Abstract
Cytochrome P450 (CYP or P450) enzymes are ubiquitous in nature where they catalyze a vast array of oxidation reactions. The active oxidants in P450s have long been assumed to be iron(IV)-oxo porphyrin radical cations termed Compounds I, but P450 Compounds I have proven difficult to prepare. The recent development of an entry to these transients by photo-oxidation of the corresponding iron(IV)-oxo neutral porphyrin species (Compounds II) permits spectroscopic and kinetic studies. We report here application of the photo-oxidation method for production of Compound I from the heme domain of CYP102A1 (cytochrome P450BM-3), and product and kinetic studies of reactions of styrene with this Compound I transient and also Compound I from CYP119. The studies were performed at low temperatures in 1:1 (v:v) mixtures of glycerol--phosphate buffer. Single turnover reactions at 0 °C gave styrene oxide in good yields. In kinetic studies conducted between −10 and −50 °C, both Compounds I displayed saturation kinetics permitting determinations of binding constants and first-order oxidation rate constants. Temperature-dependent functions for the binding constants and rate constants were determined for both Compounds I. In the temperature range studied, the Compound I transient from CYP102A1 heme domain bound styrene more strongly than Compound I from CYP119, but the rate constants for oxidations of styrene by the latter were somewhat larger than those for the former. The temperature dependent functions for the first-order oxidation reactions are log k = 13.2 – 15.2/2.303RT and log k = 13.3 – 14.6/2.303RT (kcal/mol) for Compounds I from CYP102A1 heme domain and CYP119, respectively.
Cytochrome P450 (P450 or CYP) enzymes are heme-containing enzymes that are broadly distributed in nature (1). The P450s differ from other heme enzymes by the presence of a thiolate from protein cysteine as the fifth ligand to iron. They are catalysts for many reactions, but serve primarily as catalysts for oxidation reactions, including alkene epoxidations and high energy demand oxidations of C-H bonds. The active oxidants in P450s have long been assumed to be iron(IV)-oxo porphyrin radical cations termed Compounds I by analogy to the known Compounds I formed in peroxidase and catalase enzymes (2), but production of P450 Compounds I has proven to be difficult. Rapid mixing studies of P450s with chemical oxidants dating back three decades (3) have yielded UV-visible spectroscopic evidence for production of P450 Compounds I transients (4–7), but rapid freeze-quench mixing studies of P450s with peroxy acids followed by EPR, ENDOR, and Mössbauer spectroscopic analyses found no evidence for accumulation of Compounds I from P450cam (8,9) and P450BM-3 (10,11). Accordingly, no kinetic studies of P450 Compounds I produced by chemical oxidations of the enzymes exist.
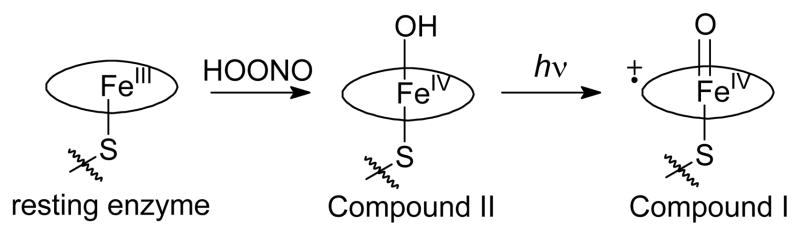
Given the difficulty in production of P450 Compounds I by chemical oxidations, our group developed an alternative entry to these long-sought intermediates. Oxidation of some P450s in the resting state with peroxynitrite gave the corresponding iron(IV)-oxo neutral porphyrin species, termed Compounds II, and subsequent photo-oxidations of Compounds II gave Compounds I. Initially, the method was developed for short time scales using laser flash photolysis (LFP) methods at ambient temperature (12), and it was applied for production of Compounds I from CYP119 (13,14), a P450 from a thermophile, and CYP2B4 (15,16), a mammalian hepatic P450. More recently, the method was extended to quantitative production of CYP119 Compound I in low temperature reactions using a high-power lamp for photolysis (17).
With the development of the photo-oxidation entry to P450 Compounds I, kinetic studies of these elusive transients became possible. The kinetics of C-H hydroxylation and alkene epoxidation reactions by CYP119 and CYP2B4 Compounds I were studied at ambient temperature using LFP methods (14–16). Variable temperature kinetic studies now also are possible, and oxidations of benzyl alcohol at varying temperatures by CYP119 Compound I were reported (17). The evolving kinetic data is expected eventually to provide valuable information regarding the reactivity of P450 Compounds I and might aid in the design and evaluation of P450 inhibitors.
In the present work, we report the low temperature production of a second P450 Compound I using the heme domain of CYP102A1, henceforth referred to as CYP102HD. CYP102A1, also known as P450BM-3, is a widely studied fusion protein containing a heme domain and a reductase domain (18), and the expressed heme domain is the equivalent of a typical P450 enzyme (19). We also report low temperature kinetic studies of oxidations of styrene by Compounds I from both CYP102HD and CYP119, a P450 from a thermophile, which permitted determinations of both binding constants for styrene in the Compound I species and first-order oxidation rate constants. The binding constants for the two Compounds I differed, as one would expect, but the first-order oxidation rate constants differed only slightly. The results indicate that oxidation velocities can be strongly affected by binding but only modestly modulated in terms of Compound I reactivity.
MATERIALS AND METHODS
Materials
The expression in E. coli and purification of CYP119 were as previously reported (13,20,21). The expression of CYP102HD in E. coli strain JM109(DE3) and purification followed the methods of Yeom et al. (22). Both P450 enzymes had RZ > 1.5. Sodium peroxynitrite solutions were prepared by the method of Uppu and Pryor (23). Glycerol and styrene were from Sigma-Aldrich Chemical Co. Styrene was passed through a column of alumina before us to remove stabilizers. Authentic samples of CYP102HD nitrosyl complex were prepared by mixing the enzyme with a commercial sample of the NO donor diethylamine NONOate (Sigma-Aldrich).
Instrumentation
UV-visible spectra were acquired an Ocean Optics USB4000 diode array spectrometer using fiber optics cables. Photolyses were performed with an EFOS Novacure 2001 spot lamp employing a mercury bulb and a 365 ± 5 nm cutoff filter; the lamp was set to deliver 5 W of light in pulses as short as 0.1 s. GC was accomplished on an Agilent 6890N gas chromatograph using flame ionization detection and separation on a 0.5 mm × 15 m wide-bore capillary DB-5 column. GC mass spectra were obtained on an Agilent 6890N gas chromatograph affixed to an Agilent 5973 mass selective detector on a 0.2 mm × 30 m narrow-bore capillary DB-5 column.
Preparation of P450 Compounds I
The method used for CYP102HD was similar to that reported for preparation of CYP119 Compound I (17). Thus, 200 μL solutions of 5 μM P450 enzyme in 100 mM phosphate buffer (pH 7.0) containing 50% by volume glycerol were cooled to ca. −10 °C in a microcuvette in a temperature-regulated cuvette holder containing leads for the fiber optics cables of the UV-visible spectrometer and for a flexible waveguide from the spot lamp. A basic solution (ca. 1 μL) of 75 mM peroxynitrite was added via pipette raising the pH of the solution to pH 7.4. The formation of Compounds II (λmax ≈ 331 nm) and the decay of PN (λmax = 308 nm) were followed spectroscopically. Compound II formation was complete within seconds. When PN decay was complete, the solutions were cooled to the desired temperature and allowed to equilibrate for several minutes. The solutions were irradiated with 365 nm light until conversion of Compound II to Compound I was complete as determined by UV-visible spectroscopic monitoring.
Photolyses Products
Solutions containing the P450 enzyme (3 nmol) and styrene (0.2 μmol) in 200 μL of a 1:1 (v:v) mixture of glycerol and 50 mM phosphate buffer (pH 7.0) in a microcuvette in the apparatus described above were cooled to ca. 0 °C. Sodium peroxynitrite solution (0.225 μmol in 3 μL) was added via pipette, and the reaction mixture was monitored spectroscopically. When PN decay was complete, the mixtures were irradiated with 365 nm light for ca. 2 s (CYP102HD) or 0.5 s (CYP119) at which point decay of Compound II monitored at λmax of the Soret band was complete. The mixtures were extracted into methylene chloride, and the organic layer was washed with saturated aqueous sodium chloride and dried (MgSO4). An internal standard of 1-phenylethanol was added, and the mixtures were analyzed by GC and GC-mass spectrometry. Styrene oxide was the only significant product detected. It was identified by comparison of its GC retention time and mass spectrum to those of an authentic commercial sample (Sigma-Aldrich). The yields of styrene oxide were determined by comparison to the internal standard using predetermined response factors in duplicate runs.
Kinetic Studies
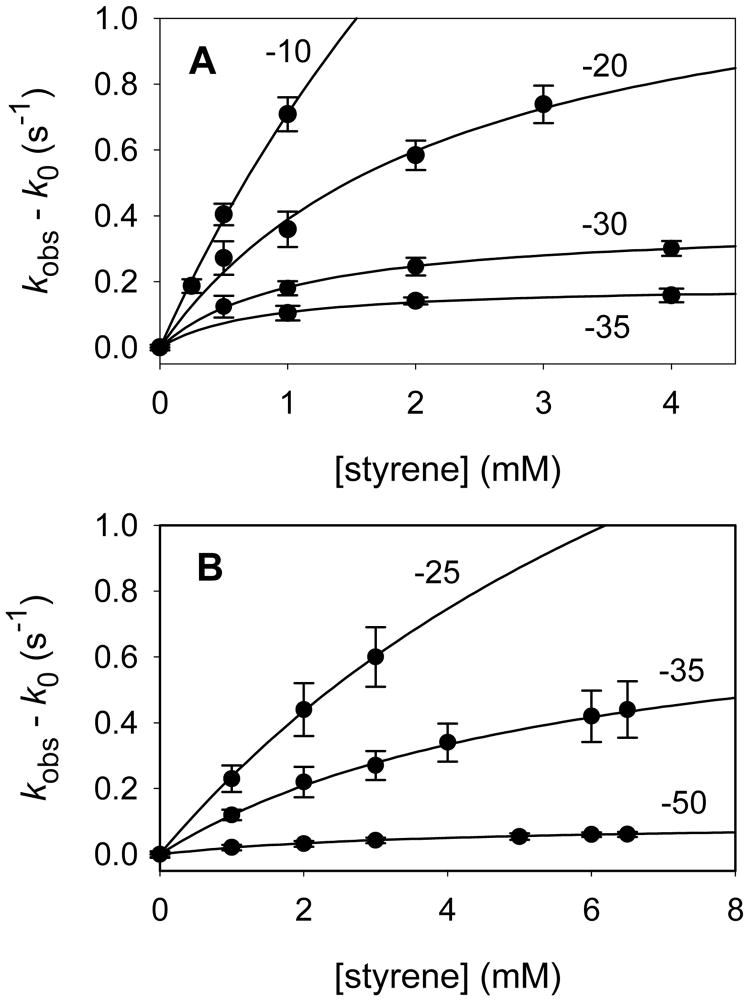
Beer’s law studies were performed to ensure that styrene was soluble in 1:1 (v:v) glycerol--100 mM phosphate buffer at the concentrations necessary for kinetic studies; at all temperatures of the kinetic studies, linear Beer’s law behavior was observed up to concentrations of styrene >8 mM. For kinetic studies, the reaction mixtures were prepared as described above with the exception that the initial enzyme solutions contained styrene at specific desired concentration. Following the irradiation step, the reactions of Compounds I were followed spectroscopically by increases in absorbance at λmax of the Soret band of the resting enzyme. The kinetic traces were solved for double exponential signal growth using SigmaPlot 2001 software. The major exponential increased with increasing styrene concentration, and the minor exponential varied randomly. The individual kinetic results are listed in the Supporting Information. Plots of the observed pseudo-first-order rate constants showed curvature indicative of saturation kinetics behavior as illustrated in Figure 3. The binding constants (Kbind) and first-order oxidation rate constants (kox) were solved according to Eq 1 and are listed in Table 1.
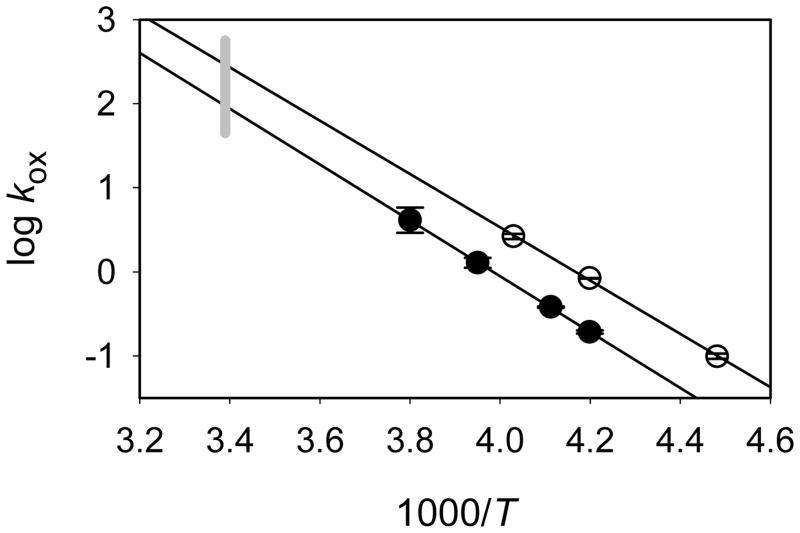
Figure 3.
Rate constants for oxidation of styrene by Compounds I from (A) CYP102HD and (B) CYP119. Temperatures for the reactions are shown next to the plots.
Table 1.
Binding constants and first-order rate constants for oxidations of styrene by cytochrome P450 Compounds I.a
| Enzyme | Temp (°C)b | Kbind (M−1) | kox (s−1) |
|---|---|---|---|
| CYP102HD | −35 | 1,250 ± 190 | 0.192 ± 0.008 |
| − 30 | 920 ± 43 | 0.381 ± 0.007 | |
| −20 | 440 ± 120 | 1.28 ± 0.19 | |
| −10 | 210 ± 100 | 4.1 ± 1.7 | |
| 22c | 36 | 90 | |
| CYP119 | −50 | 254 ± 16 | 0.098 ± 0.003 |
| −35 | 170 ± 15 | 0.84 ± 0.04 | |
| −25 | 99 ± 9 | 2.64 ± 0.20 | |
| 22c | 26 | 300 |
Errors listed are at 1σ.
± 0.2 °C.
Values for 22 °C are extrapolated from the low temperature results.
RESULTS AND DISCUSSION
The P450 enzymes used in this study were CYP119 and the heme domain of CYP102A1. CYP119 was originally believed to be from the thermophile Sulfolobus solfataricus, but more recently it was reported to be from the related species S. acidocaldarius (24). CYP119 was expressed in Escherichia coli and purified as previously described (13,20,21). The samples of CYP119 used in this study had RZ ratios > 1.5 indicating high purity. CYP102A1, also known as P450BM-3, is a fatty acid hydroxylase from the bacterium Bacillus megaterium; it is a fusion enzyme that contains both a reductase domain and a heme domain of a P450 (18). In this work, we used the heme domain portion (19), CYP102HD, that was expressed in E. coli and purified by the methods of Yeom et al. (22). Samples of CYP102HD had RZ ratios > 1.5.
The method used for production of P450 Compounds I is shown in Scheme 1 (14,17). A basic solution of peroxynitrite (PN) was added to a buffer solution containing the P450 enzyme. The buffer solution was initially at pH = 7.0, and the basic PN solution reduced the acid concentration to give pH ≈ 7.4. At this pH, the PN is protonated to peroxynitrous acid (HOONO), which decomposes rapidly at ambient temperature (25). Some P450s, including CYP119, react with PN (or HOONO) to give Compound II, presumably by oxygen transfer to iron to give a formal iron(V)-oxo species followed by an electron transfer from the by-product nitrite ion. For CYP119, Compound II was studied by X-ray absorbance spectroscopy (XAS), and the extended X-ray absorbance fine structure (EXAFS) spectrum showed a long iron-oxygen bond length (1.82 Å) indicating that the oxygen atom was protonated as shown in Scheme 1 (26). Subsequent photolysis of Compound II gives Compound I in a photo-oxidation involving loss of an electron and a proton (12). The mechanistic details of the photo-oxidation reaction, i.e. whether the loss of an electron and proton are distinct steps or coupled, are not known.
Scheme 1.
Production of Compound II from CYP102HD
After the report of production of CYP119 Compound I by the route shown in Scheme 1 (13), Green and co-workers claimed that P450BM-3 reacted with PN to give a nitrosyl product and not Compound II (27). That report contradicted a detailed earlier study by Daiber et al. concerning reactions of P450BM-3 that found that the Compound II species was formed from reaction with PN and made no mention of formation of a nitrosyl complex (28). Unfortunately, the Daiber et al. work was not discussed in the paper by Green and co-workers. Because of the conflicting claims concerning the formation of Compound II from PN reaction with CYP102, we compared the putative Compound II product formed in the PN reaction with authentic samples of the nitrosyl complex in terms of the lifetimes of the two species and their behavior when photolyzed. Details of these experiments are discussed later in this section and in the section immediately following this section, but the summary of the results is that we verified that CYP102HD reacts with PN to give the Compound II intermediate as reported in the early work by Daiber et al. (28), and we were unable to find any evidence for formation of the nitrosyl complex from this reaction.
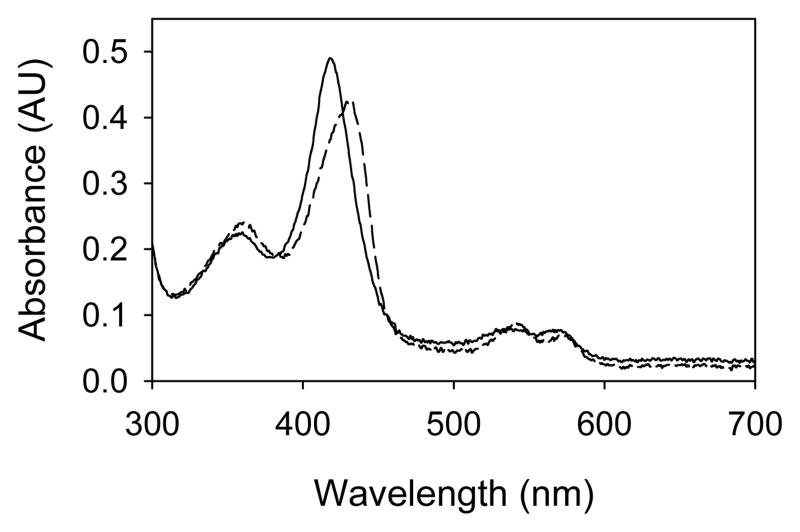
The reported formation of Compound II by reaction of CYP102 with PN involved studies with the full length enzyme, which contains a heme domain and a reductase domain (28). In the present work, when CYP102HD was allowed to react with PN, the absorbance maximum for the Soret band of the resting enzyme (λmax = 419 nm) decreased, and an absorbance with λmax = 431 nm grew in from Compound II. Figure 1 shows spectra of the resting enzyme and the Compound II intermediate, which are similar to those published for PN oxidation of the full enzyme (28). After depletion of the PN, Compound II decayed rapidly as previously reported (28) and as seen with other P450 enzymes that produce Compound II intermediates (29). At reduced temperatures in 1:1 (v:v) solutions of glycerol and buffer, CYP102HD Compound II showed good stability; the half-life for decay of this intermediate at −10 °C was ca. 9 min.
Figure 1.
UV-visible spectra of CYP102HD resting enzyme (solid line) and Compound II intermediate from reaction with peroxynitrite (dashed line).
The thermal behavior of the CYP102HD nitrosyl complex is much different than that of the Compound II species. The two complexes have similar UV-visible spectra (30), and both are readily distinguished from ferric resting enzyme. Whereas the Compound II species decayed at room temperature with a lifetime of seconds, the nitrosyl complex prepared from reaction of the resting enzyme with an NO donor was stable for minutes. Such stability was previously reported for the nitrosyl complex of CYP102 full enzyme, which was reported to have a decay lifetime of minutes or more depending on the presence of oxygen (30).
Peroxynitrite and peroxynitrous acid are highly reactive with biological molecules. Protein nitration by PN is well known, often involving nitration of tyrosine groups in the protein, and tyrosine nitration upon PN treatment of CYP102 was reported by Daiber et al. (28). In addition, PN treatment of the full length CYP102 enzyme was reported to effect enzyme inactivation eventually, but the conclusion of that study (28) was that CYP102 inactivation involved thiol oxidation in the reductase domain. At the relatively low concentrations of PN used in our work, we previously found that the mammalian hepatic P450 CYP2B4 was partially nitrated but that the activity of the enzyme under turnover conditions was not affected by PN treatment (15). In a similar manner, treatment of CYP119 by the low concentration of PN used for the present studies was shown to have no effect on catalysis by the enzyme under hydrogen peroxide shunt conditions (14). To test the effect of PN on CYP102HD, we analyzed a sample of the enzyme after PN treatment in a product yield reaction. Thus, a sample of CYP102HD was treated with PN at 0 °C, and the PN and P450 Compound II were allowed to decay. The enzyme was then mixed with styrene and a bulk styrene oxidation was conducted at 0 °C as discussed below. The yield of styrene oxide was the same as that obtained with untreated enzyme.
Production of CYP102HD Compound I
The method used for production of CYP102HD Compound I at low temperature followed that reported for production of CYP119 Compound I (17). Thus, CYP102HD in a 1:1 mixture of glycerol and phosphate buffer (pH = 7.0) was cooled to −10 °C, and a basic aqueous solution of PN was added to give a solution with pH − 7.4. Compound II formed rapidly. The decay of excess PN was followed spectroscopically by monitoring the signal at 308 nm. After the excess PN had decayed, the samples were cooled to the desired reaction temperature (−35 to −10 °C) and then irradiated with 365 ± 5 nm light to photo-oxidize the Compound II to Compound I using a pulsed lamp that was set to deliver 10 W power. The irradiation step resulted in a decrease in the absorbance at 431 nm, λmax of the Soret band of Compound II, and an increase in the absorbance centered at 419 nm from Compound I.
For CYP102HD, the amount of light necessary for complete conversion of Compound II to Compound I increased markedly as the temperature was reduced. For the 1.0 nmol enzyme samples used in this study, the approximate amount of light necessary for complete conversion of CYP102HD Compound II to Compound I was 4 J at −10 °C and 8 J at −35 °C. This behavior is unlike that observed with CYP119, where the amount of light needed for complete conversion of Compound II to Compound I did not change appreciably when the temperature was reduced. The different photochemical behavior of the two Compounds II is an interesting subject for future study. Our speculative interpretation of the effect is that such behavior is consistent with a proton-coupled electron transfer (PCET) in the oxidation step where reduced temperature could have a major influence on the rate or efficiency of deprotonation of the iron-hydroxy group of Compound II.
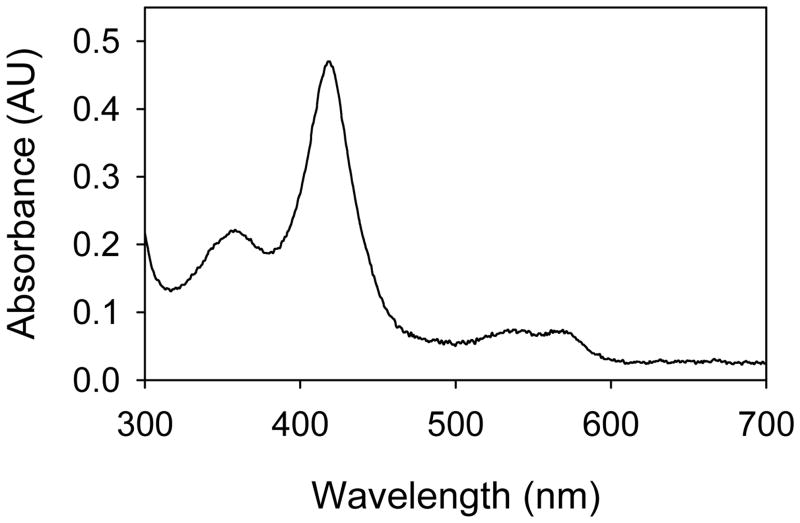
Figure 2 shows the UV-visible spectrum of CYP102HD Compound I. For CYP119, the UV-visible spectra of Compound I and Compound II were previously reported (14,17) and are shown in Figure S1 in Supporting Information. These Compound I spectra are quite similar to that of Compound I of CYP2B4 produced by the photo-oxidation method (15). The spectrum of Compound I of CYP102 F87G mutant from reaction of that enzyme with m-chloroperoxybenzoic acid (mCPBA) was recently reported (7), and that published spectrum is similar to the spectrum in Figure 2, although the ratios of the Soret bands differ somewhat suggesting that the enzyme was partially degraded in the mCPBA reaction. The UV-visible spectrum of iNOSoxy (the P450 heme domain of inducible nitric oxide synthase) also has a similar spectrum when one considers that the Soret band absorbances of iNOS and its intermediates are red-shifted in comparison to those of P450s (31).
Figure 2.
UV-visible spectrum of CYP102HD Compound I.
The UV-spectra, and especially the Soret band absorbances, of the P450 Compounds I are similar to the UV-visible spectra of the resting enzymes. This similarity is undoubtedly one of the major difficulties in attempting to detect Compounds I under turnover conditions or in reactions of the enzymes with chemical oxidants. This property also makes kinetic studies difficult because the intensity change observed upon conversion of Compound I to the resting enzyme is on the order of 0.01 AU at λmax of the Soret bands for CYP102HD and 0.02 AU at λmax of the Soret bands for CYP119.
The same irradiation procedure as described above was conducted with a sample of the CYP102HD nitrosyl complex. The UV-visible spectrum of that complex is similar to the spectrum of Compound II (30), and both are readily distinguished from the resting enzyme and Compound I. Upon irradiation of the NO complex with 365 nm light, no change was apparent in the UV-visible spectrum when the light dose was greater than that used for quantitative conversion of Compound II to Compound I. The apparent photo-stability on the relatively slow time scale of our studies was expected because heme-enzyme nitrosyl complexes are well known to photo-dissociate with small quantum efficiencies, at least in part because the NO in the active site recombines with the ferric enzyme on the picosecond to nanosecond time scale (32).
Styrene Oxidations
We studied low temperature oxidations of styrene by the Compound I intermediates from CYP102HD and CYP119 (17). For product studies, bulk oxidations were conducted by generating Compounds I in the presence of excess styrene at 0 °C. After several minutes, the reactions were worked up and analyzed by GC. The only detectable product was styrene oxide formed in 70% and 50% yields from Compounds I of CYP119 and CYP102HD, respectively. When the sequence was performed with CYP102HD that was pre-treated with PN, the yield of styrene oxide was comparable to that obtained with untreated enzyme. In control reactions, irradiation of CYP119 and CYP102HD resting enzymes in the presence of styrene at 0 °C gave no styrene oxide. In a previous study of CYP119 Compound I, the origin of the oxygen atom in styrene oxide was shown to be from the Compound I intermediate by oxygen-labeling experiments (14).
Kinetic studies of styrene oxidations were conducted over the temperature range −10 to −50 °C. Compounds I were formed in the presence of excess styrene, and kinetics were obtained by following the increase in absorbance at 413–416 nm as Compounds I reacted to give resting enzymes. Typical results are shown in the Supporting Information. As observed previously (14–17), the kinetic traces were well fit by double exponential functions where the predominant exponential (70–85% for CYP119 and 74–97% for CYP102HD) increased as a function of substrate concentration, and the minor exponential was independent of substrate concentration. The minor exponential was typically one order of magnitude smaller than the major exponential, and it is possible that this term is a random variable that serves mainly to improve the double exponential fit.
The kinetic results are listed in the Supporting Information. At a given temperature, the observed rate constants increased with substrate concentration in a nonlinear manner as shown in Figure 3, indicating that the reactions involved saturation kinetics as described in Scheme 2. Styrene forms a complex rapidly with Compound I with equilibrium constant Kbind, and the rate-limiting step in the sequence is a first-order oxidation reaction, with rate constant kox. Note that we analyze the kinetics in terms of an equilibrium constant for formation of complex as opposed to a dissociation constant of the complex typically used in Michaelis-Menten enzyme kinetics. In part, this is to emphasize the fact that we are studying single-turnover kinetic events that cannot be controlled by the rates of product release. We note also that the binding constants for formation of a complex with Compound I obtained from the kinetics are not expected to be equal to the equilibrium constants for formation of complexes between the substrate and resting enzymes.
Scheme 2.
The limit of the kinetic method we are using is illustrated in Figure 3. The lower temperature limit is established when the mixture of glycerol/buffer becomes cloudy, precluding spectroscopic detection, at temperatures below −50 °C. The upper limit is established by the rate of the reaction; because the lamp irradiations require tenths of seconds, observed rate constants must be less than 1 s−1 so that the major portion of the reaction does not occur while the lamp is on.
The observed rate constants were analyzed for saturation kinetics according to Eq 1, where kobs is the observed pseudo-first-order rate constant, k0 is the background decay rate constant in the absence of substrate, Kbind is the equilibrium binding constant, kox is the first-order rate constant, and [Subs] is the concentration of substrate. Non-linear regression analysis gave the values listed in Table 1, which were used to generate the fitting lines shown in Figure 3. Fast, reversible substrate binding in the Compound I species is important for meaningful analyses of the data because it permits solution of binding constants and first-order rate constants that can be analyzed separately. If binding were not reversible, or if the binding constant was small, then much less informative second-order (or apparent second-order) rate constants for the oxidations, which are the products of the binding constant times the oxidation rate constant, would be obtained experimentally.
| (1) |
Arrhenius plots for styrene epoxidation by the Compounds I are shown in Figure 4. The kinetics are described by the functions in Eq 2 and 3 for CYP102HD Compound I and CYP119 Compound I, respectively, where the errors are at 2σ and the activation energies are in kcal/mol. The oxidation rate constants for CYP102HD Compound I are somewhat smaller than those for CYP119 Compound I because the activation energy for CYP102HD Compound I is slightly greater than that for CYP119 Compound I. When extrapolated to ambient temperature (22 °C), the predicted rate constant for CYP119 Compound I epoxidation of styrene is a factor of 3 greater than that for CYP102 Compound I.
Figure 4.
Arrhenius functions for styrene oxidations by CYP102 Compound I (filled circles) and CYP119 Compound I (open circles). The gray bar indicates room temperature.
| (2) |
| (3) |
The entropy demand for the oxygen insertion reactions are effectively zero for both Compounds I (log A = 13.1 when ΔS‡ = 0 e.u. at ambient temperature). In general, a small entropy demand is expected for such first-order reactions because the reactive complex needs only to be oriented properly for oxygen delivery to the alkene. If, for example, half of the orientations are reactive, then the effect on log A would be a reduction by only 0.3.
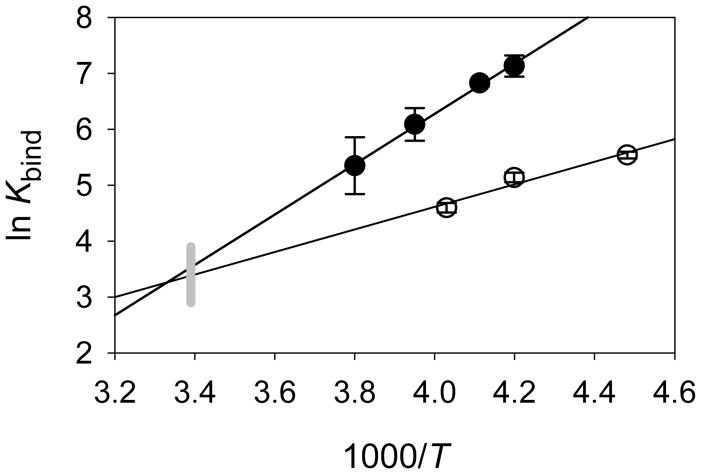
The binding constants were analyzed by van’t Hoff relationships to give the results shown graphically in Figure 5, where it is interesting to note that the extrapolated values at room temperature are nearly equal. The binding constants for CYP102 Compound I give the values in Eq 4, and the binding constants for CYP119 Compound I give the values in Eq 5, where the errors are at 2σ. The entropies of binding are slightly negative, and the enthalpies of binding are favorable with ΔH = − 9 kcal/mol (CYP102HD) and − 4 kcal/mol (CYP119).
Figure 5.
Temperature dependence for binding constants for CYP102 Compound I (filled circles) and CYP119 Compound I (open circles). The gray line indicates ambient temperature.
| (4) |
| (5) |
It is interesting to compare the effects of binding on the velocity of styrene oxidations. At low concentrations of styrene such that saturation is not achieved, CYP102HD Compound I oxidizes styrene faster than CYP119 Compound I because the binding constant for the former is much greater than that for the latter. At room temperature, however, where the binding constants for the two Compounds I are similar, CYP119 Compound I will oxidize styrene nearly three times faster than CYP102HD Compound I. It is well established that the potential dominant effects of the binding constants on observed kinetics are important for understanding enzyme-catalyzed reactions, and this example demonstrates that, for predictions of reaction velocities, estimates of the binding constants for P450 Compounds I are as important (if not more important) as estimates of the rate constants.
The extrapolated results at 22 °C for CYP119 Compound I can be compared to the value for this reaction obtained in laser flash photolysis studies at this temperature. Due to the small binding constants for styrene at room temperature, kinetic studies give observed rate constants that display an effectively linear dependence on substrate concentration; i.e. the denominator on the right side of Eq 1 approaches unity. Such a linear dependence on styrene concentration was observed for CYP119, and the apparent second-order rate constant was 7,600 M−1 s−1 (14). That value is in excellent agreement with the extrapolated value from the present work, kapp = koxKbind = 7,800 M−1.
It is also informative to compare the results for P450 Compounds I with those from Compound I of another heme-thiolate enzyme. Chloroperoxidase (CPO) is a heme-thiolate enzyme exuded from the fungus Caldariomyces fumago (33). CPO is oxidized to a detectable Compound I intermediate by hydrogen peroxide, and rapid mixing kinetic studies of CPO Compound I are possible. The apparent second-order rate constant for reaction of CPO Compound I with styrene at room temperature was found to be kapp = 61,000 M−1 s−1 (34), which is about 1 order of magnitude greater than the kapp values for P450 Compounds I obtained by extrapolation of the results in this work. Given the similarity of CPO and P450 enzymes, it seems possible that the kox value for Compound I of CPO will be similar to those for the P450 Compounds I, in which case the binding constant for styrene in CPO Compound I would appear to be an order of magnitude greater than those for the P450 Compounds I studied in this work. Nonetheless, we caution that differences in the environment of the thiolate are known to have an effect on the rate constants for Compound I reactions (15), and the kinetic differences between CPO Compound I and the P450 Compounds I might reflect such environment effects.
The reactivities of Compounds I from CYP119 and CYP102HD are similar in their epoxidations of styrene, and it was of interest to compare their reactivities in a hydroxylation reaction. For that purpose, we conducted a cursory study of the hydroxylation of benzyl alcohol-d2 by CYP102HD Compound I at −20 °C, which can be compared to the results from detailed oxidation studies of this substrate by CYP119 Compound I (17). For the CYP102HD Compound I oxidation, we obtained Kbind = 420 ± 170 M−1 and kox = 1.9 ± 0.6 s−1 (results are in the Supporting Information). The rate constant for oxidation of this substrate by CYP119 Compound I at −20 °C is kox = 2.0 s−1 (17). Thus, the oxidation rate constants in the hydroxylation reactions of the two Compounds I are essentially indistinguishable. Previously, hydroxylation reactions of the Compound I intermediates from CYP119 and CYP2B4 were found to be quite similar for two substrates (15,16).
It seems to be increasingly clear that one can generalize regarding the reactivities of various P450 Compounds I, which fall within a narrow range for a given substrate. Indeed, studies with a series of CYP2B4 mutants containing mutations in the heme region indicated that the reactivities of Compounds I were not altered unless the electronic properties of the thiolate ligand were affected by a mutation (15). It is especially noteworthy that CYP119 Compound I does not react “slowly” in comparison to other P450 Compounds I even though the enzyme is from a thermophile. Optimization of reactivity of CYP119 at elevated temperatures could be a function of many processes in the P450 reaction sequence (35), but it is not due to differences in reactivities of Compound I in comparison to Compounds I of other P450s.
The demonstration of highly equivalent reactivities of P450 Compounds I in hydroxylation reactions suggests that the seemingly small differences in reactivities of the two Compounds I studied here in styrene epoxidation reactions (differences of about 1 kcal/mol in activation energies) might reflect steric effects. Perhaps an element of strain in the active site is incurred for styrene to assume a reactive orientation in CYP102HD Compound I that is not present in CYP119 Compound I. This speculation might be tested in modeling studies that are not within the scope of the present work, but we introduce the concept here to illustrate the utility of the very precise kinetic data that can now be obtained for P450 Compounds I.
In a recent study of P450 Compound I oxidations, the estimated activation energies for oxidations of four hydrocarbons were shown to display a good correlation with the C-H bond dissociation energies of the oxidized positions (17). Those results are reproduced in Table 2 where we have incorporated the activation energies for styrene oxidations found in this work. Although the general perception might be that epoxidations should be more facile than any hydoxylation, the energy barriers for styrene epoxidations are actually slightly greater than that for hydroxylation of benzyl alcohol and comparable to those for oxidation of the aminomethyl group in benzphetamine. In terms of the correlation of the reactivities found in hydroxylation reactions, the π-bond of styrene behaves as if it is a C-H bond with a ca. 85 kcal/mol BDE.
Table 2.
Activation Energies for Oxidation Reactions of P450 Compounds I.a
| Substrate | BDE (kcal/mol) | P450 | Ea (kcal/mol) |
|---|---|---|---|
| lauric acidb | 98 | CYP119 | 17.9 |
| cyclopropylmethylc | 95 | CYP119 | 16.4 |
| 95 | CYP2B4 | 16.3 | |
| benzphetamimed | 89 | CYP119 | 15.7 |
| 89 | CYP2B4 | 15.5 | |
| styrene | CYP102HD | 15.2 | |
| CYP119 | 14.6 | ||
| benzyl alcohole | 79 | CYP119 | 13.7 |
The data for hydroxylation activation energies and hydrocarbon BDEs are from ref (17); the styrene activation energies are from this work.
Unactivated CH positions in lauric acid.
CH of methyl group adjacent to cyclopropyl ring.
CH of the methyl group adjacent to amine nitrogen.
Benzylic CH position in benzyl alcohol.
Conclusion
The production of Compound I from CYP102HD represents the second example of quantitative formation of a P450 Compound by photo-oxidation of the Compound II intermediate at reduced temperatures. P450 Compounds I are reactive transients, but not extraordinarily reactive as one might have concluded from some previous studies that failed to detect Compound I; for example, the half-life for CYP102HD at −35 °C is greater than 10 minutes, providing ample time for a variety of manipulations. The activation energies in styrene epoxidations by Compounds I from CYP102HD and CYP119 are similar, and those for a hydroxylation reaction appear to be equivalent, indicating that a simple general model for P450 Compounds I is appropriate for predictions of first-order rate constants. The binding constants of substrates in the Compound I transients studied in this work are quite different for styrene and benzyl alcohol, however, leading to large differences in apparent second-order rate constants and the velocities of oxidations. Accordingly, useful predictions of the velocities of P450 oxidation reactions will require sophisticated models for active site interactions that accurately predict binding constants.
Supplementary Material
Acknowledgments
We thank Prof. S. G. Sligar for a gift of the DNA for expression of CYP102HD.
Abbreviations
- CPO
chloroperoxidase
- CYP
cytochrome P450
- ENDOR
electron nuclear double resonance
- EPR
electron paramagnetic resonance
- EXAFS
extended X-ray absorbance fine structure
- GC
gas chromatography
- GC-MS
gas chromatography-mass spectrometry
- iNOSoxy
heme domain of inducible nitric oxide synthase
- LFP
laser flash photolysis
- mCPBA
m-chloroperoxybenzoic acid
- P450
cytochrome P450
- PCET
proton-coupled electron transfer
- PN
peroxynitrite
- XAS
X-ray absorbance spectroscopy
Footnotes
This work was supported by a grant from the National Institutes of Health (GM48722).
Supporting Information Available. UV-visible spectra of CYP119 species, representative kinetic traces, detailed kinetic results. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ortiz de Montellano PR, editor. Cytochrome P450 Structure, Mechanism, and Biochemistry. 3. Kluwer; New York: 2005. [Google Scholar]
- 2.Sono M, Roach MP, Coulter ED, Dawson JH. Heme-containing oxygenases. Chem Rev. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 3.Coon MJ, Blake RC, II, Oprian DD, Ballou DP. Mechanistic studies with purified components of the liver microsomal hydroxylation system: spectral intermediates in reaction of cytochrome P-450 with peroxy compounds. Acta Biol Med Ger. 1979;38:449–458. [PubMed] [Google Scholar]
- 4.Egawa T, Shimada H, Ishimura Y. Evidence for Compound I formation in the reaction of cytochrome-P450cam with m-chloroperbenzoic acid. Biochem Biophys Res Commun. 1994;201:1464–1469. doi: 10.1006/bbrc.1994.1868. [DOI] [PubMed] [Google Scholar]
- 5.Kellner DG, Hung SC, Weiss KE, Sligar SG. Kinetic characterization of Compound I formation in the thermostable cytochrome P450 CYP119. J Biol Chem. 2002;277:9641–9644. doi: 10.1074/jbc.C100745200. [DOI] [PubMed] [Google Scholar]
- 6.Spolitak T, Dawson JH, Ballou DP. Reaction of ferric cytochrome P450cam with peracids - Kinetic characterization of intermediates on the reaction pathway. J Biol Chem. 2005;280:20300–20309. doi: 10.1074/jbc.M501761200. [DOI] [PubMed] [Google Scholar]
- 7.Raner GM, Thompson JI, Haddy A, Tangham V, Bynum N, Reddy GR, Ballou DP, Dawson JH. Spectroscopic investigations of cytochrome P450(BM3)-F87G of intermediates in the reaction with surrogate oxygen atom donors. J Inorg Biochem. 2006;100:2045–2053. doi: 10.1016/j.jinorgbio.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Schünemann V, Jung C, Trautwein AX, Mandon D, Weiss R. Intermediates in the reaction of substrate-free cytochrome P450(cam) with peroxy acetic acid. FEBS Lett. 2000;479:149–154. doi: 10.1016/s0014-5793(00)01886-x. [DOI] [PubMed] [Google Scholar]
- 9.Schünemann V, Trautwein AX, Jung C, Terner J. Mossbauer and EPR study of reaction intermediates of cytochrome P450. Hyperfine Interact. 2002;141:279–284. [Google Scholar]
- 10.Jung C, Schünemann V, Lendzian F, Trautwein AX, Contzen J, Galander M, Bottger LH, Richter M, Barra AL. Spectroscopic characterization of the iron-oxo intermediate in cytochrome P450. Biol Chem. 2005;386:1043–1053. doi: 10.1515/BC.2005.120. [DOI] [PubMed] [Google Scholar]
- 11.Jung C, Schünemann V, Lendzian F. Freeze-quenched iron-oxo intermediates in cytochromes P450. Biochem Biophys Res Commun. 2005;338:355–364. doi: 10.1016/j.bbrc.2005.08.166. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Chandrasena REP, Martinez E, Horner JH, Newcomb M. Formation of Compound I by photo-oxidation of Compound II. Org Lett. 2005;7:1193–1195. doi: 10.1021/ol050296j. [DOI] [PubMed] [Google Scholar]
- 13.Newcomb M, Zhang R, Chandrasena REP, Halgrimson JA, Horner JH, Makris TM, Sligar SG. Cytochrome P450 Compound I. J Am Chem Soc. 2006;128:4580–4581. doi: 10.1021/ja060048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng X, Horner JH, Newcomb M. Spectra and kinetic studies of the Compound I derivative of cytochrome P450 119. J Am Chem Soc. 2008;130:13310–13320. doi: 10.1021/ja802652b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng X, Zhang H, Im SC, Horner JH, Waskell L, Hollenberg PF, Newcomb M. Kinetics of oxidation of benzphetamine by Compounds I of cytochrome P450 2B4 and its mutants. J Am Chem Soc. 2009;131:2971–2976. doi: 10.1021/ja808982g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng X, Zhang HM, Hollenberg PF, Newcomb M. Kinetic isotope effects in hydroxylation reactions effected by cytochrome P450 Compounds I implicate multiple electrophilic oxidants for P450-catalyzed oxidations. Biochemistry. 2009;48:1620–1627. doi: 10.1021/bi802279d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Sheng X, Horner JH, Newcomb M. Quantitative production of Compound I from a cytochrome P450 enzyme at low temperatures. Kinetics, activation parameters, and kinetic isotope effects for oxidation of benzyl alcohol. J Am Chem Soc. 2009;131:0000–0000. doi: 10.1021/ja9031105. 0010.1021/ja9031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narhi LO, Fulco AJ. Characterization of a catalytically self-sufficient 119,000-Dalton Cytochrome-P-450 monooxygenase induced by barbiturates in Bacillus-megaterium. J Biol Chem. 1986;261:7160–7169. [PubMed] [Google Scholar]
- 19.Li HY, Darwish K, Poulos TL. Characterization of recombinant Bacillus megaterium cytochrome P-450BM-3 and its two functional domains. J Biol Chem. 1991;266:11909–11914. [PubMed] [Google Scholar]
- 20.McLean MA, Maves SA, Weiss KE, Krepich S, Sligar SG. Characterization of a cytochrome P450 from the acidothermophilic archaea Sulfolobus solfataricus. Biochem Biophys Res Commun. 1998;252:166–172. doi: 10.1006/bbrc.1998.9584. [DOI] [PubMed] [Google Scholar]
- 21.Maves SA, Sligar SG. Understanding thermostability in cytochrome P450 by combinatorial mutagenesis. Protein Sci. 2001;10:161–168. doi: 10.1110/ps.17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeom H, Sligar SG, Li HY, Poulos TL, Fulco AJ. The role of Thr268 in oxygen activation of cytochrome P450(BM-3) Biochemistry. 1995;34:14733–14740. doi: 10.1021/bi00045a014. [DOI] [PubMed] [Google Scholar]
- 23.Uppu RM, Pryor WA. Synthesis of peroxynitrite in a two-phase system using isoamyl nitrite and hydrogen peroxide. Anal Biochem. 1996;236:242–249. [PubMed] [Google Scholar]
- 24.Rabe KS, Kiko K, Niemeyer CM. Characterization of the peroxidase activity of CYP119, a thermostable P450 from Sulfolobus acidocaldarius. ChemBioChem. 2008;9:420–425. doi: 10.1002/cbic.200700450. [DOI] [PubMed] [Google Scholar]
- 25.Kissner R, Koppenol WH. Product distribution of peroxynitrite decay as a function of pH, temperature, and concentration. J Am Chem Soc. 2002;124:234–239. doi: 10.1021/ja010497s. [DOI] [PubMed] [Google Scholar]
- 26.Newcomb M, Halgrimson JA, Horner JH, Wasinger EC, Chen LX, Sligar SG. X-ray absorption spectroscopic characterization of a cytochrome P450 compound II derivative. Proc Nat’l Acad Sci U S A. 2008;105:8179–8184. doi: 10.1073/pnas.0708299105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behan RK, Hoffart LM, Stone KL, Krebs C, Green MT. Reaction of cytochrome P450(BM3) and peroxynitrite yields nitrosyl complex. J Am Chem Soc. 2007;129:5855–5859. doi: 10.1021/ja064590y. [DOI] [PubMed] [Google Scholar]
- 28.Daiber A, Herold S, Schöneich C, Namgaladze D, Peterson JA, Ullrich V. Nitration and inactivation of cytochrome P450BM-3 by peroxynitrite - Stopped-flow measurements prove ferryl intermediates. Eur J Biochem. 2000;267:6729–6739. doi: 10.1046/j.1432-1033.2000.01768.x. [DOI] [PubMed] [Google Scholar]
- 29.Mehl M, Daiber A, Herold S, Shoun H, Ullrich V. Peroxynitrite reaction with heme proteins. Nitric Oxide Biol Chem. 1999;3:142–152. doi: 10.1006/niox.1999.0217. [DOI] [PubMed] [Google Scholar]
- 30.Quaroni LG, Seward HE, McLean KJ, Girvan HM, Ost TWB, Noble MA, Kelly SM, Price NC, Cheesman MR, Smith WE, Munro AW. Interaction of nitric oxide with cytochrome P450BM3. Biochemistry. 2004;43:16416–16431. doi: 10.1021/bi049163g. [DOI] [PubMed] [Google Scholar]
- 31.Tejero JS, Biswas A, Wang ZQ, Page RC, Haque MM, Hemann C, Zweier JL, Misra S, Stuehr DJ. Stabilization and characterization of a heme-oxy reaction intermediate in inducible nitric-oxide synthase. J Biol Chem. 2008;283:33498–33507. doi: 10.1074/jbc.M806122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoshino M, Ozawa K, Seki H, Ford PC. Photochemistry of nitric oxide adducts of water-soluble iron(III) porphyrin and ferrihemoproteins studied by nanosecond laser photolysis. J Am Chem Soc. 1993;115:9568–9575. [Google Scholar]
- 33.Morris DR, Hager LP. Chloroperoxidase. I Isolation and properties of the crystalline glycoprotein. J Biol Chem. 1966;241:1763–1768. [PubMed] [Google Scholar]
- 34.Zhang R, Nagraj N, Lansakara DSP, Hager LP, Newcomb M. Kinetics of two-electron oxidations by the Compound I derivative of chloroperoxidase, a model for cytochrome P450 oxidants. Org Lett. 2006;8:2731–2734. doi: 10.1021/ol060762k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puchkaev AV, Ortiz de Montellano PR. The Sulfolobus solfataricus electron donor partners of thermophilic CYP119: an unusual non-NAD(P)H-dependent cytochrome P450 system. Arch Biochem Biophys. 2005;434:169–177. doi: 10.1016/j.abb.2004.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.