Abstract
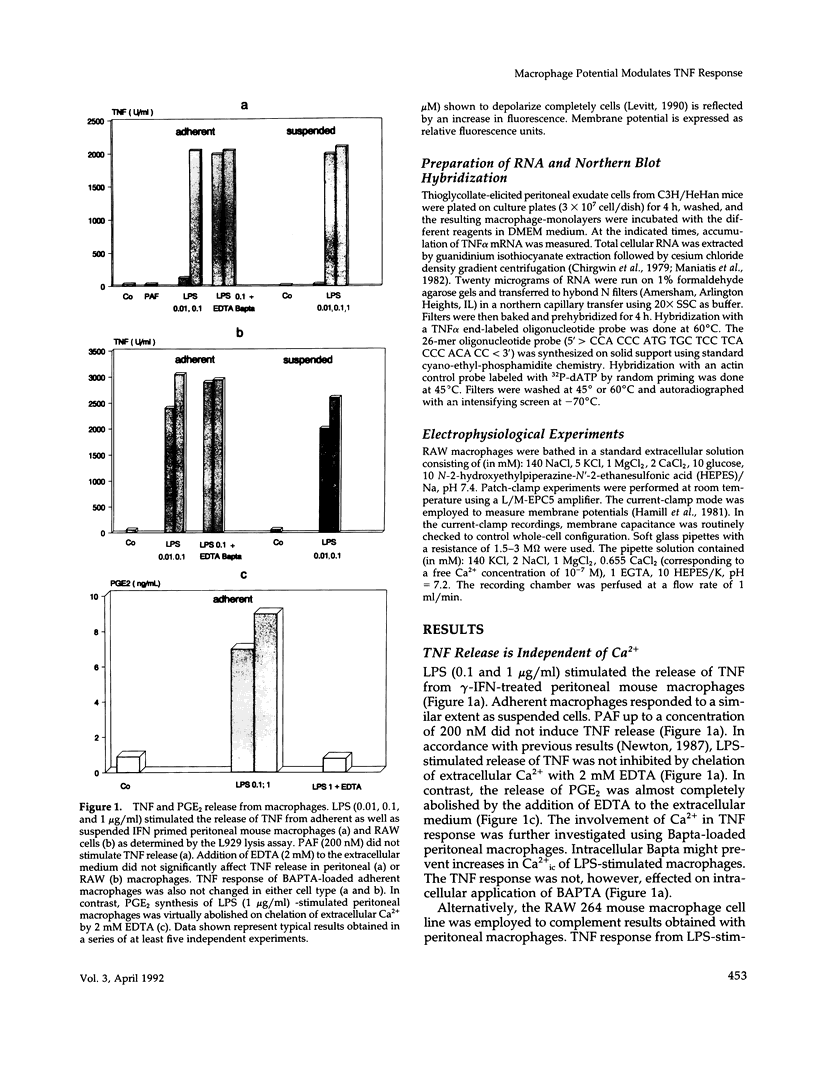
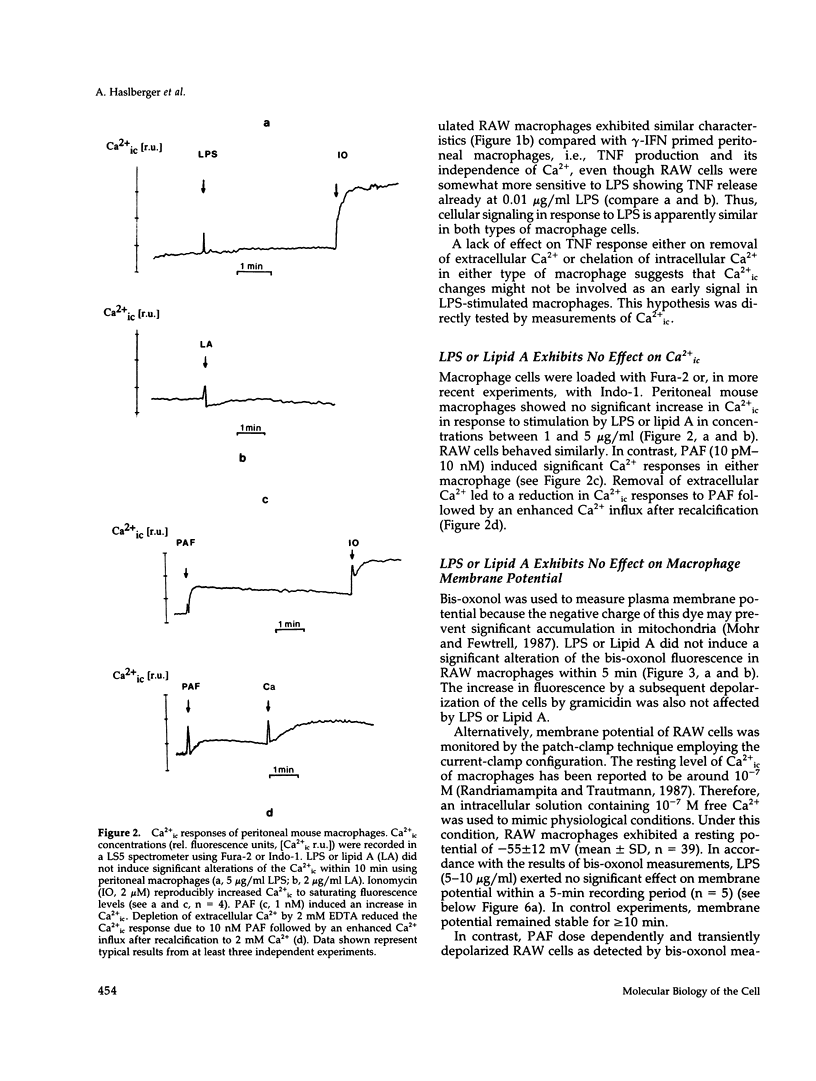
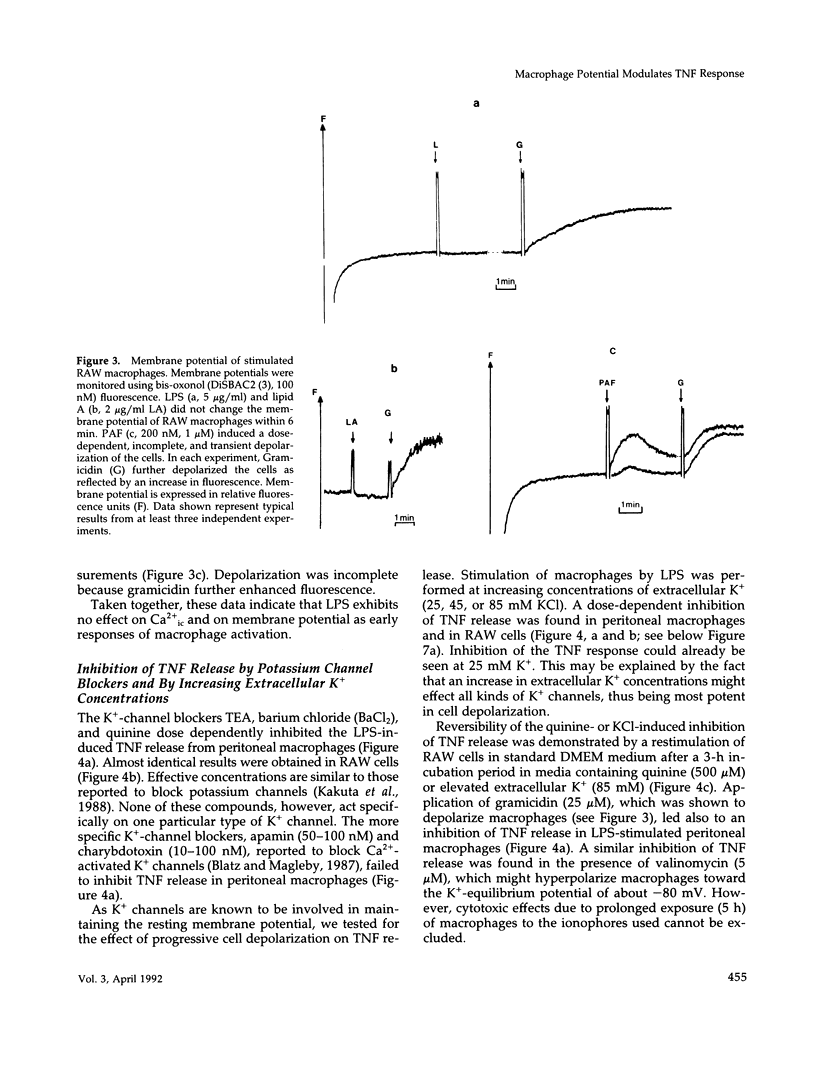
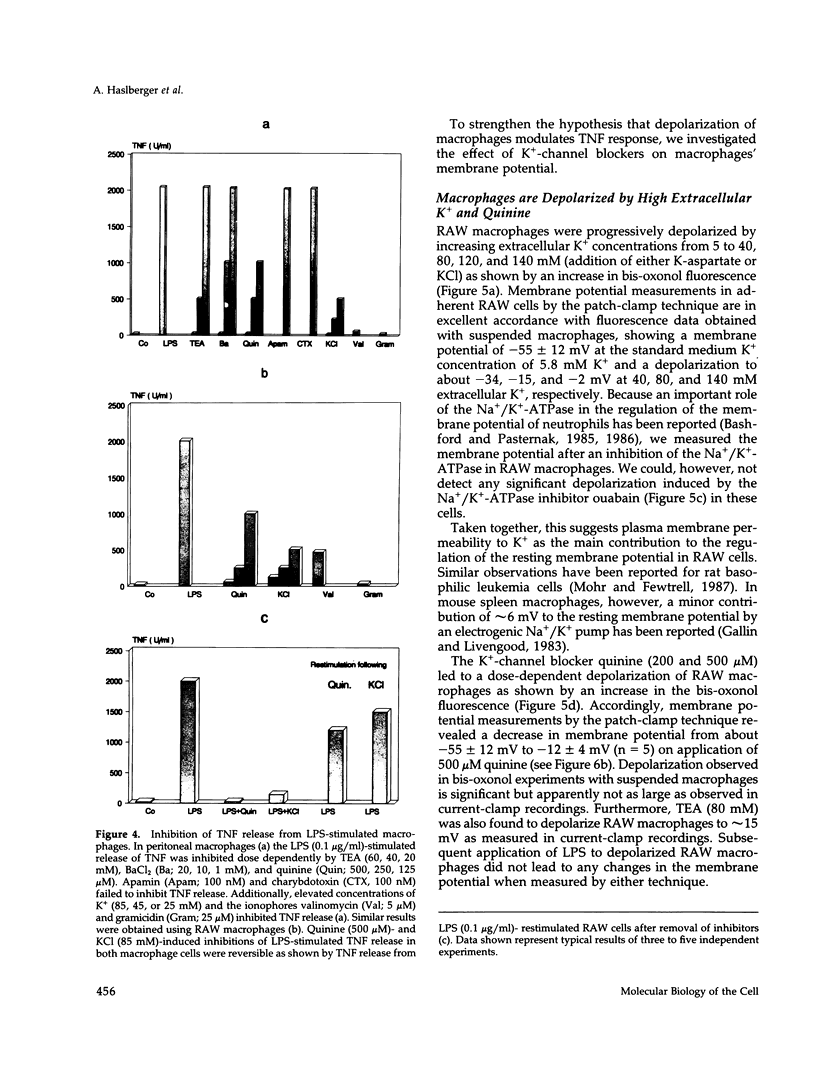
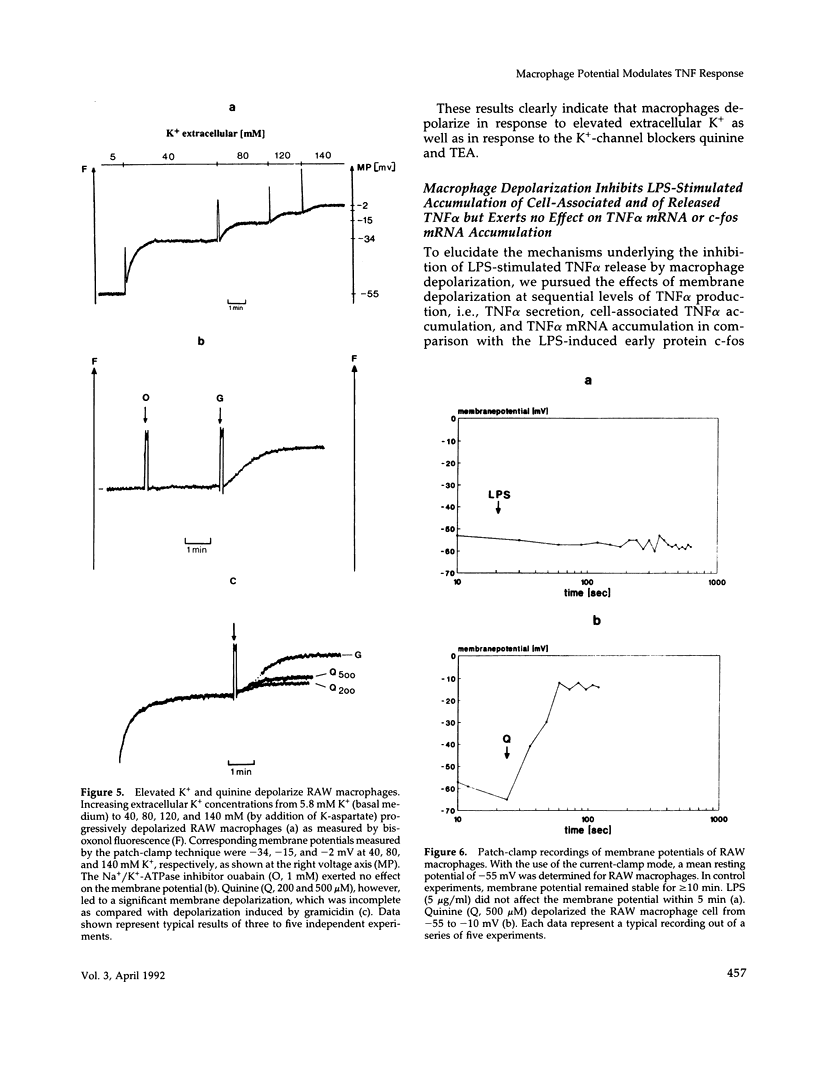
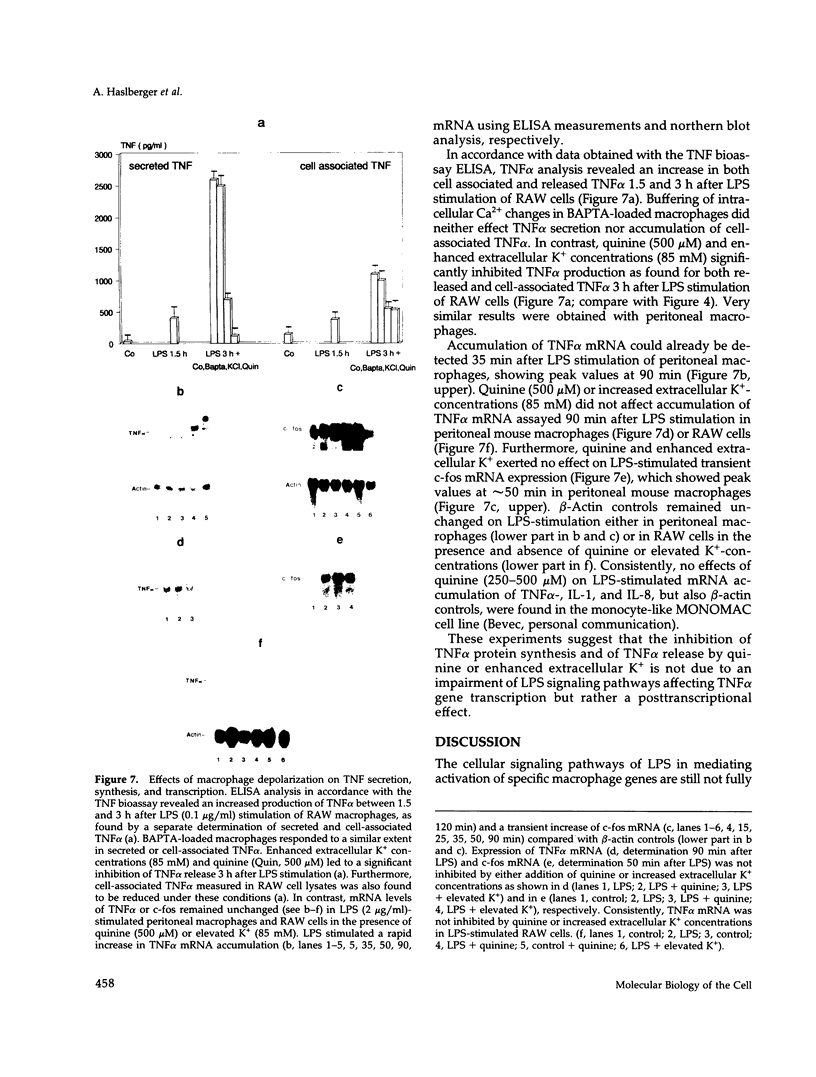
Lipopolysaccharide (LPS)-mediated synthesis of macrophage gene products such as tumor necrosis factor (TNF) is controlled by different signaling pathways. We investigated intracellular free Ca2+ (Ca2+ic) and the membrane potential as early cellular responses to LPS and their role in the synthesis and release of TNF. In peritoneal macrophages and in the RAW 269 mouse macrophage cell line, LPS and its biologically active moiety lipid A stimulated TNF synthesis but exerted no significant effects on these early cellular responses using Fura-2/Indo-1 to measure Ca2+ic and bis-oxonol, as well as the patch-clamp technique to monitor membrane potential. In contrast, the platelet-activating factor transiently induced both an increase in Ca2+ic and cell membrane depolarization but no significant TNF release. Increased extracellular K+ concentrations or K(+)-channel blockers, such as quinine, tetraethylammonium, or barium chloride, inhibited the LPS-stimulated release of TNF alpha, as well as the accumulation of cell-associated TNF alpha as found by enzyme-linked immunosorbent assay analysis, but did not inhibit TNF alpha mRNA accumulation. Concentrations of quinine (greater than 125 microM) or of enhanced extracellular K+ (25-85 mM) required to inhibit TNF production both significantly depolarized macrophages. These results indicate a lack of ion transport activities as early cellular responses of macrophages to LPS but suggest an important regulatory role of the membrane potential on the posttranscriptional synthesis and release of TNF in macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford C. L., Pasternak C. A. Plasma membrane potential of neutrophils generated by the Na+ pump. Biochim Biophys Acta. 1985 Jul 11;817(1):174–180. doi: 10.1016/0005-2736(85)90080-x. [DOI] [PubMed] [Google Scholar]
- Chandy K. G., DeCoursey T. E., Cahalan M. D., McLaughlin C., Gupta S. Voltage-gated potassium channels are required for human T lymphocyte activation. J Exp Med. 1984 Aug 1;160(2):369–385. doi: 10.1084/jem.160.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy K. G., DeCoursey T. E., Fischbach M., Talal N., Cahalan M. D., Gupta S. Altered K+ channel expression in abnormal T lymphocytes from mice with the lpr gene mutation. Science. 1986 Sep 12;233(4769):1197–1200. doi: 10.1126/science.2426784. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choquet D., Korn H. Modulation of voltage-dependent potassium channels in B lymphocytes. Biochem Pharmacol. 1988 Oct 15;37(20):3797–3802. doi: 10.1016/0006-2952(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Pandiella A., Meldolesi J., Pozzan T. Generation of inositol phosphates, cytosolic Ca2+, and ionic fluxes in PC12 cells treated with bradykinin. J Biol Chem. 1988 Nov 25;263(33):17350–17359. [PubMed] [Google Scholar]
- Gallin E. K., Livengood D. R. Demonstration of an electrogenic Na+-K+ pump in mouse spleen macrophages. Am J Physiol. 1983 Sep;245(3):C184–C188. doi: 10.1152/ajpcell.1983.245.3.C184. [DOI] [PubMed] [Google Scholar]
- Gallin E. K., McKinney L. C. Patch-clamp studies in human macrophages: single-channel and whole-cell characterization of two K+ conductances. J Membr Biol. 1988 Jul;103(1):55–66. doi: 10.1007/BF01871932. [DOI] [PubMed] [Google Scholar]
- Gelfand E. W., Cheung R. K., Mills G. B., Grinstein S. Role of membrane potential in the response of human T lymphocytes to phytohemagglutinin. J Immunol. 1987 Jan 15;138(2):527–531. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hampton R. Y., Golenbock D. T., Raetz C. R. Lipid A binding sites in membranes of macrophage tumor cells. J Biol Chem. 1988 Oct 15;263(29):14802–14807. [PubMed] [Google Scholar]
- Haslberger A., Sayers T., Reiter H., Chung J., Schütze E. Reduced release of TNF and PCA from macrophages of tolerant mice. Circ Shock. 1988 Oct;26(2):185–192. [PubMed] [Google Scholar]
- Introna M., Bast R. C., Jr, Tannenbaum C. S., Hamilton T. A., Adams D. O. The effect of LPS on expression of the early "competence" genes JE and KC in murine peritoneal macrophages. J Immunol. 1987 Jun 1;138(11):3891–3896. [PubMed] [Google Scholar]
- Jakway J. P., DeFranco A. L. Pertussis toxin inhibition of B cell and macrophage responses to bacterial lipopolysaccharide. Science. 1986 Nov 7;234(4777):743–746. doi: 10.1126/science.3095921. [DOI] [PubMed] [Google Scholar]
- Kakuta Y., Okayama H., Aikawa T., Kanno T., Ohyama T., Sasaki H., Kato T., Takishima T. K channels of human alveolar macrophages. J Allergy Clin Immunol. 1988 Feb;81(2):460–468. doi: 10.1016/0091-6749(88)90918-9. [DOI] [PubMed] [Google Scholar]
- Labrecque G. F., Holowka D., Baird B. Antigen-triggered membrane potential changes in IgE-sensitized rat basophilic leukemia cells: evidence for a repolarizing response that is important in the stimulation of cellular degranulation. J Immunol. 1989 Jan 1;142(1):236–243. [PubMed] [Google Scholar]
- LeFever A., Liepins A. Kinetic analysis of K+ ion channel function in lymphokine-activated killer (LAK) cells. Immunopharmacol Immunotoxicol. 1990;12(1):23–38. doi: 10.3109/08923979009006459. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Levitt D. Gramicidin, VDAC, porin and perforin channels. Curr Opin Cell Biol. 1990 Aug;2(4):689–694. doi: 10.1016/0955-0674(90)90112-r. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Cahalan M. D. The plasticity of ion channels: parallels between the nervous and immune systems. Trends Neurosci. 1988 May;11(5):214–218. doi: 10.1016/0166-2236(88)90129-4. [DOI] [PubMed] [Google Scholar]
- MacDougall S. L., Grinstein S., Gelfand E. W. Activation of Ca2+-dependent K+ channels in human B lymphocytes by anti-immunoglobulin. J Clin Invest. 1988 Feb;81(2):449–454. doi: 10.1172/JCI113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon D., Ceredig R. Changes in the expression of potassium channels during mouse T cell development. J Exp Med. 1986 Dec 1;164(6):1846–1861. doi: 10.1084/jem.164.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. IgE receptor-mediated depolarization of rat basophilic leukemia cells measured with the fluorescent probe bis-oxonol. J Immunol. 1987 Mar 1;138(5):1564–1570. [PubMed] [Google Scholar]
- Newton R. C. Lack of a central role for calcium in the induction and release of human interleukin-1. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1027–1033. doi: 10.1016/s0006-291x(87)80173-0. [DOI] [PubMed] [Google Scholar]
- Price M., Lee S. C., Deutsch C. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10171–10175. doi: 10.1073/pnas.86.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prpic V., Weiel J. E., Somers S. D., DiGuiseppi J., Gonias S. L., Pizzo S. V., Hamilton T. A., Herman B., Adams D. O. Effects of bacterial lipopolysaccharide on the hydrolysis of phosphatidylinositol-4,5-bisphosphate in murine peritoneal macrophages. J Immunol. 1987 Jul 15;139(2):526–533. [PubMed] [Google Scholar]
- Randriamampita C., Trautmann A. Biphasic increase in intracellular calcium induced by platelet-activating factor in macrophages. FEBS Lett. 1989 Jun 5;249(2):199–206. doi: 10.1016/0014-5793(89)80624-6. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Trautmann A. Ionic channels in murine macrophages. J Cell Biol. 1987 Aug;105(2):761–769. doi: 10.1083/jcb.105.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Tordai A., Gárdos G. Membrane depolarization selectively inhibits receptor-operated calcium channels in human T (Jurkat) lymphoblasts. Biochim Biophys Acta. 1990 Aug 24;1027(2):130–140. doi: 10.1016/0005-2736(90)90076-z. [DOI] [PubMed] [Google Scholar]
- Schell S. R., Nelson D. J., Fozzard H. A., Fitch F. W. The inhibitory effects of K+ channel-blocking agents on T lymphocyte proliferation and lymphokine production are "nonspecific". J Immunol. 1987 Nov 15;139(10):3224–3230. [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C. S., Koerner T. J., Jansen M. M., Hamilton T. A. Characterization of lipopolysaccharide-induced macrophage gene expression. J Immunol. 1988 May 15;140(10):3640–3645. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Wightman P. D., Raetz C. R. The activation of protein kinase C by biologically active lipid moieties of lipopolysaccharide. J Biol Chem. 1984 Aug 25;259(16):10048–10052. [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]