Abstract
Nicotine addiction begins with high-affinity binding of nicotine to acetylcholine (ACh) receptors in the brain. The end result is over 4,000,000 smoking-related deaths annually worldwide and the largest source of preventable mortality in developed countries. Stress reduction, pleasure, improved cognition, and other CNS effects are strongly associated with smoking. But, if nicotine activated ACh receptors found in muscle as potently as it does brain receptors, smoking would cause intolerable and perhaps fatal muscle contractions. Despite extensive pharmacological, functional, and structural studies of ACh receptors, the basis for the differential action of nicotine on brain vs. muscle ACh receptors has not been determined. Here we show that at the α4β2 brain receptors thought to underlie nicotine addiction, the high affinity of nicotine is the result of a strong cation-п interaction to a specific aromatic amino acid of the receptor, TrpB. In contrast, the low affinity of nicotine at the muscle-type receptor is largely due to the fact that this key interaction is absent, even though the immediate binding site residues, including the key TrpB, are identical in the brain and muscle receptors. At the same time a hydrogen bond from nicotine to the backbone carbonyl of TrpB is enhanced in the neuronal receptor relative to the muscle-type. A point mutation near TrpB that differentiates α4β2 and muscle-type receptors appears to influence the shape of the binding site, allowing nicotine to interact more strongly with TrpB in the neuronal receptor. ACh receptors are established therapeutic targets for Alzheimer’s disease, schizophrenia, Parkinson’s disease, smoking cessation, pain, attention deficit-hyperactivity disorder, epilepsy, autism, and depression1. Along with solving a chemical mystery in nicotine addiction, our results provide guidance for efforts to develop drugs that target specific types of nicotinic receptors.
Nicotinic acetylcholine receptors (nAChR) comprise a family of ≥ 20 homologous subtypes that mediate fast synaptic transmission throughout the central and peripheral nervous systems2. The neuronal receptors are found in the central nervous system (CNS) and autonomic ganglia. Of these, the subtype most strongly associated with nicotine addiction and the target of recently developed smoking cessation drugs is termed α4β23–7. The high nicotine affinity of α4β2 receptors, when combined with the ability of nicotine to cross the blood-brain barrier and its favorable pharmacokinetics, allows nicotine at the submicromolar concentrations in tobacco smoke to acutely activate these receptors, providing reward, cognitive sensitization, and perhaps other effects. In addition, the high-affinity interaction allows smoked nicotine to act as an intracellular pharmacological chaperone of α4β2 receptors, leading to the upregulation of receptors thought to underlie effects of chronic exposure6,8.
In previous studies of the nAChR of the neuromuscular junction (muscle-type), we showed that an important contributor to ACh binding is a cation-π interaction to a specific tryptophan (TrpB, residue 149, Fig. 1)9. These results were subsequently supported by the important series of crystal structures of ACh binding proteins (AChBP)10,11. These structures revealed the “aromatic box” structural motif of Fig. 1, and the aligning residues are predominantly aromatic throughout the Cys-loop family of neurotransmitter-gated ion channels. In other Cys-loop receptors, a cation-π interaction between the natural agonist and one of the aromatics is always seen, although its precise location varies12. Interestingly, when nicotine activates the muscle-type nAChR, there is no cation-π interaction13, consistent with its relatively low affinity for this receptor. This suggested that a cation-π interaction could discriminate between high affinity neuronal receptors and low affinity muscle-type receptors. However, subtle effects must be involved, as the nAChRs of the CNS and neuromuscular junction are homologous throughout most regions of sequence and are essentially identical in the immediate vicinity of the agonist binding site (Supplementary Fig. 1).
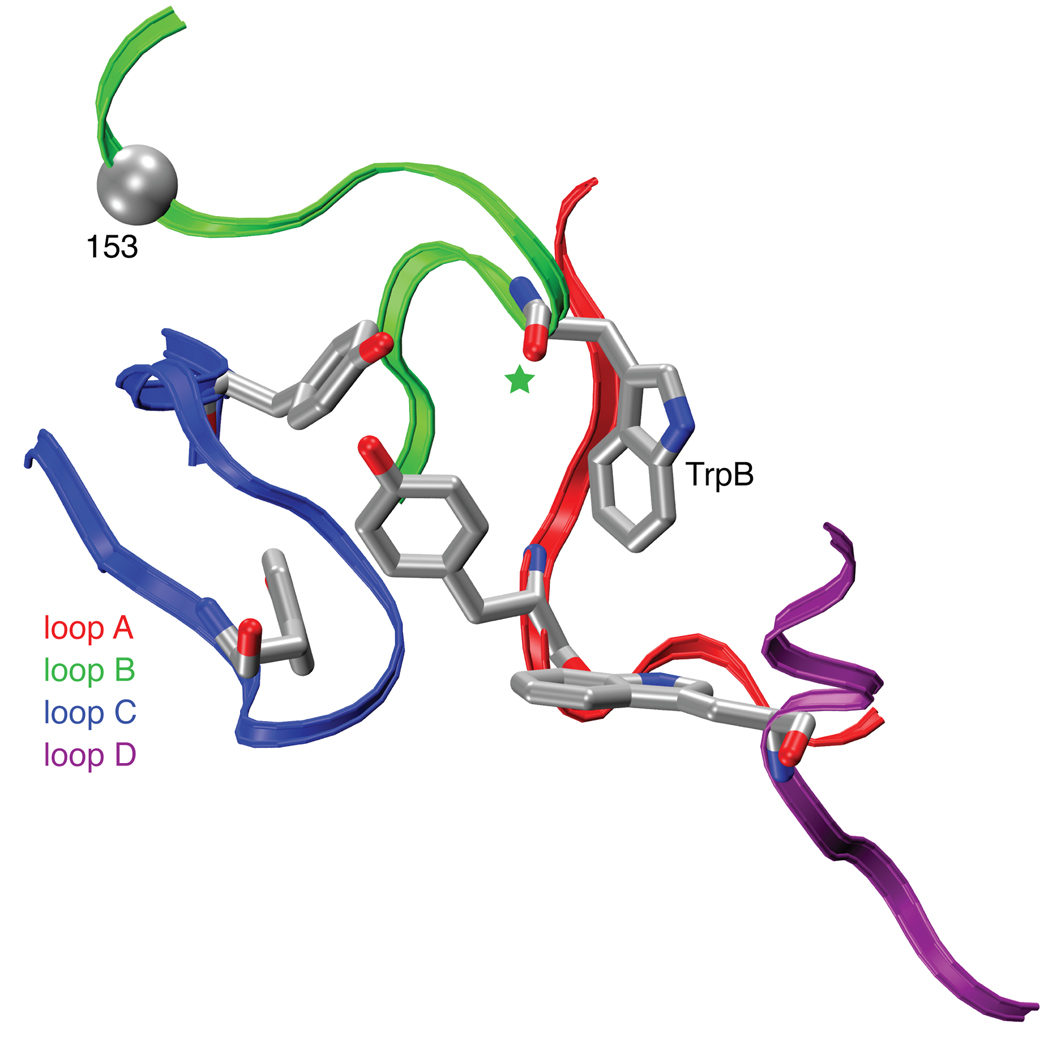
Figure 1.
The binding site of AChBP, thought to resemble that of nAChRs. Shown are the four principal “loops” that define the binding site2. Also highlighted are TrpB (149) studied here; its backbone carbonyl (green star); and the α carbon on position 153, which has also been mutated here. Note that loop C contributes two aromatic residues; the other loops each contribute one. Image is of pdb file 1I9B10.
Here we describe studies of the α4β2 neuronal receptor. We find a remarkable alteration of binding behavior: both ACh and nicotine make a strong cation-π interaction to TrpB. In addition, a hydrogen bond from nicotine to the backbone carbonyl of TrpB that is weak in the muscle-type is much stronger in the α4β2 receptor. Taken together, these two noncovalent interactions fully rationalize the differential affinity of nicotine in the brain vs. the neuromuscular junction.
A cation-π interaction between a drug and a receptor can be revealed by incorporation of a series of fluorinated amino acid analogues (Fig. 2); a consistent trend in receptor response indicates a binding interaction. Such an experiment is enabled by the nonsense suppression methodology for incorporation of unnatural amino acids into receptors and channels expressed in Xenopus oocytes. While we have found the nonsense suppression methodology to be broadly applicable14,15, implementing the methodology for study of the α4β2 neuronal nAChRs proved to be especially challenging, requiring new strategies. The α4β2 receptors express in Xenopus oocytes at inadequately low levels for nonsense suppression experiments. However, recent studies showed that the Leu9’Ala (L9’A) mutation in the M2 transmembrane helix of the α4 subunit greatly improves expression without altering the pharmacological selectivity of the receptor16. Therefore, all studies of α4β2 described here included this mutation. As with other mutations of L9’, the L9′A mutation lowers EC50 by influencing receptor gating in ways that are fairly well understood and that do not distort the present analysis of the binding site (some 60Å from the 9’ position)17,18. In addition, previous studies of the muscle-type receptor used a comparable mutation at L9’, and control experiments established that it did not alter binding trends9,19.
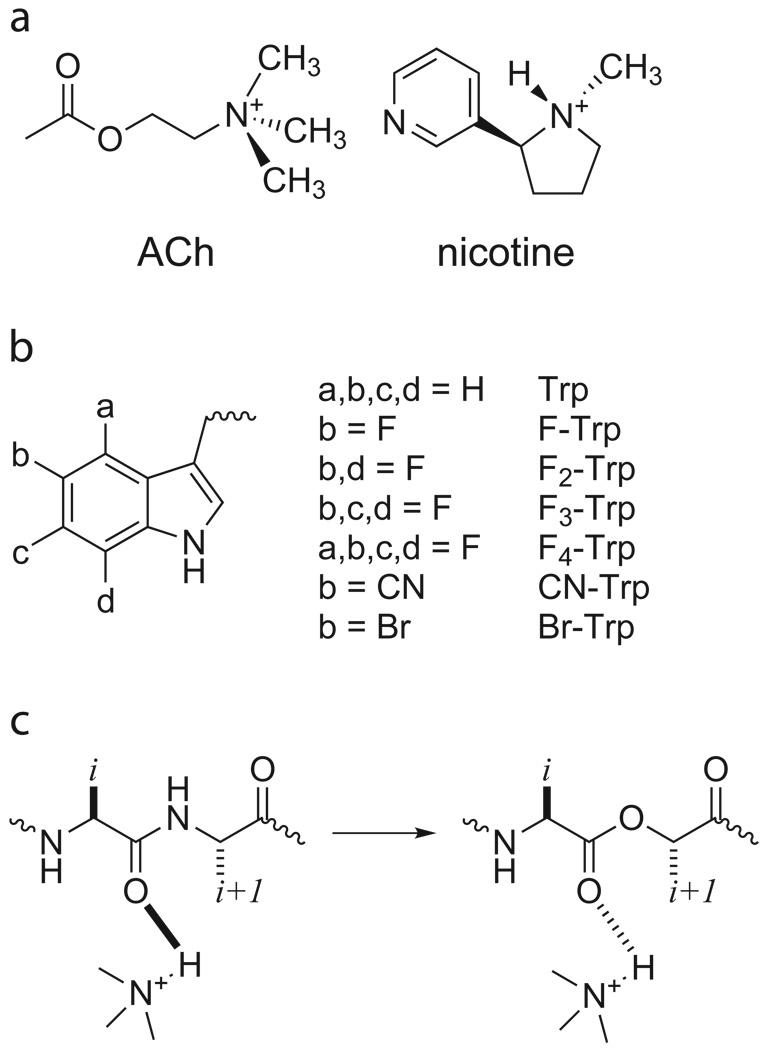
Figure 2.
Agonists and unnatural amino acids considered here. a. Structures of ACh and nicotine. b. Unnatural amino acids considered here. If not indicated, an a, b, c, or d group is H. c. The backbone ester strategy for modulating a hydrogen bond.
The nAChRs are pentameric. The muscle-type receptor has a precise stoichiometry of (α1)2β1γδ. However, the α4β2 receptor can have variable stoichiometry. In particular, there are two forms of α4β2, (α4)2(β2)3 and (α4)3(β2)2, which we will refer to as A2B3 and A3B28,20,21. Agonist binding sites are at the appropriate α-β interfaces. The A2B3 form has higher sensitivity for nicotine and may be upregulated during chronic exposure to nicotine; our studies have focused on it. Controlling the ratios of mRNAs injected into the oocyte can reliably control subunit stoichiometry in the wild type receptor. However, in a nonsense suppression experiment, the subunit that contains the stop codon where the unnatural amino acid has been incorporated can exhibit low and variable expression levels. Therefore we sought a second, independent indicator of the stoichiometry of the α4β2 receptor. We now report that the A2B3 and A3B2 forms of the α4(L9’A)β2 receptor show markedly different rectification behaviors. As indicated by either voltage ramp or voltage jump experiments, A2B3 is substantially more inward rectifying than A3B2 (Supplementary Fig. 2). Thus, in all our experiments with unnatural amino acids, the stoichiometries of mutant receptors are monitored by measuring I-V relations with voltage jumps. For each mutant receptor studied, we determined the fraction, (outward current at +70 mV/inward current at −110 mV), and a value ≤ 0.1 establishes the desired A2B3 stoichiometry (Supplementary Table 1, Supplementary Discussion). With these methodological developments in hand, incorporation of unnatural amino acids into the α4β2 receptor becomes feasible (Fig. 3).
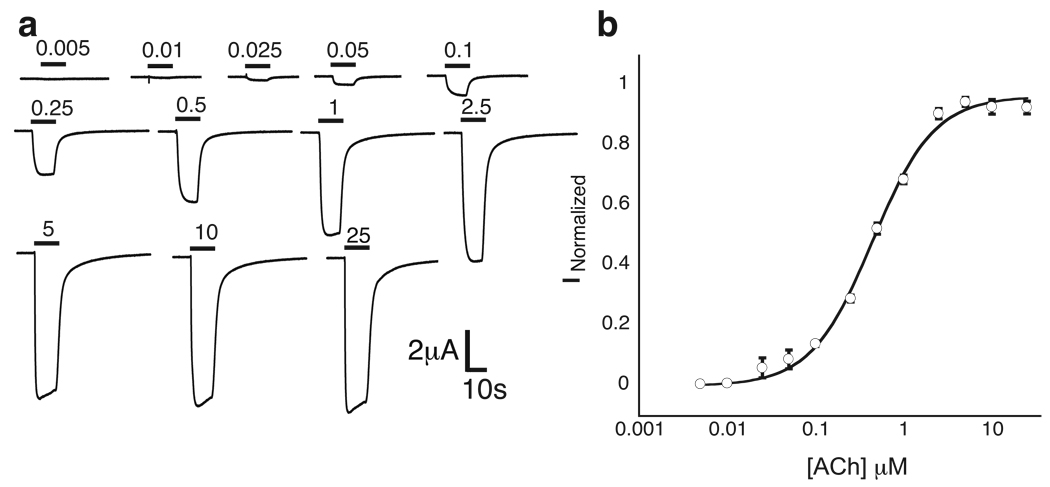
Figure 3.
Nonsense suppression in the α4β2 receptor. Shown is a wild type recovery experiment, in which Trp is incorporated at the TrpB position. a. Representative traces of voltage-clamp currents. Bars represent application of ACh at concentrations noted. b. Fit of data in A to the Hill equation.
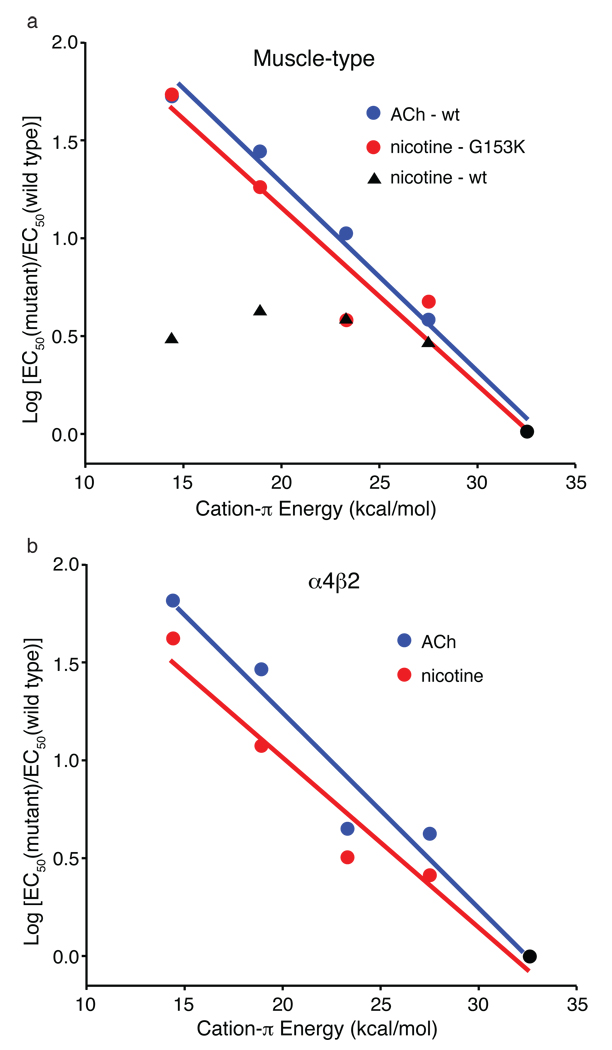
As shown in Supplementary Table 1 and Fig. 4, a compelling “fluorination” trend is seen for both ACh and nicotine at TrpB of the α4β2 receptor. This is in sharp contrast to the results at the muscle-type receptor, in which no such trend is seen for nicotine activation. Further support for an important cation-π interaction for both agonists is provided by the large perturbation induced by a cyano (CN) group – which is strongly deactivating in a cation-π interaction – compared to a bromo (Br) group, which is roughly isosteric to CN but much less deactivating.
Figure 4.
Fluorination plots. Note that in both plots, all data sets share the point at x = 32.6 kcal/mol (cation-π energy for Trp); y = 0 (black circle). Moving to the left then corresponds to monofluoro-, difluoro-, trifluoro- and tetrafluoro-TrpB. Cation-π binding energies (x-axes) are from 9. a. Muscle-type receptor. The designation “wt" indicates G at position 153. b. α4β2 receptor.
We have also evaluated other residues that constitute the aromatic box of the ACh binding site (Supplementary Table 1). The results for α4β2 very much parallel our previous findings for the muscle-type receptor (Supplementary Discussion). This indicates that it is specifically the interaction with TrpB that discriminates the two receptor subtypes.
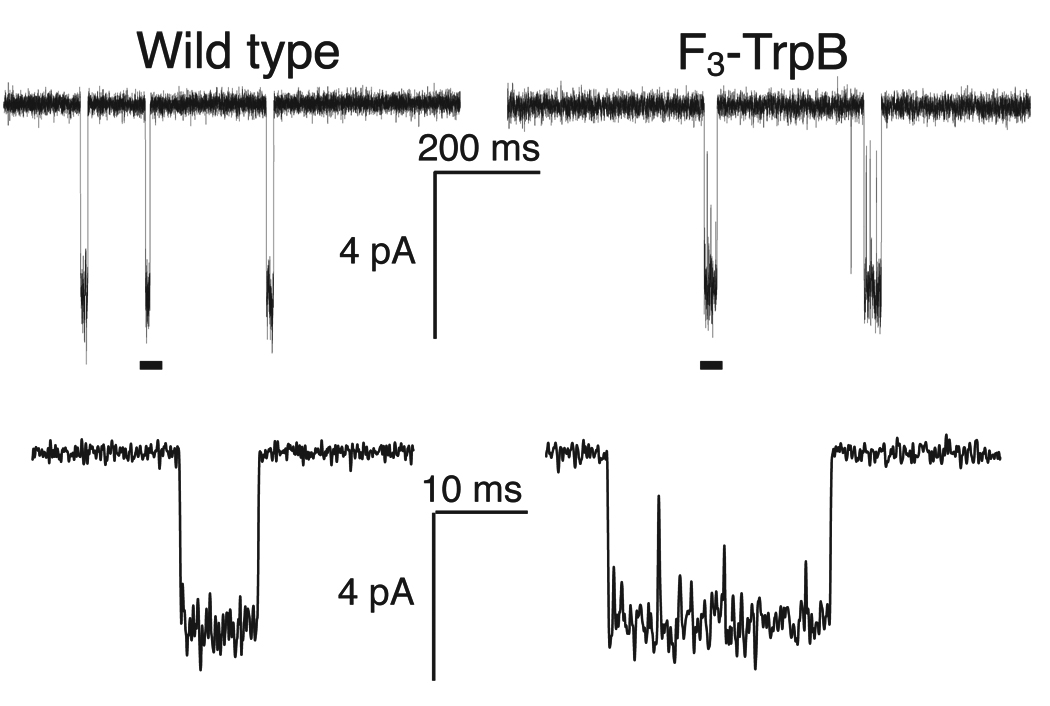
The EC50 values reported here represent a measure of receptor function; shifts in EC50 can result from changes in ligand binding and/or receptor gating properties. By ascribing the results to attenuation of a cation-π interaction, we are effectively concluding that it is ligand binding that is being modulated by fluorination, but that conclusion is not incontrovertible. To resolve this ambiguity, we evaluated the gating behaviors of key receptors using single-channel recording. For the wild type and the receptor with F3-Trp at TrpB, we compared the probabilities that the channel is open (Popen) at nicotine concentrations that evoke half-maximal macroscopic steady-state currents (EC50 = 0.08 µM and 1.2 µM, respectively). Any differences between the two Popen values must result from differences in gating behaviors. As suggested by Fig. 5 and as confirmed by further single-channel analysis (Supplementary Fig. 3 and Supplementary Discussion), the wild type and mutant receptors have Popen values that are essentially indistinguishable. Thus, the shift in EC50 for F3-Trp is primarily, if not exclusively, a consequence of changes in binding. Fluorination of TrpB of the α4β2 (A2B3) receptor primarily impacts sensitivity to nicotine by decreasing nicotine’s cation-π interaction with this residue.
Figure 5.
Single-channel recordings from wild type α4β2 (conventional expression) and the F3-Trp mutant (nonsense suppression) at site B, with nicotine applied at EC50 values (0.080 and 1.2 µM, respectively). Lower traces are expansions of the regions marked by a bar in the upper trace. Records were obtained in the cell-attached configuration with a pipette potential of +100 mV and are shown at 2 kHz bandwidth. Channel openings are shown as downward deflections.
These results indicate that nicotine is positioned more closely to TrpB in the α4β2 agonist binding site than in the muscle-type. This suggested that another nicotine binding interaction could also be altered. An important chemical distinction between ACh and nicotine is that only the latter can act as a hydrogen bond donor, through the pyrrolidine N+-H (Fig. 2A). Examination of the AChBP crystal structures (Fig. 1)22 suggested that the backbone carbonyl associated with TrpB could act as the hydrogen bond acceptor, and several groups have shown the importance of this interaction22–24. Previously, we probed this potential hydrogen bond in the muscle-type receptor by replacing the (i+1) residue with its α-hydroxy analogue (Fig. 2C). This converts the backbone amide to a backbone ester, which is well-established to be a substantially poorer hydrogen bond acceptor. In the muscle-type receptor, this change raised the nicotine EC50 by a modest factor of 1.625. We now find that for precisely the same change in the α4β2 receptor, the nicotine EC50 increases 19-fold, a relatively large effect for such a subtle mutation26–28. Recall that the backbone ester substitution does not destroy the hydrogen bond, it simply attenuates it. Importantly, ACh, which cannot make a conventional hydrogen bond to the carbonyl, shows no shift in EC50 in response to this mutation (Supplementary Table 1). This establishes that the ester mutation does not globally alter the binding/gating characteristics of the receptor.
The differential affinity of nicotine for α4β2 vs. muscle-type receptors results from stronger interactions in the former with TrpB – both cation-π and hydrogen bonding. Since the two receptors are identical with regard to the five residues that make up the aromatic box, a factor “outside the box” must be influencing its precise geometry, such that nicotine can approach TrpB more closely in α4β2 than in muscle-type nAChR. In pioneering work, Grutter et al. sought residues responsible for the fact that α4β2 receptors show consistently higher affinity than the homopentameric α7 neuronal receptors29. At a particular residue in loop B – position 153, just four residues from TrpB – mutations strongly influence affinity. In high affinity α4β2 receptors this residue is a Lys, and this residue is proposed to help shape the aromatic box by forming a backbone hydrogen bond between loops B and C (Fig. 1). In the lower affinity α7 neuronal receptor, residue 153 is a Gly, and molecular dynamics simulations of α7 suggest that a Gly at 153 discourages the formation of the hydrogen bond between loops B and C. Interestingly, the aligned residue in the muscle-type receptor is also Gly, and a naturally occurring G153S mutation is gain-of-function and associated with a congenital myasthenic syndrome30. We now report that the muscle-type α1 G153K mutant shows much higher affinity for nicotine, and that, when this mutation is present, the cation-π interaction to TrpB is strong. The data are summarized in Supplementary Table 1 and Figure 4. As expected, the ACh cation-π interaction is maintained in the muscle-type receptor with the G153K mutation. These data indicate that the loop B-loop C hydrogen bond that is naturally present in α4β2 shapes the aromatic box so that nicotine can make a closer contact to TrpB, and that this structural feature is absent or weaker in the muscle-type receptor.
Taken together, the present results indicate that the higher affinity of nicotine in the brain relative to the neuromuscular junction is a consequence of enhanced interactions with TrpB. A cation-π interaction that is absent in the muscle-type receptor is quite strong in α4β2. In addition, a hydrogen bond to a backbone carbonyl that is weak in the muscle-type is enhanced in α4β2. Both effects are quite substantial, and in combination they are more than adequate to fully account for the differential sensitivity to nicotine of the two receptors. The side chain of residue 153 in loop B distinguishes the two receptor types and apparently influences the shape of the binding site aromatic box, allowing a stronger interaction between nicotine and TrpB in high affinity receptors.
Methods Summary
Whole-cell electrophysiological characterization of agonist-induced responses
Rat α4β2 and mouse (α1)2β1γδ ion channels were expressed in Xenopus laevis oocytes. For α4β2 receptors, subunit stoichiometry was controlled by varying the α4:β2 subunit ratio and verified by voltage jump experiments. Dose-response measurements for these channels were performed with a holding potential of −60 mV.
Unnatural amino acid / α-hydroxy acid incorporation
Unnatural amino acids and α-hydroxy acids were prepared, coupled to dCA and ligated to 74-mer THG73 as described previously15.
Single-channel characterization of α4β2
Single-channel recording was performed in the cell-attached configuration with a pipette potential of +100 mV as described previously27. Popen values were calculated from event-detected data using Clampfit 9.2 single-channel search.
Full methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Material
Acknowledgements
We thank B.N. Cohen for advice on single-channel recording and analysis. This work was supported by the NIH (NS 34407; NS 11756) and the California Tobacco-Related Disease Research Program of the University of California, Grant No. 16RT-0160. J.A.P.S. was partially supported by an NRSA training grant.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature/.
References and Notes
- 1.Romanelli MN, et al. Central Nicotinic Receptors: Structure, Function, Ligands, and Therapeutic Potential. ChemMedChem. 2007;2:746–767. doi: 10.1002/cmdc.200600207. [DOI] [PubMed] [Google Scholar]
- 2.Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 3.Coe JW, et al. Varenicline: An alpha 4 beta 2 nicotinic receptor partial agonist for smoking cessation. J. Med. Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 4.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharm. Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Mansvelder HD, Keath JR, McGehee DS. Synaptic Mechanisms Underlie Nicotine-Induced Excitability of Brain Reward Areas. Neuron. 2002;33:905. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 6.Nashmi R, et al. Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapper AR, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. 2004 Nov 5;306(5698):1029-32. [DOI] [PubMed] [Google Scholar]
- 8.Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol. Pharm. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- 9.Zhong W, et al. From ab initio Quantum Mechanics to Molecular Neurobiology: A Cation-π Binding Site in the Nicotinic Receptor. Proc. Natl. Acad. Sci. (USA) 1998;95:12088–12093. doi: 10.1073/pnas.95.21.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brejc K, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 11.Sixma TK, Smit AB. Acetylcholine binding protein (AChBP): a secreted glial protein that provides a high-resolution model for the extracellular domain of pentameric ligand-gated ion channels. Annu Rev Biophys Biomol Struct. 2003;32:311–334. doi: 10.1146/annurev.biophys.32.110601.142536. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty DA. Cys-loop Neuroreceptors: Structure to the Rescue? Chem. Rev. 2008;108:1642–1653. doi: 10.1021/cr078207z. [DOI] [PubMed] [Google Scholar]
- 13.Beene DL, et al. Cation-pi interactions in ligand recognition by serotonergic (5-HT3A) and nicotinic acetylcholine receptors: The anomalous binding properties of nicotine. Biochemistry. 2002;41:10262–10269. doi: 10.1021/bi020266d. [DOI] [PubMed] [Google Scholar]
- 14.Dougherty DA. Physical organic chemistry on the brain. J Org Chem. 2008;73:3667–3673. doi: 10.1021/jo8001722. [DOI] [PubMed] [Google Scholar]
- 15.Nowak MW, et al. In vivo incorporation of unnatural amino acids into ion channels in a Xenopus oocyte expression system. Methods Enzymol. 1998;293:504–529. doi: 10.1016/s0076-6879(98)93031-2. [DOI] [PubMed] [Google Scholar]
- 16.Fonck C, et al. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive alpha 4* nicotinic receptors. J Neurosci. 2005;25(49):11396–11411. doi: 10.1523/JNEUROSCI.3597-05.2005. 2005 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filatov GN, White MM. The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Mol Pharmacol. 1995;48:379–384. [PubMed] [Google Scholar]
- 18.Labarca C, et al. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- 19.Kearney P, et al. Dose-response relations for unnatural amino acids at the agonist binding site of the nicotinic acetylcholine receptor: Tests with novel side chains and with several agonists. Mol. Pharmacol. 1996;50:1401–1412. [PubMed] [Google Scholar]
- 20.Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006 Aug;70(2):755–768. doi: 10.1124/mol.106.023044. Epub 2006 May 23. (2006) [DOI] [PubMed] [Google Scholar]
- 21.Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate Stoichiometries of alpha 4beta 2 Nicotinic Acetylcholine Receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- 22.Celie PH, et al. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 2004;41:907–914. doi: 10.1016/s0896-6273(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 23.Hansen SB, et al. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talley TT, et al. Spectroscopic analysis of benzylidene anabaseine complexes with acetylcholine binding proteins as models for ligand-nicotinic receptor interactions. Biochemistry. 2006;45:8894–8902. doi: 10.1021/bi060534y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cashin AL, Petersson EJ, Lester HA, Dougherty DA. Using physical chemistry to differentiate nicotinic from cholinergic agonists at the nicotinic acetylcholine receptor. J. Am. Chem. Soc. 2005;127:350–356. doi: 10.1021/ja0461771. [DOI] [PubMed] [Google Scholar]
- 26.Deechongkit S, et al. Context-dependent contributions of backbone hydrogen bonding to beta-sheet folding energetics. Nature. 2004;430:101–105. doi: 10.1038/nature02611. [DOI] [PubMed] [Google Scholar]
- 27.England PM, Zhang Y, Dougherty DA, Lester HA. Backbone mutations in transmembrane domains of a ligand-gated ion channel: implications for the mechanism of gating. Cell. 1999;96:89–98. doi: 10.1016/s0092-8674(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 28.Koh JT, Cornish VW, Schultz PG. An experimental approach to evaluating the role of backbone interactions in proteins using unnatural amino acid mutagenesis. Biochemistry. 1997;36:11314–11322. doi: 10.1021/bi9707685. [DOI] [PubMed] [Google Scholar]
- 29.Grutter T, et al. An H-bond between two residues from different loops of the acetylcholine binding site contributes to the activation mechanism of nicotinic receptors. EMBO J. 2003;22:1990–2003. doi: 10.1093/emboj/cdg197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sine SM, et al. Mutation of the acetylcholine receptor alpha subunit causes a slow-channel myasthenic syndrome by enhancing agonist binding affinity. Neuron. 1995;15:229–239. doi: 10.1016/0896-6273(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 31.McManus OB, Blatz AL, Magleby KL. Sampling, Log Binning, Fitting, and Plotting Durations of Open-and-Shut Intervals from Single Channels and the Effects of Noise. Pflugers Archiv-Eur. J. of Physiol. 1987;410:530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.