Abstract
It has been previously proposed that nitric oxide (NO) is the only biologically relevant nitrogen oxide capable of activating the enzyme soluble guanylate cyclase (sGC). However, recent reports implicate HNO as another possible activator of sGC. Herein, we examine the affect of HNO donors on the activity of purified bovine lung sGC and find that, indeed, HNO is capable of activating this enzyme. Like NO, HNO activation appears to occur via interaction with the regulatory ferrous heme on sGC. Somewhat unexpectedly, HNO does not activate the ferric form of the enzyme. Finally, HNO-mediated cysteine thiol modification appears to also affect enzyme activity leading to inhibition. Thus, sGC activity can be regulated by HNO via interactions at both the regulatory heme and cysteine thiols.
Nitric oxide (NO)2 is the most studied of the endogenously generated nitrogen oxides and is well known to mediate many aspects of cardiovascular function including the regulation of vascular tone and platelet aggregation (for example, see Ref. 1). These responses are in large part due to the interaction of NO with its most established endogenous receptor, soluble guanylate cyclase (sGC) (2). This 150-kDa heterodimeric heme protein catalyzes the production of the second messenger molecule cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP) (3). The basal activity of sGC is enhanced several hundred fold upon binding of NO to the single regulatory heme site. This stimulation of activity is a result of a conformational change induced by cleavage of the proximal histidine heme ligand upon formation of the ferrous nitrosyl complex, which is preferentially pentacoordinate (4). In addition to heme site regulation of sGC, there are numerous reports indicating that oxidation of cysteine thiol residues on this protein can also alter/regulate both the basal activity and the degree of NO-mediated activation (5–10).
Recently, the one-electron reduced and protonated congener of NO, nitroxyl (HNO) has received significant interest as a cardiovascular agent whose actions are independent of NO formation (11). For example, a study by Ellis and co-workers (12) suggests that HNO is a vital component of endothelium-derived relaxing factor along with NO in rat aorta. HNO is also able to mediate murine aorta vasorelaxation even in the presence of NO scavengers (13). Furthermore, the vasodilation produced by HNO was inhibited by the sGC heme site inhibitor, 1H-[1,2,4]oxadiazolo[4,3-a]-quinoxalin-1-one implicating sGC activation in this HNO-mediated effect. In addition to its effects on large conduit vessels like the aorta, HNO also dilates rat small mesenteric resistance-like arteries through sGC-dependent and voltage-dependent K+ channel-dependent mechanisms (14). Nitroxyl (derived from the HNO-donor Angeli's salt) is also a potent dilator of feline pulmonary vasculature equal to that of the NO donors SPER/NO, DETA/NO, and SULFI/NO (15). Most recently, HNO was found to be a potent dilator of rat coronary arteries through an sGC-mediated mechanism (16). The evidence presented in these studies suggests that HNO is able to modulate cGMP levels through an interaction with sGC, an idea in conflict with a previous report showing that NO is the only nitrogen oxide capable of directly activating sGC (17).
HNO forms a stable adduct with the ferrous heme of deoxymyoglobin (18, 19) providing precedence for a possible interaction between HNO and sGC that is akin to the interaction of NO with ferrous sGC. In light of all the reports indicating possible HNO-mediated activation of sGC, an examination of the direct interaction of HNO with purified sGC was carried out to evaluate the possibility that HNO may be capable of directly interacting with sGC to elicit activation. Moreover, due to the previously reported thiol redox regulation of sGC (see above) and the known thiophilicity of HNO (20), we also examined the effects of HNO-mediated thiol modification on enzyme activity.
EXPERIMENTAL PROCEDURES
Materials
Sodium trioxodinitrate (Angeli's salt, AS) was synthesized by the general method of Smith and Hein (21). Diethylamine NONOate (DEA/NO) was synthesized following the method of Drago and Paulik (22). 1-Nitrosocyclohexyl trifluoroacetate (NCTFA) was synthesized according to Sha et al. (23). Triethanolamine hydrochloride (TEA), dl-dithiothreitol (DTT), l-cysteine, EDTA, guanosine triphosphate sodium salt (GTP), protoprophyrin IX (PPIX), hemin chloride, 3-isobutyl-1-methylxanthine, hydroxylamine·HCl, K3Fe(CN)6, and sodium nitrite were all purchased from Sigma, in the highest purity available. MgCl2·6H2O was purchased from Mallinckrodt (Hazelwood, MO). Tween 20 (enzyme grade) was purchased from Fisher (Pittsburgh, PA). 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one was purchased from Cayman Chemical (Ann Arbor, MI). NAP-10 and NAP-5 size exclusion columns and EIA kit (RPN226) were purchased from GE Healthcare. All experiments were performed anaerobically unless otherwise stated.
Methods
Purification of Soluble Guanylate Cyclase from Bovine Lung
Bovine sGC was isolated and purified as described previously (24). The procedure produced sGC that was bovine serum albumin-free by Western blot analysis. The enzyme had a specific activity of 28.3 ± 0.4 nmol of cGMP min−1 mg−1 and was activated 27-fold by the NO donor S-nitroso-N-acetylpenicillamine.
Soluble Guanylate Cyclase Activity Assay
The activity of sGC was determined as previously reported (25) with slight modifications. The enzyme reaction mixture (40 mm TEA, pH 7.4, 1 mm GTP, 3 mm MgCl2, and 0.3 mm 3-isobutyl-1-methylxanthine) was premixed in bulk in a sealed round bottom flask and deoxygenated by purging the headspace with argon for 30 min while stirring. All further manipulations were performed in an anaerobic chamber (Plas Labs, Lansing MI). sGC was diluted to the appropriate concentration with cold deoxygenated buffer (25 mm TEA, pH 7.4) and manipulated further as specified. All reactions were initiated by the addition of the enzyme reaction mixture. The reaction was carried out for 10–20 min at 37 °C and stopped by adding 10 μl of 0.5 m EDTA to chelate the Mg2+ cofactor. Samples were stored at −20 °C until ready to assay. Accumulated cGMP was quantified with the cGMP EIA kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Samples were diluted 100–1000-fold with EIA assay buffer for quantification.
Preparation of NO and HNO Donors and Porphyrin Solutions
NO and HNO donors were taken into the anaerobic chamber as solids or pure liquid and diluted with deoxygenated solvent inside the chamber. All donors were made into a ×200 stock to be added in 1-μl aliquots to the reaction mixture to minimize solvent pH effects. DEA/NO and Angeli's salt were diluted with 10 mm NaOH, NCTFA was diluted with deoxygenated dimethyl sulfoxide, PPIX was diluted with deoxygenated 25 mm TEA buffer, pH 7.4, containing 5% Tween 20. Decomposed controls for AS and NCTFA were made by allowing the appropriate concentration of donor to decompose overnight in 25 mm TEA buffer, pH 7.4, at 37 °C. All dilutions were performed just prior to the beginning of the experiment.
Removal of sGC Heme
Based on a procedure by Schmidt et al. (26), ferrous sGC was mixed with a solution containing 2% Tween 20 and 25 mm TEA, pH 7.4, for 10 min at 37 °C. The mixture was diluted to 0.5 ml and loaded onto a NAP-5 column previously equilibrated with deoxygenated buffer (25 mm TEA, pH 7.4) to remove unbound heme and Tween 20.
Oxidation of Ferrous sGC with K3Fe(CN)6
Ferrous sGC was mixed with 100 μm K3Fe(CN)6 and incubated for 1 min at 25 °C. This mixture was diluted to 0.5 ml and passed through a NAP-5 size exclusion column previously equilibrated with deoxygenated buffer (25 mm TEA, pH 7.4) to remove excess K3Fe(CN)6. Of the 1 ml collected, half was used as the ferric form and the other half was re-reduced with 2 mm DTT.
RESULTS
sGC Is Activated by HNO Donors
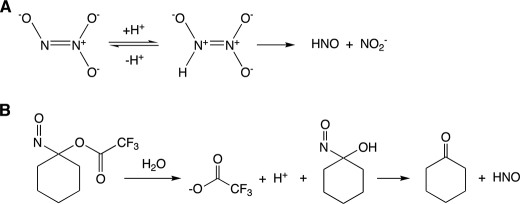
To determine whether sGC is activated by HNO, we measured the activity of purified sGC treated with the HNO donors AS and the acyloxynitroso compound NCTFA. These HNO donors are structurally dissimilar but decompose predictably in aqueous solution to give HNO and nitrite (AS) or HNO and cyclohexanone (NCTFA) (Fig. 1) (23, 27).
FIGURE 1.
Schematic of Angeli's salt (A) and NCTFA (B) decomposition to yield HNO.
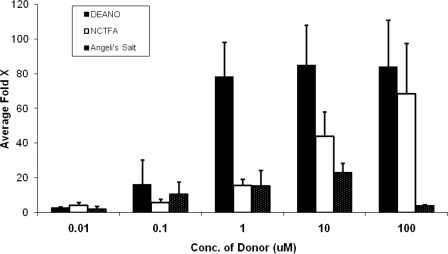
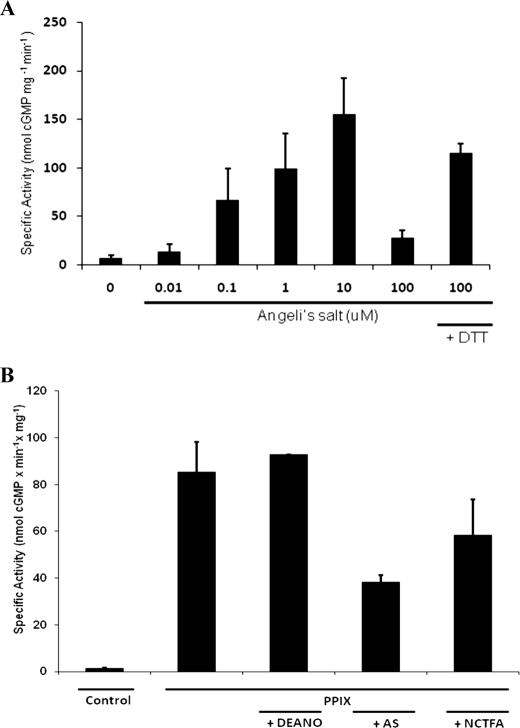
As a positive control we also examined the effect of the NO donor DEA/NO on enzyme activity. sGC was maximally activated nearly 80-fold by DEA/NO (100 μm), whereas AS (10 μm) activated sGC by up to 20-fold and NCTFA (100 μm) activated 60-fold (Fig. 2).
FIGURE 2.
sGC is activated by HNO donors. sGC (3.2 μg final: 0.11 μm) activity was measured in the presence of NO (DEANO) or HNO (NCTFA, Angeli's salt) donors for 16 min at 37 °C. Data represents the average of triplicate measurements reported as fold-activation over basal activity (no donor).
To verify that sGC activation is directly dependent on HNO release from AS and NCTFA (as opposed to other donor byproducts), we measured sGC activity in the presence of decomposed donors or sodium nitrite (AS decomposition co-product). Under the same incubation conditions as before, no activation was observed (data not shown).
Because AS and NCTFA are structurally distinct HNO donors, enzyme activation likely occurs via HNO generation and is not due to the donors themselves. These results thus suggest that HNO produced from AS and NCTFA activate sGC. We propose that HNO-mediated sGC activation can occur in one of at least three possible ways: 1) HNO could be converted to a very small amount of NO which activates sGC; 2) HNO could modify critical thiols on sGC to affect its activity; or 3) HNO could activate sGC via a direct interaction with the heme of sGC, akin to NO-mediated activation. Of course, the net observed activation of sGC by HNO could be a combination of some or all of these effects. The following experiments were carried out to specifically address these mechanistic possibilities.
HNO (and Not NO) Is the Species Activating sGC
To test if HNO indirectly activates sGC through conversion to NO, we measured sGC activity in the presence of various species that prevent HNO to NO conversion. First, HNO can be converted to NO by adventitious oxidants present in the reagents, such as Fe3+ or Cu2+ (Reaction 1) (28).
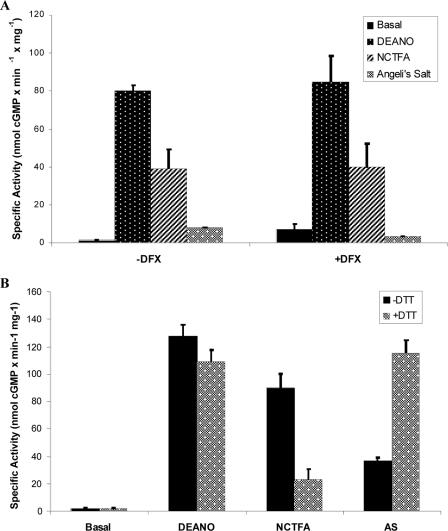
To eliminate contamination from metals that could lead to the conversion of HNO to NO, the highest purity water available was used (double distilled from glass), all solutions were passed through Chelex resin and metal chelators were used. Traditional chelators including EDTA, EGTA, and diethylene triamine pentacetic acid cannot be used in this case because of their high affinity for magnesium, a required factor for the cyclization reaction catalyzed by sGC. Therefore we used desferrioxamine for its preferential chelation of iron and copper over magnesium (Fe3+, Kb = 1031; Cu2+, Kb = 1014, versus Mg2+, Kb = 104 (29, 30)). Desferrioxamine did not effect the ability of DEA/NO, AS, or NCTFA to activate sGC (Fig. 3A) indicating that HNO is directly binding to sGC.
FIGURE 3.
Activation of sGC by HNO donors is not due to NO. A, effect of desferoxamine on sGC activation. The effect of excess NO (DEANO) or HNO (NCTFA, Angeli's salt) donors (100 μm) on sGC (3.6 μg final) activity was measured in the presence or absence of 10 μm of the metal chelator (desferrioxamine, DFX) for 15 min at 37 °C. Data represents the average of triplicate measurements reported as specific activity (nmol of cGMP mg−1 min−1). B, effect of DTT on sGC activation. We measured sGC (19 μg final) activation by 100 μm NO (DEANO) or HNO (NCTFA, Angeli's salt) donors in the presence or absence of 10 mm DTT for 15 min at 37 °C. Data represents the average of triplicate measurements reported as specific activity (nmol of cGMP mg−1 min−1).
The HNO donor Angeli's salt is known to produce trace amounts of NO as a minor decomposition side product (31, 32). Because the reaction of NO with HNO is fast (5.8 × 106 m−1 s−1 (33)), in most cases NO should be scavenged by excess HNO, precluding any biological activity associated with NO. However, given the very high affinity of sGC for NO, it may still be possible to observe some activity associated with NO. NCTFA, however, does not generate NO in an alternative decomposition pathway and, therefore, represents a strict HNO donor. Although it would be difficult to definitively validate that production of minute levels of NO from Angeli's salt were not responsible for the observed activity enhancements, the problem can be refocused to the effect of HNO levels on sGC activity.
It is established that HNO is efficiently scavenged by thiols (compared with NO), an effect used previously to differentiate between HNO- versus NO-mediated biological activity (34). Thus, to determine whether the activation of sGC by HNO donors is due to HNO release or to the small amount of NO produced as a byproduct, DTT was co-incubated with the donor to see if HNO scavenging by DTT can inhibit sGC activation (Fig. 3B). DTT does not react with NO under the conditions of our reactions (anaerobic) and, expectedly, has very little effect on sGC activation by DEANO. However, DTT significantly reduces the NCTFA-mediated sGC activation, suggesting that NCTFA primarily activates sGC through HNO generation. Interestingly, DTT does not inhibit the ability of Angeli's salt to activate sGC, but rather potentiated the Angeli's salt-mediated sGC activation to the level of DEA/NO. This result is not necessarily surprising because DDT will scavenge the HNO generated from Angeli's salt and will not react with the trace amount of NO (see above). Because the trace amount of NO made by Angeli's salt should normally be scavenged by HNO, in the presence of DTT, NO will “survive” to activate sGC (indeed, small amounts of NO are observed in a decomposing solution of AS only in the presence of DTT).3 This observation could also be due to the differing kinetics of AS versus NCTFA decomposition (see below).
The reaction of DTT with HNO produces hydroxylamine according to the Reaction 2 (35).
As a control, we verified that hydroxylamine did not activate sGC (data not shown), thus ruling out a possible reaction of DTT with HNO to generate another species (H2NOH) with possible activity.
HNO Activates sGC by Directly Interacting with the Heme
The established chemistry of HNO (36) predicts at least two possible types of interaction with sGC. HNO can coordinate to the heme site (18) and/or react with one or more of the numerous cysteine thiols (20) of sGC. Because both heme coordination (by NO) and cysteine thiol redox processes are known to be capable of regulating sGC activity (6, 37) it is clearly possible that the observed HNO-mediated changes in sGC activity could be due to either interaction or a combination of interactions at these two targets.
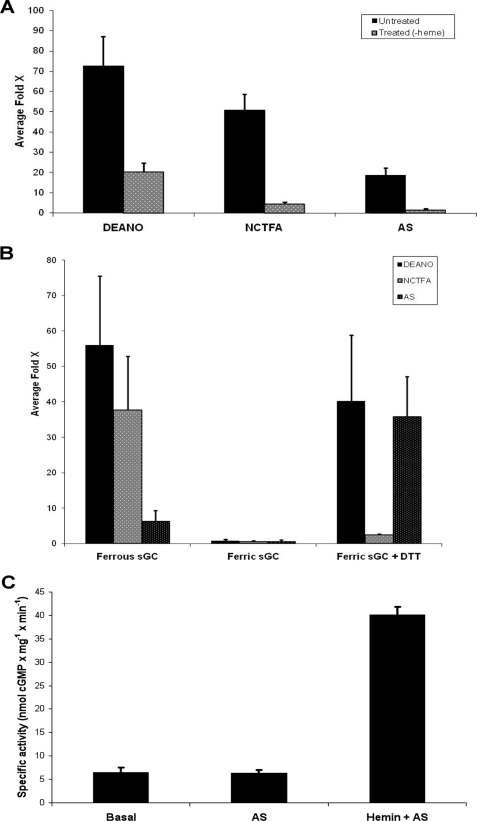
Although removal of heme from sGC does not substantially alter basal activity (26), it drastically affected activation by both DEA/NO and HNO donors (Fig. 4A). Heme removal from sGC decreased its sensitivity to activation by DEA/NO (from 70-fold activation down to 20-fold) and markedly reduced the HNO-mediated activation. The small degree of activation still observed for both NO and HNO even after depletion of heme was undoubtedly the result of the incomplete removal of heme. Complete heme removal from sGC proved to be very difficult and complete loss of DEA/NO-dependent activation could not be attained without significant loss of basal activity (data not shown). These results suggest that sGC activation by HNO donors occurs via direct interaction of HNO with the sGC heme. The loss of HNO activation after partial heme removal from sGC also implies that any significant activation by HNO is not due to any interaction between HNO and the cysteine thiols on sGC.
FIGURE 4.
HNO activation of sGC depends on the presence of ferrous heme. A, effect of heme removal on sGC activation. We measured the ability of NO (DEANO) or HNO (NCTFA, Angeli's salt) donors to activate sGC with and without heme. Untreated (heme bound) and treated (heme removed) sGC (19 μg final) were assayed for activity in the presence of 100 μm donors for 15 min at 37 °C. Data represents the average of triplicate measurements reported as fold-activation over basal activity (no donor). B, effect of heme redox state on sGC activation. We compared the ability of NO (DEANO) or HNO (NCTFA, Angeli's salt) donors to activate reduced and oxidized sGC. sGC was treated with ferricyanide to oxidize heme and re-reduced with DTT. Ferrous (reduced, untreated), ferric (oxidized, treated with ferricyanide), and ferric + DTT (re-reduced, treated with ferricyanide then DTT) sGC (3.5 μg final) were assayed for activity in the presence of 100 μm donors for 12 min at 37 °C. Data represents the average of triplicate measurements reported as fold-activation over basal activity (no donor). C, effect of ferric heme and HNO on sGC activation. Heme was removed from sGC and activity was measured in the presence of Angeli's salt (100 μm) alone or Angeli's salt (100 μm) and hemin chloride (0.1 μm). Data represents the average of triplicate measurements reported as specific activity (nmol of cGMP mg−1 min−1).
HNO Activates Ferrous Heme sGC
Under normal physiological conditions, the heme in sGC exists in the ferrous state. Because NO activates sGC via binding to the ferrous state of this enzyme (Reaction 3), oxidation of sGC yields an NO-insensitive ferric form, a species that may be relevant in diseased tissues (38).
HNO has been shown to reductively nitrosylate other ferric heme proteins (39, 40) suggesting that HNO could interact with the ferric heme of sGC to give the ferrous nitrosyl adduct, the same product generated from the reaction of NO with the ferrous form of the enzyme (Reaction 4). Thus, it may be postulated that HNO could activate the ferric enzyme to levels similar to what is observed when NO binds to the ferrous enzyme. To determine whether HNO binds to ferrous or ferric heme sGC, we measured sGC activation when the enzyme was in different oxidation states (Fig. 4B). Our results show that NO and HNO donors only activate ferrous sGC, as heme oxidation with ferricyanide renders sGC insensitive to activation by both NO and HNO donors. To test if this effect could be due to heme loss, the oxidized sGC was then re-reduced with DTT. The re-reduced sGC was again sensitive to DEANO and AS (but not to NCTFA, likely because of the scavenging action of millimolar levels of DTT, as shown before (see Fig. 3B)).
It has been reported that heme-bound sGC can exchange with an exogenous ferrous nitrosyl heme source resulting in activation via formation of the sGC-Fe2+-NO complex (41). Also, heme-free enzyme is capable of incorporating a ferrous-nitrosyl heme, resulting in activation (41). Interestingly, when hemin chloride is mixed with AS, to generate the corresponding ferrous nitrosyl heme adduct in situ, and incubated with heme-free sGC, the enzyme is activated 6-fold. This suggests that HNO nitrosylated hemin (the ferrous nitrosyl species) can be made in solution and capable of incorporating into heme-free sGC resulting in enzyme activation (Fig. 4C).
sGC Activity Is Inhibited by an HNO-Thiol Interaction
The activation of sGC by Angeli's salt shown in Figs. 2 and 5A represents a dose-response relationship up to 10 μm. However, at the highest concentration of donor (100 μm) Angeli's salt begins to inhibit activity. The activation is likely occurring via HNO ligation to the heme iron (see above). The inhibition at high concentration could be due to HNO interacting with a second lower affinity inhibitory site on sGC. Significantly, the inhibition of sGC activity at the high Angeli's salt concentration was protected against by the presence of DTT, a reagent known to reduce oxidized thiol species. Because thiol modification is known to inhibit the NO-activated form of sGC, we investigated the possibility that at high concentrations HNO was modifying sGC thiols and leading to inhibition of activation of sGC.
FIGURE 5.
HNO inhibits activated sGC in a thiol dependent manner. A, effect of HNO (Angeli's salt) on sGC activity. We measured sGC (19 μg final) activation by an HNO donor (Angeli's salt) in the presence or absence of 10 mm DTT for 15 min at 37 °C. Data represents the average of triplicate measurements reported as specific activity (nmol of cGMP mg−1 min−1). B, effect of HNO donors on PPIX-activated sGC. Heme was removed from sGC and it was incubated in the absence (control) or presence of PPIX (100 nm) for 5 min at 37 °C. Activity was measured in the absence of PPIX, the presence of PPIX alone, or PPIX co-incubated with DEANO (100 μm), Angeli's salt (100 μm), or NCTFA (100 μm). Data represents the average of triplicate measurements reported as specific activity (nmol of cGMP mg−1 min−1).
We isolated the interaction between HNO and the thiols (from possible heme interactions) by removing the iron heme and substituting it with PPIX. PPIX binds the enzyme to activate it to the same level as the NO ferrous heme-bound form of sGC and is kinetically indistinguishable from this NO-activated form (42). The iron-free PPIX thus eliminates the possible interaction of HNO with the heme iron. Thus, apo-sGC activated with 100 nm PPIX (incubated for 5 min at 37 °C) was then co-incubated with HNO and NO donors (Fig. 5B). PPIX alone activated apo-sGC 52-fold over basal activity (specific activity of 85.17 versus 1.63 nmol of cGMP min−1 mg−1). This was unchanged in the presence of NO (100 μm DEA/NO). However, when apo-sGC activated with PPIX was co-incubated with Angeli's salt, it was inhibited by 50% (38.21 nmol of cGMP min−1 mg−1). Likewise, when the same treatment was done with NCTFA, PPIX-activated apo-sGC was inhibited by 33% (58.49 nmol of cGMP min−1 mg−1).
DISCUSSION
These experiments show that the HNO donors AS and NCTFA significantly activate purified bovine sGC through a direct interaction of HNO with the ferrous heme of sGC. To date, NO has been considered to be the only nitrogen oxide capable of activating sGC (17). However, due to a variety of studies alluding to possible biological actions of HNO occurring via cGMP pathways (12–15, 43, 44) it appears that HNO may in fact activate sGC. It has been proposed that HNO conversion to NO by various physiological oxidants (28) could be responsible for the increase in cGMP observed in vitro. However, the observation that HNO also elicits cGMP-dependent effects in the presence of NO scavengers (45) indicates that there may be a direct interaction between HNO and sGC. In contrast to the results presented herein, Dierks and Burstyn (17) previously showed that HNO derived from AS had no direct stimulatory effect on sGC and did not alter the ability of NO donors to activate sGC. However, these early experiments examined the interaction of HNO and sGC in the presence of 10 mm DTT, a vital component of most aerobic sGC studies due to the oxidative instability of the heme and protein thiols (46). This amount of DTT is sufficient to scavenge the HNO produced from AS and prevent any direct interaction (47). To eliminate the potentially confounding effect of high levels of exogenous thiols, the studies described herein were performed anaerobically and in the absence of high levels of exogenous thiols.
It is clear that sGC can be activated by HNO donors AS and NCTFA (Fig. 2). As stated earlier, the fact that these two donors are structurally distinct and release HNO via completely different mechanisms indicates that HNO is the activating species and not impurities, the donors themselves, or other decomposition by-products.
In anaerobic solution, the concentration of an NO donor can be reasonably correlated to the amount of available NO in solution. Thus, the maximum enhancement of sGC activity by DEA/NO, which was observed at ≥1 μm (Fig. 2), corresponds to a threshold of ∼2 μm NO because DEA/NO releases 2 eq of NO (48). The minimum threshold for both AS and NCTFA is >100 μm, however, correlation of donor concentration to available HNO concentration is significantly hampered by the fact that HNO self-consumes (Reaction 5) (27).
Despite a relatively high rate constant (8 × 106 m−1 s−1; Ref. 33), the rate of dimerization is highly coupled to the HNO concentration given the second-order dependence.
The kinetics of HNO release from AS and NCTFA are quite different. The decomposition of AS is very fast (t½ = 2–3 min, pH 7.4, 37 °C) (27) and will result in a large, initial spike in the concentration of HNO during the time course of the assay. In contrast, the longer half-life of NCTFA (t½ > 1 h, pH 7.4, at room temperature (23)) will produce a lower steady state concentration of HNO. Although these two donors are expected to release equal amounts of HNO (one equivalent of HNO per donor), the faster release of HNO from AS will facilitate greater self-consumption and thus reduce the overall amount of HNO available to react with other targets such as the sGC heme (49). This reduction in available HNO at high AS concentrations likely contributes to the biphasic nature of the activation profile of AS compared with those of DEA/NO and NCTFA (49).
The higher peak concentration of HNO with AS may also contribute to the biphasic nature of its activation of sGC as opposed to NCTFA. In Fig. 2 the activation of sGC by Angeli's salt is attenuated at the highest concentration (100 μm). Further experiments probing this effect revealed that HNO inhibits sGC activity by interacting at a second site distinct from the heme (Fig. 5B). That is, in the absence of a metal ligation site, NO has no effect on sGC activity. However, under these same conditions, HNO significantly inhibits enzyme activity, implicating a second site of interaction. Based on the known chemistry of HNO this is most likely a thiol cysteine target, consistent with the observed protection by DTT (Fig. 3B) (20, 50). Significantly, recent data by Sayed et al. (51) characterized the formation of S-nitrosated cysteine residues in sGC, indicating possible thiol modification by nitrogen oxides. Thus, there may be a subset of sGC thiols that are subject to biochemical modification and are important to the physiological regulation of sGC activity. Significantly, cysteine thiol modification of numerous proteins by HNO has been reported, further establishing protein thiols as a significant target for HNO biology (see for example, Refs. 52 and 53). Moreover, identification of HNO-thiol adducts has been established via mass spectral analysis (54).
Addition of DTT enhanced the activation of sGC by AS while reducing the effect of NCTFA (Fig. 3B). This apparently selective inhibition of the NCTFA versus AS is likely due to the fact that AS generates NO (as a minority product) along with HNO (31). Because the trace amounts of NO generated rapidly reacts with excess HNO (33), this typically precludes any biological activity associated with NO from AS. However, when HNO is selectively scavenged by DTT (which is a faster reaction than the HNO-NO reaction (49)), NO is allowed to survive and activate sGC. Because NCTFA decomposition is not known to generate significant amounts of NO, this is not an issue with this donor. Another possible explanation that can also contribute to this observation is related to the dissimilar kinetics of donor decomposition. We have shown that HNO at low concentrations can activate sGC via the ferrous heme, and, as the concentration is increased, it attenuates its own activation by reacting at a second site (via thiol interaction). In the case of AS, when DTT is added it competitively scavenges enough HNO to prevent the inhibitory interaction with the thiols or simply reverses the HNO-mediated thiol modifications, but is unable to prevent HNO from activating via the heme (implying rapid association of HNO to the ferrous heme of sGC). During the decomposition of NCTFA, however, DTT is sufficient to scavenge the low, sustained concentration of HNO, thereby preventing activation. Despite the kinetic differences in decomposition, these results highlight the fact that HNO is mediating the sGC effects.
Based on known HNO chemistry, we hypothesize that HNO can modulate sGC activity by binding to the sGC heme, modifying critical sGC thiols or a combination of both effects. Although our data are consistent with the idea that HNO can modify sGC thiols and result in sGC inhibition, the experiments presented here unambiguously show that HNO directly interacts with the ferrous heme of sGC (Fig. 4). It was unexpected that HNO activated the ferrous but not the ferric form of sGC because HNO readily forms ferrous nitrosyl complexes by reductive nitrosylation of a variety of ferric heme- and non-heme proteins (40, 55–58). Reductive nitrosylation of sGC may be kinetically slow compared with dimerization or reaction with protein thiols. The fact that many of the ferric systems that undergo reductive nitrosylation do not contain thiols, whereas bovine lung sGC contains multiple cysteine residues may be a significant factor in the resistance of sGC to this reaction. Furthermore, ferric sGC has been suggested to be unusually substitutionally inert because addition of very high concentrations of azide and cyanide (150 and 4 mm, respectively) were required to induce changes in ferric sGC spectra (59). Because dimerization of HNO inhibits the ability to produce millimolar concentrations of HNO in solution, a similar study with an HNO donor is not possible.
Interaction between HNO and the ferrous heme of sGC generates activated enzyme. Previous reports suggest that HNO forms a stable complex with ferrous heme proteins (18, 60–62) and this may also be the case with sGC. Indeed, in current studies utilizing a truncated sGC protein containing only the heme domain, a HNO-ferrous heme adduct was been observed.4 According to the currently accepted mechanism of sGC activation by NO (63), binding of NO to the pentacoordinate ferrous histidyl heme results in cleavage of the axial His105 bond and associated conformational changes. The preference of ferrous porphyrin nitrosyl complexes for pentacoordination has been long established (for example, see Refs. 64 and 65). The strong trans effect of NO is a result of the donation of the π* electron from NO into the Fe2+ dz2 orbital, which weakens the trans ligand bond enough to cleave (66). In contrast, CO complexes are preferentially hexacoordinate because CO is a weaker trans director due to both the absence of ligand π* electrons and the importance of π backbonding compared with σ bonding in CO association (66). Unlike NO, there is no occupancy of the π* orbital in HNO thus predicting no analogous trans-activation. However, in the Mb-HNO complex characterized by Immoos et al. (61) the iron-axial His distance was significantly longer for the HNO-bound complex compared with the NO-bound complex. Moreover, ferrous nitroxyl complexes are isoelectronic with cobalt nitrosyl complexes, which are generally considered to have a charge distribution of CoIIINO− (67). The equilibrium constant for addition of a second axial ligand to cobalt (II) porphyrin complexes is small (68), supporting the possibility that ferrous nitroxyl complexes can be pentacoordinate.
It should be noted that HNO can be considered as the simplest form of an alkyl-nitroso molecule and sGC is not significantly activated by a variety of short and long chain length alkyl-nitroso compounds (69, 70). Furthermore, the current study cannot exclude the possibility that the ferrous nitroxyl complex of sGC is oxidized to the active ferrous nitrosyl complex. The analogous Mb-HNO complex is a good reductant as indicated by its facile oxidation by methyl viologen (71). HNO is also a fairly good one-electron oxidant (0.1 V versus NHE for HNO, H+/H2NO, pH 7) (72) and excess HNO may oxidize bound HNO to NO. Further spectral analyses are currently underway to investigate this possibility.
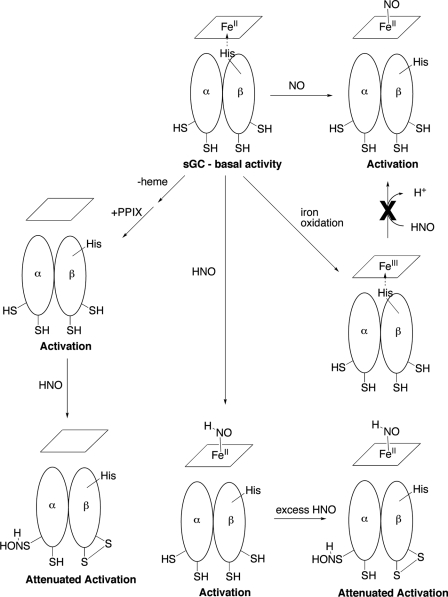
The combined results presented herein are summarized as follows: 1) HNO activates sGC via coordination to the ferrous enzyme, akin to NO-mediated activation, 2) HNO does not activate the oxidized ferric enzyme, 3) HNO modification of regulatory thiols on sGC results in loss of activity, resulting in a biphasic activity curve, low HNO gives significant activation via heme coordination and higher levels begin to attenuate this activity. Fig. 6 schematically depicts these interactions of HNO with sGC.
FIGURE 6.
Proposed mechanisms for the effects of HNO on sGC activity. Note, the possible and/or likely products of sGC thiol oxidation by excess HNO are shown. The exact nature of the products and the sites of these cysteine thiols are not known.
One of the most pressing and provocative questions regarding HNO remains: is HNO an endogenously generated physiological signaling agent? To date, there is no clear evidence that this is the case. Although a variety of biologically accessible chemical processes have been reported that are capable of generating HNO (36), there is no evidence that any of these processes represent an endogenous source of HNO for signaling purposes. However, considering the extreme potency and specificity of the actions of HNO in, for example, the cardiovascular system (see below) it is intriguing to speculate that HNO is generated endogenously for the purpose of regulating cardiac function. Regardless, proper examination of this issue is clearly warranted.
There is growing interest in the cardiovascular actions of HNO (for example, see Ref. 73). Studies suggest that the use of HNO to treat heart failure has numerous advantages over NO-releasing drugs because HNO causes increased left ventricular contractility and reduced cardiac preload while decreasing peripheral vascular resistance; it is also additive to the beneficial actions of β-agonists (74). Further still, one of the principal pharmacological interventions for heart failure, administration of organic nitrates, suffers from the development of tachyphylaxis, which severely limits the clinical use of this family of therapeutics. Interestingly, HNO donors do not induce tolerance and remain equipotent in the circulation of animals made tolerant to glyceryl trinitrate (75), suggesting that HNO will maintain its beneficial activity long after NO-based treatment has diminished. There is also a risk of systemic hypotension and headache (dilatation of the cerebral vasculature) with the use of organic nitrates (76) that may be avoided with the use of an HNO donor.
Our data provide a potential explanation for this favorable profile of HNO donors; the mechanism by which they activate sGC is self-limiting. Thus, we have demonstrated unequivocally that HNO can activate sGC (e.g. dilate the vasculature) via interaction at the heme site, but at higher concentrations will attenuate its own activation (via interactions at the regulatory thiol sites), thus minimizing the possibility of excessive stimulation and tolerance. This work therefore clearly demonstrates a potential advantage of using HNO, compared with NO donors as vasodilatory agents in a number of cardiovascular disorders.
Acknowledgment
We thank Dr. Bernd Mayer for helpful discussions regarding the release of NO from Angeli's salt in the presence of thiols.
B. Mayer, personal communication.
A. Rajapakshe, S. T. Massey, X. Hu, T. W. Miller, J. M. Fukuto, W. R. Montfort, and K. M. Miranda, manuscript in preparation.
- NO
- nitric oxide
- NCTFA
- 1-nitrosocyclohexyl trifluoroacetate
- DEA/NO
- diethylamine NONOate
- TEA
- triethanolamine
- PPIX
- protoprophyrin IX
- AS
- Angeli's salt
- DTT
- dithiothreitol
- SGC
- soluble guanylate cyclase.
REFERENCES
- 1.Hobbs A. J., Ignarro L. J. (1996) Methods Enzymol. 269, 134–148 [DOI] [PubMed] [Google Scholar]
- 2.Denninger J. W., Marletta M. A. (1999) Biochim. Biophys. Acta 1411, 334–350 [DOI] [PubMed] [Google Scholar]
- 3.Ignarro L. J., Degnan J. N., Baricos W. H., Kadowitz P. J., Wolin M. S. (1982) Biochim. Biophys. Acta 718, 49–59 [DOI] [PubMed] [Google Scholar]
- 4.Burstyn J. N., Yu A. E., Dierks E. A., Hawkins B. K., Dawson J. H. (1995) Biochemistry 34, 5896–5903 [DOI] [PubMed] [Google Scholar]
- 5.DeRubertis F. R., Craven P. A. (1977) J. Biol. Chem. 252, 5804–5814 [PubMed] [Google Scholar]
- 6.Craven P. A., DeRubertis F. R. (1978) Biochim. Biophys. Acta 524, 231–244 [DOI] [PubMed] [Google Scholar]
- 7.Haddox M. K., Stephenson J. H., Moser M. E., Goldberg N. D. (1978) J. Biol. Chem. 253, 3143–3152 [PubMed] [Google Scholar]
- 8.Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. (1981) J. Pharmacol. Exp. Ther. 218, 739–749 [PubMed] [Google Scholar]
- 9.Brandwein H. J., Lewicki J. A., Murad F. (1981) J. Biol. Chem. 256, 2958–2962 [PubMed] [Google Scholar]
- 10.Kamisaki Y., Waldman S. A., Murad F. (1986) Arch. Biochem. Biophys. 251, 709–714 [DOI] [PubMed] [Google Scholar]
- 11.Paolocci N., Jackson M. I., Lopez B. E., Miranda K., Tocchetti C. G., Wink D. A., Hobbs A. J., Fukuto J. M. (2007) Pharmacol. Ther. 113, 442–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis A., Li C. G., Rand M. J. (2000) Br. J. Pharmacol. 129, 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanstall J. C., Jeffery T. K., Gambino A., Lovren F., Triggle C. R. (2001) Br. J. Pharamacol. 134, 463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irvine J. C., Favaloro J. L., Kemp-Harper B. K. (2003) Hypertension 41, 1301–1307 [DOI] [PubMed] [Google Scholar]
- 15.De Witt B. J., Marrone J. R., Kaye A. D., Keefer L. K., Kadowitz P. J. (2001) Eur. J. Pharmacol. 430, 311–315 [DOI] [PubMed] [Google Scholar]
- 16.Favaloro J. L., Kemp-Harper B. K. (2007) Cardiovasc. Res. 73, 587–596 [DOI] [PubMed] [Google Scholar]
- 17.Dierks E. A., Burstyn J. N. (1996) Biochem. Pharmacol. 51, 1593–1600 [DOI] [PubMed] [Google Scholar]
- 18.Sulc F., Immoos C. E., Pervitsky D., Farmer P. J. (2004) J. Am. Chem. Soc. 126, 1096–1101 [DOI] [PubMed] [Google Scholar]
- 19.Farmer P. J., Sulc F. (2005) J. Inorg. Biochem. 99, 166–184 [DOI] [PubMed] [Google Scholar]
- 20.Bartberger M. D., Fukuto J. M., Houk K. N. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2194–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith P. A. S., Hein J. (1960) J. Am. Chem. Soc. 82, 5731–5740 [Google Scholar]
- 22.Drago R. S., Paulik F. E. (1960) J. Am. Chem. Soc. 82, 96–98 [Google Scholar]
- 23.Sha X., Isbell T. S., Patel R. P., Day C. S., King S. B. (2006) J. Am. Chem. Soc. 128, 9687–9692 [DOI] [PubMed] [Google Scholar]
- 24.Serfass L., Carr H. S., Aschenbrenner L. M., Burstyn J. N. (2001) Arch. Biochem. Biophys. 387, 47–56 [DOI] [PubMed] [Google Scholar]
- 25.Kim T. D., Burstyn J. N. (1994) J. Biol. Chem. 269, 15540–15545 [PubMed] [Google Scholar]
- 26.Schmidt P., Schramm M., Schröder H., Stasch J. P. (2003) Protein Expr. Purif. 31, 42–46 [DOI] [PubMed] [Google Scholar]
- 27.Bonner F. T., Ravid B. (1975) Inorg. Chem. 14, 558–563 [Google Scholar]
- 28.Fukuto J. M., Hobbs A. J., Ignarro L. J. (1993) Biochem. Biophys. Res. Commun. 196, 707–713 [DOI] [PubMed] [Google Scholar]
- 29.Halliwell B., Gutteridge J. M. (1989) Free Radicals in Biology and Medicine, 2nd Edition, pp. 15–19, Clarendon Press, Oxford [Google Scholar]
- 30.Anderegg G. (1963) Helv. Chim. Acta 46, 2397–2410 [Google Scholar]
- 31.Dutton A. S., Fukuto J. M., Houk K. N. (2004) J. Am. Chem. Soc. 126, 3795–3800 [DOI] [PubMed] [Google Scholar]
- 32.Lopez B. E., Shinyashiki M., Han T. H., Fukuto J. M. (2007) Free Radic. Biol. Med. 42, 482–491 [DOI] [PubMed] [Google Scholar]
- 33.Shafirovich V., Lymar S. V. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 7340–7345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pino R. Z., Feelisch M. (1994) Biochem. Biophys. Res. Commun. 201, 54–62 [DOI] [PubMed] [Google Scholar]
- 35.Turk T., Hollocher T. C. (1992) Biochem. Biophys. Res. Commun. 183, 983–988 [DOI] [PubMed] [Google Scholar]
- 36.Miranda K. M. (2005) Coord. Chem. Rev. 249, 433–455 [Google Scholar]
- 37.Craven P. A., DeRubertis F. R. (1983) Biochim. Biophys. Acta 745, 310–321 [DOI] [PubMed] [Google Scholar]
- 38.Stasch J. P., Schmidt P. M., Nedvetsky P. I., Nedvetskaya T. Y., Arun K. H. S., Meurer S., Deile M., Taye A., Knorr A., Lapp H., Müller H., Turgay Y., Rothkegel C., Tersteegen A., Kemp-Harper B., Müller-Esterl W., Schmidt H. H. (2006) J. Clin. Invest. 116, 2552–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bari S. E., Martí M. A., Amorebieta V. T., Estrin D. A., Doctorovich F. (2003) J. Am. Chem. Soc. 125, 15272–15273 [DOI] [PubMed] [Google Scholar]
- 40.Doyle M. P., Mahapatro S. N., Broene R. D., Guy J. K. (1988) J. Am. Chem. Soc. 110, 593–599 [Google Scholar]
- 41.Ignarro L. J., Adams J. B., Horwitz P. M., Wood K. S. (1986) J. Biol. Chem. 261, 4997–5002 [PubMed] [Google Scholar]
- 42.Wolin M. S., Wood K. S., Ignarro L. J. (1982) J. Biol. Chem. 257, 13312–13320 [PubMed] [Google Scholar]
- 43.Fukuto J. M., Wallace G. C., Hszieh R., Chaudhuri G. (1992) Biochem. Pharmacol. 43, 607–613 [DOI] [PubMed] [Google Scholar]
- 44.Bermejo E., Sáenz D. A., Alberto F., Rosenstein R. E., Bari S. E., Lazzari M. A. (2005) Thromb. Haemost. 94, 578–584 [DOI] [PubMed] [Google Scholar]
- 45.Ellis A., Lu H., Li C. G., Rand M. J. (2001) Br. J. Pharmacol. 134, 521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ignarro L. J., Wood K. S., Wolin M. S. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 2870–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeMaster E. G., Redfern B., Nagasawa H. T. (1998) Biochem. Pharmacol. 55, 2007–2015 [DOI] [PubMed] [Google Scholar]
- 48.Maragos C. M., Morley D., Wink D. A., Dunams T. M., Saavedra J. E., Hoffman A., Bove A. A., Isaac L., Hrabie J. A., Keefer L. K. (1991) J. Med. Chem. 34, 3242–3247 [DOI] [PubMed] [Google Scholar]
- 49.Miranda K. M., Paolocci N., Katori T., Thomas D. D., Ford E., Bartberger M. D., Espey M. G., Kass D. A., Feelisch M., Fukuto J. M., Wink D. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9196–9201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong P. S., Hyun J., Fukuto J. M., Shirota F. N., DeMaster E. G., Shoeman D. W., Nagasawa H. T. (1998) Biochemistry 37, 5362–5371 [DOI] [PubMed] [Google Scholar]
- 51.Sayed N., Baskaran P., Ma X., van den Akker F., Beuve A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12312–12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Väänänen A. J., Salmenperä P., Hukkanen M., Miranda K. M., Harjula A., Rauhala P., Kankuri E. (2008) Free Radic. Biol. Med. 45, 749–755 [DOI] [PubMed] [Google Scholar]
- 53.Väänänen A. J., Kankuri E., Rauhala P. (2005) Free Radic. Biol. Med. 38, 1102–1111 [DOI] [PubMed] [Google Scholar]
- 54.Shen B., English A. M. (2005) Biochemistry 44, 14030–14044 [DOI] [PubMed] [Google Scholar]
- 55.Hoshino M., Maeda M., Konishi R., Seki H., Ford P. C. (1996) J. Am. Chem. Soc. 118, 5702–5707 [Google Scholar]
- 56.Rusche K. M., Spiering M. M., Marletta M. A. (1998) Biochemistry 37, 15503–15512 [DOI] [PubMed] [Google Scholar]
- 57.Shahidullah M., Duncan A., Strachan P. D., Rafique K. M., Ball S. L., McPate M. J., Nelli S., Martin W. (2002) Eur. J. Pharmacol. 435, 93–101 [DOI] [PubMed] [Google Scholar]
- 58.Miranda K. M., Katori T., Torres de Holding C. L., Thomas L., Ridnour L. A., McLendon W. J., Cologna S. M., Dutton A. S., Champion H. C., Mancardi D., Tocchetti C. G., Saavedra J. E., Keefer L. K., Houk K. N., Fukuto J. M., Kass D. A., Paolocci N., Wink D. A. (2005) J. Med. Chem. 48, 8220–8228 [DOI] [PubMed] [Google Scholar]
- 59.Stone J. R., Sands R. H., Dunham W. R., Marletta M. A. (1996) Biochemistry 35, 3258–3262 [DOI] [PubMed] [Google Scholar]
- 60.Sulc F., Fleischer E., Farmer P. J., Ma D., La Mar G. N. (2003) J. Biol. Inorg. Chem. 8, 348–352 [DOI] [PubMed] [Google Scholar]
- 61.Immoos C. E., Sulc F., Farmer P. J., Czarnecki K., Bocian D. F., Levina A., Aitken J. B., Armstrong R. S., Lay P. A. (2005) J. Am. Chem. Soc. 127, 814–815 [DOI] [PubMed] [Google Scholar]
- 62.Pervitsky D., Immoos C., Veer W., Farmer P. J. (2007) J. Am. Chem. Soc. 129, 9590–9591 [DOI] [PubMed] [Google Scholar]
- 63.Poulos T. L. (2006) Curr. Opin. Struct. Biol. 16, 736–743 [DOI] [PubMed] [Google Scholar]
- 64.Wayland B. B., Newman A. R. (1979) J. Am. Chem. Soc. 101, 6472–6473 [Google Scholar]
- 65.Yonetani T., Yamamoto H., Erman J. E., Leigh J. S., Jr., Reed G. H. (1972) J. Biol. Chem. 247, 2447–2455 [PubMed] [Google Scholar]
- 66.Wayland B. B., Minkiewixz J. V., Abd-Elmageed M. E. (1974) J. Am. Chem. Soc. 96, 2795–2801 [Google Scholar]
- 67.Scheidt W. R., Hoard J. L. (1973) J. Am. Chem. Soc. 95, 8281–8288 [DOI] [PubMed] [Google Scholar]
- 68.Niederhoffer E. C., Timmons J. H., Martell A. E. (1984) Chem. Rev. 84, 137–203 [Google Scholar]
- 69.Stone J. R., Sands R. H., Dunham W. R., Marletta M. A. (1995) Biochem. Biophys. Res. Commun. 207, 572–577 [DOI] [PubMed] [Google Scholar]
- 70.Derbyshire E. R., Tran R., Mathies R. A., Marletta M. A. (2005) Biochemistry 44, 16257–16265 [DOI] [PubMed] [Google Scholar]
- 71.Lin R., Farmer P. J. (2000) J. Am. Chem. Soc. 122, 2393–2394 [Google Scholar]
- 72.Dutton A. S., Fukuto J. M., Houk K. N. (2005) Inorg. Chem. 44, 4024–4028 [DOI] [PubMed] [Google Scholar]
- 73.Feelisch M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4978–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paolocci N., Katori T., Champion H. C., St. John M. E., Miranda K. M., Fukuto J. M., Wink D. A., Kass D. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5537–5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Irvine J. C., Favaloro J. L., Widdop R. E., Kemp-Harper B. K. (2007) Hypertension 49, 885–892 [DOI] [PubMed] [Google Scholar]
- 76.Thadani U., Ripley T. L. (2007) Expert Opin. Drug. Saf. 6, 385–396 [DOI] [PubMed] [Google Scholar]