Abstract
Cross-talk of BMP and Wnt signaling pathways has been implicated in many aspects of biological events during embryogenesis and in adulthood. A secreted protein Wise and its orthologs (Sostdc1, USAG-1, and Ectodin) have been shown to modulate Wnt signaling and also inhibit BMP signals. Modulation of Wnt signaling activity by Wise is brought about by an interaction with the Wnt co-receptor LRP6, whereas BMP inhibition is by binding to BMP ligands. Here we have investigated the mode of action of Wise on Wnt and BMP signals. It was found that Wise binds LRP6 through one of three loops formed by the cystine knot. The Wise deletion construct lacking the LRP6-interacting loop domain nevertheless binds BMP4 and inhibits BMP signals. Moreover, BMP4 does not interfere with Wise-LRP6 binding, suggesting separate domains for the physical interaction. Functional assays also show that the ability of Wise to block Wnt1 activity through LRP6 is not impeded by BMP4. In contrast, the ability of Wise to inhibit BMP4 is prevented by additional LRP6, implying a preference of Wise in binding LRP6 over BMP4. In addition to the interaction of Wise with BMP4 and LRP6, the molecular characteristics of Wise, such as glycosylation and association with heparan sulfate proteoglycans on the cell surface, are suggested. This study helps to understand the multiple functions of Wise at the molecular level and suggests a possible role for Wise in balancing Wnt and BMP signals.
Wise is a secreted protein that was isolated from a functional screen of a chick cDNA library of embryonic tissues. It was identified as being able to alter the antero-posterior character of neuralized Xenopus animal caps by promoting activity of the Wnt pathway (1). Independently, the homologous protein was isolated from a functional screen to detect genes that are preferentially expressed in the rat endometrium, which had been maximally sensitized to implantation, and named USAG-1 (uterine sensitization-associated gene-1) (2). The protein was identified a third time from the GenBankTM sequence data base of mouse as a putative secreted protein, shown to be a BMP antagonist, and named Ectodin (3). The gene has also been called Sostdc1 (Sclerostin domain-containing 1) or Sostl (Sclerostin-like) due to the homology with Sclerostin-encoding gene Sost (4, 5). USAG-1/Wise/Ectodin/Sostdc1 is expressed in various tissues, such as the surface ectoderm of the posterior axis (1, 6), branchial arches (3, 6), the dermal papilla in hair follicles (7), vibrissae (3), mammalian tooth cusps (3, 8), rat endometrium (2), developing testis (9–11), interdigital tissues (12), and embryonic and adult kidneys (13, 14).
Wise appears to have a dual role in modulating the Wnt pathway. Injection of Wnt8 RNA into a ventral vegetal blastomere of Xenopus embryos at the four-cell stage induces a full secondary axis to form, and this is blocked by the addition of Wise RNA as well as other Wnt inhibitors (1). Activation of the Wnt/β-catenin pathway in hair follicles triggers regeneration of hair growth, and expression of Wise appears to have a defined role to inhibit this (15). In this context, Wise expression is repressed by the nuclear receptor co-repressor, Hairless, which results in activation of the Wnt pathway; thus, a model of periodic regeneration of hair follicles has been proposed (15, 16). In addition, Wise and its homologue USAG-1 have been shown to block Wnt1, Wnt3a, and Wnt10b activities in reporter assays (14, 15, 17). Wise was found to bind to the Wnt co-receptor, LRP6, sharing the binding domain with Wnt ligands. Importantly, Wise was found to compete with Wnt8 for binding to LRP6, therefore suggesting a mechanism for inhibition of the Wnt pathway whereby Wise blocks the binding of ligand and receptor (1). Wise may also be retained in the endoplasmic reticulum and inhibit the trafficking of LRP6 to the cell surface (18). Wise also binds LRP4 (19), a member of the LRP family functioning inhibitory to Wnt signals (20). It is noteworthy that Wise was isolated from a screen designed to detect the activation of the Wnt/β-catenin pathway, not inhibition. The exact mechanism of how Wise exerts such a context-dependent modulation on the Wnt pathway is yet to be clarified.
Osteoblast differentiation of MC3T3-E1 cells, as measured by alkaline phosphatase activity, can be induced by a wide range of BMP molecules. In this assay, Ectodin, the mouse ortholog of Wise, was shown to inhibit differentiation induced by BMP2, -4, -6, or -7 in a dose-dependent manner (3). Similarly, Ectodin (also known as USAG-1) was also found to inhibit the bone differentiation induced by BMP2, -4, or -7 in C2C12 cells (14). Ectodin also inhibits BMP2- or BMP7-induced Msx2 expression in dissected mouse tooth buds in organ culture (3). In tooth buds, Ectodin expression is detected in the dental ectoderm and mesenchymal cells excluding from the enamel knot (3). Ectodin/USAG-1-deficient mice created by targeted-disruption show altered tooth morphology and extra teeth, indicating that Ectodin and BMP tightly control tooth development and patterning in mammals (8, 21–23). Furthermore, in mouse adult kidneys, the ability of BMP7 to repair established renal injury is blocked by USAG-1 (13). All of these findings indicate that USAG-1/Wise/Ectodin has a clear antagonistic effect on BMP signaling, where it binds BMP2, -4, -6, and -7 (3, 14) and presumably prevents BMP binding to its receptors.
Analysis of the sequence of Wise reveals that it has the C1XnC2XGXC3XnC4XnC5XC6 motif of a six-membered cystine knot, where C1 forms a disulfide bond with C4, C2 with C5, and C3 with C6 (for a review of the cystine knot, see Refs. 24–27). This arrangement results in a globular protein with three loops, “finger 1,” “heel,” and “finger 2,” held together with an eight-membered ring of C2XGXC3C6XC5C2 (Fig. 1). BMP antagonists represent a subfamily in the cystine knot superfamily, and this is further subdivided into three subfamilies based on the size of the cystine knot. These are the CAN family (eight-membered ring), Twisted Gastrulation (nine-membered ring), and Chordin and Noggin (10-membered ring) (27). There is generally little sequence homology between family members in the heel, finger 1, and finger 2 regions, yet Wise does show a moderate homology with Sclerostin (28). Sclerostin is involved in regulating bone mass (4, 5) and also appears to antagonize both Wnt (29–32) and BMP (28, 33, 34) signals. This paper aims to analyze the dual role of Wise on Wnt and BMP pathways by probing the structural features of the protein and reconciling them to physiological properties. It also aims to reveal the molecular nature of the protein in view of possible glycosylation, secretion, and association with extracellular matrix.
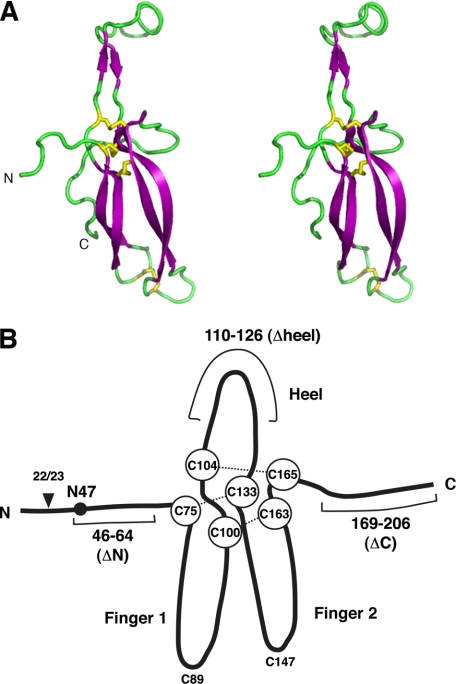
FIGURE 1.
Structure of chick Wise protein. A, stereo ribbon representation of the chick Wise three-dimensional structural model (residues 68–186). Purple, β-strands; green, loop regions. Yellow, disulfide bonds in the cystine knot plus a further disulfide (cysteines 89 and 147) linking two fingers of the structure. N- and C-terminal ends are indicated. B, schematic drawing of the full-length chick Wise structure. Arrowhead, the predicted signal sequence cleavage site for secretion; black dot, asparagine at position 47 (N47), the glycosylated site revealed in this study. Six cysteine residues forming the “cystine knot” are shown in circles, and disulfide bonds for the knot formation are shown by dotted lines. Three loops (Finger 1, Heel, and Finger 2) are indicated. The scheme also shows the deleted parts of Wise constructs ΔN, Δheel, and ΔC.
EXPERIMENTAL PROCEDURES
Prediction of Wise Structure
Secondary structure prediction was performed on the chick amino acid sequence and closely related sequences from zebrafish, Xenopus, mouse, and human using the program PHD (35). The secondary structure prediction was used to guide a manual alignment of the five sequences. The result is shown in supplemental Fig. 1. This alignment was processed by the three-dimensional fold recognition server 3DPSSM (36). The top fold was that of the cystine knot in human chorionic gonadotropin (37) with 90% certainty. A three-dimensional structural model was built of the chick Wise sequence from residue 68 to 186 based on the human chorionic gonadotropin structure (Protein Data Bank code 1HCN) extracted from the Protein Data Bank (38). The molecular modeling program QUAFNTA (Accelrys Inc.) was employed on a Silicon Graphics O2 computer running the IRIX operating system. An alignment from the chick, human, rat, and mouse amino acid sequences was made using the program ClustalX (39) with default multiple alignment parameters. Secondary structure regions (β-strands) obtained from the structural model of chick Wise is shown in supplemental Fig. 2.
Propagation and Transfection of Cells
HEK293 cells (ATCC) were propagated in Dulbecco's modified Eagle's medium with 10% fetal calf serum at 37 °C and 5% CO2. Cells were transfected with DNA constructs using Polyfect (Qiagen) according to the manufacturer's guidelines.
DNA Constructs and Protein Reagents
FLAG-tagged chick Wise was subcloned into pCS2+, and deletions and point mutations were introduced into this clone using PCR with specific primers. All clones containing mutated constructs were sequenced throughout the Wise gene insert before being used in protein production and assays. The BMP4 construct is a fusion of a pro-region from the BMP2 and the mature region of BMP4 tagged with Myc (40) subcloned into pCS2+. Recombinant BMP4 protein (R&D Systems) and anti-BMP4 antibody (R&D Systems) were also used. A clone containing a fusion of the extracellular domain of LRP6 and Fc domain of IgG (LRP6-IgG) was a gift from Xi He (41). LRP6 extracellular domain (LRP6ECD, amino acids 1–1370) was subcloned into pCS2 after attaching a Myc tag and a stop codon.
Production of Wise Conditioned Medium
HEK293 cells were grown to 80% confluence in 80-cm2 flasks and transfected with expression plasmids. Aspirated medium was replaced with Opti-MEM (Invitrogen) after 24 h to provide a serum-free environment for protein collection. Condition medium was then collected after 3 and 5 days or after each further 24-h period for the next 4 days. They were clarified to remove debris and then applied to a 10-kDa cut-off centrifugal filter device (Millipore). The supernatant was concentrated 5 or 50 times, respectively, and stored in aliquots at −80 °C.
Immunoprecipitation and Western Blotting
HEK293 cells in T25 flasks were transfected with expression plasmids of FLAG-tagged Wise and Myc-tagged LRP6 extracellular domain (ECD)3 or BMP4. After 24 h, the medium was replaced by OptiMEM (Invitrogen). After a further 24 h, medium was collected, added to anti-FLAG-agarose affinity gel beads (Sigma), and incubated at 4 °C for 6 h. In some experiments, Wise and LRP6ECD-IgG were individually transfected, and the conditioned media were mixed with bovine serum albumin or recombinant BMP4, followed by mixing with protein A beads (GE Healthcare). Beads were then washed five times with wash buffer (150 ml of NaCl, 50 mm Tris-HCl, pH 7.5, 0.1% Triton X-100). Protein was eluted from the beads in modified Laemmli buffer (2% SDS, 10% glycerol, 100 mm dithiothreitol, 60 mm Tris-HCl, pH 6.8, 10% 2-mercaptoethanol, 0.1% bromphenol blue) at 100 °C for 5 min before loading onto a denaturing SDS-polyacrylamide gel. For immunoprecipitation of LRP6ECD, a 4–15% gradient gel (Bio-Rad) was used to detect both Wise and LRP6ECD. For detecting Wise and BMP4, a 15% gel was used. Protein samples run on polyacrylamide gels were transferred onto polyvinylidene difluoride membrane. Membranes were then blocked in 10% milk protein in phosphate-buffered saline plus 0.1% Tween 20 before exposing to antibodies: anti-FLAG M2-horseradish peroxidase (Sigma), anti-Myc (Upstate Biotechnology, Inc.), or anti-Fc (Sigma) and anti-mouse horseradish peroxidase (Amersham Biosciences). Detection was by ECL (Amersham Biosciences; SuperSignal West Pico/Femto, Thermo) on x-ray film.
Protein Analyses
Deglycosylation of Wise protein was carried out by treating concentrated Wise-conditioned medium with peptide:N-glycosidase F (PNGase F; New England Biolabs), O-glycosidase (Roche Applied Science), endo-β-N-acetylglucosaminidase H (New England Biolabs), and endo-β-N-acetylglucosaminidase D (Merck) according to the manufacturer's guidelines. Typically, 50 times concentrated protein in conditioned medium was first denatured at 100 °C in 0.5% SDS, 1% 2-mercaptoethanol and then digested with the enzyme in manufacturer's buffer in a total of 50 μl at 37 °C for 60 min.
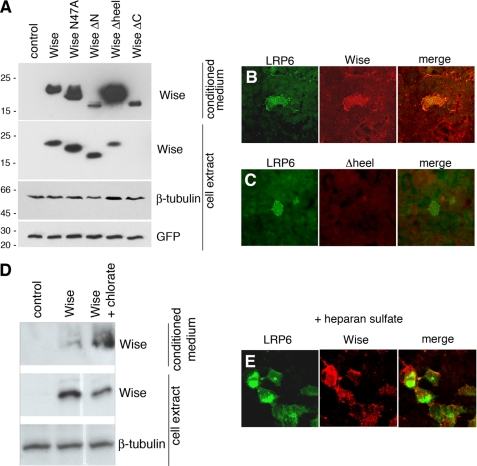
For heparan sulfate treatment, HEK293 cells were transfected with Myc-tagged LRP6, and the medium was replaced on the following day with Wise conditioned medium together with 2 μg/ml heparan sulfate (Sigma). The cells were incubated for 1 h at 4 °C before the immunostaining procedure with anti-Myc and anti-FLAG antibodies. For the sodium chlorate experiment, HEK293 cells were transfected with Wise, split, and incubated with or without 20 nm of sodium chlorate for 2 days, after which the conditioned medium and cell extracts were collected for Western analyses.
Luciferase Reporter Assay
The Wnt pathway activity was detected by transfecting reporter constructs of 0.08 μg of TOPflash (Upstate Biotechnology) (42) and 0.02 μg of Renilla luciferase reporter (pRL-TK; Promega) plasmids in each well of 24-well plates. For the BMP pathway, 0.15 μg of BMP response element (BRE) reporter, which contains Smad binding elements identified in the Id1 promoter (43) or the Xvent2 promoter (44), and 0.1–2 ng of Renilla reporter (pRL-CMV; Promega) were used. HEK293 cells were transfected with expression plasmids (Fig. 5C, BMP4 (0.05 μg), Wise (0.1 μg); Fig. 6C, Wnt1 (0.1 μg), Wise (0.1 μg), BMP4 (0.1 or 0.2 μg); Fig. 6D, BMP4 (0.05 μg), Wise (0.3 μg), LRP6 (0.005 or 0.01 μg)), each well receiving a total of 0.5 μg of DNA, using a control vector where necessary. After 24 h, medium was replaced with a serum-free medium, OptiMEM (Invitrogen), and cells were lysed 48 h post-transfection. The normalized luciferase activity was determined using the Stop and Glo Dual Luciferase System (Promega) on a Turner Luminometer. Bar graphs show the average of triplicate samples in one experiment, and the error bars display S.D. values. The same experiments were repeated at least three times.
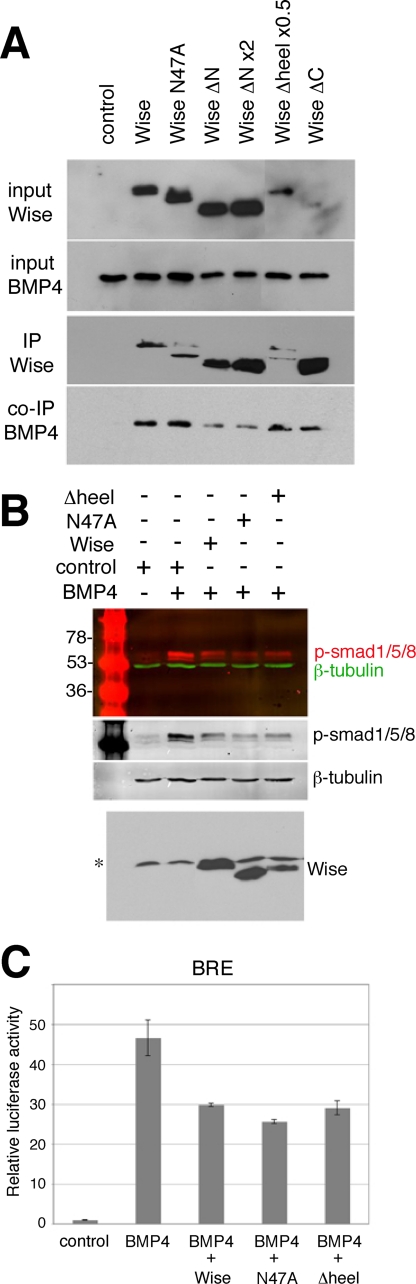
FIGURE 5.
Effect of Wise deletion constructs on BMP4. A, immunoprecipitation (IP) assay. HEK293 cells were transfected with control or FLAG-tagged Wise constructs, as indicated above, together with a construct encoding Myc-tagged BMP4. The amounts of DNA used for Wise(ΔN) and Wise(Δheel) were adjusted in a manner similar to that in Fig. 4A. The conditioned media were collected from each and used for immunoprecipitation with anti-FLAG antibody. Input samples are shown in the top two panels. Immunoprecipitation of Wise and BMP4 is shown in the bottom two panels. There was no significant difference in the precipitation efficiency except that Wise(ΔN) showed less precipitation of BMP4 relative to the abundant amount of Wise(ΔN) inputs. This trend was not consistently seen in other experiments. B, phospho-Smad1/5/8 assay. HEK293 cells were treated with a mixture of recombinant BMP4 protein and Wise conditioned medium (Wise, N47A, or Δheel), as indicated, and the cells were collected to detect phosphorylated Smad (p-Smad)1/5/8 (red) on a Western blot with a fluorescent secondary antibody. β-Tubulin (green) is a loading control. Each channel is also shown in black and white. The bottom panel shows a Western blot of input Wise media. *, a nonspecific band. C, BRE reporter assay. HEK293 cells were transfected with BMP4 and Wise constructs, as indicated, together with BRE and control Renilla reporters. The graph shows the relative luciferase units, normalized to the control sample (no BMP4). Wise, N47A, and Δheel suppress BMP4 activity.
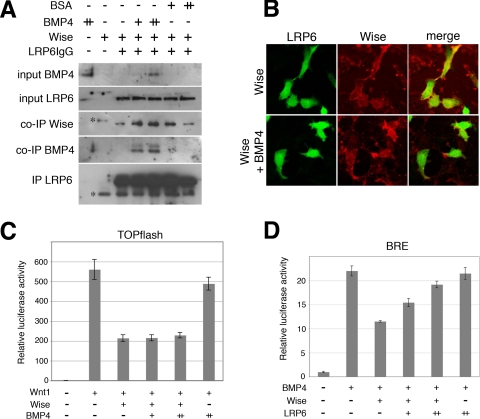
FIGURE 6.
Function of Wise on LRP6 and BMP4. A, immunoprecipitation (IP) assay. Conditioned media of HEK293 cells separately transfected with Wise or LRP6IgG were mixed together with bovine serum albumin or recombinant BMP4, as indicated (+, 100 ng; ++, 200 ng/700 μl) and immunoprecipitated with LRP6IgG, and the precipitated samples were analyzed on Western blots using anti-FLAG (Wise), anti-BMP4, or anti-Fc antibodies. Inputs of BMP4 and LRP6 indicate the presence of these proteins in the mix of relevant conditioned media and proteins. Input Wise is not shown because it was undetectable after diluting with other conditioned media. Concentrated Wise medium was checked on a separate blot prior to use, and an equal volume of the medium from the same batch was used for each of the immunoprecipitation samples. Immunoprecipitation of Wise (upper panel) is not compromised by the presence of BMP4. *, nonspecific bands. B, immunostaining of HEK293 cells stably expressing Myc-tagged LRP6 (green), treated with conditioned medium of FLAG-tagged Wise (red), without or with recombinant BMP4 (100 ng/ml). Additional BMP4 does not interfere with the binding of Wise to LRP6. C, TOPflash reporter assay. HEK293 cells were transfected with Wnt1 (0.1 μg), Wise (0.1 μg), and/or BMP4 (+, 0.1 μg; ++, 0.2 μg) constructs, as indicated, together with TOPflash and control Renilla reporters. Activation of TOPflash reporter by Wnt1 is suppressed by Wise. The addition of BMP4 does not affect the function of Wise in Wnt1 inhibition. D, BRE reporter assay. HEK293 cells were transfected with BMP4 (0.05 μg), Wise (0.3 μg), and/or LRP6 (+, 0.005 μg; ++, 0.01 μg) constructs, as indicated, together with BRE and control Renilla reporters. Suppression of BMP4 activity by Wise is largely prevented by additional LRP6. BSA, bovine serum albumin.
Smad Phosphorylation Assay
To detect phosphorylation of Smad1/5/8 proteins, HEK293 cells were plated in 35-mm dishes 24 h prior to experimentation. Recombinant BMP4 (5 ng/ml) was premixed with 5 times concentrated Wise conditioned medium or similarly concentrated control conditioned medium and incubated for 2 h at 4 °C before applying to the cells. The cells were incubated at 37 °C for 1 h and then collected on ice with 200 μl of modified Laemmli buffer by scraping. The samples were boiled for 5 min and sonicated before loading onto polyacrylamide gels. Anti-phospho-Smad1/5/8 antibody (Cell Signaling) was used on Western blots together with anti-β-tubulin antibody, which serves as a loading control. The signals were detected by the fluorescent Western system for quantification (LI-COR).
Immunostaining
Cells transfected with Myc-tagged LRP6 were treated with conditioned medium of FLAG-tagged Wise- or Wise(Δheel)-expressing cells overnight. Cells were then fixed with 3% paraformaldehyde in phosphate-buffered saline, and immunostaining was carried out with anti-FLAG and anti-Myc antibodies.
RESULTS
Wise Is Likely to Form a Cystine Knot
The predicted three-dimensional structural model of the core part of the Wise protein revealed that Wise is very likely to form a cystine knot (Fig. 1A). In addition, the two other cysteines at positions 89 and 147 are located very close to the tip of two “fingers” and are likely to anchor the fingers by a disulfide bond, analogous to other cystine knot proteins (27). The two fingers curve in the same direction in parallel (toward the back of the plane in Fig. 1A). The domain between the C terminus and the 165th cysteine residue turns around and comes close to the first finger.
Wise Is Glycosylated
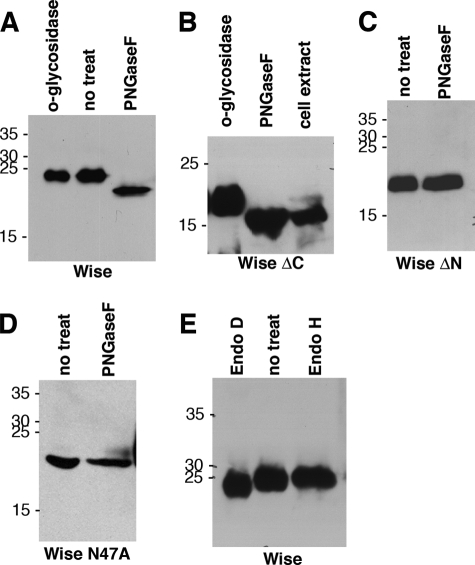
Inspection of the amino acid sequence of chick Wise revealed two putative N-glycosylation sites, at positions 47 and 173. To investigate glycosylation of Wise, concentrated Wise conditioned medium was treated either with PNGase F, which cleaves most N-glycosylations between the innermost GlcNAc and the asparagine residue, or with O-glycosidase, which cleaves most O-glycosylations. Only PNGase F was found to produce a band shift on a Western blot of treated proteins (Fig. 2A). A similar result was found with a version of Wise with a C terminus deletion from position 169 onward, Wise(ΔC) (Fig. 2B). In contrast, a Wise construct with a deletion in the N terminus of amino acids 46–64, Wise(ΔN), was insensitive to PNGase F (Fig. 2C), all of which suggested that the asparagine residue at the position 173 is not involved in glycosylation. To confirm that the glycosylation of Wise is at amino acid 47, the asparagine at this position was mutated to alanine. Expression of this protein, Wise(N47A), was found in the conditioned medium of transfected cells and was insensitive to PNGase F (Fig. 2D). These results indicate that the asparagine at position 47, not 173, is the N-glycosylation site. Full-length Wise protein was also found to be sensitive to endo-β-N-acetylglucosaminidase D but insensitive to endo-β-N-acetylglucosaminidase H (Endo H; Fig. 2E), showing that Wise protein has a complex oligosaccharide processed by Golgi Mannosidase II in the Golgi apparatus. In addition, Fig. 2B illustrates that Wise(ΔC) protein from cell extracts (i.e. nonsecreted protein) appears to be non-glycosylated, since it ran at the same size as PNGase F-treated protein. This was also found to be the case with full-length Wise protein (data not shown).
FIGURE 2.
Glycosylation of Wise. A–D, conditioned media from cells transfected with Wise (A), Wise(ΔC) (B), Wise(ΔN) (C), or Wise(N47A) (D), treated with O-glycosidase or PNGase F. Wise and Wise(ΔC) are sensitive to PNGase F and not to O-glycosidase. In B, the cell extract was also loaded, showing that the majority of Wise protein in the cell extract is a non-glycosylated form, and only a small amount of the glycosylated form exists. Wise(ΔN) and Wise(N47A) are not sensitive to PNGase F. E, conditioned medium of Wise treated with endo-β-N-acetylglucosaminidase D (Endo D) or endo-β-N-acetylglucosaminidase H (Endo H). Wise is sensitive to endo-β-N-acetylglucosaminidase D and resistant to endo-β-N-acetylglucosaminidase H.
Various Wise Constructs Show Different Secretion Efficiencies
Wise protein has six cysteine residues that putatively form a cystine knot, a common structure found in small globular proteins (27) (Fig. 1). When this structure forms, three loops (finger 1, heel, and finger 2) protrude out. In addition to the above described mutants, another deletion construct was made where the tip of the “heel” (the second loop), incorporating amino acids 110–126, was removed and replaced by three glycine/serine repeats (Wise(Δheel)). The secretion efficiency of all mutated constructs was examined together with full-length Wise, by expressing each of the constructs in HEK293 cells and collecting the conditioned media. Although all constructs of proteins were detectable in the conditioned media, expression of Wise(Δheel) protein was significantly abundant in the conditioned medium (Fig. 3A). On the other hand, Wise(ΔN) was secreted only at a low level. It was noted that WiseN47A, a non-glycosylation mutant, was not affected in secretion, suggesting that glycosylation is not critical for Wise secretion. Wise(ΔC) was secreted but hardly detectable in the cell extract.
FIGURE 3.
Secretion and proteoglycan binding of Wise. A, HEK293 cells were transfected with DNA constructs, as indicated above the blots, together with green fluorescent protein DNA, and the cell extracts and conditioned media were analyzed on Western blots. β-Tubulin is a loading control for the cell extract, and green fluorescent protein serves as a control for transfection efficiency. Secretion efficiency of Wise(ΔN) is significantly low, whereas that of Wise(Δheel) is high. Wise(ΔC) in the cell extract is below the detectable level. B and C, HEK293 cells were transfected with Myc-LRP6 (green) and treated with either Wise (B, red) or Wise(Δheel) (C, red) conditioned medium. Wise is bound strongly to the LRP6-transfected cells in addition to non-transfected cells. Wise(Δheel) does not bind to LRP6-transfected cells or to non-transfected cells. D, release of Wise protein into the conditioned medium after treatment with sodium chlorate. Wise-transfected HEK293 cells treated with sodium chlorate show an increased amount of Wise protein in the conditioned medium and a lesser amount in the cell extract. E, HEK293 cells were transfected with Myc-LRP6 (green) and treated with Wise (red) medium along with heparan sulfate. In contrast to B, Wise is bound specifically to LRP6-transfected cells.
Wise Binds Cell Surface Proteoglycans through the Heel Domain
To examine the distribution of secreted Wise proteins, HEK293 cells were transfected with LRP6, treated with Wise conditioned medium, and processed for immunostaining (Fig. 3B). When cells were treated with the conditioned medium of full-length Wise, Wise was detected on the entire cell surface in addition to LRP6-transfected cells (Fig. 3B). Other Wise mutants, such as N47A, ΔN, and ΔC, showed patterns similar to that of full-length Wise (data not shown). Conversely, when Wise(Δheel) medium was applied, the staining was not only absent on LRP6-expressing cells but also unseen in any other cells (Fig. 3C). Possible explanations for these findings are as follows. 1) Wise not only binds LRP6 but also non-specifically to the surface of all cells, whereas Wise(Δheel) does not have the features to do so. For instance, Wise might be attached to cell surface proteoglycans like many other signaling molecules. 2) Wise binds endogenously expressed LRP6 as well as overexpressed forms, and the heel region is responsible for the binding. To test the first possibility, HEK293 cells transfected with Wise were cultured either in the presence or absence of sodium chlorate, a suppressor of sulfate attachment to glycosaminoglycans (45, 46). This treatment affects the function of sulfated glycosaminoglycans by reducing sulfate chains. The conditioned medium was collected from each group of cells and examined for the release of secreted Wise into the conditioned medium. The sodium chlorate treatment caused an increase of Wise released into the medium, whereas the cell extract showed a reduction of Wise (Fig. 3D), suggesting that at least some of the secreted Wise is attached to cell surface proteoglycans on HEK293 cells.
To further test the binding of Wise to cell surface proteoglycans, LRP6-transfected cells were treated with Wise together with exogenous heparan sulfate. If Wise binds proteoglycans, the addition of heparan sulfate would compete with endogenous heparan sulfate chains for binding to Wise (47). Indeed, this resulted in Wise binding specifically to LRP6-overexpressing cells (Fig. 3E), suggesting that secreted Wise tends to bind heparan sulfate proteoglycans on the cell surface. However, the extracellular matrix does not seem to be required for Wise-LRP6 interaction since Wise binding to LRP6-expressing cells is seen after heparinase treatment (supplemental Fig. 3). Collectively, the above results suggest that the abundant secretion of Wise(Δheel) into the conditioned medium is likely to be, at least in part, due to the involvement of the heel region in binding to cell surface proteoglycans.
Wise Binds to LRP6 via the Heel Region
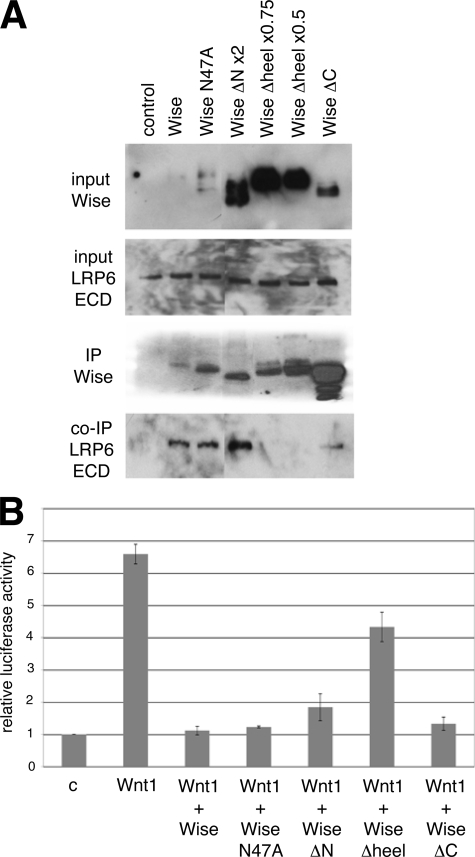
The above Wise constructs were further tested to determine an interaction with LRP6. Conditioned medium of cells transfected with various Wise mutants and LRP6 extracellular domain (LRP6ECD) was used for the immunoprecipitation assay. In order to adjust the amount of secreted Wise in the medium to as comparable a level as possible, the amount of Wise(ΔN) DNA was doubled, whereas that of Wise(Δheel) was reduced to half or to three-quarters. Input Wise was also monitored on Western blots along with LRP6ECD. Despite a large amount of Wise(Δheel) in the input, LRP6ECD was not detectable in the immunoprecipitated complex, whereas a small amount of full-length Wise was sufficient to co-precipitate LRP6ECD (Fig. 4A). Other Wise deletion mutants of either Wise(ΔN) or Wise(ΔC), as well as the non-glycosylated mutant Wise(N47A), were all able to bind to LRP6 (Fig. 4A). This result shows that the heel region is essential for the binding of Wise to LRP6.
FIGURE 4.
Effect of Wise deletion constructs on LRP6. A, immunoprecipitation assay. HEK293 cells were transfected with control or FLAG-tagged Wise constructs, as indicated, together with a construct encoding the LRP6 extracellular domain with a Myc tag (LRP6ECD). The amount of DNA used for Wise(ΔN) and Wise(Δheel) was either increased or decreased, respectively, due to different secretion efficacies seen in Fig. 3A. The conditioned media were collected and used for immunoprecipitation (IP) with anti-FLAG antibody. A small volume of the media from each sample was loaded separately to examine the expression of proteins (top two panels). Immunoprecipitation of Wise and LRP6ECD is shown in the bottom panels. Despite the large amount of Wise(Δheel) in the conditioned medium, it fails to precipitate LRP6ECD. B, TOPflash reporter assay. HEK293 cells were transfected with TOPflash and control Renilla reporters and Wnt1 construct and then treated with conditioned media of various Wise mutants. The conditioned media were first concentrated five times and checked on Western blots. The required samples were then diluted so that each of the media contained a comparable amount of Wise proteins. After the adjustment, the media were applied to the transfected cells and also reexamined on Western blots (shown in supplemental Fig. 4). The graph shows the relative luciferase units, normalized to the control sample (no Wnt1). Wise(Δheel) shows a decreased ability to block Wnt1-dependent reporter activity.
The conditioned media of various Wise constructs were further tested for the functional assay of Wnt1 inhibition. The TOPflash reporter assay (42) was performed by transfecting HEK293 cells with reporters and Wnt1, followed by treatment of cells with concentrated Wise conditioned medium, which was checked on Western blots (Fig. 4B and supplemental Fig. 4). In this system, Wise inhibits Wnt1 function, as reported previously (14, 15, 18) (Fig. 4B). It was consistently observed that Wise constructs that are able to bind LRP6 (WiseN47A, ΔN, and ΔC) showed a similar inhibitory action on Wnt1, whereas Wise(Δheel), which is unable to bind LRP6, showed a lesser extent of inhibition (Fig. 4B). This result confirms that the function of Wise on the Wnt pathway is executed by its binding to LRP6. Although it was noticeable that WiseΔheel showed moderate inhibition on Wnt1 activity, the mechanism by which this occurs is uncertain. One explanation is that WiseΔheel may be able to interact with LRP6 at a very low level that is not detectable by immunoprecipitation, whereas the reporter assay is so sensitive that it may detect subtle effects caused by a weak interaction between WiseΔheel and LRP6. It is also possible that unidentified mechanism(s) may be involved.
The Wise Heel Domain Is Not Required for Its Binding to BMP4
Using a sensor tip and the BIAcore system (3) and in co-immunoprecipitation of transfected COS-7 cell lysates and recombinant BMP proteins (14), Wise homologues were reported to bind to BMP2, -4, -6, and -7. The binding of Wise to BMP4 was further confirmed here by co-immunoprecipitation using concentrated conditioned medium from cells transfected separately to express Wise proteins tagged with FLAG and BMP4 tagged with Myc, using recombinant BMP4 protein and anti-BMP4 antibody (data not shown) or using conditioned medium from cells co-transfected with Wise and BMP4 (Fig. 5A). However, it was noticed that specific Wise-BMP4 binding was not consistently observed, in comparison with Wise-LRP6 binding.4 The immunoprecipitation conditions could easily affect the binding such that any observation of binding is lost following a highly stringent wash condition, whereas more gentle wash conditions often show nonspecific binding interactions in the negative controls. In a series of immunoprecipitation experiments using the above five Wise constructs, we did not find a mutant that significantly and consistently affects the binding with BMP4, suggesting that the Wise-BMP4 interaction is either through a domain outside of our deletions, such as in either of the two fingers, or the precise three-dimensional structure is required for interaction with BMP4. Since two finger loops are suggested to form a disulfide bond by two cystines (27), we did not make further deletion constructs that may destroy the overall structure of Wise. Nevertheless, it was clear that a lack of the heel region, to which LRP6 binds, does not affect the binding of Wise to BMP4 (Fig. 5A).
The function of Wise on BMP4 was further examined in a biochemical assay. One of the immediate early responses of cells to BMP signals is phosphorylation of Smad1/5/8 (48). We observed attenuation of phosphorylation of Smad1/5/8 by Wise as well as by WiseN47A and Wise(Δheel) (Fig. 5B). However, the attenuation was seen only when a small amount of BMP4 protein, such as 5 nm, was used and when phosphorylated Smad1/5/8 was mildly increased by BMP4.5 This can be explained by the model in which Wise inhibits BMP signals by preventing it from binding to the receptor; once all available Wise is bound to BMP ligands, the excess BMP is able to bind the receptor and activate the pathway.
The inhibitory activity of Wise on the BMP pathway was further confirmed with the BMP reporter assay, where BMP signals were detected using the BRE reporter (43). Results similar to those of the phospho-Smad assay were obtained using Wise, Wise(N47A), and Wise(Δheel) constructs, showing a suppression of BMP4 activity (Fig. 5C).
It is of interest to determine whether Wise is able to interact with both LRP6 and BMP4 at the same time or if LRP6 and BMP4 compete for binding to Wise, with the result that Wise is only able to bind BMP4 and LRP6 in a mutually exclusive fashion. To test this, Wise-LRP6 co-immunoprecipitation was reassessed in the presence of BMP4. Wise and LRP6ECD-IgG were separately transfected, and the conditioned media were mixed together with recombinant BMP4. As shown in Fig. 6A, the presence of BMP4 did not affect the binding of Wise to LRP6. Moreover, BMP4 was detectable in the immunoprecipitated complex, suggesting that Wise is able to bind both LRP6 and BMP4 without significant competition. It was confirmed by a separate experiment that BMP4 is not co-immunoprecipitated with LRP6 when Wise is absent (supplemental Fig. 5). Supporting the immunoprecipitation result, binding of Wise and LRP6 on the surface of HEK293 cells was not affected by additional BMP4 in the medium (Fig. 6B).
To further examine whether the Wise-LRP6 binding is affected by BMP4, the TOPflash reporter assay was performed in the presence of additional BMP4. BMP4 did not interfere with the inhibitory function of Wise on Wnt1 (Fig. 6C), supporting the immunoprecipitation finding that additional BMP4 does not interfere with Wise-LRP6 interaction.
A contrary experiment was also performed using the BRE reporter assay to see whether the inhibitory function of Wise on the BMP pathway can be affected by additional LRP6. The ability of Wise to inhibit BMP4 was largely prevented by additional LRP6 (Fig. 6D). This is again consistent with the above idea that Wise-LRP6 interaction is not affected by BMP4. The result also suggests that Wise may preferably bind LRP6 to BMP4.
DISCUSSION
There has been increasing evidence of cross-talk between Wnt and BMP signals at the promoter level, in the cytoplasm or in the extracellular space (49). Some secreted proteins from each of the pathways can form a complex and modulate signal activities. For example, Cerberus, another cystine knot protein, binds to BMP, Wnt, and Nodal ligands via independent sites (50). Wise appears to be similar to this, such that it has presumably separate domains responsible for binding to LRP6 and BMP4. The question is raised as to whether these interactions can occur independently, synergistically, or competitively. Judging by the immunoprecipitation finding that Wise-LRP6 binding is not disrupted by additional BMP4 (Fig. 6A), the binding capability of Wise to each of LRP6 and BMP4 seems to be independent. It is not clear whether or not the binding capability is synergistic by the in vitro analyses. Supporting the in vitro data indicating that Wise-LRP6 binding is not affected by additional BMP4 (Fig. 6A), Wise is able to inhibit the Wnt1 activation of TOPflash in the presence of additional BMP4 (Fig. 6C). However, contrary to this, the ability of Wise to inhibit BMP4 signals is impeded by the addition of LRP6 (Fig. 6D), suggesting that Wise may not exert its function to inhibit BMP4 in a full capability when it is bound to LRP6. It is yet to be clarified for this mechanism, for example, whether Wise binds to LRP6 more strongly than to BMP4.
There remains a possible indirect mechanism that may occur in the context where, for example, BMP signals alter transcription of components of the Wnt pathway, as seen in other cell types (51–54). Thus, the effect of Wise on the Wnt pathway can be influenced secondarily though modulation of the BMP pathway, or vice versa.
With regard to BMP antagonism, although it is certain that USAG-1/Wise/ectodin antagonizes BMP signals, the efficiency does not appear to be comparable with other BMP antagonists, such as Noggin, in Xenopus assays. For example, USAG-1/Wise induces a neural marker NCAM in Xenopus animal cap explants when 1–2 ng of RNA is injected (7, 14), whereas 200 pg of Noggin is sufficient to induce the neural marker (55). The inhibitory effect of Wise on BMP4 in the reporter assay is not as significant as the one exerted by Noggin (data not shown). Another example is that, in the assay of secondary axis induction by injecting RNA of BMP inhibitor into the Xenopus ventral marginal zone, although 50 pg of noggin is sufficient (56), it requires 500 pg of USAG-1 (10 times as much) to induce the secondary axis (14). In fact, while the affinity (Kd) of Noggin and BMP4 is 1.9 × 10−11 m (57), the affinity between Ectodin/Wise and BMP2, -4, -6, and -7 is in the range of 3.93–9.96 × 10−9 m (3). This may explain why the antagonistic activity of USAG-1/Wise/ectodin on BMP signals is marginal in Xenopus contexts. In order to quantify binding affinity of Wise to LRP6 and thus to compare it with that to BMPs, we attempted to purify Wise proteins. However, when expressed in bacteria, Wise was found in the insoluble fraction (further information available upon request). Despite relatively low affinity, the function of USAG-1/Wise/Ectodin on the BMP pathway is evident in other contexts, such as tooth bud formation and renal cell differentiation and repair (3, 8, 13, 14, 21, 22, 58). In these contexts Ectodin/USAG-1/Wise is found to exhibit a significant role in regulating BMP signals. We were also able to detect inhibition of Smad1/5/8 phosphorylation induced by BMP4 (Fig. 5B), consistent with another group's result using BMP7 (17). Hence the effect of USAG-1/Wise/Ectodin is likely to depend on cellular contexts that express different arrays of genes, such as BMPs, LRP6, and other BMP-binding proteins and also extracellular matrix in different degrees.
Physical interactions among cystine knot proteins have been reported in many cases. Sclerostin, which has a structure similar to that of Wise, has been shown to bind to various BMP proteins with an affinity of 5.71–22.4 × 10−8 m (28) or 0.9–3.4 × 10−9 m (34) as well as to Noggin at 2.92 × 10−9 m (59). Connective tissue growth factor, a protein that may contain a cystine knot structure (60, 61) but is not homologous to Wise, has also been shown to have antagonistic activity on BMP and TGFβ signals by directly binding to BMP4 (Kd = 5 × 10−9) and TGFβ1 (Kd = 3 × 10−8), respectively (62). Other examples of physical interaction between cystine knot proteins include Noggin and BMP (57, 63), Cerberus and BMP (50), and Gremlin and BMP (64). Given the wide range of combinations of possible interactions, regulation of signaling must involve tight control of expression and local concentrations of each ligand as well as their relative affinities.
Most cystine knot proteins are known to form either homo- or heterodimers, and dimerization appears to be critical for their function (65). Point mutations in human noggin causing multiple synostosis syndrome or proximal symphalangism are due to the failure of Noggin to be secreted or to form homodimers, respectively (56). A requirement of the cystine knot structure for secretion and dimerization is also shown in rat mucin (66). In addition, N-linked glycosylation in mucin is further required for dimerization (67). BMP proteins are known to form either homo- or heterodimers, and the heterodimers show a more potent activity than the homodimers in the case of BMP2/BMP7 (68) and BMP7/GDF7 (69). Thus, dimerization of cystine knot proteins appears to be critical for their function and would further affect the interaction with other dimerized complexes, such as noggin and BMP7, that form a tetramer complex (63). Hence, it is possible that Wise also forms a dimer to exert its function. We have found that secreted Wise is able to form a dimer in the presence of a cross-linking reagent, bis[sulfosuccinimidyl]-suberate (supplemental Fig. 6). Wise deletion constructs, such as Wise(ΔN), Wise(Δheel), and Wise(ΔC), showed similar dimerizations. One possibility is that dimerization, if it occurs, is mediated through two fingers that were not analyzed in this study. Since cystines at the tip of these fingers are suggested to make an intramolecular disulfide bond (27), mutations in these regions may cause destruction of the whole structure, and hence further analyses were not performed. In studies with the rat homolog USAG-1 only the monomer was observed in the cell lysate when analyzed on a non-reducing SDS gel (14). Hence, whether or not Wise forms a dimer in a physiological condition is yet to be clarified.
Various heparan sulfate proteoglycans (HSPGs) modulate the activities of signaling molecules by enhancing the ligand-receptor interactions (reviewed in Refs. 47, 70, and 71). For instance, Syndecan-1 enhances activity of Wnt1 in mammary glands (72), whereas Syndean-4 is required in non-canonical Wnt signaling (73). In Drosophila imaginal discs, two members of the Glypican family, Dally and Dally-like, promote and restrict the movement of signaling molecules, such as Dpp, Hh, and Wg, on the cell surface, thus regulating the spatial distribution of these ligands, allowing them to function as morphogens (74–77). A possible role of HSPGs is to sequester ligands on the cell surface, thus increasing the concentration of the ligand near receptors. Alternatively, HSPGs may form a complex with a ligand and a receptor thus functioning as a co-receptor. The finding that secreted Wise binds on the cell surface via HSPGs (Fig. 3, B, D, and E) suggests that interaction of Wise with LRP6 and/or BMP may be facilitated by HSPGs. Nevertheless, at least for the binding of Wise to LRP6, HSPGs may not be prerequisite, since Wise-LRP6 binding is seen in cell-free conditions (Fig. 6A) and in heparinase-treated cell surface (supplemental Fig. 3).
There remain further questions to be investigated. For example, the function of the C-terminal end and two finger domains is yet to be determined. In addition, the molecular mechanism of how Wise affects LRP6 to modulate the Wnt pathway is yet to be clarified. Since BMP and Wnt signals may affect each other depending on the cellular context (49), the reporter assay might not reflect all in vivo contexts. Thus, although we have identified a domain responsible for interacting with LRP6 that does not seem to be involved in the interaction with BMP4, the effect of Wise on Wnt and BMP signals may not be completely separable. Indeed, studies on Sclerostin have shown that, although Sclerostin physically interacts with LRP5/6 (29–31) and BMPs (28, 34) and inhibits both Wnt and BMP signals, the primary function of Sclerostin appears to be arguable, either BMP antagonism (33) or Wnt antagonism (32, 78). The fact that Wnts and BMPs are co-expressed in many developmental contexts suggests that these pathways closely interact with each other.
Supplementary Material
Acknowledgments
We thank Drs. K. W. Y. Cho and T. Dijke for the BRE reporter, I. Araki for Wnt1, X. He for LRP6 and LRP6ECD-IgG, and S. Butler for BMP4 constructs. We thank I. Fairclough for the work on bacterial expression of Wise.
This work was supported by the Medical Research Council (United Kingdom).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
K. Lintern and S. Guidato, unpublished data.
K. Lintern, S. Guidato, and N. Itasaki, data not shown.
- ECD
- extracellular domain
- BRE
- BMP response element
- PNGase F
- peptide:N-glycosidase F
- HSPG
- heparan sulfate proteoglycan.
REFERENCES
- 1.Itasaki N., Jones C. M., Mercurio S., Rowe A., Domingos P. M., Smith J. C., Krumlauf R. (2003) Development 130,4295–4305 [DOI] [PubMed] [Google Scholar]
- 2.Simmons D. G., Kennedy T. G. (2002) Biol. Reprod. 67,1638–1645 [DOI] [PubMed] [Google Scholar]
- 3.Laurikkala J., Kassai Y., Pakkasjärvi L., Thesleff I., Itoh N. (2003) Dev. Biol. 264,91–105 [DOI] [PubMed] [Google Scholar]
- 4.Brunkow M. E., Gardner J. C., Van Ness J., Paeper B. W., Kovacevich B. R., Proll S., Skonier J. E., Zhao L., Sabo P. J., Fu Y., Alisch R. S., Gillett L., Colbert T., Tacconi P., Galas D., Hamersma H., Beighton P., Mulligan J. (2001) Am. J. Hum. Genet. 68,577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balemans W., Ebeling M., Patel N., Van Hul E., Olson P., Dioszegi M., Lacza C., Wuyts W., Van Den Ende J., Willems P., Paes-Alves A. F., Hill S., Bueno M., Ramos F. J., Tacconi P., Dikkers F. G., Stratakis C., Lindpaintner K., Vickery B., Foernzler D., Van Hul W. (2001) Hum. Mol. Genet. 10,537–543 [DOI] [PubMed] [Google Scholar]
- 6.Shigetani Y., Itasaki N. (2007) Dev. Dyn. 236,2277–2284 [DOI] [PubMed] [Google Scholar]
- 7.O'Shaughnessy R. F., Yeo W., Gautier J., Jahoda C. A., Christiano A. M. (2004) J. Invest. Dermatol. 123,613–621 [DOI] [PubMed] [Google Scholar]
- 8.Kassai Y., Munne P., Hotta Y., Penttilä E., Kavanagh K., Ohbayashi N., Takada S., Thesleff I., Jernvall J., Itoh N. (2005) Science 309,2067–2070 [DOI] [PubMed] [Google Scholar]
- 9.Coveney D., Ross A. J., Slone J. D., Capel B. (2007) Gene. Expr. Patterns 7,82–92 [DOI] [PubMed] [Google Scholar]
- 10.Bouma G. J., Hart G. T., Washburn L. L., Recknagel A. K., Eicher E. M. (2004) Gene Expr. Patterns 5,141–149 [DOI] [PubMed] [Google Scholar]
- 11.Menke D. B., Page D. C. (2002) Gene Expr. Patterns 2,359–367 [DOI] [PubMed] [Google Scholar]
- 12.Knosp W. M., Saneyoshi C., Shou S., Bächinger H. P., Stadler H. S. (2007) J. Biol. Chem. 282,6843–6853 [DOI] [PubMed] [Google Scholar]
- 13.Yanagita M., Okuda T., Endo S., Tanaka M., Takahashi K., Sugiyama F., Kunita S., Takahashi S., Fukatsu A., Yanagisawa M., Kita T., Sakurai T. (2006) J. Clin. Invest. 116,70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanagita M., Oka M., Watabe T., Iguchi H., Niida A., Takahashi S., Akiyama T., Miyazono K., Yanagisawa M., Sakurai T. (2004) Biochem. Biophys. Res. Commun. 316,490–500 [DOI] [PubMed] [Google Scholar]
- 15.Beaudoin G. M., 3rd, Sisk J. M., Coulombe P. A., Thompson C. C. (2005) Proc. Natl. Acad. Sci. U.S.A. 102,14653–14658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson C. C., Sisk J. M., Beaudoin G. M., 3rd (2006) Cell Cycle 5,1913–1917 [DOI] [PubMed] [Google Scholar]
- 17.Blish K. R., Wang W., Willingham M. C., Du W., Birse C. E., Krishnan S. R., Brown J. C., Hawkins G. A., Garvin A. J., D'Agostino R. B., Jr., Torti F. M., Torti S. V. (2008) Mol. Biol. Cell 19,457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidato S., Itasaki N. (2007) Dev. Biol. 310,250–263 [DOI] [PubMed] [Google Scholar]
- 19.Ohazama A., Johnson E. B., Ota M. S., Choi H. Y., Choi H. J., Porntaveetus T., Oommen S., Itoh N., Eto K., Gritli-Linde A., Herz J., Sharpe P. T. (2008) PLoS ONE 3,e4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson E. B., Hammer R. E., Herz J. (2005) Hum. Mol. Genet 14,3523–3538 [DOI] [PubMed] [Google Scholar]
- 21.Murashima-Suginami A., Takahashi K., Sakata T., Tsukamoto H., Sugai M., Yanagita M., Shimizu A., Sakurai T., Slavkin H. C., Bessho K. (2008) Biochem. Biophys. Res. Commun. 369,1012–1016 [DOI] [PubMed] [Google Scholar]
- 22.Murashima-Suginami A., Takahashi K., Kawabata T., Sakata T., Tsukamoto H., Sugai M., Yanagita M., Shimizu A., Sakurai T., Slavkin H. C., Bessho K. (2007) Biochem. Biophys. Res. Commun. 359,549–555 [DOI] [PubMed] [Google Scholar]
- 23.Munne P. M., Tummers M., Järvinen E., Thesleff I., Jernvall J. (2009) Development 136,393–402 [DOI] [PubMed] [Google Scholar]
- 24.Yanagita M. (2005) Cytokine Growth Factor Rev. 16,309–317 [DOI] [PubMed] [Google Scholar]
- 25.Vitt U. A., Hsu S. Y., Hsueh A. J. (2001) Mol. Endocrinol. 15,681–694 [DOI] [PubMed] [Google Scholar]
- 26.Balemans W., Van Hul W. (2002) Dev. Biol. 250,231–250 [PubMed] [Google Scholar]
- 27.Avsian-Kretchmer O., Hsueh A. J. (2004) Mol. Endocrinol. 18,1–12 [DOI] [PubMed] [Google Scholar]
- 28.Kusu N., Laurikkala J., Imanishi M., Usui H., Konishi M., Miyake A., Thesleff I., Itoh N. (2003) J. Biol. Chem. 278,24113–24117 [DOI] [PubMed] [Google Scholar]
- 29.Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., Harris S. E., Wu D. (2005) J. Biol. Chem. 280,19883–19887 [DOI] [PubMed] [Google Scholar]
- 30.Semënov M., Tamai K., He X. (2005) J. Biol. Chem. 280,26770–26775 [DOI] [PubMed] [Google Scholar]
- 31.Ellies D. L., Viviano B., McCarthy J., Rey J. P., Itasaki N., Saunders S., Krumlauf R. (2006) J. Bone Miner. Res. 21,1738–1749 [DOI] [PubMed] [Google Scholar]
- 32.van Bezooijen R. L., Svensson J. P., Eefting D., Visser A., van der Horst G., Karperien M., Quax P. H., Vrieling H., Papapoulos S. E., ten Dijke P., Löwik C. W. (2007) J. Bone Miner. Res. 22,19–28 [DOI] [PubMed] [Google Scholar]
- 33.Winkler D. G., Sutherland M. S., Ojala E., Turcott E., Geoghegan J. C., Shpektor D., Skonier J. E., Yu C., Latham J. A. (2005) J. Biol. Chem. 280,2498–2502 [DOI] [PubMed] [Google Scholar]
- 34.Winkler D. G., Sutherland M. K., Geoghegan J. C., Yu C., Hayes T., Skonier J. E., Shpektor D., Jonas M., Kovacevich B. R., Staehling-Hampton K., Appleby M., Brunkow M. E., Latham J. A. (2003) EMBO J. 22,6267–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rost B. (1996) Methods Enzymol. 266,525–539 [DOI] [PubMed] [Google Scholar]
- 36.Kelley L. A., MacCallum R. M., Sternberg M. J. (2000) J. Mol. Biol. 299,499–520 [DOI] [PubMed] [Google Scholar]
- 37.Wu H., Lustbader J. W., Liu Y., Canfield R. E., Hendrickson W. A. (1994) Structure 2,545–558 [DOI] [PubMed] [Google Scholar]
- 38.Berman H., Henrick K., Nakamura H. (2003) Nat. Struct. Biol. 10,980. [DOI] [PubMed] [Google Scholar]
- 39.Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. (1997) Nucleic Acids Res. 25,4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Augsburger A., Schuchardt A., Hoskins S., Dodd J., Butler S. (1999) Neuron 24,127–141 [DOI] [PubMed] [Google Scholar]
- 41.Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J. P., He X. (2000) Nature 407,530–535 [DOI] [PubMed] [Google Scholar]
- 42.Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. (1997) Science 275,1784–1787 [DOI] [PubMed] [Google Scholar]
- 43.Korchynskyi O., ten Dijke P. (2002) J. Biol. Chem. 277,4883–4891 [DOI] [PubMed] [Google Scholar]
- 44.von Bubnoff A., Peiffer D. A., Blitz I. L., Hayata T., Ogata S., Zeng Q., Trunnell M., Cho K. W. (2005) Dev. Biol. 281,210–226 [DOI] [PubMed] [Google Scholar]
- 45.Safaiyan F., Kolset S. O., Prydz K., Gottfridsson E., Lindahl U., Salmivirta M. (1999) J. Biol. Chem. 274,36267–36273 [DOI] [PubMed] [Google Scholar]
- 46.Keller K. M., Brauer P. R., Keller J. M. (1989) Biochemistry 28,8100–8107 [DOI] [PubMed] [Google Scholar]
- 47.Belting M. (2003) Trends Biochem. Sci. 28,145–151 [DOI] [PubMed] [Google Scholar]
- 48.Whitman M. (1998) Genes Dev. 12,2445–2462 [DOI] [PubMed] [Google Scholar]
- 49.Itasaki N., Hoppler S. (2009) Dev. Dyn., in press [DOI] [PubMed] [Google Scholar]
- 50.Piccolo S., Agius E., Leyns L., Bhattacharyya S., Grunz H., Bouwmeester T., De Robertis E. M. (1999) Nature 397,707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kratochwil K., Dull M., Farinas I., Galceran J., Grosschedl R. (1996) Genes Dev. 10,1382–1394 [DOI] [PubMed] [Google Scholar]
- 52.Dassule H. R., McMahon A. P. (1998) Dev. Biol. 202,215–227 [DOI] [PubMed] [Google Scholar]
- 53.Fischer L., Boland G., Tuan R. S. (2002) J. Cell. Biochem. 84,816–831 [DOI] [PubMed] [Google Scholar]
- 54.Bonafede A., Kohler T., Rodriguez-Niedenfuhr M., Brand-Saberi B. (2006) Dev. Biol. 299,330–344 [DOI] [PubMed] [Google Scholar]
- 55.Sasai Y., Lu B., Piccolo S., De Robertis E. M. (1996) EMBO J. 15,4547–4555 [PMC free article] [PubMed] [Google Scholar]
- 56.Marcelino J., Sciortino C. M., Romero M. F., Ulatowski L. M., Ballock R. T., Economides A. N., Eimon P. M., Harland R. M., Warman M. L. (2001) Proc. Natl. Acad. Sci. U.S.A. 98,11353–11358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zimmerman L. B., De Jesús-Escobar J. M., Harland R. M. (1996) Cell 86,599–606 [DOI] [PubMed] [Google Scholar]
- 58.Yanagita M. (2007) Ther. Apher. Dial 11, Suppl. 1, S38–S43 [DOI] [PubMed] [Google Scholar]
- 59.Winkler D. G., Yu C., Geoghegan J. C., Ojala E. W., Skonier J. E., Shpektor D., Sutherland M. K., Latham J. A. (2004) J. Biol. Chem. 279,36293–36298 [DOI] [PubMed] [Google Scholar]
- 60.Bork P. (1993) FEBS Lett. 327,125–130 [DOI] [PubMed] [Google Scholar]
- 61.Moussad E. E., Brigstock D. R. (2000) Mol. Genet. Metab. 71,276–292 [DOI] [PubMed] [Google Scholar]
- 62.Abreu J. G., Ketpura N. I., Reversade B., De Robertis E. M. (2002) Nat. Cell Biol. 4,599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Groppe J., Greenwald J., Wiater E., Rodriguez-Leon J., Economides A. N., Kwiatkowski W., Affolter M., Vale W. W., Belmonte J. C., Choe S. (2002) Nature 420,636–642 [DOI] [PubMed] [Google Scholar]
- 64.Hsu D. R., Economides A. N., Wang X., Eimon P. M., Harland R. M. (1998) Mol. Cell 1,673–683 [DOI] [PubMed] [Google Scholar]
- 65.Isaacs N. W. (1995) Curr. Opin. Struct. Biol. 5,391–395 [DOI] [PubMed] [Google Scholar]
- 66.Bell S. L., Xu G., Forstner J. F. (2001) Biochem. J. 357,203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell S. L., Xu G., Khatri I. A., Wang R., Rahman S., Forstner J. F. (2003) Biochem. J. 373,893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu W., Kim J., Cheng C., Rawlins B. A., Boachie-Adjei O., Crystal R. G., Hidaka C. (2006) Bone 39,61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butler S. J., Dodd J. (2003) Neuron 38,389–401 [DOI] [PubMed] [Google Scholar]
- 70.Perrimon N., Bernfield M. (2000) Nature 404,725–728 [DOI] [PubMed] [Google Scholar]
- 71.Park P. W., Reizes O., Bernfield M. (2000) J. Biol. Chem. 275,29923–29926 [DOI] [PubMed] [Google Scholar]
- 72.Alexander C. M., Reichsman F., Hinkes M. T., Lincecum J., Becker K. A., Cumberledge S., Bernfield M. (2000) Nat. Genet. 25,329–332 [DOI] [PubMed] [Google Scholar]
- 73.Muñoz R., Moreno M., Oliva C., Orbenes C., Larraín J. (2006) Nat. Cell Biol. 8,492–500 [DOI] [PubMed] [Google Scholar]
- 74.Baeg G. H., Lin X., Khare N., Baumgartner S., Perrimon N. (2001) Development 128,87–94 [DOI] [PubMed] [Google Scholar]
- 75.Fujise M., Takeo S., Kamimura K., Matsuo T., Aigaki T., Izumi S., Nakato H. (2003) Development 130,1515–1522 [DOI] [PubMed] [Google Scholar]
- 76.Han C., Belenkaya T. Y., Wang B., Lin X. (2004) Development 131,601–611 [DOI] [PubMed] [Google Scholar]
- 77.Belenkaya T. Y., Han C., Yan D., Opoka R. J., Khodoun M., Liu H., Lin X. (2004) Cell 119,231–244 [DOI] [PubMed] [Google Scholar]
- 78.van Bezooijen R. L., Roelen B. A., Visser A., van der Wee-Pals L., de Wilt E., Karperien M., Hamersma H., Papapoulos S. E., ten Dijke P., Löwik C. W. (2004) J. Exp. Med. 199,805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.