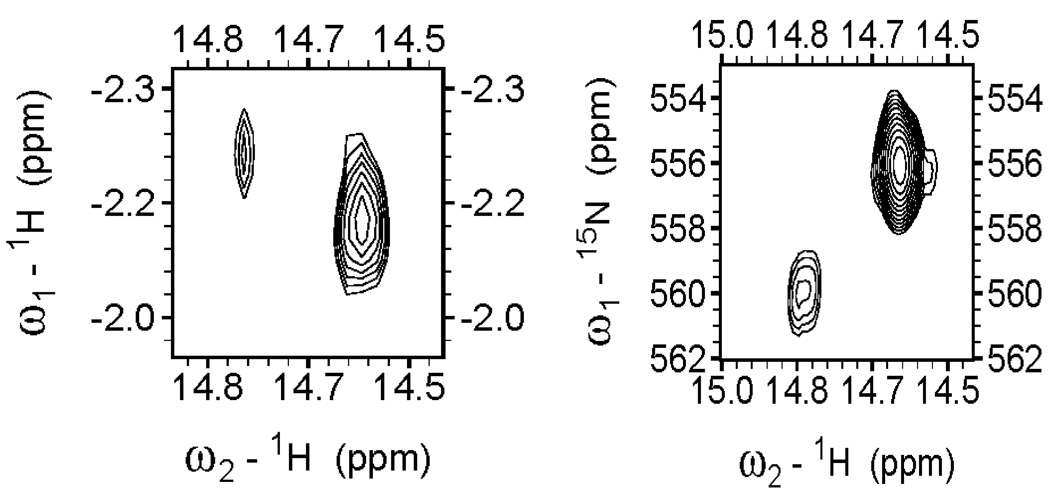
During initial characterizations of HNO-Mb, 1H-1H NOESY experiments showed a strong cross-peak at –2.67 ppm due to dipolar relaxation of the nitroxyl hydride with a close by valine methyl group at one side of the heme pocket;17 subsequently, more detailed experiments identified some twenty NOEs between the nitrosyl hydride and protein and heme-based signals, which were used to solve the three dimensional solution structure.19 In a similar manner, initial 1H-1H NOESY experiments on HNO-hHb exhibit strong cross-peaks to the distal valine methyl in the two subunits, Figure 7a suggesting the orientation of the nitrosyl hydride within the heme pocket is analogous to that of HNO-Mb. 1H-15N HSQC experiments are also useful for HNO adducts as rapid characterizations of both the 15N isomer shifts and JNH coupling values, Figure 7b. The 15N isomer shifts provide an additional unique identifer of a nitrosyl hydride adduct and its electronic environment, Table 2.
Figure 7.
Left) 1H- 1H NOESY spectrum of HNO-hHb showing crosspeak between nitrosyl hydride and distal pocket valine methyl groups; right) 15N-1H HSQC spectrum of HNO-hHb showing both 1H and 15N chemical shifts for the two subunits.
Table 2.
1H and 15N NMR chemical shifts and coupling constants of H15NO adducts of globins
| Protein | δ, ppm |
J, Hz | |
|---|---|---|---|
| 1H | 15Na | ||
| H15NO-Mb | 14.87, 14.99 | 549.1 | 71.2 |
| H15NO-hHb | 14.57, 14.65 | 560.0 | 70.39 |
| 14.75, 14.84 | 556.2 | 66.37 | |
| H15NO-lgHb | 14.94, 15.05 | 553.0 | 71.19 |
| H15NO-cHb | 15.55, 15.44 | n/a | 70.1 |
from 1H-15N HSQC experiments, referenced to 15NH4Cl.
Quantification of HNO adduct yield
The described reactions generate the HNO-adduct in mixtures with other species, the paramagnetic deoxy and nitrosyl adduct species, and accurate quantification is problematic. For the trapping reactions of deoxy globins with HNO precursors, NMR analysis confirms the generation of the diamagnetic HNO adduct, but often smaller paramagnetic peaks due to the NO adduct are seen.17 During the early stages of these reactions, the deoxy Soret absorbance is rapidly lost; addition of CO to the headgas over the product mixture produces no change, thus implying minimal deoxy impurity. As given in Table 1, the HNO-adduct and NO-adducts have absorbance maxima shifted by only 1 to 3 nm; therefore EPR analysis of the product solutions are used to quantify the nitrosyl adduct concentration by comparison with authentic samples, and these values used to estimate yield of HNO adduct (supplemental). By this analysis, the reaction of PA with deoxyMb, ca. 4:1 stoichiometry, gives a yield of 70–80% HNO-Mb.18 By comparison, that of HNO-hHb under analogous reactions with PA is ca. 25%, but yields of up to 48% were obtained in slower reactions with the precursor MSHA. The overall yield of HNO adduct from reactions of PA with other monomeric globins is also low, e.g.. 30–40% by EPR calibration of the NO-adduct content for cHb.
Reductive titrations of the ferrous nitrosyl adduct samples using DTDP/Zn-Hg method generates higher purity samples of HNO adduct. These titrations are monitored by the typical increase and shift of the Soret absorbance as the HNO adduct is formed; loss of Soret absorbance indicates an over-reduction generating the deoxy species, observable in the absorbance spectra or by CO trapping. This method has been used to generate HNO-Mb in greater than 90% purity,27 HNO-hHb in 80% yield, and of other globins in an estimated range of 60–90%.
Kinetics of HNO trapping
The rate of the trapping reactions of ferrous globins were obtained by measuring loss of the ferrous Soret absorbance in sequential UV-vis absorbance spectra taken during reactions with PA over ca. 500 seconds, as shown in Figure 1 for lgHb. As the generation of free HNO by PA is rate-limiting, the rate constants are modeled with a kinetic simulations program37 utilizing a reaction sequence analogous to that previously reported for Mb.18 This sequence includes the release of HNO from PA (Eq. 4), its dimerization (Eq. 5), its trapping by the ferrous globin to form the HNO adduct (Eq.6).
| (4) |
| (5) |
| (6) |
| (7) |
The bimolecular rate constants for HNO trapping resulting from this analysis are on the order of 105 M−1 s−1 for all the monomeric globins, Table 3. For tetrameric hHb the loss of the deoxy absorbance during HNO trapping reactions is multiphasic over the same period, likely due to allosteric interactions between subunits as well as a competing reaction of HNO with the β̃ Cys93;38 the value given in Table 3 was obtained by comparison of loss of deoxy Soret absorbance over 50 seconds in comparison to Mb under conditions of equivalent heme and PA concentrations (supplemental).
Table 3.
Initial rate constants of trapping of HNO by ferrous globins.
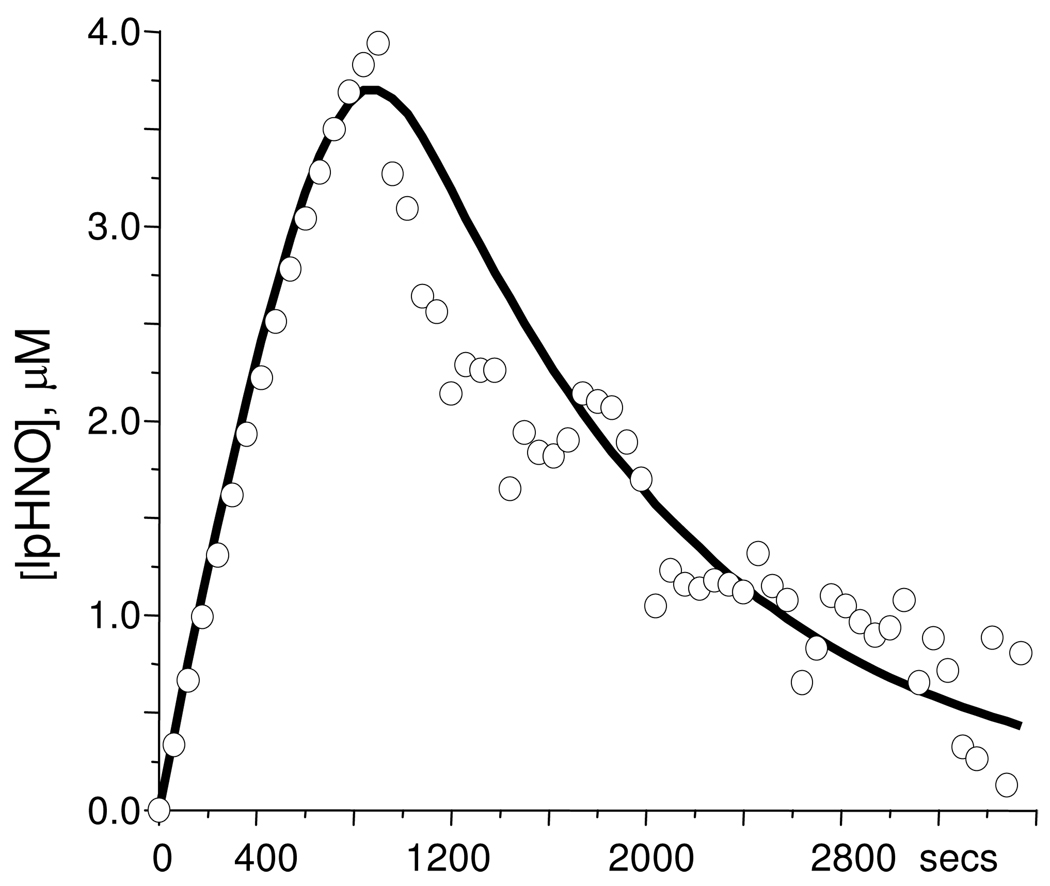
A key factor limiting HNO-adduct yield in trapping reactions is a subsequent reactivity of the adduct with free HNO, Eq.7, previously described for HNO-Mb.18, 39 As comparison, a more complete modeling was performed for the reaction of cHb and PA over an hour, Figure 8, using the transient changes at the Soret absorbance of HNO-cHb at 421 nm. Multiple iterations were performed using fixed rate constants for PA decomposition (Eq. 4) and HNO dimerization (Eq. 5) until experimental and calculated concentration profiles gave an acceptable match. EPR quantification of the NO-cHb content of the final reaction mixture (supplemental) at ca. 80% is in good agreement with the calculated speciation of 85 % by the kinetic analysis. The modeled bimolecular rate constant of Eq. 7 for cHb, at 7 × 104 M−1 sec−1, is considerably faster than that determined for Mb, at 4 × 103 M−1 sec−1,39 which may explain the lower yield of HNO-cHb obtained under equivalent reaction conditions.
Figure 8.
HNO binding kinetics Experimental (circle) and calculated (line) concentration profile of the HNO-cHb obtained using Soret absorbance changes at 421 nm during the reaction of 5 µM deoxy cHb with 25 µM PA in carbonate buffer at pH 9.4.
Conclusion
This work demonstrates that several oxygen-binding globins react with free HNO in solution to form long-lived HNO adducts, and that such adducts can be alternatively formed by careful reduction of the ferrous NO adducts of these proteins. The HNO adducts are diamagnetic and have a unique 1H NMR HNO hydride signal well away from other protein residues. The location of nitrosyl hydride peaks is sensitive to the protein environment, and thus differentiates subunits and isoforms within protein mixtures. More broadly, these diamagnetic FeII-HNO adducts may be of value as models of ferrous oxycomplexes, and allow characterizations of structural changes accompanying oxygen binding and activation in similar proteins.
Supplementary Material
ACKNOWLEDGMENT
Electrospray LC-MS was performed by the UCI Center for Virus Research Mass Spec Facility. We also thank Fraser Bergeson for the generous donation of native lgHb isoform mixture.
Abbreviations
- Mb
myoglobin
- hHb
human hemoglobin
- cHb
clam Hemoglobin I from Lucina pectinata
- lgHb
leghemoglobin
- MSHA
methylsulfonylhydroxylamic acid
- PA
Piloty’s acid
- NO
nitric oxide
- HNO
nitrosyl hydride
- NO−
nitroxyl anion
- DTDP
4,4’-dimethyl-1,1’-trimethylene-2,2’dipyridinium
- Zn-Hg
zinc amalgam
- NMR
nuclear magnetic resonance
- HSQC
hetero nuclear single quantum coherence
- EPR
electron paramagnetic resonance
- UV-vis
ultra violet visible spectroscopy
Footnotes
This research was supported by the National Science Foundation (PJF CHE-0100774) and the National Institutes of Health (PJF 1R21ES016441-01).
SUPPORTING INFORMATION AVAILABLE. Experimental details available include descriptions of peak fitting for isoforms of native legHb mixtures, timecourse UVvis spectra during the formation of HNO-hHb, EPR characterization of ferrous NO adduct impurities in HNO adduct samples and data for initial rate analysis of HNO trapping by deoxy Mb and hHb. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Fukuto JM, Bartberger MD, Dutton AS, Paolocci N, Wink DA, Houk KN. The Physiological Chemistry and Biological Activity of Nitroxyl (HNO): The Neglected, Misunderstood, and Enigmatic Nitrogen Oxide. Chem. Res. Toxicol. 2005;18:790–801. doi: 10.1021/tx0496800. [DOI] [PubMed] [Google Scholar]
- 2.Fukuto JM, Switzer CH, Miranda KM, Wink DA. Nitroxyl (HNO): chemistry, biochemistry, and pharmacology. Ann. Rev. Pharmacol. Toxicol. 2005;45:335–355. doi: 10.1146/annurev.pharmtox.45.120403.095959. [DOI] [PubMed] [Google Scholar]
- 3.Shafirovich V, Lymar SV. Spin-forbidden deprotonation of aqueous nitroxyl (HNO) J. Am. Chem. Soc. 2003;125:6547–6552. doi: 10.1021/ja034378j. [DOI] [PubMed] [Google Scholar]
- 4.Lymar SV, Shafirovich V, Poskrebyshev GA. One-electron reduction of aqueous nitric oxide: a mechanistic revision. Inorg. Chem. 2005;44:5212–5221. doi: 10.1021/ic0501317. [DOI] [PubMed] [Google Scholar]
- 5.Shafirovich V, Lymar SV. Nitroxyl and its anion in aqueous solutions: Spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc. Natl. Acad. Sci. USA. 2002;99:7340–7345. doi: 10.1073/pnas.112202099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Richter-Addo GB. Binding of Organic Nitroso Compounds to Metalloporphyrins. Acc. Chem. Res. 1999;32:529–536. [Google Scholar]; b) Lee J, Chen L, West AH, Richter-Addo GB. Interactions of Organic Nitroso Compounds with Metals. Chem. Rev. 2002;102:1019–1065. doi: 10.1021/cr0000731. [DOI] [PubMed] [Google Scholar]
- 7.a) Mansuy D, Chottard, Chottard G. Nitrosoalkanes as Fe(II) Ligands in the Hemoglobin and Myoglobin Complexes Formed from Nitroalkanes in Reducing Conditions. Eur. J. Biochem. 1977;76:617–623. doi: 10.1111/j.1432-1033.1977.tb11632.x. [DOI] [PubMed] [Google Scholar]; b) Mansuy D, Battioni P, Chottard JC, Lange M. Nitrosoalkanes as new ligands of iron(II) porphyrins and hemoproteins. J. Am. Chem. Soc. 1977;99:6441–6443. doi: 10.1021/ja00461a046. [DOI] [PubMed] [Google Scholar]
- 8.Mansuy D, Battioni P, Chottard JC, Riche C, Chiaroni A. Nitrosoalkane complexes of iron-porphyrins: analogy between the bonding properties of nitrosoalkanes and dioxygen. J. Amer. Chem. Soc. 1983;105:455–463. [Google Scholar]
- 9.Melenkivitz R, Hillhouse GL. Synthesis, structure, and reactions of a nitroxyl complex of iridium(III), cis,trans-IrHCl2(NH=O)(PPh3)2. Chem. Commun. 2002:660–661. doi: 10.1039/b111645b. [DOI] [PubMed] [Google Scholar]
- 10.Southern JS, Green MT, Hillhouse GL, Guzei IA, Rheingold AL. Chemistry of coordinated nitroxyl. Reagent-specific protonations of trans-Re(CO)(2)(NO)(PR(3))(2) (R = Ph, Cy) that give the neutral nitroxyl complexes cis,trans-ReCl(CO)(2)(NH=O)(PR(3))(2) or the cationic hydride complex [trans,trans-ReH(CO)(2)(NO)(PPh(3))(2)(+)][SO(3)CF(3)(−)] Inorg. Chem. 2001;40:6039–6046. doi: 10.1021/ic010669m. [DOI] [PubMed] [Google Scholar]
- 11.Melenkivitz R, Southern JS, Hillhouse GL, Concolino TE, Liable-Sands LM, Rheingold AL. A new route to coordination complexes of nitroxyl (HN=O) via insertion reactions of nitrosonium triflate with transition-metal hydrides. J. Am. Chem. Soc. 2002;124:12068–12069. doi: 10.1021/ja0277475. [DOI] [PubMed] [Google Scholar]
- 12.Sellmann D, Gottschalk-Gaudig T, Haussinger D, Heinemann FW, Hess BA. [Ru(HNO)('py(bu)S4')], the first HNO complex resulting from hydride addition to a NO complex ('pybuS4'2-=2,6-Bis(2-mercapto-3,5-di-tert-butylphenylthio)dime thylpyridine(2–1)) Chem. Eur. J. 2001;7:2099–2103. doi: 10.1002/1521-3765(20010518)7:10<2099::aid-chem2099>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.a) Marchenko AV, Vedernikov AN, Dye DF, Pink M, Zaleski JM, Caulton KG. An electron-excessive nitrosyl complex: reactivity of a ligand-centered radical leading to coordinated HNO. Inorg. Chem. 2002;41:4087–4089. doi: 10.1021/ic025699j. [DOI] [PubMed] [Google Scholar]; b) Marchenko AV, Vedernikov AN, Dye DF, Pink M, Zaleski JM, Caulton KG. Reactivity of the hydrido/nitrosyl radical MHCl(NO)(CO)(P(i)Pr(3))(2), M = Ru, Os. Inorg. Chem. 2004;43:351–360. doi: 10.1021/ic0349407. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Richter-Addo GB. A nitrosyl hydride complex of a heme model [Ru(ttp)(HNO)(1-MeIm)] (ttp=tetratolylporphyrinato dianion) J. Inorg. Biochem. 2004;98:1247–1250. doi: 10.1016/j.jinorgbio.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Farmer PJ, Sulc F. Coordination chemistry of the HNO ligand with hemes and synthetic coordination complexes. J. Inorg. Biochem. 2005;99:166–184. doi: 10.1016/j.jinorgbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Bayachou M, Lin R, Cho W, Farmer PJ. Electrochemical Reduction of NO by Myoglobin in Surfactant Film: Characterization and Reactivity of the Nitroxyl (NO-) Adduct. J. Am. Chem. Soc. 1998;120:9888–9893. [Google Scholar]
- 17.Lin R, Farmer PJ. The HNO Adduct of Myoglobin: Synthesis and Characterization. J. Am. Chem. Soc. 2000;122:2393–2394. [Google Scholar]
- 18.Sulc F, Immoos CE, Pervitsky D, Farmer PJ. Efficient Trapping of HNO by Deoxymyoglobin. J. Am. Chem. Soc. 2004;126:1096–1101. doi: 10.1021/ja0376184. [DOI] [PubMed] [Google Scholar]
- 19.Sulc F, Ma D, Fleischer E, Farmer PJ, La Mar GN. 1H NMR Structure of the Heme Pocket of HNO-Myoglobin. J. Biol. Inorg. Chem. 2003;8:348–352. doi: 10.1007/s00775-002-0422-7. [DOI] [PubMed] [Google Scholar]
- 20.King SB, Nagasawa HT. Chemical Approaches Toward the Generation of Nitroxyl (HNO. Methods in Enzymology. 1998;301:211–221. doi: 10.1016/s0076-6879(99)01084-8. [DOI] [PubMed] [Google Scholar]
- 21.Sulc F. Ph.D. dissertation. Irvine: University of California; 2006. Nitrosyl hydride adduct of deoxymyoglobin: Structure, reactivity and biological importance of Mb-nitrosyl hydride. [Google Scholar]
- 22.Appleby CA. Leghemoglobin and Rhizobim Respiration. Ann. Rev. Plant Physiol. 1984;35:443–478. [Google Scholar]
- 23.Navarro AM, Maldonado M, Gonzales-Lagoa J, Lo'pez-Mejia R, Lopez-Garriga J, Colo'n JL. Control of carbon monoxide binding states and dynamics in hemoglobin I of Lucina pectinata by nearby aromatic residues. Inorg. Chim. Act. 1996;243:161–166. [Google Scholar]
- 24.Park SY, Yokoyama T, Shibayama N, Shiro Y, Tame JR. 1.25 angstrom resolution crystal structures of human haemoglobin in the oxy, deoxy and carbonmonoxy forms. J. Mol. Biol. 2006;360:690–701. doi: 10.1016/j.jmb.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 25.Brink K, Gombler W, Bliefert CZ. Methylsulfonylhydroxylamine. Anorg. Allg. Chem. 1977;429:255–260. [Google Scholar]
- 26.Hargrove MS, Barry1 JK, Brucker EA, Berry1 MB, Phillips GN, Jr, Olson JS, Arredondo-Peter R, Dean JM, Klucas RV, Sarath G. Characterization of recombinant soybean leghemoglobin a and apolar distal histidine mutants. J. Mol. Biol. 1997;266:1032–1042. doi: 10.1006/jmbi.1996.0833. [DOI] [PubMed] [Google Scholar]
- 27.Natera SHA, Guerreiro N, Djordjevic MA. Proteome analysis of differentially displayed proteins as a tool for the investigation of symbiosis. Mol. Plant-Micr. Interact. 2000;13:995–1009. doi: 10.1094/MPMI.2000.13.9.995. [DOI] [PubMed] [Google Scholar]
- 28.Pervitsky D, Immoos C, van der Veer W, Farmer PJ. Photolysis of the HNO Adduct of Myoglobin: Transient Generation of the Aminoxyl Radical. J. Am. Chem. Soc. 2007;129:9590–9591. doi: 10.1021/ja073420y. [DOI] [PubMed] [Google Scholar]
- 29.Kapp OH, Moens L, Vanfleteren J, Trotman CN, Suzuki T, Vinogradov SN. Alignment of 700 globin sequences: extent of amino acid substitution and its correlation with variation in volume. Protein Sci. 1995;4:2179–2190. doi: 10.1002/pro.5560041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appleby CA. The origin and functions of haemoglobin in plants. Sci. Progress. 1992;76:365–398. [Google Scholar]
- 31.Atanasov BP, Dimitrova EA, Kudryatseva NN, Zhiznevskaya GY, Appleby CA. Structure of Ferric Soybean Leghemoglobin a Nicotinate at 2.3 Å Resolution. Biochim. Biophys. Acta. 1989;998:80–84. [Google Scholar]
- 32.Nguyen BD, Zhao X, Vyas K, La Mar GN, Lile RA, Brucker EA, Phillips JR, Jr, Olson JS, Wittenberg JB. Solution and crystal structures of a sperm whale myoglobin triple mutant that mimics the sulfide-binding hemoglobin from Lucina pectinata. J Biol Chem. 1998;273:9517–9526. doi: 10.1074/jbc.273.16.9517. [DOI] [PubMed] [Google Scholar]
- 33.Saalbach G, Erik P, Wienkoop S. Characterisation by proteomics of peribacteroid space and peribacteroid membrane preparations from pea (Pisum sativum) symbiosomes. Proteomics. 2002;2:325–337. doi: 10.1002/1615-9861(200203)2:3<325::aid-prot325>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 34.Mihailescu MR, Russu IM. A signature of the T→R transition in human hemoglobin. Proc. Nat. Acad. Sci. 2001;98:3773–3777. doi: 10.1073/pnas.071493598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yonetani T, Tsuneshige A, Zhou Y, Chen X. Electron Paramagnetic Resonance and Oxygen Binding Studies of α-Nitrosyl Hemoglobin. J. Biol. Chem. 1998;273:20323–20333. doi: 10.1074/jbc.273.32.20323. [DOI] [PubMed] [Google Scholar]
- 36.Hille R, Olson JS, Palmer G. Spectral transitions of nitrosyl hemes during ligand binding to hemoglobin. J. Biol. Chem. 1979;254:12110–12120. [PubMed] [Google Scholar]
- 37.Manka MJ, editor. Alchemy Software. Chapel, FL: Wesley; 2001. REACT for Windows, Version 1.2. [Google Scholar]
- 38.Doyle MP, Mahapatro SN, Broene RD, Guy JK. Oxidation and Reduction of Hemoproteins by Trioxodinitrate(II). The Role of Nitrosyl Hydride and Nitrite. J. Am. Chem. Soc. 1988;110:593–599. [Google Scholar]
- 39.Pervitsky D. Ph.D. dissertation. Irvine: University of California; 2008. Generation and reactivity of HNO adducts of horse skeletal myoglobin and human hemoglobin. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.