Abstract
Diabetes is characterized by decreased function of insulin-producing insulin β cells and insufficient insulin output resulting from an absolute (Type 1) or relative (Type 2) inadequate functional β cell mass. Both forms of the disease would greatly benefit from treatment strategies that could enhance β cell regeneration and/or function. Successful and reliable methods of generatingβ cells or whole islets from progenitor cells in vivo or in vitro could lead to restoration of β cell mass in individuals with Type 1 diabetes and enhanced β cell compensation in Type 2 patients. A thorough understanding of the normal developmental processes that occur during pancreatic organogenesis, e.g., transcription factors, cell signaling molecules, and cell-cell interactions that regulate endocrine differentiation from the embryonic pancreatic epithelium, is required in order to successfully reach these goals. This review summarizes our current understanding of pancreas development, with particular emphasis on factors intrinsic or extrinsic to the pancreatic epithelium that are involved in regulating the development and differentiation of the various pancreatic cell types. We also discuss the recent progress in generating insulin-producing cells from progenitor sources.
Keywords: pancreas development, pancreas progenitors, endocrine differentiation, lineage allocation
Introduction to pancreas development
The mature pancreas is comprised of two functionally distinct tissue types. The exocrine pancreas, consisting of acinar cells that secrete digestive enzymes into a complex ductal network, makes up approximately 98 percent of the adult organ. Interspersed within the acinar parenchyma are the islets of Langerhans containing hormone-producing endocrine cells, which play a critical role in maintaining glucose homeostasis within the organism. Each islet is a microorgan containing at least four different hormone-producing cell types includingβ (insulin),α (glucagon), δ (somatostatin), ε (ghrelin), and PP (pancreatic polypeptide) cells. Diabetes mellitus results from insulin insufficiency caused by either a selective autoimmune destruction ofβ cells (type 1) or a failure of β cells to compensate for peripheral insulin resistance, usually associated with obesity (type 2). There has been some therapeutic success with transplanted cadaveric islets into patients with type 1 diabetes; several patients achieved insulin independence for a limited period of time. However, lack of sufficient donor tissue and ongoing autoimmunity have prevented islet transplantation from becoming a widely available treatment option. Consequently, researchers are currently trying to develop ways to generate replacement sources of β cells by expanding existing β cells in vivo or generating them de novo, in vitro. One current avenue of study involves the directed differentiation of human embryonic stem (ES) cells or induced pluripotent stem (iPS) cells down the normal path of pancreas development into glucose-responsive β cells (Figure 1). A thorough understanding of how pancreas organogenesis occurs in the embryo should provide insight into how β cells can be generated more efficiently and effectively using these differentiation protocols. Of particular interest are the transcription factors, signaling molecules, and cell-cell interactions that regulate the cell fate decisions directing multipotent progenitor cells to the proper pancreatic cell lineages.
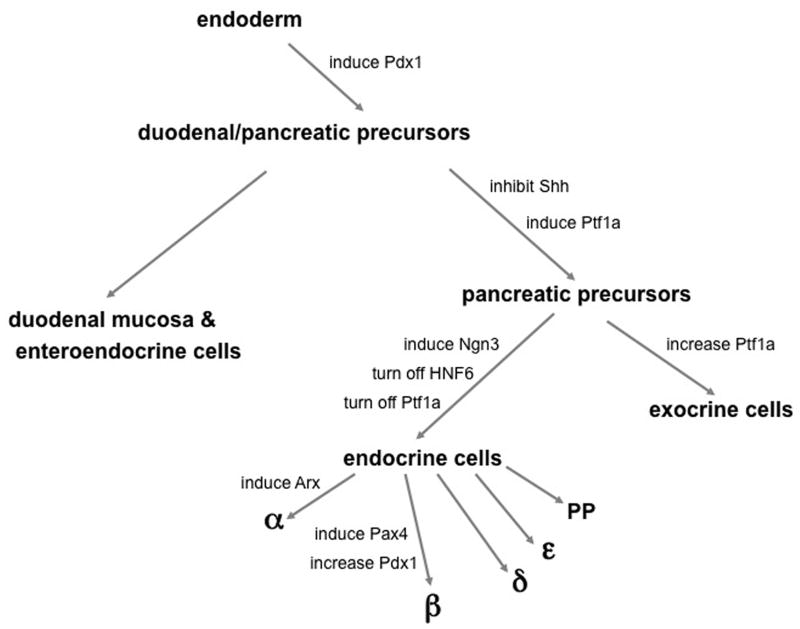
Figure 1. Lineage bifurcations leading to pancreatic endocrine differentiation.
Beginning with definitive embryonic endoderm, cell fate specification within the posterior foregut can be thought of simply as a series of binary decisions that lead to the formation of the different cell types in the pancreas. Certain transcription factors and secreted factors are activated or inhibited along this developmental pathway, directing pluripotent cells toward one lineage or another. While not comprehensive, this schematic highlights some of the factors critical for pancreatic cell fate specification and differentiation. See text for more details.
Pancreas development can be thought of simplistically as a series of bifurcating lineage decisions: (1) endoderm versus mesoderm and ectoderm, (2) pancreas versus Duodenum, (3) exocrine versus Endocrine, and (4)β cell versus other hormone positive cell types (Figure 1). The pancreas develops from a region of the foregut endoderm located posterior to the developing liver and anterior to the duodenum. The developing dorsal bud lies in close proximity to the notochord and later to the dorsal aorta, and these structures are thought to produce signals that are important for early bud formation (Kim et al., 1997; Lammert et al., 2001). At embryonic day (e) 9.5 in the mouse (gestational day 25 in humans), the dorsal and ventral buds of the pancreas begin to evaginate. Subsequently, the pancreatic buds undergo elongation and branching within the pancreatic mesenchyme to yield a highly branched ductal network. At e12.5 the dorsal and ventral buds fuse to form a single organ. Both extrinsic and intrinsic factors regulate the differentiation and proliferation of endocrine cells in order to generate the normal proportions of each of the cell types within the pancreas. Glucagon and insulin expression can be detected in the developing pancreatic anlagen as early as e10.5, prior to epithelial branching. However, these “first wave” endocrine cells do not express markers of mature endocrine cells and it is thought that they do not contribute to mature islets; the ultimate fate of these cells is still unclear (Herrera, 2000; Herrera et al., 1994; Lee et al., 1999; Pang et al., 1994; Wilson et al., 2002). Additionally, these early endocrine cells seem to differentiate in a distinct manner from other endocrine cells since they form independently of the critical transcription factors, pancreatic duodenal homeobox 1 (Pdx1) and pancreas transcription factor 1a (Ptf1a), each of which are required at early stages of pancreatic bud development (Ahlgren et al., 1996; Burlison et al., 2008; Kawaguchi et al., 2002; Offield et al., 1996). At approximately e13 to 16 in the mouse, a stage known as the “secondary transition”, there is a dramatic increase in the number of endocrine cells budding from the ductal epithelium. These endocrine cells contribute to the mature islet. At e18.5, the differentiated endocrine cells begin to separate from the ductal epithelium from which they originate, migrate into the surrounding acinar tissue, and organize into islets. Although many of the cellular events required for this process have not been identified, they likely include changes in expression of cell adhesion molecules, modifications in extracellular matrix proteins, and paracrine and juxtacrine cell-cell communication.
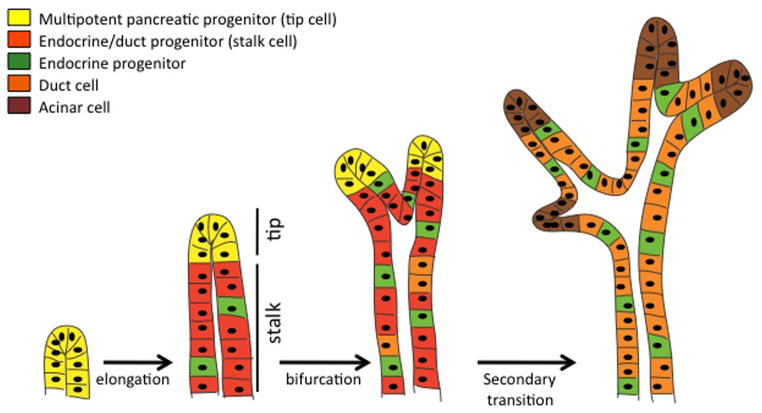
It is thought that the progenitor cells that will give rise to all the endocrine and exocrine cells reside within the ductal epithelium; however, it is unclear whether any or all of these progenitor cells are truly multipotent, with the capacity to differentiate into any or all of the different pancreatic cell types, or whether these progenitors are already specified to a particular pancreatic cell lineage(s) at an early stage within the undifferentiated epithelium. Molecular marker analyses and lineage tracing studies suggest that the branching pancreatic epithelium consists of functionally distinct microdomains termed “tip cells” and “stalk cells” (Zhou et al., 2007). Undifferentiated progenitors are located within growing branch tips prior to the secondary transition, and as these cells leave the tip domain and occupy the stalk, they gain the ability to generate endocrine progenitors (Figure 2). These stalk cells will also give rise to differentiated duct cells. Later-forming tip cells give rise to acinar cells.
Figure 2. Location of pancreatic progenitors during branching morphogenesis.
Multipotent pancreatic progenitors (yellow) are localized to tips of developing epithelial branches. As branches elongate at the tips, cells remaining in the stalks (red) lose the potential to differentiate as acinar cells. Cells that will give rise to definitive duct cells (orange) and endocrine progenitors (green) become specified in the stalks as development proceeds. Finally, during the secondary transition, cells at the tips begin to differentiate as acinar cells (brown).
This review focuses on the extrinsic and intrinsic signals that are important for cell fate decisions during pancreas development. Much work has been done to identify the factors that are involved in proliferation and expansion of pancreatic cell types during development and postnatally. Although these aspects of pancreas organogenesis will not be discussed here, the reader is referred to excellent reviews on these topics (Ackermann and Gannon, 2007; Bonner-Weir, 2000; Cozar-Castellano et al., 2006).
Regulation of initial pancreas bud formation: Factors intrinsic to the epithelium
Regionalization of the endoderm
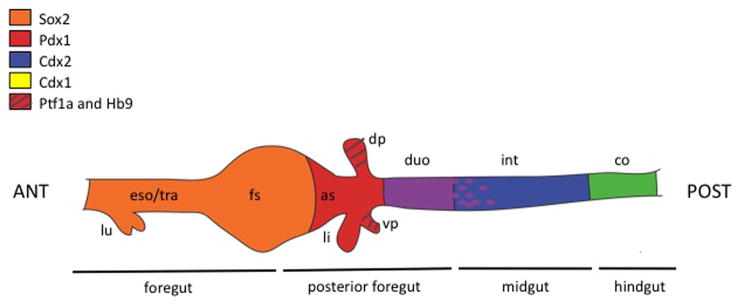
Overlapping expression domains of transcription factors initially broadly pattern the endoderm along the anterior to posterior axis (Figure 3). For example, Sox2 is expressed in the anterior domain of endoderm that will give rise to the esophagus and stomach; Pdx1 expression is found in the antral stomach, presumptive pancreas, common bile duct, and rostral duodenum (Offield et al., 1996), and its expression coincides with the earliest stage that the presumptive pancreatic foregut can be cultured ex vivo and still develop into pancreas tissue (Wessels, 1967); Cdx2 is expressed in the entire post gastric epithelium in the regions which will form intestine (Silberg et al., 2000). Initially, the presumptive pancreatic domain is marked by overlapping expression of Pdx1, Ptf1a, and homeobox gene 9 (Hlxb9/Hb9) (Harrison et al., 1999; Kawaguchi et al., 2002; Li et al., 1999; Offield et al., 1996). In the absence of Hb9, the dorsal pancreatic bud fails to develop, although the adjacent endoderm and surrounding mesenchyme are patterned normally, indicating Hb9 is absolutely required for pancreatic bud specification and outgrowth (Harrison et al., 1999; Li et al., 1999).
Figure 3. Anterior/posterior patterning of the digestive tract.
Expression of various transcription factors is regionalized along the anterior-posterior axis of the developing gut tube endoderm. Regions of common expression are shown in blended colors: purple, overlapping expression of Pdx1 and Cdx2; green, overlapping expression of Cdx1 and Cdx2 in the hindgut. The posterior boundary of Pdx1 expression is diffuse (purple dots). Abbreviations: ANT, anterior; as, antral stomach; co, colon; dp, dorsal pancreas; duo, duodenum; eso, esophagus; fs, forestomach; int, intestine; li, liver; lu, lung; POST, posterior; tra, trachea; vp, ventral pancreas.
Many factors involved in regionalization of the endoderm early in development also have roles in later stages of organogenesis as well as in mature organ function. For example, at late gestation, the initial broad expression of Pdx1 becomes selectively elevated in developing β cells with only low levels of expression in the surrounding acinar tissue (Guz et al., 1995; Offield et al., 1996; Wu et al., 1997). Global inactivation of Pdx1 leads to pancreas agenesis, indicating that Pdx1 is necessary for pancreas development (Jonsson et al., 1994; Offield et al., 1996). Interestingly, however, Pdx1 is not required for the specifcation of pancreatic endoderm or for the formation of first wave endocrine cells, since Pdx1 null embryos have a minimally branched dorsal ductule and low numbers of insulin- and glucagon-expressing cells (Ahlgren et al., 1996; Offield et al., 1996). Conditional inactivation of Pdx1, specifically in insulin-producing cells during embryonic development, revealed a role for Pdx1 in regulating the numbers of the different endocrine cell types (Gannon et al., 2008). Additionally, Pdx1 is required for the maturation and function of β cells; Pdx1 heterozygosity or conditional inactivation of Pdx1 in adult β cells leads to the development of glucose intolerance or type 2 diabetes, respectively in both mice and humans (Ahlgren et al., 1998; Dutta et al., 1998; Stoffers et al., 1997; Stoffers et al., 1998). Consistent with these results, Pdx1 has been shown to regulate the expression of many genes that are important for β cell development and mature β cell function, such as Pax4, MafA, insulin, glut2, IGRP, and glucokinase (Chakrabarti et al., 2002; Martin et al., 2004; Ohlsson et al., 1993; Raum et al., 2006; Smith et al., 2000). The fact that Pdx1 is expressed in regions of the stomach, duodenum, and common bile duct suggest that these tissues may be competent to become pancreas if the correct combination of factors are induced or inhibited at the right developmental stage. Indeed, inactivation of Hes-1, a transcriptional repressor downstream of Notch signaling, is sufficient to allow cells within the common bile duct to become pancreatic endocrine and exocrine cells (Fukuda et al., 2006; Sumazaki et al., 2004).
Specification of pancreas from posterior foregut
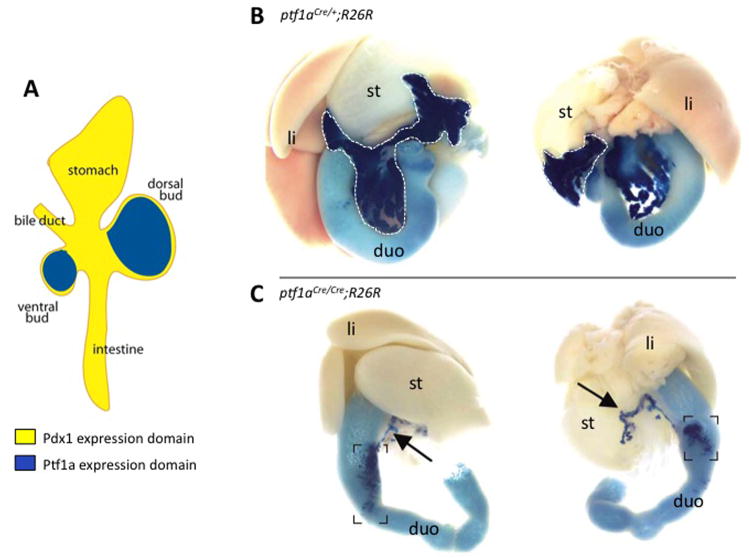
At e9.5, a subset of cells within the Pdx1 expression domain begins to express the bHLH transcription factor, Ptf1a/p48 (Burlison et al., 2008). It is the Ptf1a/Pdx1 double-positive cells that will give rise to the pancreatic anlagen, while Pdx1+/Ptf1a− cells contribute instead to the antral stomach and rostral duodenum (Figure 3). Ptf1a is the tissue-specific component of a heterotrimeric transcription factor complex known as PTF1 that also includes the proteins p65 and p75 (Krapp et al., 1996; Rose et al., 2001). Although it was originally thought to be required solely for acinar cell development, Ptf1a is now known to be indispensable for specification of the pancreas as well as for the development of both endocrine and exocrine cell types (Kawaguchi et al., 2002; Krapp et al., 1998). Lineage tracing analysis showed that Ptf1a-expressing progenitors give rise to all of the cell types in the pancreas (Kawaguchi et al., 2002). Similar to the Pdx1 null phenotype, mice lacking Ptf1a are apancreatic except for a severely hypoplastic dorsal bud (Kawaguchi et al., 2002; Krapp et al., 1998). The similarities between the null phenotypes suggest that PTF1 and Pdx1 may each regulate the expression of the other. Indeed, PTF1 binds and activates the Pdx1 promoter in early development; however, Ptf1a is not required for Pdx1 expression (Burlison et al., 2008; Wiebe et al., 2007). Interestingly, in the absence of Ptf1a, cells that would normally become ventral pancreas survive and proliferate; they are found within the duodenum and express intestinal cell markers, suggesting a role for Ptf1a in directing bipotential endodermal progenitor cells towards the pancreatic fate and away from the intestinal lineage (Kawaguchi et al., 2002) (Figure 4). That Ptf1a mutant cells adopt an intestinal fate rather than undergoing apoptosis, and that ectopic expression of Ptf1a in the Pdx1 domain can convert the duodenum and stomach to pancreas, indicates the highly plastic nature of the endoderm during gut formation (Afelik et al., 2006; Jarikji et al., 2007).
Figure 4. Ptf1a expression defines the pancreas fate.
(A) Ptf1a expression (blue) overlaps with Pdx1 expression (yellow) in the dorsal and ventral pancreatic buds. Ptf1a is excluded from other areas of Pdx1 expression. (B) Lineage tracing (X-gal staining, dark blue) of Ptf1a expressing cells using neonatal mice in which Cre recombinase has been knocked into the Ptf1a locus (ptf1aCre/+) bred with the R26R strain of mice reveals that all pancreatic cells types are derived from a Ptf1a-expressing progenitor. The limits of the pancreas are outlined with a white dotted line. (C) Homozygosity for the ptf1aCre allele results in a Ptf1a null animal. Lineage tracing reveals that in the absence of Ptf1a, the dorsal pancreatic bud forms only a small ductule (arrows), while presumptive ventral pancreatic cells become incorporated into the duodenum (dark blue in brackets). Abbreviations: duo, duodenum; li, liver; st, stomach. (Adapted with permission from Kawaguchi et al., Nature Genetics 32: 128, 2002).
Regulation of initial pancreas bud formation: Extrinsic mesodermally-derived factors
Regionalization of the foregut/midgut endoderm
Suppression of Wnt signaling in the anterior endoderm is required for both liver and pancreas development (McLin et al., 2007). As development proceeds, the mesodermal tissues surrounding the posterior foregut provide secreted signals that promote either liver or pancreas development from a common region of endoderm. In the ventral foregut, bone morphogenetic proteins (BMP2 in zebrafish and chick, and BMP4 in mouse) secreted by the septum transversum mesenchyme and FGF1 and 2 produced by the cardiac mesoderm promote liver development while concomitantly suppressing the pancreatic differentiation program (Chung et al., 2008; Deutsch et al., 2001; Rossi et al., 2001; Shin et al., 2007; Zhang et al., 2004). When cultured in the absence of cardiac mesoderm, ventral foregut endoderm normally fated to become liver instead expresses Pdx1, suggesting that in vivo, fibroblast growth factors (FGFs) direct bipotent cells away from the “default” pancreatic identity towards the liver fate (Deutsch et al., 2001). Recent work in Xenopus has identified the co-repressor, transforming growth factor-β (TGF-β) induced factor 2 (TGIF2), as a factor that acts to limit BMP signaling within the endoderm and promote the expression of pro-pancreas genes (Spagnoli and Brivanlou, 2008).
Retinoic acid (RA) produced by the foregut mesoderm may be involved in setting up the anterior and posterior boundaries of the posterior foregut within the endoderm. Treatment of both zebrafish and Xenopus embryos with exogenous RA expands the pancreatic field anteriorly, although a conserved role for RA in endoderm patterning has not yet been shown in mammals (Stafford et al., 2004). The ability of RA to act as a posteriorizing agent has also been shown in organs derived from the other germ layers, including the chick heart (mesoderm) and neural tube (ectoderm) (Blumberg, 1997; Yutzey et al., 1994).
The pancreatic epithelium lies in close proximity to the splenic mesenchyme during early bud formation; however, after e11 the splenic mesenchyme condenses and separates from the pancreatic anlagen. In embryos lacking the transcription factor Bapx1 (Nkx3.2), the splenic mesenchyme fails to condense and remains associated with the dorsal pancreas (Asayesh et al., 2006). The pancreatic epithelium developed cyst-like structures, which became surrounded by the presumptive splenic mesenchyme. However, this mesenchyme displayed characteristics of differentiated intestinal mesenchyme. Interestingly, pancreatic epithelium cultured adjacent to wild type splenic mesenchyme also develops cysts, suggesting that separation of the mesoderm surrounding the pancreas and spleen is required for normal development of the epithelium, and the inhibition of the duodenal program within the pancreatic mesenchyme (Asayesh et al., 2006).
Induction of pancreas development
Dorsally, signals from the notochord induce pancreas formation. From the time it is formed, the notochord is in contact with the prepancreatic endoderm until e8 in the mouse, at which time the dorsal aortae fuse between the notochord and endoderm. In experiments with chick embryos, removal of the notochord at a time when it normally contacts the presumptive pancreatic endoderm results in a reduction in epithelial branching, as well as a loss of expression of pancreas/endocrine transcription factors, such as Pdx1, Islet 1 (Isl1), and Pax6, as well as insulin (Hebrok et al., 1998; Kim et al., 1997). Interestingly, while recombination of the notochord with prepancreatic endoderm induced pancreatic gene expression, placing the notochord in contact with endoderm isolated from a more posterior location failed to induce pancreas gene expression, suggesting that the endoderm is already patterned at this stage and only a particular domain is competent to form pancreas in response to notochord signals. The notochord promotes pancreas formation by repressing the expression of the secreted morphogen, sonic hedgehog (Shh), in the underlying endoderm (Hebrok et al., 1998). Although Shh is highly expressed in the endoderm rostral and caudal to the developing pancreas, it is markedly absent from the presumptive pancreas epithelium. Studies from the Edlund lab (Apelqvist et al., 1997) suggest that exclusion of Shh from the pancreatic endoderm is required to inhibit intestinal fates. Ectopic expression of Shh in the Pdx1 expression domain is incompatible with normal pancreas development; pancreatic mesoderm in Pdx1-Shh transgenic mice expressed markers of intestinal mesoderm, including smooth muscle α-actin. FGF2 and Activin-βB are likely the endogenous signals secreted from the notochord which mediate its suppressive effects on Shh in the dorsal pancreatic region (Hebrok et al., 1998). In contrast, the ventral pancreas develops in the absence of any contact with the notochord. This is just one example of the divergent developmental programs resulting in dorsal versus ventral pancreatic bud formation.
At e9 the two dorsal aortae fuse, disrupting the contact of the notochord with the prepancreatic endoderm. Signals from blood vessel endothelial cells are also important for pancreas development in vitro and in vivo, although the relevant endothelial-derived molecule(s) await identification. Co-culture experiments demonstrated that signals from the endothelium are required for maintenance of Pdx1 expression, dorsal bud outgrowth, and initiation of Ptf1a and insulin gene expression (Lammert et al., 2001; Yoshitomi and Zaret, 2004). Conversely, transgenic mice over-expressing vascular endothelial growth factor A (VEGFA) using the Pdx1 promoter displayed an increase in pancreatic blood vessels with a concomitant increase in pancreatic islets and ectopic insulin positive cells within the posterior stomach (Lammert et al., 2001). Since Pdx1 is also expressed in this region of the stomach early in development, these data suggest that endothelial cells are able to induce the pancreatic endocrine cell fate in competent regions of endoderm. Signals from the dorsal aorta may be acting in part by influencing epithelial-mesenchymal interactions as the endothelium induces expression of FGF10, a mesodermally-derived factor required for proliferation of pancreatic progenitors and branching of the epithelium (Jacquemin et al., 2006). Consistent with other data suggesting that the ventral pancreas develops quite differently from the dorsal pancreas, ventral bud evagination was not affected in the absence of endothelium.
In addition to its role in anterior/posterior patterning, RA signals to the underlying endoderm to induce pancreas differentiation in zebrafish (Stafford et al., 2006). Interfering with RA signaling using an RA-receptor antagonist or antisense morpholino oligonucleotides directed against the RA synthesis enzyme, RALDH2, leads to a loss of Pdx1 expression, the early endocrine marker Isl1, and ultimately, insulin (Stafford and Prince, 2002; Stafford et al., 2006). Studies in quail and Xenopus provide support for these data by showing that blocking RA receptor signaling results in a failure of dorsal pancreas bud formation (Figure 5) (Chen et al., 2004; Stafford et al., 2004). An important role for RA in pancreas bud formation is also conserved in mammals. Mice lacking Raldh2 displayed dorsal pancreas agenesis (Martin et al., 2005; Molotkov et al., 2005), while mice expressing a dominant negative form of the RA receptor α driven by the Pdx1 promoter showed loss of both dorsal and ventral pancreatic buds (Ostrom et al., 2008), suggesting that another RA synthesizing enzyme (perhaps Raldh1) acts within the ventral pancreas. The expression patterns of other factors involved in early steps of pancreas development, such as Hb9 and Shh, however, are normal suggesting that RA acts prior to the initiation of Pdx1 expression but after pancreas specification (Martin et al., 2005).
Figure 5. Retinoic acid signaling affects dorsal pancreas differentiation.
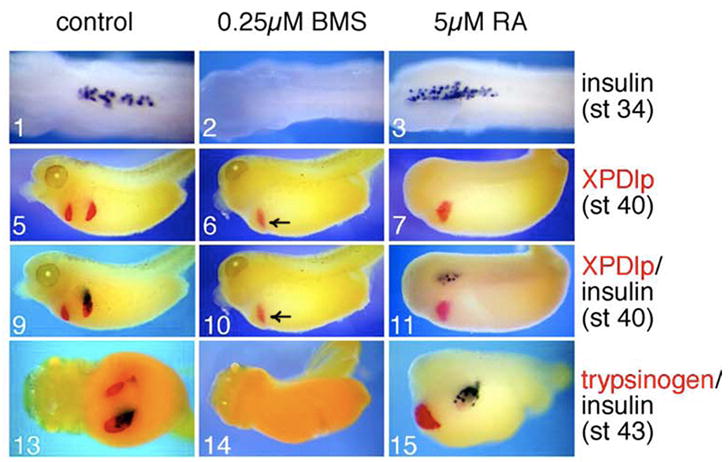
Stage 11 Xenopus embryos were treated with either retinoic acid (RA) or the RA receptor inhibitor, BMS453 (BMS). BMS inhibited both endocrine and exocrine development in the dorsal pancreas as assayed by insulin (panels 2, 10, and 14), XPDlp (panels 6 and 10), and trypsinogen (panel 14), respectively. Ventral pancreas differentiation was unaffected (arrows in panels 6 and 10). Exogenous RA resulted in an expansion of insulin-positive cells (panels 3, 11, and 15) at the expense of acinar differentiation (panels 7, 11, and 15) in the dorsal pancreas. RA expands exocrine differentiation in the ventral pancreas (panel 15). (Adapted with permission from Chen et al., Dev. Biol. 271: 17, 2004).
Regulation of exocrine versus endocrine differentiation: Factors intrinsic to the epithelium
Location of exocrine and endocrine progenitors
During branching morphogenesis, the pancreatic epithelium repeatedly evaginates to form new branches with a lumen that remains contiguous with the main pancreatic duct. In other branching tissues, such as the lung and the kidney, multipotent progenitors are physically separated within the developing branch from the more differentiated populations of cells (Costantini, 2006; Hogan et al., 1997). Similarly, recent lineage tracing evidence in the pancreas suggests that much of branch growth and elongation occurs at the tips of the developing ductal tree, while cells left behind in the wake of the growing tip become incorporated into the “stalk” of the branch (Zhou et al., 2007). The tips of the branching epithelium are marked by expression of Carboxypeptidase A1 (Cpa1), Pdx1, Ptf1a, and c-Myc. Lineage tracing of tip cells revealed that progeny of these cells contribute to endocrine, duct, and acinar lineages, and can thus be considered multipotent progenitors (Figure 2). The first cells to be deposited in the stalk or trunk region give rise to endocrine and ductal cells. Tip cell progenitors become restricted as development proceeds such that by e14.5 the majority differentiate into acinar cells. Thus, in addition to being spatially regulated, the decision between endocrine and exocrine differentiation is also temporally regulated with the endocrine progenitors being specified earlier and the exocrine cells later.
Within the ductal epithelium, juxtacrine Notch-Delta signaling regulates the differentiation of progenitor cells (Apelqvist et al., 1999; Jensen et al., 2000a). It is hypothesized that this occurs in a manner similar to what occurs within equivalence groups during Drosophila neurogenesis (Fisher and Caudy, 1998). Cells initially express low levels of both the Notch receptor and its ligand, Delta. Interactions between adjacent cells lead to stochastic up-regulation of either Notch or Delta. Notch signaling leads to activation of the target gene, Hes1, which represses expression of the pro-endocrine bHLH transcription factor, neurogenin 3 (Ngn3) (Jensen et al., 2000b). Cells that fail to activate Ngn3 remain in an undifferentiated state while cells in which Ngn3 becomes activated initiate the endocrine differentiation program and delaminate from the ductal epithelium (Gradwohl et al., 2000; Gu et al., 2002). All endocrine cell types arise from a Ngn3-expressing progenitor; currently Ngn3 is the earliest known marker of a definitive endocrine progenitor (Gradwohl et al., 2000; Gu et al., 2002).
Transcription factors regulating exocrine versus endocrine cell fate
Hepatic nuclear factor 6 (Hnf6/OC-1) is a member of the ONECUT family of transcription factors and regulates genes involved in liver and pancreas development. Hnf6 expression is colocalized with Pdx1 in the pancreatic epithelium at e10.5 but is not expressed in hormone-positive cells; however, expression is maintained throughout adulthood in the ducts and at low levels in exocrine tissue (Figure 6) (Landry et al., 1997; Rausa et al., 1997) (H. Zhang, E.T. Ables, and M. Gannon, manuscript in preparation). Hnf6 is required for pancreatic endocrine development, as global null mice have a dramatic decrease in Ngn3 expression as well as a marked reduction in insulin and glucagon expression (Jacquemin et al., 2000). After birth, these mice have impaired glucose homeostasis. Consistent with these results, Hnf6 binds to and activates the Ngn3 and Pdx1 promoters (Jacquemin et al., 2000; Jacquemin et al., 2003). Maintenance of Hnf6 in the endocrine lineage leads to disrupted islet architecture and diabetes caused by defects in insulin granule maturation and glucose-stimulated insulin secretion (Gannon et al., 2000; Tweedie et al., 2006). Hnf6 is also required for exocrine duct differentiation. In the absence of Hnf6, the pancreatic ducts are dilated and develop cysts due to the lack of primary cilia on ductal epithelial cells (Pierreux et al., 2006) (H. Zhang, E.T. Ables, and M. Gannon, manuscript in preparation). These data indicate that dynamic expression of Hnf6 is critical for pancreas development proper levels are required for endocrine and exocrine cell differentiation while down-regulation before birth is essential for β cell maturation and islet morphogenesis.
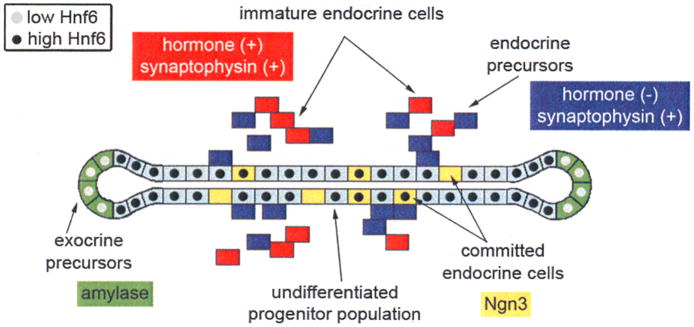
Figure 6. Schematic representation of Hnf6 expression in the developing pancreas.
Hnf6 is expressed at high levels (black circles) in the undifferentiated pancreatic stalk epithelium (light blue), and at lower levels (gray circles) in differentiating acinar cells (green) at branch tips at the secondary transition. Hnf6 is not expressed in definitive endocrine cells (blue and red), but is detected in a sub-population of Ngn3-positive endocrine progenitors (yellow).
In vivo studies have also demonstrated an important role for Ngn3 in regulating the endocrine differentiation program within the pancreatic epithelium. Ngn3 expression is biphasic, correlating with the first and second waves of endocrine cell differentiation (Villasenor et al., 2008). Expression of Ngn3 begins around e9.5 and peaks during the secondary transition; by birth Ngn3 expression is nearly undetectable (Gradwohl et al., 2000). Consistent with the hypothesis that Ngn3 marks endocrine progenitors, Ngn3-positive cells do not co-express pancreatic hormones and are found within or adjacent to the ductal epithelium. Ngn3 null embryos lack all endocrine cell types and die two to three days after birth due to diabetes (Gradwohl et al., 2000). The role of Ngn3 in promoting endocrine differentiation is further demonstrated by over-expressing Ngn3 throughout the pancreas using the Pdx1 promoter. Pdx1-Ngn3 transgenic embryos have a hypoplastic pancreas, with a dramatic decrease in carboxypeptidase-positive acinar cells and an increase in differentiated (mainly glucagon-expressing) endocrine cells (Apelqvist et al., 1999; Schwitzgebel et al., 2000). Ectopic expression of Ngn3 throughout the pancreatic epithelium thus leads to a loss of multipotent pancreatic progenitors and specifically favors the development of α cells with very few insulin-positive cells (Apelqvist et al., 1999; Grapin-Botton et al., 2001; Schwitzgebel et al., 2000). A recent report from the Grapin-Botton laboratory (Johansson et al., 2007) demonstrates that the competence of Ngn3-expressing progenitors changes with time, and that early differentiating endocrine cells are more likely to become α cells.
Studies from our laboratory using a conditional Hnf6 allele demonstrate that irreversible commitment to the endocrine fate requires a threshold of Hnf6-dependent Ngn3 expression. Ngn3-Cre-mediated inactivation of Hnf6 in putative endocrine progenitors results in a significant decrease in total endocrine area at birth (H. Zhang, E.T. Ables, and M. Gannon, manuscript in preparation). Lineage tracing analyses indicated that in the absence of sustained Hnf6 expression, a subset of cells (14%) that had activated Ngn3 became diverted to the exocrine lineage and expressed markers of terminally differentiated acinar and ductal cells, while in normal development, approximately 1% of cells that activate the Ngn3 promoter become incorporated into exocrine tissue (Schonhoff et al., 2004). Thus, Ngn3 gene activation does not necessarily commit a cell to the endocrine fate. We propose that continued Hnf6 activity at the Ngn3 promoter is required in order for Ngn3 protein to reach a required level within the cell and commit multipotent pancreatic progenitors to an endocrine fate (Figure 7). These data indicate that Ngn3-expressing cells have a high degree of plasticity, and in the absence of sufficient levels of Ngn3, intrinsic or extrinsic signals may influence these cells to adopt the exocrine differentiation program. Alternatively, Ngn3 may need to be expressed for a certain window of time to allow these cells to activate endocrine-specific Ngn3 target genes. Studies using a hypomorphic Ngn3 allele or partial attenuation of Ngn3 activity (for example with partial knockdown) would confirm the hypothesis that low levels of Ngn3 expression during development are insufficient for endocrine differentiation and result in cells committing to the exocrine lineage.
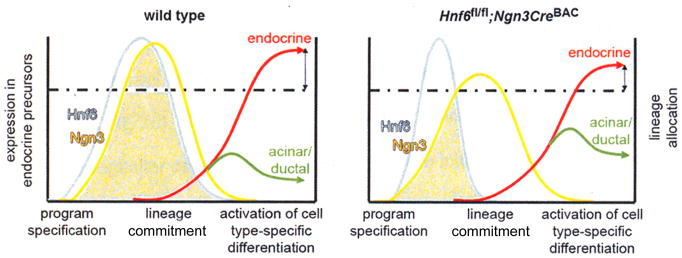
Figure 7. A threshold of Ngn3 is required to generate fully committed endocrine cells.
Inactivation of Hnf6 subsequent to Ngn3 gene activation (Hnf6fl/fl;Ngn3CreBAC) results in a reduced number of differentiated endocrine cells. In our model, in wild type mice (left graph), Hnf6 expression (light blue) precedes Ngn3 expression (yellow). Hnf6 expression must be maintained for a certain period of time to allow Ngn3 expression to exceed a critical threshold (dotted line), allowing for adequate endocrine differentiation (red). When Hnf6 expression is prematurely extinguished (right graph), Ngn3 levels reach this threshold in fewer cells, resulting in reduced endocrine mass.
Ptf1a is expressed broadly throughout the pancreatic domain early in development but becomes enriched in acinar cells after e13.5, and has been shown to bind the promoters of exocrine genes, such as elastase and trypsin (Cockell et al., 1989; Krapp et al., 1996). Although lineage tracing studiesg revealed that Ptf1a is expressed in multipotent pancreatic progenitor cells that give rise to the islet endocrine cells (Kawaguchi et al., 2002), Ptf1a is not essential for endocrine formation; a small number of hormone-producing cells are generated in the absence of Ptf1a (Krapp et al., 1998). However, Ptf1a is absolutely required for exocrine development as no acinar tissue is found in Ptf1a null mice.
Ptf1a plays both early and late roles in pancreas development, which are mediated by its interaction with two different isoforms of the vertebrate suppressor of hairless protein, RBPJ (Masui et al., 2007). The Notch-dependent, RBPJκ form of the protein is found in the PTF1 complex early in pancreas development and is swapped for the Notch-independent, RBPJL form at the onset of acinar cell development (Masui et al., 2007). It is exclusively the RBPJL form that is found bound to the promoters of acinar-specific genes (Beres et al., 2006; Masui et al., 2007). Several studies indicate that the dosage of Ptf1a can affect pancreas cell fate decisions. Mice hemizygous for a hypomorphic allele of Ptf1a and a null allele display decreased pancreatic size and impaired acinar cell differentiation (Fukuda et al., 2008). While Pdx1 is normally only found at low levels in acinar tissue postnatally, whereas persistent exocrine Pdx1 expression is detected in Ptf1a hypomorphic pancreata, indicating impaired acinar differentiation. Interestingly, Ptf1a gene dosage may also play a role in determining whether pancreatic progenitors adopt an exocrine or endocrine fate in zebrafish (Dong et al., 2008). In fish carrying a hypomorphic Ptf1a allele, cells normally fated to become exocrine cells co-express the endocrine cell marker, Isl1. These data suggest that high levels of Ptf1a are necessary to fully commit pancreatic progenitor cells towards the exocrine lineage while concomitantly suppressing the endocrine lineage.
Conversely, the prospero-related transcription factor, Prox1, is expressed at higher levels in endocrine progenitors with lower levels of expression detected in differentiating exocrine cells (Burke and Oliver, 2002). Loss of Prox1 results in a decrease in secondary transition endocrine cells, with a concomitant increase in differentiated acinar cells (Wang et al., 2005). Since Prox1 is required for normal branching morphogenesis, it is possible that Prox1-deficient embryos lack sufficient numbers of stalk cells required for endocrine progenitor specification, and thus more of the epithelium differentiates as acinar cells.
Regulation of exocrine versus endocrine differentiation: Extrinsic mesodermally-derived factors
Short-range mesenchymal signals promote exocrine differentiation
Mesenchyme envelops the early pancreatic bud separating it from the dorsal aorta and other surrounding tissues. Accordingly, the mesenchyme has been shown to be important for steps of pancreas differentiation subsequent to initial bud formation. Classic in vitro co-culture experiments demonstrated that mesenchyme plays an essential, permissive role in branching morphogenesis of the pancreas. Subsequent experiments culturing pancreas buds on basement membrane matrices or collagen gels suggested that mesenchymal components may also play an instructive role in regulating cell fate decisions (Gittes et al., 1996; Golosow and Grobstein, 1962; Miralles et al., 1999b). Transplantation or culture of mid-gestation rodent pancreatic buds with their associated mesenchyme results in normal acinar and endocrine development. In comparison, buds from which the mesenchyme has been removed differentiate only into endocrine cells (Gittes et al., 1996; Miralles et al., 1999b). These experiments led to the concept that endocrine tissue is the “default” lineage, and that mesenchymally-derived factors are required to promote acinar differentiation while simultaneously inhibiting endocrine differentiation.
The character of the mesenchyme seems to change with developmental age, altering the differentiation capacity of the epithelium in recombination experiments (Rose et al., 1999). Mesenchyme from e10.5 pancreatic buds is less efficient than e12.5 pancreatic mesenchyme at promoting acinar differentiation from e11.5 epithelium, even after a week in culture. In addition, the proexocrine effects of pancreatic mesenchyme may depend upon juxtacrine signaling; no acinar differentiation was seen when the mesenchyme was separated from the epithelium by a filter (Li et al., 2004). Studies from the Scharfmann lab (Zertal-Zidani et al., 2007) revealed that sulfated proteoglycan components of the pancreatic mesenchyme are required for its inhibitory effects on endocrine differentiation. Chemical inhibition of proteoglycan sulfation resulted in increased Ngn3 expression as well as expression of more mature endocrine lineage markers. These results support a role for short-range or juxtacrine signaling between the mesenchyme and epithelium.
Several additional factors have been identified which can reproduce the acinar-promoting mesenchymal effect on cultured pancreatic bud epithelium, including laminin, follistatin, and FGF1, 7, and 10 (Li et al., 2004; Miralles et al., 1999a; Miralles et al., 1998). Inactivation and over-expression analyses of FGF10 function in the pancreas indicate that FGF10 may be the relevant factor in vivo for branching and regulating the amount of pancreatic progenitors; it does not, however, seem to influence lineage fate decisions in vivo (Bhushan et al., 2001; Hart et al., 2003; Norgaard et al., 2003). Differences in these results may reflect separate roles for FGF10 at different times throughout development. While in vivo inactivation and over-expression assess functions of FGF10 signaling in early developmental events, the ex vivo bud culture system tests the ability of FGF signaling to influence the differentiation of the epithelium after some degree of branching morphogenesis has occurred.
Retinoic acid promotes endocrine differentiation
RA plays multiple, stage-specific roles in pancreas development. In addition to its early roles in endodermal patterning and pancreas bud specification described above, RA influences the exocrine versus endocrine lineage decision. Treatment of Xenopus embryos with exogenous RA leads to a dose dependant reduction in exocrine cells and an increase in endocrine cells in the dorsal pancreas, suggesting that RA may be important for regulating the proportions of exocrine and endocrine cells during later stages of development (Figure 5) (Chen et al., 2004). The fact that RA signaling is required for initial pancreas bud outgrowth precludes an in vivo analysis of exocrine and endocrine tissue in RA-deficient mice; however, exogenously added RA stimulated the differentiation of endocrine and duct cells at the expense of acinar cells in murine bud cultures (Kadison et al., 2001; Kobayashi et al., 2002; Tulachan et al., 2003). Similarly, Ostrom et al. (2008) found that buds cultured in the presence of RA have a 2.5-fold increase in the number of insulin-positive cells compared to control buds, although the amount of exocrine tissue present was not examined in these studies. An increase in the number of Ngn3-expressing cells preceded the increase in hormone expression, suggesting that RA alters the fate of multipotent pancreatic progenitors.
Vascular endothelium and endocrine differentiation
Adult islets are highly vascularized, and proper vascular organization and function are required for maintaining glucose homeostasis in mice. As mentioned above, the close relationship between vascular endothelial cells and the pancreatic epithelium originates early in pancreas morphogenesis and continues as development progresses. The angiogenic factor, VEGFA, is expressed by developing endocrine cells as early as e13.5, and is required for formation of the normal vasculature within the islet (Brissova et al., 2006). Reciprocally, endothelial cells play an inductive role in endocrine development (Lammert et al., 2001). Endocrine cells form adjacent to developing capillaries and endothelial cells have been shown to produce basement membrane components for islets (Brissova et al., 2006; Nikolova et al., 2006). The increase in islet area observed with increased expression of VEGF is accompanied by a decrease in acinar tissue, suggesting that endothelial cells produce factors that promote the endocrine lineage; however, the mechanism by which this occurs is not yet known. VEGFR2/flk-1, the main VEGF receptor, is not expressed in endocrine cells, therefore the increase in endocrine mass in Pdx1-VEGFA transgenic mice is likely due to a secondary consequence of the increase in inductive endothelial cells, rather than a direct effect of VEGF on multipotent pancreatic progenitors. In support of this conclusion, loss of VEGFA from embryonic β cells has no effect on islet mass or architecture. However, islet endocrine cell-derived VEGFA is required to generate normal islet vasculature, and therefore is required for islet function in vivo (Brissova et al., 2006).
Regulation of endocrine lineage allocation: Factors intrinsic to the epithelium
Temporal regulation of endocrine differentiation
All endocrine cells are derived from Ngn3-expressing progenitors, but the mechanisms by which these endocrine progenitors are specified to the individual hormone-positive lineages are not well characterized. Genetic analyses have revealed that α and β cells develop from independent lineages, while β and PP cells may arise from a common endocrine precursor (Herrera, 2000). Hormone-expressing cells differentiate in the pancreas in a cell type-specific pattern with α cells appearing earliest, followed by β, δ, and PP cells. The sequential pattern of hormone expression suggests that endocrine differentiation is temporally regulated and that competence of the Ngn3-positive progenitors changes as development proceeds. To test this hypothesis, the Grapin-Botton laboratory (Johansson et al., 2007) used an “add back” strategy, expressing a tamoxifen-inducible Ngn3-ER fusion protein throughout the pancreatic epithelium using the Pdx1 promoter at different developmental time points in the Ngn3 null background (Figure 8). These studies identified specific windows of competence for the differentiation of the individual endocrine cell types. The pancreatic epithelium becomes competent to form glucagon-positive cells at the earliest stages of pancreas development, consistent with previous findings that over-expression of Ngn3 using the Pdx1 promoter induces the differentiation of mostly α cells (Apelqvist et al., 1999; Grapin-Botton et al., 2001; Johansson et al., 2007). Competency to form insulin-producing cells appears to occur between e10.5 and 14.5, with the greatest number of β cells formed when Ngn3 is induced at e12.5. Later in development, the epithelium loses the ability to differentiate into glucagon-positive cells and acquires the competence to form PP and δ cells. Interestingly, these shifts in developmental competence appear to be mediated by intrinsic changes within epithelium, rather than in the character of the surrounding mesenchyme (Johansson et al.).
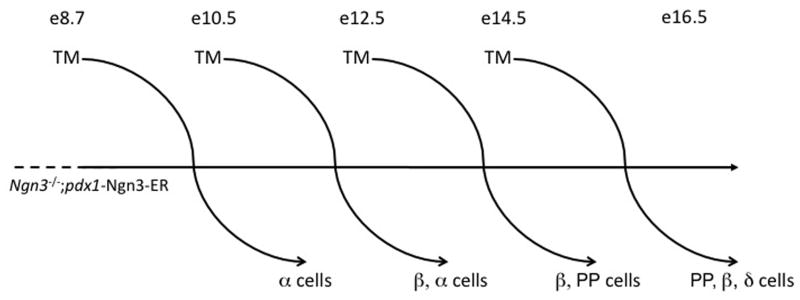
Figure 8. Temporally-dependent specification of Ngn3-expressing endocrine progenitors.
Using the Pdx1 promoter to drive expression of a tamoxifen (TM)-inducible Ngn3-estrogen receptor (ER) fusion protein, Ngn3 expression was restored to Ngn3 null mutant embryos at different developmental time points with the addition of TM. Examination of endocrine differentiation two days after a single TM injection revealed that endocrine progenitors preferentially differentiate as a particular hormone-producing cell depending on when during development they were generated. α cells are formed from the earliest Ngn3-producing cells, while β, PP, and δ cells subsequently form, in that order.
Specification and differentiation of α and β cells
The formation of β and α cells is thought to be regulated by the opposing actions of the transcription factors, Pax4 and Arx. Pax4 is a paired homeodomain factor, and its mRNA can be detected within cells in the pancreas beginning at e9.5 (Sosa-Pineda et al., 1997). Its expression is restricted to first and second wave insulin cells, but becomes down-regulated soon after birth and is not detected in adult islets (Smith et al., 1999). Pancreata from Pax4 null embryos express Pdx1 and Hb9 and contain first wave insulin-producing cells, but lack mature β cells, indicating that Pax4 is required during the secondary transition for β cell differentiation (Sosa-Pineda et al., 1997; Wang et al., 2004). Additionally, Pax4 null mutant mice have a decrease in δ cells and an increase in the numbers of glucagon- and ghrelin-expressing cells (Prado et al., 2004; Sosa-Pineda et al., 1997). Since no changes in endocrine cell proliferation or apoptosis were observed in Pax4 mutants, it is likely that a common progenitor of both β and δ cells is directed toward alternate endocrine lineages in the absence of Pax4 (Wang et al., 2004). Many of the glucagon-expressing cells in Pax4 null pancreata co-expressed ghrelin (Heller et al., 2005; Prado et al., 2004). Small numbers of glucagon/ghrelin co-expressing cells are normally found in wild type embryos; however, the number of these cells is significantly increased in Pax4 mutants.
Pax4 is thought mainly to function as a transcriptional repressor. It directly binds and represses both the glucagon and ghrelin promoters, thus providing a mechanism for the increased expression of these two hormones observed in Pax4 mutants (Collombat et al., 2003; Ritz-Laser et al., 2002; Wang et al., 2008). In addition, Pax4 inhibits the expression of Arx, a transcription factor that promotes α cell differentiation. Although Pax4 is dispensable for the formation of α and PP cell types, lineage tracing experiments have shown that Pax4-expressing cells give rise to α, β, and ε cells, suggesting that Pax4 is expressed in pluripotent endocrine progenitors (Wang et al., 2008).
In contrast to the role of Pax4, Arx expression within the endocrine progenitor population promotes the development of α cells. Arx acts downstream of Ngn3 and Arx null embryos show a complete loss of α cells with a concomitant increase in β cells and δ cells (Collombat et al., 2003). The ability of Arx to direct cells towards the α cell lineage is further evidenced by studies in which Arx was over-expressed under control of the Pdx1 promoter (Collombat et al., 2007). These mice have an increased number of α and PP cells at the expense of the β and δ lineages; the total number of endocrine cells was unchanged. Additionally, the authors used an inducible system to drive Arx expression in mature β cells and found that insulin-positive cells were converted to the α and PP lineages (Collombat et al., 2007). Arx mutants have an increase in Pax4 expression, and Arx expression is up-regulated in Pax4 mutants, indicating that Arx and Pax mutually inhibit each other’s expression (Collombat et al., 2005; Collombat et al., 2003). Indeed, binding sites for Arx were found in the Pax4 promoter and vice versa (Collombat et al., 2005). However, Arx is not sufficient to repress Pax4 within the α cell lineage, since Pax4 levels are not reduced in transgenic mice over-expresing Arx. These results underscore the importance of the balance of Pax4 and Arx in establishing the β and α cell lineages. Inactivation of both Pax4 and Arx leads to a loss of both β and α cell lineages along with a dramatic increase in somatostatin-producing cells, suggesting that glucagon-producing cells may normally inhibit the development of δ cells.
Pax6, another paired homeodomain transcription factor, is expressed at e9.5 and e10.5 in a subset of cells in the pancreatic epithelium, and is later expressed in cells committed to the endocrine lineage (Jensen et al., 2000a; St-Onge et al., 1997; Turque et al., 1994). Although Pax6 is expressed in both insulin-positive and glucagon-positive cells, it appears to be essential only for the formation of α cells. Pax6 mutant mice have a dramatic loss of glucagon-expressing cells and a lesser reduction in the other islet cell types (Sander et al., 1997; St-Onge et al., 1997). Thus, Pax6 may be important not only for allocation to the α cell lineage, but for expansion of the endocrine population as a whole (Heller et al., 2005; Heller et al., 2004). In Pax6 mutants there is an increase in ghrelin-expressing cells without an increase in proliferation of this cell population, suggesting that a reduction in Pax6 levels may direct endocrine progenitors towards the ε cell fate (Heller et al., 2005).
The NK homeodomain factor, Nkx2.2, regulates β cell differentiation in a pathway parallel to Pax4 (Wang et al., 2004). Nkx2.2 is expressed throughout the whole pancreatic bud at early developmental stages, as well as in Ngn3-expressing endocrine progenitors (Schwitzgebel et al., 2000; Sussel et al., 1998). Although Nkx2.2 expression is detected in all hormone-positive cells except δ cells during late gestation, it is only essential for β cell differentiation. Nkx2.2 null embryos completely lack β cells but have reduced numbers of α and PP cells (Sussel et al., 1998). The function of Nkx2.2 appears to be conserved; morpholino knockdown in zebrafish leads to a similar phenotype as seen in mice (Pauls et al., 2007). The fact that the expression of early endocrine markers, such as Isl1 and synaptophysin, were normal in Nkx2.2 mutant mice, suggests that cells lacking Nkx2.2 are specified correctly as endocrine cells. Similar to embryos lacking Pax4, Nkx2.2 mutants have an increase in ghrelin-expressing cells (Prado et al., 2004). Using an Nkx2.2 repressor-fusion construct, the Sussel laboratory (Doyle et al., 2007) demonstrated that the repressor function of Nkx2.2 can partially rescue the defects in β and α cell development, as well as the increase in ghrelin expression found in null mice. Thus, Nkx2.2 acts as a repressor during endocrine specification, however, its activating function appears to be required for later steps of β cell maturation. The rescued β cells in these transgenic mice did not express MafA (a marker of mature β cells and a transactivator of the insulin promoter) (Matsuoka et al., 2004; Olbrot et al., 2002; Zhang et al., 2005). Consistent with these results, Nkx2.2 was found in other studies to bind and activate the MafA promoter (Doyle et al., 2007; Raum et al., 2006).
MafB is a member of the large Maf family of basic leucine zipper transcription factors that also includes MafA. MafB is expressed in some Ngn3-positive cells, as well as in first and second wave insulin- and glucagon-expressing cells. In the adult pancreas, MafB is only detected in α cells (Artner et al., 2006). MafB is not required for endocrine specification; embryos lacking MafB still express markers of the endocrine lineage, such as Isl1 and Pax6. However, first wave endocrine cells are absent in MafB mutant pancreata and MafB is required for the differentiation of second wave α and β cells (Artner et al.). MafB mutant embryos have a 50% decrease in insulin- and glucagon-expressing cells compared to wild type littermates (Artner et al.). Consistent with the reduction of α and β cells, MafB was shown to bind to the insulin, glucagon, and MafA promoters (Artner et al., 2007).
Pdx1 expression becomes elevated in β cells at late gestation during islet formation. The Pdx1 promoter contains a conserved cis-regulatory region, termed “Areas I-II-III”, which mediates this islet-specific expression. Deletion of this region from the endogenous Pdx1 locus generates a hypomorphic allele (Fujitani et al., 2006). When placed in trans to a Pdx1 null allele, the hypomorphic allele is incapable of promoting normal pancreas development; defects similar to those observed in Pdx1 null mice are observed. Mice heterozygous for the hypomorphic allele displayed altered islet architecture, an increase in α and PP cells, and impaired glucose tolerance. The reduction of β cells found in these mice suggests that, similar to Ngn3, a threshold of Pdx1 is required to activate the full β cell differentiation program; reduced Pdx1 levels may favor the differentiation of α and PP cells.
Regulation of endocrine lineage allocation: Extrinsic mesodermally-derived factors
TGF-β signaling and endocrine differentiation
Connective tissue growth factor (CTGF) is a member of the CCN family of secreted proteins. These proteins modulate multiple growth factor signaling pathways (TGF-β, BMP, Wnt, VEGF) and function in proliferation and differentiation of many tissues, including bone and blood vessels (Abreu et al., 2002; Inoki et al., 2002; Mercurio et al., 2004). In the pancreas, CTGF expression is detected in β cells, ducts, and endothelial cells as early as e12.5 (Crawford et al., 2009). While CTGF is down-regulated in β cells by postnatal day 3, its expression is maintained in ducts and blood vessels into adulthood. Global inactivation of CTGF in mice leads to an increase in α cells and a decrease in β cells beginning at the secondary transition, with no change in total endocrine tissue (Figure 9) (Crawford et al., 2009). No alterations in β or α cell proliferation or apoptosis were observed in CTGF mutants until e18.5, at which point there was a marked decrease in β cell proliferation. These data suggest that, in addition to regulating embryonic β cell proliferation, CTGF is required for lineage allocation specifically during the secondary transition. CTGF may act downstream of HNF6 to promote proper allocation to the α and β cell lineages, as it was found to be down-regulated in a transgenic model of endocrine HNF6 over-expression (Wilding Crawford et al., 2008). Since CTGF is expressed in multiple cell types within the pancreas, it is currently unclear whether CTGF is acting in an autocrine and/or paracrine manner during pancreas development. There is no known receptor for CTGF; however, CTGF does bind to integrins extracellularly, and blocking integrin signaling in human fetal pancreas anlagen transplanted under the kidney capsule results in a similar alteration in endocrine cell ratio as is seen in the CTGF mutants (Cirulli et al., 2000). In addition, CTGF may act by modulating the TGF-β signaling pathway, which has also been implicated in endocrine differentiation.
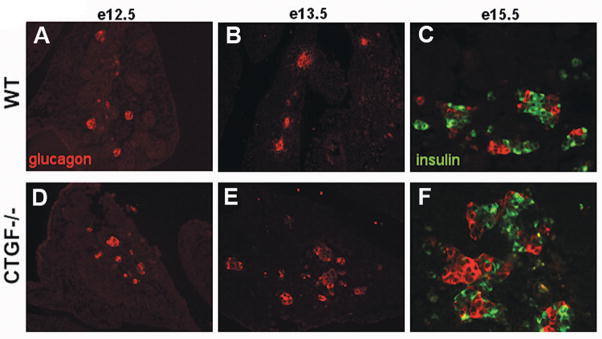
Figure 9. Altered endocrine lineage allocation in CTGF null pancreata.
When compared with controls (A), CTGF null mutant embryos showed no difference in glucagon area (red) at e12.5 (D). However, an increase in glucagon-positive cells was seen in CTGF mutant embryos beginning at e13.5 (compare E with B) and continuing throughout embryonic development. By e15.5. there was clearly a decrease in insulin-positive cells (green) in the mutant pancreata, with a concomitant increase in glucagon-expressing cells (compare C and F). (Used with permission from Crawford et. al. Mol. Endo. 23: 324, 2009. Copyright 2009, The Endocrine Society).
The TGF-β superfamily of signaling molecules includes TGF-β, Activin, Nodal, and BMP ligands. These ligands bind to type I and type II serine-threonine kinase receptors, resulting in phosphorylation of downstream receptor Smad (R-Smad) proteins. TGF-β, Activins, and Nodal activate Smads 2 and 3, while ligands in the BMP family have been shown to activate Smads 1, 5, and 8 (Massague, 2000). R-Smad phosphorylation leads to interaction with Smad 4, and subsequent nuclear localization where the complex interacts with co-factors to activate and repress transcription. There is conflicting in vitro evidence that TGF-β signaling regulates the ratio of exocrine and endocrine cell types during pancreas development (Crisera et al., 2000; Sanvito et al., 1994). The number of ligands and receptors make isolating specific effects of a given ligand difficult. Thus, despite several studies inactivating specific ligands or making use of dominant negative receptors, a clear requirement for this family of growth factors in endocrine lineage allocation has yet to be revealed in vivo (Bottinger et al., 1997; Goulley et al., 2007; Tulachan et al., 2007).
Pancreas-wide over-expression of Smad7, which inhibits signaling mediated by Smads 2/3 and 1/5/8, causes a dramatic loss of β cells and an increase in the number of α cells without any changes in proliferation or apoptosis, thereby supporting a role for the TGF-β signaling pathway in lineage allocation (Smart et al., 2006). It must be remembered, however, that Smad7 inhibits signaling by multiple ligands. Inactivation of the TGF-β ligand, growth differentiation factor 11 (GDF11), leads to an increase in undifferentiated Ngn3-expressing progenitors and a decrease in the α to β cell ratio, similar to what was seen in Smad7 transgenic mice (Harmon et al., 2004). GDF11 acts through Smad2/3 and, consistent with these data, the pancreatic defects in Smad2 heterozygous mice phenocopy those found in GDF11 mutants (Harmon et al., 2004; Oh et al., 2002). In contrast, an alternative study of GDF11 function in pancreas development found an increase in Ngn3-positive cells, but no alterations in endocrine cell mass or ratios (Dichmann et al., 2006). The reason for the conflicting results in these studies is not clear, but it may be due to differences in genetic background of the mouse strains used. Although GDF11 interacts with the Activin A type IIB (ActRIIB) receptor, inactivation of this receptor (in combination with heterozygous deletion of the Activin A type IIA receptor) indicates that Activin receptors function to regulate pancreas and islet size, but not endocrine lineage specification, since ActR mutant mice show pancreatic and islet hypoplasia, with no change in endocrine cell ratio (Andersson et al., 2006; Kim et al., 2000; Oh et al., 2002).
Communication between endocrine cell types
Glucagon-positive cells are found in the pancreas as early as e10.5, but no metabolic function is known for these cells at this time. Therefore, some have postulated that these early hormone-expressing cells provide a signal that regulates formation of other cell types within the developing pancreas. Global inactivation of the glucagon receptor or deletion of pro-hormone convertase-2 (PC2), the enzyme responsible for converting pro-glucagon to glucagon, leads to a loss of first waveβ cells and an increase in the percentage of glucagon- and somatostatin-expressing cells in embryonic islets (Vincent et al., 2003; Vuguin et al., 2006). The increase in α cells in these models was due to an increase in proliferation of the pro-glucagon-expressing cell population, suggesting that glucagon does not affect allocation to the α cell lineage; glucagon is required for formation of early insulin-expressing cells. Loss of glucagon also affects later steps ofβ cell differentiation, since both strains of mice have a decrease in the expression of mature β cell markers; these immature β cells show increased postnatal proliferation (Vincent et al., 2003; Vuguin et al., 2006). Pancreatic bud explants dissected at e11 and treated with pre-pro-glucagon antisense oligonucleotides or anti-glucagon neutralizing antibodies also display a decrease in insulin expression, supporting the idea that glucagon has a non-cell autonomous role in early β cell differentiation (Prasadan et al., 2002).
β cells may also provide signals regulating the numbers of other cell types, in particular the α cell population. Loss of Pdx1 from embryonic insulin producing cells, for example, results in a dramatic decrease in the number of β cells with a concomitant increase in α cell number, due to increased α cell proliferation (Gannon et al., 2008). These data suggest that embryonicβ cells normally provide a signal to the α cell population, inhibiting its expansion.
Perspectives and future directions
The factors regulating cell fate specification and differentiation of pancreatic lineages continue to be elaborated. A thorough understanding of normal pancreas development and endocrine differentiation will facilitate the generation of functional islets for use in the treatment of diabetes. The ability to induce β cells or whole islets from embryonic or pancreatic stem cells in vivo or in vitro or embryonic stems in vitro would provide an alternative source of transplantable tissue to cadaver islets.
There are several potential sources for generating the large number of insulin-producing cells needed to make “islet” transplantation a therapeutic reality. These include: (1) proliferation and expansion of existing β cells in vivo or in vitro; (2) proliferation and expansion of cadaver-derived islets; (3) induction of β cell differentiation from endogenous progenitors (embryonic ductal cells) or from adult ductal epithelium; (4) induction of β cell differentiation from ES or induced pluripotent stem cells (iPS cells); and (5) transdifferentiation of closely related cell types, such as acinar, liver, and intestinal enteroendocrine cells.
Multiple studies on isolated ductal epithelium suggest that adult ducts retain the capacity for the production of new β cells (neogenesis) (Bonner-Weir, 2000; Ogata et al., 2004; Ramiya et al., 2000; Trivedi et al., 2001). In addition, recent in vivo evidence shows that, following pancreatic injury, mature ducts can reactivate Ngn3 expression and initiate islet neogenesis (Ackermann Misfeldt et al., 2008; Xu et al., 2008). Thus, it seems that at least a facultative stem cell exists in adult pancreatic ducts that, when properly activated, is capable of giving rise to new, functional β cells. In addition, acinar cells have recently been shown to trans-differentiate in vivo directly to insulin-producing cells following the expression of known developmental regulators of the β cell fate (Pdx1, Ngn3, and MafA) (Zhou et al., 2008), while expression of Ngn3 in hepatic stem cells in vivo leads to reprogramming to the pancreatic endocrine lineage (Yechoor et al., 2009).
Directed differentiation of human ES or iPS cells into islet endocrine cells has also made some recent progress. Using a step-wise approach of adding exogenous factors to the culture medium, ES or iPS cells can be directed down the same developmental pathway that endogenous endocrine progenitors follow: definitive endoderm → foregut endoderm → pancreas progenitor → endocrine progenitor →β cell. In general, the percentage of hormone-positive cells in these cultures is extremely low, there are many other cell types are present in the cultures, and the insulin-producing cells generated are immature in their gene expression patterns and in their ability to regulate insulin secretion in response to glucose (D’Amour et al., 2006; Tateishi et al., 2008). Transplantation of immature cells under the kidney capsule allows for completion of the β cell differentiation pathway in vivo from these stem cell-like progenitors (Kroon et al., 2008). Thus, the critical factors required to promote the formation of mature functional β cells in culture have yet to be identified. Ultimately, one would like to be able to use small, cell-permeable molecules to generate fully functional mature β cells from stem cells or alternative cellular sources using entirely exogenously added factors (growth factors, hormones, small molecules, drugs), rather than viral transduction of critical pancreatic transcription factors. We may actually not be too far away from this reality; high throughput screening of chemical libraries has already yielded compounds that can direct human ES cells toward a pancreatic fate (Chen et al., 2009).
Acknowledgments
We thank the members of the Gannon laboratory for critical reading of the manuscript. We would like to thank Dr. Elizabeth Ables for help with preparation of figures. We especially thank the many different laboratories that have contributed greatly to the study of pancreas development and cell fate regulation. We apologize for not being able to include all of the contributions of the investigators in this field. M.A.G. was supported by the Vanderbilt Molecular Endocrinology Training Program (5 T 32 DK07563). M.G. is supported by NIH Grants, DK065131 and DK071052, and grant #1-2007-548 from the JDRFI.
References
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann AM, Gannon M. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol. 2007;38:193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]
- Ackermann Misfeldt A, Costa RH, Gannon M. β-Cell Proliferation, but not Neogenesis, Following 60% Partial Pancreatectomy is Impaired in the Absence of FoxM1. Diabetes. 2008;57:3069–3077. doi: 10.2337/db08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afelik S, Chen Y, Pieler T. Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 2006;20:1441–6. doi: 10.1101/gad.378706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–16. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–8. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson O, Reissmann E, Ibanez CF. Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep. 2006;7:831–7. doi: 10.1038/sj.embor.7400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7:801–4. doi: 10.1016/s0960-9822(06)00340-x. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–81. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A. 2007;104:3853–8. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artner I, Le Lay J, Hang Y, Elghazi L, Schisler JC, Henderson E, Sosa-Pineda B, Stein R. MafB: An Activator of the Glucagon Gene Expressed in Developing Islet {alpha}- and {beta}-Cells. Diabetes. 2006;55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- Asayesh A, Sharpe J, Watson RP, Hecksher-Sorensen J, Hastie ND, Hill RE, Ahlgren U. Spleen versus pancreas: strict control of organ interrelationship revealed by analyses of Bapx1−/− mice. Genes Dev. 2006;20:2208–13. doi: 10.1101/gad.381906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol. 2006;26:117–30. doi: 10.1128/MCB.26.1.117-130.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–17. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- Blumberg B. An essential role for retinoid signaling in anteroposterior neural specification and neuronal differentiation. Semin Cell Dev Biol. 1997;8:417–28. doi: 10.1006/scdb.1997.0165. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S. Islet growth and development in the adult. J Mol Endocrinol. 2000;24:297–302. doi: 10.1677/jme.0.0240297. [DOI] [PubMed] [Google Scholar]
- Bottinger EP, Jakubczak JL, Roberts IS, Mumy M, Hemmati P, Bagnall K, Merlino G, Wakefield LM. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. Embo J. 1997;16:2621–33. doi: 10.1093/emboj/16.10.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, et al. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–85. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- Burke Z, Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev. 2002;118:147–55. doi: 10.1016/s0925-4773(02)00240-x. [DOI] [PubMed] [Google Scholar]
- Burlison JS, Long Q, Fujitani Y, Wright CV, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti SK, James JC, Mirmira RG. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem. 2002;277:13286–93. doi: 10.1074/jbc.M111857200. [DOI] [PubMed] [Google Scholar]
- Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–65. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–60. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Chung WS, Shin CH, Stainier DY. Bmp2 signaling regulates the hepatic versus pancreatic fate decision. Dev Cell. 2008;15:738–48. doi: 10.1016/j.devcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, Beattie GM, Klier G, Ellisman M, Ricordi C, Quaranta V, Frasier F, Ishii JK, Hayek A, Salomon DR. Expression and function of alpha(v)beta(3) and alpha(v)beta(5) integrins in the developing pancreas: roles in the adhesion and migration of putative endocrine progenitor cells. J Cell Biol. 2000;150:1445–60. doi: 10.1083/jcb.150.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell M, Stevenson BJ, Strubin M, Hagenbuchle O, Wellauer PK. Identification of a cell-specific DNA-binding activity that interacts with a transcriptional activator of genes expressed in the acinar pancreas. Mol Cell Biol. 1989;9:2464–76. doi: 10.1128/mcb.9.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132:2969–80. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007;117:961–70. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–21. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev. 2006;27:356–70. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- Crawford LA, Guney MA, Oh YA, Deyoung RA, Valenzuela DM, Murphy AJ, Yancopoulos GD, Lyons KM, Brigstock DR, Economides A, et al. Connective Tissue Growth Factor (CTGF) Inactivation Leads to Defects in Islet Cell Lineage Allocation and {beta}-Cell Proliferation during Embryogenesis. Mol Endocrinol. 2009;23:324–36. doi: 10.1210/me.2008-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisera CA, Maldonado TS, Kadison AS, Li M, Alkasab SL, Longaker MT, Gittes GK. Transforming growth factor-beta 1 in the developing mouse pancreas: a potential regulator of exocrine differentiation. Differentiation. 2000;65:255–9. doi: 10.1046/j.1432-0436.2000.6550255.x. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–81. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Dichmann DS, Yassin H, Serup P. Analysis of pancreatic endocrine development in GDF11-deficient mice. Dev Dyn. 2006;235:3016–25. doi: 10.1002/dvdy.20953. [DOI] [PubMed] [Google Scholar]
- Dong PD, Provost E, Leach SD, Stainier DY. Graded levels of Ptf1a differentially regulate endocrine and exocrine fates in the developing pancreas. Genes Dev. 2008;22:1445–50. doi: 10.1101/gad.1663208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MJ, Loomis ZL, Sussel L. Nkx2.2-repressor activity is sufficient to specify alpha-cells and a small number of beta-cells in the pancreatic islet. Development. 2007;134:515–23. doi: 10.1242/dev.02763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Bonner-Weir S, Montminy M, Wright C. Regulatory factor linked to late-onset diabetes? Nature. 1998;392:560. doi: 10.1038/33311. [DOI] [PubMed] [Google Scholar]
- Fisher A, Caudy M. The function of hairy-related bHLH repressor proteins in cell fate decisions. Bioessays. 1998;20:298–306. doi: 10.1002/(SICI)1521-1878(199804)20:4<298::AID-BIES6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev. 2006;20:253–66. doi: 10.1101/gad.1360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Kawaguchi M, Terao M, Doi R, Wright CV, et al. Reduction of Ptf1a gene dosage causes pancreatic hypoplasia and diabetes in mice. Diabetes. 2008;57:2421–31. doi: 10.2337/db07-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Koizumi M, Boyer DF, Fujimoto K, Doi R, et al. Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116:1484–93. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon M, Ray MK, Van Zee K, Rausa F, Costa RH, Wright CV. Persistent expression of HNF6 in islet endocrine cells causes disrupted islet architecture and loss of beta cell function. Development. 2000;127:2883–95. doi: 10.1242/dev.127.13.2883. [DOI] [PubMed] [Google Scholar]
- Gannon M, Tweedie Ables E, Crawford L, Lowe D, Offield MF, Magnuson MA, Wright CV. pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol. 2008;314:406–417. doi: 10.1016/j.ydbio.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debase HT. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development. 1996;122:439–47. doi: 10.1242/dev.122.2.439. [DOI] [PubMed] [Google Scholar]
- Golosow N, Grobstein C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev Biol. 1962;4:242–55. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5:207–219. doi: 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–11. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapin-Botton A, Majithia AR, Melton DA. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 2001;15:444–54. doi: 10.1101/gad.846001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–57. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–8. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- Harmon EB, Apelqvist AA, Smart NG, Gu X, Osborne DH, Kim SK. GDF11 modulates NGN3+ islet progenitor cell number and promotes {beta}-cell differentiation in pancreas development. Development. 2004;131:6163–6174. doi: 10.1242/dev.01535. [DOI] [PubMed] [Google Scholar]
- Harrison KA, Thaler J, Pfaff SL, Gu H, Kehrl JH. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nat Genet. 1999;23:71–5. doi: 10.1038/12674. [DOI] [PubMed] [Google Scholar]
- Hart A, Papadopoulou S, Edlund H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev Dyn. 2003;228:185–93. doi: 10.1002/dvdy.10368. [DOI] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–13. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller RS, Jenny M, Collombat P, Mansouri A, Tomasetto C, Madsen OD, Mellitzer G, Gradwohl G, Serup P. Genetic determinants of pancreatic epsilon-cell development. Dev Biol. 2005;286:217–224. doi: 10.1016/j.ydbio.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Heller RS, Stoffers DA, Liu A, Schedl A, Crenshaw EB, 3rd, Madsen OD, Serup P. The role of Brn4/Pou3f4 and Pax6 in forming the pancreatic glucagon cell identity. Dev Biol. 2004;268:123–34. doi: 10.1016/j.ydbio.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–22. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Herrera PL, Huarte J, Zufferey R, Nichols A, Mermillod B, Philippe J, Muniesa P, Sanvito F, Orci L, Vassalli JD. Ablation of islet endocrine cells by targeted expression of hormone-promoter-driven toxigenes. Proc Natl Acad Sci U S A. 1994;91:12999–3003. doi: 10.1073/pnas.91.26.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL, Grindley J, Bellusci S, Dunn NR, Emoto H, Itoh N. Branching morphogenesis of the lung: new models for a classical problem. Cold Spring Harb Symp Quant Biol. 1997;62:249–56. [PubMed] [Google Scholar]
- Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. Faseb J. 2002;16:219–21. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- Jacquemin P, Durviaux SM, Jensen J, Godfraind C, Gradwohl G, Guillemot F, Madsen OD, Carmeliet P, Dewerchin M, Collen D, et al. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol Cell Biol. 2000;20:4445–54. doi: 10.1128/mcb.20.12.4445-4454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemin P, Lemaigre FP, Rousseau GG. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev Biol. 2003;258:105–16. doi: 10.1016/s0012-1606(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev Biol. 2006;290:189–99. doi: 10.1016/j.ydbio.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Jarikji ZH, Vanamala S, Beck CW, Wrigh CV, Leach SD, Horb ME. Differential ability of Ptf1a and Ptf1a-VP16 to convert stomach, duodenum and liver to pancreas. Dev Biol. 2007;304:786–99. doi: 10.1016/j.ydbio.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000a;49:163–76. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000b;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12:457–65. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–9. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Kadison A, Kim J, Maldonado T, Crisera C, Prasadan K, Manna P, Preuett B, Hembree M, Longaker M, Gittes G. Retinoid signaling directs secondary lineage selection in pancreatic organogenesis. J Pediatr Surg. 2001;36:1150–6. doi: 10.1053/jpsu.2001.25734. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–34. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Li E, Oh SP, Schrewe H, Harmon EB, Lee JS, Melton DA. Activin receptor patterning of foregut organogenesis. Genes Dev. 2000;14:1866–71. [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–52. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Spilde TL, Bhatia AM, Buckingham RB, Hembree MJ, Prasadan K, Preuett BL, Imamura M, Gittes GK. Retinoid signaling controls mouse pancreatic exocrine lineage selection through epithelial-mesenchymal interactions. Gastroenterology. 2002;123:1331–40. doi: 10.1053/gast.2002.35949. [DOI] [PubMed] [Google Scholar]
- Krapp A, Knofler M, Frutiger S, Hughes GJ, Hagenbuchle O, Wellauer PK. The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. Embo J. 1996;15:4317–29. [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Knofler M, Ledermann B, Burki K, Berney C, Zoerkler N, Hagenbuchle O, Wellauer PK. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998;12:3752–63. doi: 10.1101/gad.12.23.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–7. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Landry C, Clotman F, Hioki T, Oda H, Picard JJ, Lemaigre FP, Rousseau GG. HNF-6 is expressed in endoderm derivatives and nervous system of the mouse embryo and participates to the cross-regulatory network of liver- enriched transcription factors. Dev Biol. 1997;192:247–57. doi: 10.1006/dbio.1997.8757. [DOI] [PubMed] [Google Scholar]
- Lee YC, Damholt AB, Billestrup N, Kisbye T, Galante P, Michelsen B, Kofod H, Nielsen JH. Developmental expression of proprotein convertase 1/3 in the rat. Mol Cell Endocrinol. 1999;155:27–35. doi: 10.1016/s0303-7207(99)00119-7. [DOI] [PubMed] [Google Scholar]
- Li H, Arber S, Jessell TM, Edlund H. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat Genet. 1999;23:67–70. doi: 10.1038/12669. [DOI] [PubMed] [Google Scholar]
- Li Z, Manna P, Kobayashi H, Spilde T, Bhatia A, Preuett B, Prasadan K, Hembree M, Gittes GK. Multifaceted pancreatic mesenchymal control of epithelial lineage selection. Dev Biol. 2004;269:252–63. doi: 10.1016/j.ydbio.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Martin CC, Oeser JK, O’Brien RM. Differential regulation of islet-specific glucose-6-phosphatase catalytic subunit-related protein gene transcription by Pax-6 and Pdx-1. J Biol Chem. 2004;279:34277–89. doi: 10.1074/jbc.M404830200. [DOI] [PubMed] [Google Scholar]
- Martin M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dolle P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Masui T, Long Q, Beres TM, Magnuson MA, MacDonald RJ. Early pancreatic development requires the vertebrate Suppressor of Hairless (RBPJ) in the PTF1 bHLH complex. Genes Dev. 2007;21:2629–43. doi: 10.1101/gad.1575207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka TA, Artner I, Henderson E, Means A, Sander M, Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci U S A. 2004;101:2930–3. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–17. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–47. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- Miralles F, Czernichow P, Ozaki K, Itoh N, Scharfmann R. Signaling through fibroblast growth factor receptor 2b plays a key role in the development of the exocrine pancreas. Proc Natl Acad Sci U S A. 1999a;96:6267–72. doi: 10.1073/pnas.96.11.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development. 1998;125:1017–24. doi: 10.1242/dev.125.6.1017. [DOI] [PubMed] [Google Scholar]
- Miralles F, Serup P, Cluzeaud F, Vandewalle A, Czernichow P, Scharfmann R. Characterization of beta cells developed in vitro from rat embryonic pancreatic epithelium. Dev Dyn. 1999b;214:116–26. doi: 10.1002/(SICI)1097-0177(199902)214:2<116::AID-AJA2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;28:28. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol. 2003;264:323–38. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–95. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Ogata T, Park KY, Seno M, Kojima I. Reversal of streptozotocin-induced hyperglycemia by transplantation of pseudoislets consisting of beta cells derived from ductal cells. Endocr J. 2004;51:381–6. doi: 10.1507/endocrj.51.381. [DOI] [PubMed] [Google Scholar]
- Oh SP, Yeo CY, Lee Y, Schrewe H, Whitman M, Li E. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 2002;16:2749–54. doi: 10.1101/gad.1021802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. Embo J. 1993;12:4251–9. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrot M, Rud J, Moss LG, Sharma A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci U S A. 2002;99:6737–42. doi: 10.1073/pnas.102168499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom M, Loffler KA, Edfalk S, Selander L, Dahl U, Ricordi C, Jeon J, Correa-Medina M, Diez J, Edlund H. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS ONE. 2008;3:e2841. doi: 10.1371/journal.pone.0002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang K, Mukonoweshuro C, Wong GG. Beta cells arise from glucose transporter type 2 (Glut2)-expressing epithelial cells of the developing rat pancreas. Proc Natl Acad Sci U S A. 1994;91:9559–63. doi: 10.1073/pnas.91.20.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls S, Zecchin E, Tiso N, Bortolussi M, Argenton F. Function and regulation of zebrafish nkx2.2a during development of pancreatic islet and ducts. Dev Biol. 2007;304:875–90. doi: 10.1016/j.ydbio.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Pierreux CE, Poll AV, Kemp CR, Clotman F, Maestro MA, Cordi S, Ferrer J, Leyns L, Rousseau GG, Lemaigre FP. The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology. 2006;130:532–41. doi: 10.1053/j.gastro.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing {beta} cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A. 2004;101:2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasadan K, Daume E, Preuett B, Spilde T, Bhatia A, Kobayashi H, Hembree M, Manna P, Gittes GK. Glucagon is required for early insulin-positive differentiation in the developing mouse pancreas. Diabetes. 2002;51:3229–36. doi: 10.2337/diabetes.51.11.3229. [DOI] [PubMed] [Google Scholar]
- Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–82. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- Raum JC, Gerrish K, Artner I, Henderson E, Guo M, Sussel L, Schisler JC, Newgard CB, Stein R. FoxA2, Nkx2.2, and PDX-1 regulate islet beta-cell-specific mafA expression through conserved sequences located between base pairs -8118 and -7750 upstream from the transcription start site. Mol Cell Biol. 2006;26:5735–43. doi: 10.1128/MCB.00249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausa F, Samadani U, Ye H, Lim L, Fletcher CF, Jenkins NA, Copeland NG, Costa RH. The cut-homeodomain transcriptional activator HNF-6 is coexpressed with its target gene HNF-3 beta in the developing murine liver and pancreas. Dev Biol. 1997;192:228–46. doi: 10.1006/dbio.1997.8744. [DOI] [PubMed] [Google Scholar]
- Ritz-Laser B, Estreicher A, Gauthier BR, Mamin A, Edlund H, Philippe J. The pancreatic beta-cell-specific transcription factor Pax-4 inhibits glucagon gene expression through Pax-6. Diabetologia. 2002;45:97–107. doi: 10.1007/s125-002-8249-9. [DOI] [PubMed] [Google Scholar]
- Rose MI, Crisera CA, Colen KL, Connelly PR, Longaker MT, Gittes GK. Epithelio-mesenchymal interactions in the developing mouse pancreas: morphogenesis of the adult architecture. J Pediatr Surg. 1999;34:774–9. doi: 10.1016/s0022-3468(99)90372-x. discussion 780. [DOI] [PubMed] [Google Scholar]
- Rose SD, Swift GH, Peyton MJ, Hammer RE, MacDonald RJ. The role of PTF1-P48 in pancreatic acinar gene expression. J Biol Chem. 2001;276:44018–26. doi: 10.1074/jbc.M106264200. [DOI] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–73. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- Sanvito F, Herrera P, Huarte J, Nichols A, Montesano R, Orci L, Vassalli J. TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development. 1994;120:3451–62. doi: 10.1242/dev.120.12.3451. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–54. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–42. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Shin D, Shin CH, Tucker J, Ober EA, Rentzsch F, Poss KD, Hammerschmidt M, Mullins MC, Stainier DY. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. 2007;134:2041–50. doi: 10.1242/dev.000281. [DOI] [PubMed] [Google Scholar]
- Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961–71. doi: 10.1053/gast.2000.18142. [DOI] [PubMed] [Google Scholar]
- Smart NG, Apelqvist AA, Gu X, Harmon EB, Topper JN, MacDonald RJ, Kim SK. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol. 2006;4:e39. doi: 10.1371/journal.pbio.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Ee HC, Conners JR, German MS. Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Mol Cell Biol. 1999;19:8272–80. doi: 10.1128/mcb.19.12.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Watada H, Scheel DW, Mrejen C, German MS. Autoregulation and maturity onset diabetes of the young transcription factors control the human PAX4 promoter. J Biol Chem. 2000;275:36910–9. doi: 10.1074/jbc.M005202200. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- Spagnoli FM, Brivanlou AH. The Gata5 target, TGIF2, defines the pancreatic region by modulating BMP signals within the endoderm. Development. 2008;135:451–61. doi: 10.1242/dev.008458. [DOI] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–9. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Stafford D, Hornbruch A, Mueller PR, Prince VE. A conserved role for retinoid signaling in vertebrate pancreas development. Dev Genes Evol. 2004;214:432–41. doi: 10.1007/s00427-004-0420-6. [DOI] [PubMed] [Google Scholar]
- Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–20. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–56. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–9. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Stanojevic V, Habener JF. Insulin promoter factor-1 gene mutation linked to early-onset type 2 diabetes mellitus directs expression of a dominant negative isoprotein. J Clin Invest. 1998;102:232–41. doi: 10.1172/JCI2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–7. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–21. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Tateishi K, He J, Taranova O, Liang G, D’Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–7. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- Trivedi N, Hollister-Lock J, Lopez-Avalos MD, O’Neil JJ, Keegan M, Bonner-Weir S, Weir GC. Increase in beta-cell mass in transplanted porcine neonatal pancreatic cell clusters is due to proliferation of beta-cells and differentiation of duct cells. Endocrinology. 2001;142:2115–22. doi: 10.1210/endo.142.5.8162. [DOI] [PubMed] [Google Scholar]
- Tulachan SS, Doi R, Kawaguchi Y, Tsuji S, Nakajima S, Masui T, Koizumi M, Toyoda E, Mori T, Ito D, et al. All-trans retinoic acid induces differentiation of ducts and endocrine cells by mesenchymal/epithelial interactions in embryonic pancreas. Diabetes. 2003;52:76–84. doi: 10.2337/diabetes.52.1.76. [DOI] [PubMed] [Google Scholar]
- Tulachan SS, Tei E, Hembree M, Crisera C, Prasadan K, Koizumi M, Shah S, Guo P, Bottinger E, Gittes GK. TGF-beta isoform signaling regulates secondary transition and mesenchymal-induced endocrine development in the embryonic mouse pancreas. Dev Biol. 2007;305:508–21. doi: 10.1016/j.ydbio.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turque N, Plaza S, Radvanyi F, Carriere C, Saule S. Pax-QNR/Pax-6, a paired box-and homeobox-containing gene expressed in neurons, is also expressed in pancreatic endocrine cells. Mol Endocrinol. 1994;8:929–38. doi: 10.1210/mend.8.7.7984154. [DOI] [PubMed] [Google Scholar]
- Tweedie E, Artner I, Crawford L, Poffenberger G, Thorens B, Stein R, Powers AC, Gannon M. Maintenance of hepatic nuclear factor 6 in postnatal islets impairs terminal differentiation and function of beta-cells. Diabetes. 2006;55:3264–70. doi: 10.2337/db06-0090. [DOI] [PubMed] [Google Scholar]
- Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn. 2008;237:3270–9. doi: 10.1002/dvdy.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M, Guz Y, Rozenberg M, Webb G, Furuta M, Steiner D, Teitelman G. Abrogation of protein convertase 2 activity results in delayed islet cell differentiation and maturation, increased alpha-cell proliferation, and islet neogenesis. Endocrinology. 2003;144:4061–9. doi: 10.1210/en.2003-0088. [DOI] [PubMed] [Google Scholar]
- Vuguin PM, Kedees MH, Cui L, Guz Y, Gelling RW, Nejathaim M, Charron MJ, Teitelman G. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology. 2006;147:3995–4006. doi: 10.1210/en.2005-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Elghazi L, Parker SE, Kizilocak H, Asano M, Sussel L, Sosa-Pineda B. The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic beta-cell differentiation. Dev Biol. 2004;266:178–89. doi: 10.1016/j.ydbio.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Wang J, Kilic G, Aydin M, Burke Z, Oliver G, Sosa-Pineda B. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev Biol. 2005;286:182–194. doi: 10.1016/j.ydbio.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Wang Q, Elghazi L, Martin S, Martins I, Srinivasan RS, Geng X, Sleeman M, Collombat P, Houghton J, Sosa-Pineda B. Ghrelin is a novel target of Pax4 in endocrine progenitors of the pancreas and duodenum. Dev Dyn. 2008;237:51–61. doi: 10.1002/dvdy.21379. [DOI] [PubMed] [Google Scholar]
- Wessels NK, Cohn JH. Early pancreas organogenesis: Morphogenesis, tissue interactions, and mass effects. Devopmental Biology. 1967;15:237–270. doi: 10.1016/0012-1606(67)90042-5. [DOI] [PubMed] [Google Scholar]
- Wiebe PO, Kormish JD, Roper VT, Fujitani Y, Alston NI, Zaret KS, Wright CV, Stein RW, Gannon M. Ptf1a binds to and activates area III, a highly conserved region of the Pdx1 promoter that mediates early pancreas-wide Pdx1 expression. Mol Cell Biol. 2007;27:4093–104. doi: 10.1128/MCB.01978-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding Crawford L, Tweedie Ables E, Oh YA, Boone B, Levy S, Gannon M. Gene expression profiling of a mouse model of pancreatic islet dysmorphogenesis. PLoS ONE. 2008;3:e1611. doi: 10.1371/journal.pone.0001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Kalamaras JA, German MS. Expression pattern of IAPP and prohormone convertase 1/3 reveals a distinctive set of endocrine cells in the embryonic pancreas. Mech Dev. 2002;115:171–6. doi: 10.1016/s0925-4773(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Wu KL, Gannon M, Peshavaria M, Offield MF, Henderson E, Ray M, Marks A, Gamer LW, Wright CV, Stein R. Hepatocyte nuclear factor 3beta is involved in pancreatic beta-cell- specific transcription of the pdx-1 gene. Mol Cell Biol. 1997;17:6002–13. doi: 10.1128/mcb.17.10.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van De Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H, Chan L. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell. 2009;16:358–73. doi: 10.1016/j.devcel.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;21:21. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Rhee JT, Bader D. Expression of the atrial-specific myosin heavy chain AMHC1 and the establishment of anteroposterior polarity in the developing chicken heart. Development. 1994;120:871–83. doi: 10.1242/dev.120.4.871. [DOI] [PubMed] [Google Scholar]
- Zertal-Zidani S, Bounacer A, Scharfmann R. Regulation of pancreatic endocrine cell differentiation by sulphated proteoglycans. Diabetologia. 2007;50:585–95. doi: 10.1007/s00125-006-0571-2. [DOI] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–76. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yatskievych TA, Baker RK, Antin PB. Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev Biol. 2004;268:312–26. doi: 10.1016/j.ydbio.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]