Abstract
In addition to polyamine homeostasis, it has become increasingly clear that polyamine catabolism can play a dominant role in drug response, apoptosis, response to stressful stimuli, and contribute to the etiology of several pathological states, including cancer. The highly inducible enzymes spermidine/spermine N1-acetyltransferase (SSAT) and spermine oxidase (SMO), and, the generally constitutively expressed N1-acetylpolyamine oxidase (APAO), appear to play critical roles in many normal and disease processes. The dysregulation of polyamine catabolism frequently accompanies several disease states and suggests that such dysregulation may both provide useful insight into disease mechanism and provide unique drugable targets that can be exploited for therapeutic benefit. Each of these enzymes has the potential to alter polyamine homeostasis in response to multiple cell signals and the two oxidases produce the reactive oxygen species H2O2 and aldehydes, each with the potential to produce pathologies. The activity of SSAT has the potential to provide substrates for APAO or substrates for the polyamine exporter, thus reducing the intracellular polyamine concentration, the net effect of which depends on the magnitude and rate of any increase in SSAT. SSAT may also influence cellular metabolism via interaction with other proteins and by perturbing the content of acetyl CoA and ATP. The goal of this review is to cover those aspects of polyamine catabolism that have potential to impact disease etiology or treatment and to provide a solid background in this ever more exciting aspect of polyamine biology.
Keywords: Spermine, spermidine, oxidases, acetyl CoA, oxidative damage
Introduction
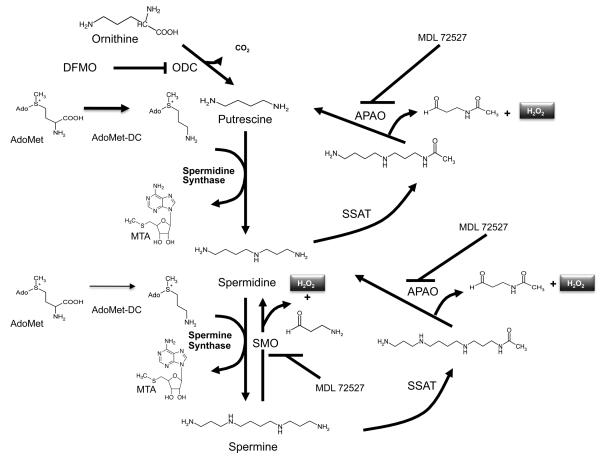
The recognition that polyamines are absolute requirements for mammalian cell growth has led to: 1) the study of the molecular mechanism of cell proliferation in which these naturally occurring alkylamines are involved and; 2) the targeting of their function and metabolism as a strategy for antiproliferative therapy [1, 2]. Intracellular polyamine concentrations are maintained at a cell type-specific level by a highly regulated metabolic pathway, an energy dependent transport system, and an export system that leads to the efflux of both modified and unmodified polyamines. Highly regulated rate-limiting enzymes facilitate the fine control of both biosynthesis and catabolism. In biosynthesis (Fig. 1), ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (AdoMetDC) collaborate to produce the higher polyamines spermidine and spermine. Putrescine, the product of ODC is used as the substrate for the constitutive spermidine synthase that adds the aminopropyl group donated from the decarboxylated S-adenosylmethionine provided by AdoMetDC. Spermine synthase performs a similar function on spermidine, producing spermine by the addition of another aminopropyl group. Inhibitors for each of the biosynthetic enzymes exist and have been extensively tested in multiple in vitro and in vivo systems [3]. Particular emphasis has focused on the rate-limiting enzymes, ODC and AdoMetDC. However, with respect to neoplastic disease none of these inhibitors has demonstrated clinical effectiveness in clinical trials. The reasons for these less than optimal results most probably include pharmacokinetic parameters and compensatory mechanisms within the tumor cells that allow compensation for loss of a single enzyme's activity. It should be noted however, that difluoromethylornithine (DFMO), an inhibitor of ODC, is an effective clinical agent against Trypanosoma brucei gambiense, one of the causative agents of African sleeping sickness [4]. Additionally, DFMO has demonstrated promising results in human trials as a chemopreventive agent [5, 6].
Figure 1.
The polyamine metabolic pathway. ODC, ornithine decarboxylase; AdoMet, S-adenosylmethionine; AdoMetDC, S-adenosylmethionine decarboxylase, this enzyme provides the aminopropyl group for the synthesis of spermidine and spermine from putrescine and spermidine, respectively; MTA, methylthioadeonsine; SMO, spermine oxidase is an inducible enzyme that produces the reactive oxygen species, H2O2 and the aldehyde, 3-aminopropanal; SSAT, spermidine/spermine N1-acetyltransferase produces the acetylated polyamines that can be oxidized by APAO or excreted from the cell; APAO, N1-acetylpolyamine oxidase is a peroxisomal enzyme that oxidizes N1-acetylated polyamines producing 3-acetoaminopropanal and H2O2; DFMO, 2-difluoromethylornithine (an inhibitor of ODC); MDL 72527, N1,N4-bis(buta-2,3-dienyl)butanediamine (an inhibitor of both APAO and SMO).
Initially, the mammalian polyamine catabolic pathway was thought to contain two enzymes, a rate-limiting acetylase, spermidine/spermine N1-acetyltransferase (SSAT) and a constitutively expressed N1-acetylpolyamine oxidase (APAO) [7]. It is generally thought that the activity of APAO is rate-limited by the availability of the acetylated product produced by SSAT (Fig. 2).
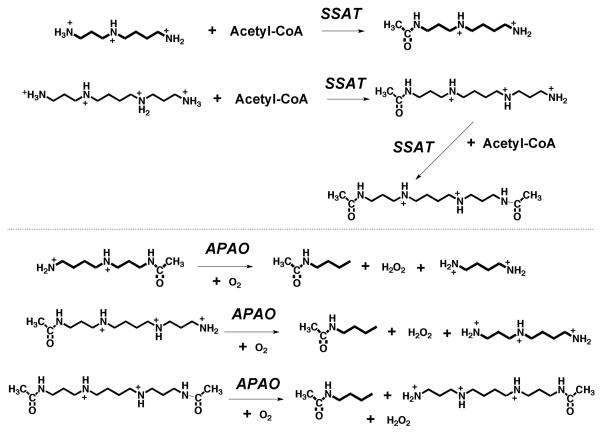
Figure 2.
Reactions catalyzed by SSAT and APAO. Physiological substrates for SSAT are spermidine, spermine and N1-acetylspermine. Physiological substrates for APAO are N1-acetylspermidine, N1-acetylspermine and N1,N12-acetylspermine.
During the development of alternatives to single enzyme inhibitors, the true significance of the of polyamine catabolism in polyamine homeostasis and cell survival became more clear. One of the earliest and most biochemically interesting class of polyamine analogues were the bis(alkylated)spermine analogues [8]. Besides down-regulating the activity of the biosynthetic enzymes they were found to massively increase the expression of SSAT [9, 10]. It was also during the studies of the molecular regulation of this massive up-regulation that both the potential therapeutic benefits of targeted up-regulation of polyamine catabolism and the potential pathological effects of aberrant increases in polyamine catabolism began to be recognized. More recently, in an attempt to more fully understand the mechanisms of cytotoxicity mediated by the up-regulation of polyamine catabolism, a new mammalian enzyme was discovered, spermine oxidase (SMO) [11, 12]. SMO is also induced by various stimuli, including polyamine analogues and pathogens and thus can have a role both as a target for antiproliferative therapy and as a potential molecular basis for specific pathologies.
The last few years has seen a steady increase in studies examining the role of intracellular polyamine catabolism in the pathologies of several human diseases as well as potential targets for chemotherapy and chemoprevention. Therefore, it is the goal of this review to summarize the most recent finding in the field related to polyamine catabolism and specifically to address those issues that are relevant to human disease.
SSAT: a highly regulated, rate-limiting step in polyamine catabolism
Since APAO has very poor activity on unacetylated polyamines, flux through this enzyme is controlled by the supply of acetylated substrates. These are produced by the action of the highly inducible enzyme SSAT. Early studies on the activity of SSAT and the regulation of its activity have been summarized in reviews [7, 13]. SSAT is a primarily cytosolic enzyme although a mitochondrial form, whose function is unknown, has also been described. Its physiological substrates are spermidine and spermine [14]. It uses acetyl-CoA to acetylate primary amines. Importantly, it strongly prefers substrates of the structure RNH(CH2)3NH2 (Fig. 2). Compounds with only two methylene groups (RNH(CH2)2NH2) are acetylated poorly, whereas those with four methylene groups (RNH(CH2)4NH2) are not substrates. Therefore, only the aminopropyl end of spermidine is attacked to form N1-acetylspermidine. Spermine, which is symmetrical, can be attacked at both ends and both N1-acetylspermine and N1-,N12-diacetylspermine have been observed as products. Under normal conditions, the content of SSAT is very low but it is rapidly and extensively induced by conditions increasing polyamines or by polyamine analogues and by a variety of pathophysiological stimuli. The detection of N1-acetylated polyamines in urine, extracellular fluids or in cell extracts is usually highly indicative of increased SSAT activity although this accumulation obviously depends on their ability to escape degradation by APAO.
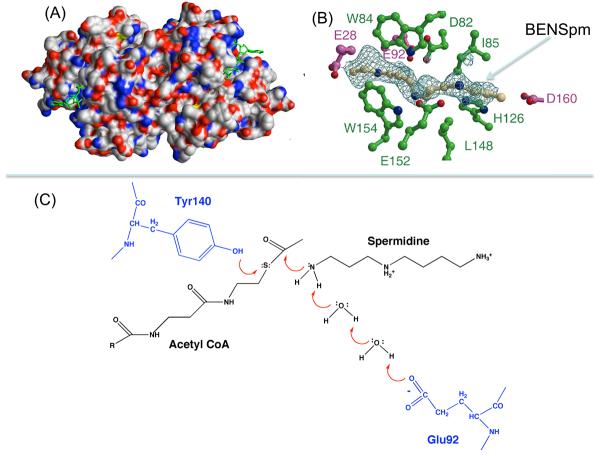
Relatively recent studies of the structure and mechanism of SSAT have shown that the enzyme is a member of the GNAT (GCN5-related N-acetyltransferase) family. It is a homodimer with a monomer of a 171 amino acids in the case of the human protein. Crystal structures of the human and mouse SSAT protein have been obtained in the presence and absence of ligands including substrates and inhibitors (Fig. 3) [15-17]. These studies provide a good understanding of the reaction mechanism and the substrate specificity. The dimeric unit contains two active sites, each located at the dimer interface and involving residues from both subunits. Tyr140, a totally conserved residue, is likely to serve as the general acid for protonation of the thiolate anion of acetyl-CoA. Glu92 interacting with water molecules acts as a general base that extracts the proton for the attacking N1- group of the polyamine substrate. Mutation of either of these residues has a profound effect on the activity. Structures of SSAT in complex with polyamines analogues such as BENSpm (N1, N11-bis(ethyl)norspermine) or BESpm (N1, N12-bis(ethyl)spermine) show that these bind to the protein using many of the same residues as those involved in substrate binding. The strong binding of these analogues accounts for their profound effect on the protein stability described below and for the fact that they act as weak inhibitors of the acetylation reaction (Fig. 3C).
Figure 3.
Structure of SSAT. (A) Molecular surface of SSAT dimer colored according to element (nitrogen, blue; oxygen, red; carbon, grey; sulfur, yellow) with acetyl CoAs shown in green. (B) BENSpm bound to SSAT. Residues from one subunit are shown in green and from the other in purple. (C) Reaction mechanism showing role of Tyr140 and Glu92.
SSAT can self-acetylate at Lys26 [15]. This is an exception to the substrate specificity rules describe above. The significance of this reaction is unknown but it could regulate to the regulation of SSAT or suggest that, under some circumstances, SSAT may acetylate associated proteins.
The Sat1 gene encodes SSAT, which is present on the X chromosome at location Xp22.1. A related gene termed Sat2 encodes another closely related GNAT family member. The human Sat2 gene product has a crystal structure similar overall to SSAT but with significant differences in charged surface residues and in the presumed amine substrate binding residues of the active site region [18]. The protein has 170 amino acids having 46% sequence identity and 64% sequence similarity with SSAT. Despite this similarity, it has negligible activity to acetylate polyamines. The kcat/Km specificity constant for spermidine and spermine was <0.001% of genuine SSAT and expression of the Sat2 product did not increase cellular acetylpolyamines or reduce free polyamine content [19]. Further characterization reveals the substrate for this human protein, and for homologs from other species, is thialysine (S-(2-aminoethyl-L-cysteine) Related compounds such as 5-hydroxy-L-lysine, S-(aminoethyl)-homocysteine and O-(aminoethyl)-L-serine [19, 20]. It is therefore more correctly described as thialysine-Ne-acetyltransferase.
Function of SSAT and interactions with other proteins
The primary function of SSAT is undoubtedly to maintain polyamine homeostasis. Induction of SSAT in response to a rise in polyamines leads to acetylation of spermidine and spermine, which not only changes their potential physiological activity due to alteration in charge, but also facilitates excretion and degradation via the SSAT/APAO pathway. Several other potential functions have been suggested based on the interaction of SSAT with important cellular proteins.
Current data suggest that there may be a direct connection between SSAT and polyamine efflux from the cell. The existence of a specific efflux mechanism for putrescine and acetylpolyamines has been known for some time. Recently, a transporter has been characterized and found to consist of SLC3A2, previously known as a glycosylated heavy chain of a cationic amino acid transporter and its partner y+ LAT light chain [21]. Efflux was coupled to arginine uptake, indicating that there is a putrescine/arginine exchange reaction. SSAT was co-localized with SLC3A2 and was co-immunoprecipitated by an anti-SLC3A2 antibody suggesting that this efflux system may be closely linked to polyamine acetylation [21].
There is a specific and functional interaction between SSAT and integrin α9β1 [22, 23]. This integrin, which mediates cell migration, binds to ligands at sites of tissue injury and inflammation. The N-terminal region of SSAT binds to the α9 cytoplasmic domain. This binding of SSAT is needed for α9β1-mediated migration and requires the SSAT catalytic activity [22]. The integrin and the Kir4.2 K+ ion channel co-localize in focal adhesions at the leading edge of migrating cells. SSAT-mediated catabolism of spermidine and/or spermine, which are known to mediate inward rectification of such channels [24], alters K+ ion efflux through the Kir4.2 channel [23]. These results strongly support a novel pathway through which an anchored SSAT molecule mediates localized polyamine catabolism and subsequent biological effects.
SSAT binds to the hypoxia-inducible factor 1 (HIF-1) α subunit [25]. HIF-1α regulates transcription at hypoxia response elements contained in genes involved in angiogenesis, glucose metabolism, erythropoiesis, inflammation, apoptosis, and cellular stress. SSAT binding stabilizes the interaction of the form of HIF-1α present in hypoxic conditions with RACK-1 and elongin C and promotes the ubiquitination and degradation of HIF-1α. Interestingly, thialysine-Ne-acetyltransferase, which, as described above, has similar overall structure to SSAT, also binds to HIF-1α and promotes degradation. However, it stabilizes the complex of hydroxylated HIF-1α that is formed under aerobic conditions with von Hippel-Lindau protein (VHL) and elongin C [26]. Thus, these two acetyltransferases promote the O2-independent and O2-dependent degradation of HIF-1α respectively. The mechanism of the effect is not clear but requires enzymatically active forms of the acetyltransferases and could involve protein acetylation. As described above, SSAT can acetylate an unmodified lysine in itself and thialysine-Ne-acetyltransferase can act on 5-hydroxylysine, which might be formed in the target HIF-1α by a lysine hydroxylase using O2.
Induction of SSAT
The content of SSAT is normally very low and in resting, uninduced cells much of the minimal activity that is detected in in vitro assays of cell extracts is not due to SSAT but to other less specific acetyltransferases and nonenzymatic reactions of acetyl-CoA. Many factors cause a large and rapid induction of SSAT activity (reviewed in: [7, 13, 27, 28]). The increase in activity results from a rise in the amount of SSAT protein. Covalent post-translational modifications or low molecular weight effector molecules increasing activity have not been described. Stimuli elevating SSAT levels include polyamines, polyamine analogues, hormones and cytokines (corticosteroids, estradiol, progesterone, calcitriol, growth hormone, PTH, catecholamines and IGF-1), stress pathways (including ischemia-reperfusion injury), cachexia, natural products (gossypol,7-β-hydroxycholesterol or 7-β-hydroxysitosterol, H2O2, resveratrol and interferons) and drugs (non-steroidal anti-inflammatory agents (NSAIDS), cisplatins, 5-fluorouracil).
Transcriptional regulation
The Sat1 gene encoding SSAT contains sites for the binding of transcription factors that can respond to these stimuli either directly or via the polyamine responsive element (PRE). Polyamines are powerful inducers of SSAT and some of the factors listed above may act indirectly by raising the content of free polyamines either by increasing synthesis or causing release from bound sites. The Sat1 promoter region lacks a TATA box but has potential binding sites for Sp-1, AP-1, C/EBPβ, CREB, NFκB and PPARs. The Sat1 gene also contains a sequence 5′-TATGACTAA-3′ which acts as a PRE. It occurs about 1500 base pairs upstream from the transcriptional start site and allows the increased transcription of SSAT mRNA when polyamine content is high [13, 27]. The transcription factor, NF-E2-related factor 2 (Nrf-2) interacts constitutively with the PRE. Binding of the leucine zipper region of Nrf-2 to a 20 kDa protein termed polyamine-modulated factor-1 (PMF-1) turns on the transcription of SSAT in response to polyamines [29]. Mutations that alter the ability of these factors to interact destroy their ability to regulate the transcription of the SSAT gene [30]. Thioredoxin-1 reduces SSAT mRNA expression by decreasing PMF-1/Nrf-2 binding [31].
NSAIDs, Tumor necrosis factor-α (TNFα), and inflammatory stress increase SSAT mRNA production via nuclear factor kB (NFκB)
There are two NFkB response elements in the Sat1 gene [32] and their occupation by NFκB is increased by NSAIDs or by TNFα [28, 33]. NSAIDs also increase Sat1 transcription via its peroxisomal proliferators response element (PPRE) and PPAR-γ [34]. The gene contains two PPREs, one of which is located at +48 bases relative to the transcription start site is specifically bound by PPAR-γ and is clearly implicated in the induction by NSAIDs. Resveratrol also acts to increase SSAT mRNA production using the PPREs [35] whereas, expression of an activated Ki-Ras oncogene gene reduces expression via this PPAR-γ mediated system [36, 37].
Post-transcriptional regulation
Induction of SSAT to high levels requires a reinforcement of the effect produced by the increased transcription. Several levels of post-transcriptional regulation are documented and all respond to increased polyamine levels by increasing the amount of SSAT. These include: SSAT mRNA stability; alternative splicing of the SSAT mRNA; translational regulation of SSAT synthesis; and stabilization of SSAT protein, which normally turns over very rapidly.
The Sat1 gene contains 6 exons: exon 1 contains the 5′UTR and the first 22 codons of the coding sequence; exons 2-5 contains parts of the coding sequence; exon 6 contains the last 56 amino acid codons, the stop codon and the 3'UTR. These exons encode mRNAs of about 1.3 and 1.5 kb. The SSAT pre-mRNA can also undergo alternative splicing by the inclusion of an additional 110bp exon between exons 3 and 4 to form SSAT-X mRNA [38]. The encoded sequence contains multiple premature termination codons thus rendering the SSAT-X variant a target for nonsense mediated mRNA decay. The inclusion of this exon is inhibited by polyamines, which therefore leads to more of the correct and stable mRNA [38]. The SSAT-X mRNA has been found to accumulate in response to X-rays, viruses and hypoxia (see [13] for references). Inhibition of host protein synthesis is known to stabilize transcripts targeted to nonsense-mediated mRNA decay.
There is also convincing evidence for translational regulation of SSAT synthesis [13, 39]. The movement of SSAT mRNA into polysomes and enhanced SSAT synthesis occurs in response to increased polyamines. This translational regulation does not require either the 5' or 3' untranslated regions of the mRNA. A cytoplasmic protein, which binds to two regions (localized to nucleotides 1-45 and 492-504 of the coding region) in the mRNA, was detected and this binding was inhibited by BENSpm [39]. This protein may therefore repress SSAT synthesis by binding to the mRNA and blocking translation, but it has not yet been fully characterized.
SSAT turns over very rapidly with a half life of approximately 15 min. Degradation is carried out by the 26S proteasome after polyubiquitination and rapid turnover requires the –MATEE sequence located at the at the C-terminal end of the protein [13, 40-42]. The site of ubiquitin addition is probably the surface Lys87 but this is not absolutely established. Binding of polyamines to the protein interferes with ubiquitination and greatly increases the half-life of SSAT to >>12 h thus causing a large amplification of the effect provided by increased mRNA content and translation.
Induction of SSAT by polyamine analogues
Regulation of SSAT levels by polyamines via effects on all of the steps described above allows cells respond to respond to increased free polyamine content and thus maintain polyamine homeostasis. Some polyamine analogues with N-terminal alkyl groups such as BENSpm or BESpm produce an even more striking and prolonged increase in SSAT because their content does not decrease in response to the activation of the SSAT/APAO pathway and they may have a stronger effect on the regulation steps. Binding of such polyamine analogues to the SSAT protein occurs at a site overlapping the polyamine-binding portion of the active site. Therefore they act as weak inhibitors of the enzymatic reaction but the massive accumulation of SSAT protein clearly increases in vivo SSAT activity leading to degradation and excretion of cellular polyamines.
The increase in SSAT activity in response to polyamine analogues occurs in many cell types but varies in magnitude with some cells showing inductions of >200-fold. The cell types able to express such high amounts of SSAT have high amounts of Nrf-2 in the nucleus [27]. Increased Sat1 gene transcription via the PRE is critical for the induction. However, even in cell lines that show large increases in SSAT protein, the increase in transcription is generally limited to less than a 10-fold increase [13, 27]. The additional increase is due to the other mechanisms described above, particularly protein stabilization. The ability of polyamine analogues to enhance SSAT by acting at these post-transcriptional steps accounts for the synergism in the induction of SSAT when they are combined with the classical cytotoxins, 5-fluorouracil, cisplatin or oxaliplatin [32, 43, 44]. Exposure of cells to each of the cytotoxins alone results in increased SSAT mRNA, but no appreciable increase in protein or activity are observed in the absence of a polyamine analogue.
The Role of SSAT in drug response
Response to cisplatin
There was a much lower induction of SSAT by BENSpm in human ovarian carcinoma cell lines resistant to cisplatin than cisplatin-sensitive lines. The ablated increased SSAT gene transcription was due to the presence of labile repressor proteins [45]. Transient transfection with a plasmid allowing SSAT expression despite the presence of these repressors increased SSAT levels and restored sensitivity to cisplatin [45]. A plausible explanation for these results is that activation of the SSAT/APAO pathway allows more extensive interaction of cisplatin with DNA through the removal of DNA-bound polyamines.
Response to polyamine analogues
Certain polyamine analogues such as BENSpm and BESpm produce a massive increase in SSAT in some tumor cells as described above. Evidence strongly supporting the hypothesis that this increase is relevant to cell killing by the analogue was obtained in experiments in which repeated exposure of CHO cells to increasing levels of BESpm was used to derive a resistant cell line [42, 46]. The resistant cells failed to increase SSAT activity in response to the BENSpm or BESpm and were found to contain a mutation in the Sat1 gene that alters the coding sequence changing Leu156 to Phe. This mutation changes the binding surface for the aliphatic carbons of the substrate/inhibitor, thus preventing the analogues from protecting the mutant protein from polyubiquitination and degradation and also reduces the enzymatic activity [15, 42]. Transfection of the resistant cells with a plasmid expressing wild type SSAT restored sensitivity to BENSpm.
The massive induction of SSAT and the ability of the analogues to repress the synthesis of ODC and AdoMetDC, as well to increase production of antizyme that causes ODC degradation [47], leads to a rapid and profound drop in cellular polyamines which may account, at least in part, for the antitumor effect. Induction of high levels of SSAT also may exert antitumor effects via toxicity associated with the oxidative damage be produced by the activated SSAT/APAO pathway [27, 48, 49] or a reduction in acetyl-CoA and malonyl-CoA, which would decrease fatty acid synthesis [50, 51]. Independent studies in which SSAT expression was increased from transfected or transduced plasmids also indicate that this is sufficient to induce cell cycle arrest in colorectal and prostate cancer cells [52, 53].
As described below, the induction of SMO is also likely to be a major cause of the antitumor effect of polyamine analogues. It is possible that other effects, such as unproductive binding to critical sites normally occupied by polyamines and via targeting mTOR-mediated protein synthesis [49] or other signaling pathways may contribute. Neoplastic cells may be selectively responsive to polyamine analogues not only via the presence of high amounts of nuclear Nrf-2 leading to the high SSAT induction, but also because they are more sensitive than normal cells. Remarkably, embryonic stem cells from Sat1 knock-out mice were actually more sensitive to BENSpm than controls [54]. Also, apoptosis is induced in chondrocytes by BENSpm independently of its ability to alter levels of natural polyamines by either SMO or the SSAT/APAO pathways [55]. It is possible that in some cells, the binding of the analogue by SSAT protein can protect from interaction with other sites.
It is important to note that many other polyamine analogues are also profoundly antiproliferative towards cancer cells but not all cause as a large an increase in SSAT [2, 56-61]. They may act at the other sites described above or through additional mechanisms including interfering with polyamine-mediated cell signaling or by acting on the cytoskeleton.
Useful animal models for the study of SSAT function
Inactivation of the Sat1 gene
Mice that have the Sat1 gene inactivated showed little change in polyamine content with only a slight increase in the spermidine:spermine ratio [54]. As expected, the back conversion of spermine and spermidine into putrescine was greatly reduced in the cells from these mice. There was some conversion of spermine into spermidine, which could be brought about by SMO. These mice were reported to have a normal life span and no major metabolic deficiencies although, at later ages, there was a development of insulin resistance [54]. Later investigations indicated that there was body fat accumulation, an increase acetyl- and malonyl-CoA pools, and a decrease in palmitate and glucose oxidation in adipose tissue [62]. Also the Sat1 knock-out mice gained more weight and lipid when placed on a high fat diet. These results indicate that, even in the basal state where SSAT expression is low, the reactions mediated via SSAT utilize significant amounts of acetyl-CoA.
Transgenic increase of SSAT
Both transgenic mice and transgenic rats overexpressing SSAT have been obtained (reviewed in: [63-65]). The constructs for expression include: a metallothionein promoter to give general inducible expression; a keratin-6 promoter to target expression to the skin (K6/SSAT); and increased Sat1 gene copy number that should give an enhanced response to physiological stimuli increasing SSAT.
The ubiquitous transgenic expression of high levels of SSAT led to changes consistent with the role of SSAT in regulating polyamine levels with a substantial reduction in spermidine/spermine pools with a large increase in putrescine and in N1-acetylspermidine and excretion of acetylated polyamines including N1, N12-diacetylspermine [64]. These animals also showed a wide variety of other defects including hair loss, female infertility, weight loss, CNS effects, a tendency to develop pancreatitis and altered susceptibility to carcinogenesis. The overstimulation of the SSAT/APAO pathway leads increased oxidative damage and a large reduction in acetyl-CoA with additional metabolic consequences including profound changes in lipid metabolism (see below and [64, 65]). It is therefore difficult, in some cases, to ascertain the extent to which the reduction in polyamines is directly responsible for the pathophysiology that results.
Effects on lipid and carbohydrate metabolism
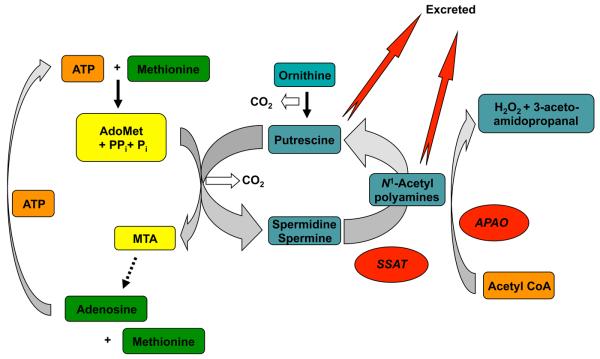
Detailed studies of carbohydrate and lipid metabolism have now been carried out in mice overexpressing SSAT due to an increase in the Sat1 gene copy number. There was a decrease in white adipose tissue, a loss of body fat with high basal metabolic rate, improved glucose tolerance and high insulin sensitivity [62, 66]. A plausible explanation for these changes is that the high levels of SSAT increase metabolic flux through the polyamine pathway because ODC (and possibly other biosynthetic enzymes) are increased in response to the reduction in polyamines caused by SSAT [62, 67]. This produces a futile cycle in which acetyl-CoA is depleted (Fig. 4). The decrease in acetyl-CoA, and the related reduction in malonyl-CoA, removing its inhibition of fatty acid oxidation, leads to a large increase in glucose and palmitate oxidation and a loss of fat. There is also a significant loss of ATP due to the utilization of AdoMet that is converted by the aminopropyltransferase reactions to 5′-methylthioadenosine (MTA), which is then recycled to AdoMet in ATP-dependent reactions. The futile cycle thus uses up ATP and acetyl-CoA forming adenosine, N-acetylaminopropanal (which is reduced to β-alanine), H2O2 and CO2. The fall in ATP increases AMPK, which activates PPAR-γg coactivator 1α (PGC-1α), in white adipose tissue [66]. The increase in PGC-1α leads to the enhanced expression of the OXPHOS genes in the SSAT transgenic mice causing a reduced fat mass and improved glucose tolerance.
Figure 4.
Futile cycle formed by SSAT. Elevations in SSAT aided by excretion of the acetylated products and putrescine formed by APAO cause a decrease in polyamine content. If this is compensated for by an increase in polyamine biosynthetic enzymes, a futile cycle, which depletes ATP and acetyl CoA, is created.
This mechanism is supported by experiments showing that treatment with DFMO to inhibit ODC and reduce flux through the pathway reversed the decrease in white adipose tissue and body fat and restored ATP and the levels of AMPK and PGC-1α towards normal [66]. Other evidence for the cycle was obtained by directly measuring polyamine flux in prostate carcinoma cells transfected with a regulatable construct for SSAT expression [52, 67]. When SSAT was increased, the spermidine and spermine pools were maintained by a compensatory increase in ODC and AdoMetDC activities, despite a large increase in acetylated polyamines and there was a substantial increase in flux through the pathway. This increase was only slightly affected by inhibition of APAO showing that excretion of the acetylated polyamines rather than oxidation was the primary reason for the increased flux in these cell cultures.
Hair loss
The loss of hair in SSAT transgenic mice with increased Sat1 gene copy number is similar to that seen in mice overexpressing ODC [68]. It may therefore be due to the harmful effects of very high levels of putrescine on hair follicle development.
Pancreatitis and SSAT
Transgenic rats with overexpression of SSAT from the metallothionein promoter may be a interesting model for pancreatitis [69]. When SSAT was increased using induction with zinc, pancreatic damage occurred very rapidly. There was an increase in cathepsin B release within 2 h after induction of SSAT and acute necrotizing pancreatitis resulted. Replacement of the natural polyamines with methylated polyamine analogueues that are not substrates for SSAT prevented the disease [69, 70]. It is likely that loss of higher polyamines from cellular structures leads to premature zymogen activation and autodigestion of acinar cells, but is also possible that inflammatory and oxidative stress mediators formed via the SSAT/APAO pathway may be involved since serum inflammatory cytokine levels and NFκB were activated [70].
Liver regeneration
Transgenic overexpression of SSAT blocks liver regeneration after partial hepatectomy [71]. Such regeneration is known to be accompanied by large increase sin ODC and AdoMetDC resulting in a rise of putrescine and spermidine Provision of 1-methylspermidine, which is not attacked by SSAT, overcame the effect of SSAT expression, confirming that spermidine rather than putrescine is the critical polyamine needed for liver regeneration.
As will be discussed below, ischemia-reperfusion injury (IJI) increases SSAT expression in kidney, heart and brain [72-75]. The Sat1 knock-out mice were protected from liver or kidney damage caused by an ischemia-reperfusion model [75].
Carcinogenesis
Several studies have shown that transgenic overexpression of SSAT influences carcinogenesis in mouse models but the results of the various studies differ strikingly. When mice with increased gene copy of Sat1 on the C57BL/6J background were bred with TRAMP mice, which develop prostate tumors due to an expression of SV40 T antigens from the probasin promoter, there was a significant reduction in tumor incidence [76]. Also, in earlier studies using these mice on a hybrid CD2F1background, it was reported that there was a decreased tumor incidence in the skin in response to a two-stage tumorigenesis protocol [64, 77].
In contrast, other studies with the same transgenic mice on the inbred C57BL/6J background showed that when they were bred with ApcMin/+ mice, which are predisposed to intestinal tumor formation, the incidence of intestinal tumors was increased [78]. Consistently, crosses of the ApcMin/+ mice with Sat1 knock-out mice led to a 75% reduction in tumor burden [78]. Similarly, a striking increase in tumor incidence and progression to carcinomas was seen in response to the two-stage tumorigenesis protocol when K6/SSAT C57BL/6J transgenic mice in which a SSAT cDNA was driven by the K6 promoter to express only in the skin were used [79]. The increased tumor incidence was reduced by treatment with MDL72527, an inhibitor of APAO suggesting that oxidative stress or putrescine or both was responsible [80].
Several factors may be relevant to reconciling these disparate results. The mice with general SSAT overexpression have such a variety of pleiotropic phenotypes relating to the general stimulation of oxidative damage, the loss of polyamines and the profound effects on carbohydrate and lipid metabolism resulting from depletion of acetyl-CoA that they are not really suitable for detailed studies in carcinogenesis. Secondly, it is well established that elevated levels of polyamines are associated with neoplastic growth and a major loss of polyamines may account for the reduction in tumors in the TRAMP model and in the skin model with Sat1 gene amplification, which led to a very high level of SSAT expression. In the K6/SSAT mice, there was a much lower expression of the SSAT in the skin as evidenced by only a slight reduction in hair growth [79] as opposed to the hairless phenotype of the general SSAT overexpressing mice. If a compensatory increase in polyamine synthesis is required to allow for sufficient of polyamines for tumor growth, these mice may be able to respond appropriately, whereas the higher level of SSAT would prevent such compensation. Evidence for the importance of continued polyamine synthesis in the K6/SSAT model is supported by the finding that treatment with DFMO resulted in the complete regression of established tumors. Breeding of the K6-SSAT mice with transgenic mice that express antizyme, a negative regulator of ODC, also blocked the development of tumors [80]. A similar compensatory increase in the biosynthesis of polyamines may occur in the intestinal tumor models [78]. These results focus attention on the possibility that oxidative damage caused by modest increases in the SSAT/APAO pathway that do not also result in a major depletion of polyamines may be a particularly hazardous condition leading to tumor development.
Pathological states that maybe associated with a change in SSAT expression
Low SSAT gene expression has been linked to anxiety, depression and a propensity to suicide [81-84]. The reduced levels of SSAT mRNA correlate with the C allele of the Sat1 342A/C polymorphism located in the vicinity of the PRE region of the Sat1 gene promoter [82, 83]. It has been suggested that BENSpm might have a therapeutic benefit in psychiatric patients with a high risk of suicide [85], but the extent to which this charged molecule can penetrate the blood brain barrier or whether cells in the brain can induce SSAT in response to it are not known. The increase in SSAT in normal tissues was much less than in tumor tissues of patients treated with BENSpm when examined by immunohistochemistry [86].
The mechanism by which SSAT is linked to behavioral effects is unknown but may be related to effects on ion channels. Polyamines influence the activity of glutamate receptor ion channels including N-methyl-D-aspartate (NMDA) receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and kainate receptors. These channels are known to be involved in many key neurological functions including sensing pain, neuronal development, and in supporting plasticity processes that are the cellular bases of learning and memory [87]. Also, an inherited defect in spermine synthase that reduces spermine content causes Snyder-Robinson syndrome, an X-linked mental retardation condition [88, 89]. Thus, SSAT-induced alterations in polyamine content could alter brain function. There are alterations in kainate receptor activity in the transgenic mice expressing high levels of SSAT from Sat1 copy number increase [64]. Transgenic overexpression of SSAT protects from kainate related epilepsy-like seizure activity induction [90] and these mice with very high SSAT expression levels had a reduced activity and spatial learning impairment [91].
Keratosis follicularis spinulosa decalvans (KFSD) is a genodermatosis characterized by follicular hyperkeratosis, progressive cicatricial alopecia and photophobia. One form of KFSD is an X-linked recessive condition, and a patient had a gene duplication that included the Sat1 region of the X chromosome [92]. Cultured fibroblasts from the patient had a 3-fold increase in SSAT, a reduction in spermidine and a two-fold increase in putrescine. It is therefore possible that gain-of-function mutants in the Sat1 gene are a general cause of this form of KFSD.
Studies of molecular diagnosis for prostate cancer detection have found that an eight gene “expression signature” provides a sensitive and reliable method for early detection of disease. SSAT, along with ODC, antizyme and AdoMetDC make up half of the eight genes [93]. Additionally, the k-Ras oncogene negatively regulates SSAT [36], which contributes to the increase in polyamines brought about by Ras activation through increased ODC activity [94], increased polyamine uptake [95] and reduced polyamine efflux [21]. It is possible that these effects are critical in the development of tumors with Ras activation.
Thus alterations in SSAT expression can have profound effects with respect to multiple pathologies including proliferative and non-proliferative diseases.
N1-Acetylpolyamine oxidase (APAO)
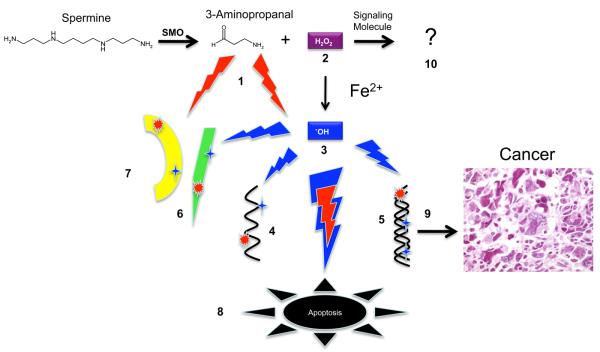
APAO is an FAD-dependent amine oxidase that was first described by Hollta [96, 97]. Although its activity was purified by Hollta in 1977, it has only recently been cloned and more completely characterized [98, 99]. This oxidase preferentially oxidizes N1-acetylated polyamines with an affinity for N1-acetylspermine > N1-acetylspermidine > than N1, N12 diacetylspermine >>> spermine [98, 99]. Consistent with the findings of Hollta, the peroxisomal signaling sequence, PRL has been identified in each of the mammalian APAOs cloned to date [98, 99]. The oxidation of the acetylated polyamines produces, spermidine or putrescine, depending on the starting substrate, 3-acetoamidopropanal, and the reactive oxygen species, H2O2 (Fig. 1).
APAO is generally constitutively expressed in most normal tissues and its activity, as stated above, is rate-limited by the activity of SSAT that provides the acetylated substrate. However, Wallace et al. have demonstrated that APAO expression can vary considerably in human breast tumor cells, suggesting it greater regulation than is typically observed in other tissues [100]. As will be seen below, altered levels of APAO can have profound cellular effects.
The role of APAO in drug response
As described above, SSAT activity can be massively up-regulated by various stimuli. This increase in SSAT activity has the potential to result in large increases in intracellular acetylated polyamines thus providing substrate for APAO. As one mechanism for selective tumor cell toxicity that has been observed with specific polyamine analogues, we originally proposed that massive increases in acetylated polyamines produced by SSAT induced apoptosis subsequent to ROS damage down stream from H2O2 production by APAO [101]. Similar results from other laboratories also suggested a role of APAO in the ROS-induced response of specific cancer cells to the polyamine analogues [102]. Although the interpretation of the data was entirely consistent with what was know about SSAT and APAO at the time, this hypothesis has required modification based on more recent results that suggest SMO oxidase may be the source of H2O2 (see below).
Even though questions remain with respect to the direct involvement of APAO in the cellular response to the cytotoxic polyamine analogues, there is at least one other mechanism by which APAO can affect drug response. Seiler proposed that APAO could detoxify the polyamine analogue N1-(n-octanesulfonyl)spermine (N1-OSSpm) based on the increased cellular toxicity when combined with N1,N4-bis(buta-2,3-dienyl)butanediamine (MDL72527), an inhibitor of APAO [103]. However, the use of the inhibitor MDL72527 is somewhat problematic as it is not specific for APAO [11, 12, 104, 105]. Bitonti and colleagues demonstrated that bis(benzyl)polyamine analogues were also substrates for an oxidizing enzyme that was inhibited by MDL72527 and demonstrated the debenzylating activity co-eluted with the N1-acetylspermine oxidizing activity [106]. Lawson et al. suggested a similar mechanism for resistance to the potent antitumor polyamine analogue, N1-ethyl-N11-[cycloheptyl)methyl]-4,8-diazaundecane (CHENSpm) [107]. In these studies, Chinese hamster ovary cells were found to be highly resistant to CHENSpm. Chromatographic results suggested that CHENSpm was metabolized, indicating that the cells were detoxifying the analogue. With the recent cloning and characterization of APAO it has been confirmed that APAO is indeed capable of oxidizing several polyamine analogues, including CHENSpm [98, 99, 108]. Thus APAO has the capacity to significantly alter the response to various analogues and it is possible that additional unidentified drugs are substrates for APAO.
Spermine oxidase (SMO)
The last enzyme to be cloned in the intracellular polyamine catabolic enzyme was APAO. However, in our attempt to clone APAO we made a fortunate mistake, the discovery of a FAD-dependent oxidase that oxidized spermine as its preferred substrate [12]. The products of this enzyme are spermidine, 3-aminopropanal, and H2O2 (Fig. 1). We initially named the gene PAOh1 as the first human polyamine oxidase to be cloned; however, Vujcic et al. subsequently confirmed the preference for spermine and renamed it spermine oxidase with the abbreviation SMO [11]. Unlike APAO, SMO is highly inducible by a variety of stimuli including the antitumor polyamine analogues [11, 12, 60, 109]. Importantly, all of the analogues that induce SSAT also induce SMO expression; the significance of this will be discussed below.
The human gene for SMO (GenBank name SMOX) is located on chromosome 20p13 and codes for several splice variants [110, 111]. Similarly, the mouse gene has been demonstrated to have multiple splice variants [112]. Active splice variants are found both in the cytoplasm and the nucleus; however, the structural requirements for nuclear translocation is controversial [111, 112]. The predominant human splice variant, SMO1 (SMO/PAOh1), codes for a 61 kD protein containing 555 amino acids. The purified, recombinant protein demonstrates a Km for spermine of 8 μM and Kcat of 7.2 s−1 [113].
As indicated above, SSAT expression is the result of considerable post-transcriptional regulation, with the greatest increases in protein expression being a result of enhanced translation and protein stabilization [41, 114, 115]. By contrast, SMO expression appears to be regulated predominantly at the level of transcription and somewhat by message stabilization [116], but there is currently no evidence of post-translation regulation in response to any of the antitumor polyamines analogues examined.
SMO activity and cellular response to polyamine analogues
As the ROS H2O2 is a major product of SMO activity, the rapid increase in SMO expression subsequent to analogue exposure has the potential to play a significant role in the tumor cell response to analogue treatment. We first proposed the role of H2O2 in the selective cytotoxic response of human non-small cell lung cancers to the bis(ethyl)polyamine analogues [101]; however, this hypothesis was formulated before the awareness of the existence of SMO. The original model proposed that the massive increase in SSAT in response to analogue produced sufficient substrate for the constitutively expressed APAO to produce cytotoxic H2O2 within the tumor cells. This interpretation was based on the accepted view at that time that the polyamine oxidase inhibitor, MDL72,527 was specific for APAO [117, 118]. As MDL72,527 is clearly a potent inhibitor of SMO as well as APAO, a re-evaluation of the role of APAO in the production of cytotoxic H2O2 was necessary [113].
Although it is clear that induction of polyamine catabolism by polyamine analogues resulted in both H2O2 production and oxidative DNA damage, it was not clear as to the origin of the H2O2. Using a stable, short hairpin RNA (shRNA) knockdown strategy it was demonstrated in a human breast cancer model that SMO was the primary source of the cytotoxic H2O2 produced in response to BENSpm treatment. These data suggest that APAO plays no role in the oxidative damage produced by analogue-induced increases in polyamine catabolism in the breast model examined [48].
It is also important to note that the induction of SSAT by specific polyamine analogues leads to a reduction in intracellular polyamine pools by acetylation and export, and that this process occurs in combination with increased ROS through SMO induction. The depletion of polyamines by SSAT activity and subsequent secretion also makes cells more sensitive to ROS damage as the polyamines have been demonstrate to act as free radical scavengers that can protect DNA from H2O2-induced damage [119-121]. These results indicate that the most effective polyamine analogues, with respect to tumor cell toxicity, are those that induce both SSAT and SMO.
It is not known whether APAO serves as a source for ROS in response to other stimuli; however, as APAO is a peroxisomal enzyme it is possible that any H2O2 produced is immediately detoxified by catalase, which is also located in the peroxisome.
Induction of SMO by various stimuli associated with human pathologies
The rapid production of H2O2 and/or 3-aminopropanal clearly can play a significant role in tumor cell response to the antitumor polyamine analogues. However, as ROS are thought to play a role in the etiology of several pathologies including cancer, the potential that SMO activity has other physiological relevance must be considered.
Parchment and Pierce originally suggested that polyamine oxidation and the subsequent production of H2O2 was an important tissue-remodeling event in early embryogenesis [122, 123]. They postulated that polyamine oxidase-produced H2O2 lead to apoptosis of cells as a natural process during development. Although this process is obviously necessary for normal development it also has the potential to produce a pathological outcome if not carefully controlled.
Ischemia reperfusion injury
IRI is associated with the etiology of myocardial infarct, stroke, and acute renal tubular necrosis [124-127]. These pathologies are associated with decreased energy production and the accumulation of toxic metabolites, including ROS, which are thought to play a direct role in the injuries produced. Recent work in several laboratories indicates that the polyamine catabolic enzymes, possibly including SSAT/APAO and SMO, play a significant role in the production of ROS in myocardial infarct, kidney IRI, and in stroke. Zahedi, Solemani, and colleagues have implicated H2O2 production through polyamine catabolism as a source of injurious ROS in kidney IRI [128]. Using a mouse model of renal IRI, SSAT was identified as one of the genes that was significantly up-regulated and suggested that polyamine catabolism may be a source of ROS either through APAO or SMO [129]. Initial follow-up in vitro studies confirmed ROS production and injury using a Tet-inducible SSAT in kidney epithelial cells and clearly demonstrated that the addition of catalase could reduce the cytotoxic effects of SSAT expression suggesting that H2O2 production by polyamine catabolism was involved in the observed cytotoxicity. However, the precise source of H2O2 is still in question as SMO is also significantly upregulated in the Tet-inducible SSAT transfected cells (unfortunately, misidentified in the manuscript as PAO [72]). More recent in vitro studies confirm H2O2 production through polyamine catabolism and demonstrate DNA damage, and suggest that this leads to decreased proliferation and repair capability in injured kidneys [72]. Interestingly, the authors conclude that the H2O2 production by polyamine oxidation does not play a role in the observed inhibition of cell proliferation or DNA damage. However, this conclusion was solely based on the use of the polyamine oxidase inhibitor, MDL72527, at the concentration of 50 μM, a concentration that is not sufficient to inhibit either APAO or SMO in some cell types [101]. Therefore, the role that the polyamine oxidases play in kidney IRI awaits genetic based studies; this is particularly true in light of the work of Igarashi and colleagues who have demonstrated high concentrations of polyamine catabolites in the plasma of renal failure patients [130].
It is, however, now abundantly clear that SSAT plays an important role in kidney IRI as has been confirmed, as stated above, in the SSAT knockout mouse [75]. The loss of SSAT expression was found to significantly protect both the liver and kidney from IRI. These results clearly have important implications in the treatment of IRI.
Similar to their results in patients with kidney failure, Igarashi and colleagues have also found evidence of polyamine catabolism being involved in cerebral stroke [131, 132]. Although the studies do not provide data to show a mechanistic link between increased polyamine catabolism and stroke injury, the studies do suggest that the polyamine oxidases, APAO and SMO, in addition to the metabolite, acrolein provide useful biomarkers for the diagnosis of stroke. It will, however, be necessary to define any existing molecular links between ischemic stroke and polyamine catabolism to determine if it represents a useful therapeutic target for treatment or prevention of cerebral strokes.
Infection and SMO
Several recent lines of evidence suggest that SMO plays a significant role in carcinogenesis and maybe one of the direct molecular links between infection, inflammation, and carcinogenesis. The first evidence of this possibility was the observation that gut macrophages infected with Helicobacter pylori, the causative agent in gastric ulcers and stomach cancer, highly induced SMO and resulted in apoptosis of the affected macrophages [133]. These results suggested a mechanism by which H. pylori infection could escape the immune system by killing the immune cells responsible for eradicating the infection. The mechanism of apoptotic death induced by H. pylori in the macrophages was directly associated with the H2O2 produced by SMO and subsequent fatal DNA damage [133]. It is also of note that in the affected macrophages respond to H. pylori with a substantial increase in ODC activity thus leading to a greater production of polyamines that possibly contribute to enhanced catabolism and subsequent apoptotic cell death [134].
Although these findings alone are highly significant by indicating a means by which low level H. pylori infections can persist, it also suggested a direct role in which H. pylori infection could result in carcinogenic DNA damage. Specifically, if H. pylori infection leads to increased SMO expression in the gastric epithelial cells, the chronic, sub-lethal production of H2O2 could result in the necessary mutagenic DNA damage required for neoplastic transformation.
Similar to the observations in macrophages, when the human gastric epithelial line AGS cells are exposed to H. pylori, there is a significant increase in SMO mRNA, protein, and activity that results in sufficient H2O2 production to result in oxidative DNA damage as measure by the production of 8-hydroxy deoxyguanosine (Fig. 5) [135]. Inhibition of SMO by MDL 72527 or the reduction of its expression through the use of siRNA targeting SMO greatly reduced the DNA damage produced by H. pylori exposure. Most importantly, when gastric tissue of H. pylori infected patients was examined for SMO expression, uniformly elevated SMO mRNA levels were observed from tissues of infected individuals. However, when tissues of the same individuals were examined after eradication of H. pylori infection by antibiotics, the levels of SMO mRNA were uniformly and markedly reduced. When taken together, these results point directly to an association of H. pylori infection, SMO induction, and DNA damage and the data strongly suggest a molecular mechanism directly linking infection with the production of carcinogenic ROS. It is also important to note that the source of the ROS can be the affected epithelial cells themselves, as no immune cells are present in the in vitro cultures of AGS cells.
Figure 5.
Potential damage resulting from polyamine oxidation. Intracellular polyamines can be oxidized by both SMO and APAO. Here the potential damage resulting from spermine oxidation by SMO is illustrated. The red lightening bolts represent potentially damaging insults from reactive aldehydes and the blue lightening bolts represent potentially damaging reactive oxygen species (1) The reactive aldehyde, 3-aminopropanal, can damage ( ) RNA (4), DNA (5), proteins (6), and membranes (7). Similarly, the readily diffusible H2O2 (2) can damage (
) RNA (4), DNA (5), proteins (6), and membranes (7). Similarly, the readily diffusible H2O2 (2) can damage ( ) RNA, DNA, membranes, and proteins, once converted to the highly reactive hydroxyl radical (3) through Fenton or Fenton-like catalysis. If oxidative damage to cells is severe enough, apoptotic cell death may ensue (8). Heritable damage to DNA may result in transformation, carcinogenesis (9) and metastatic disease. Although unexplored, H2O2 produced by polyamine catabolism may play a role as a signaling molecule (10).
) RNA, DNA, membranes, and proteins, once converted to the highly reactive hydroxyl radical (3) through Fenton or Fenton-like catalysis. If oxidative damage to cells is severe enough, apoptotic cell death may ensue (8). Heritable damage to DNA may result in transformation, carcinogenesis (9) and metastatic disease. Although unexplored, H2O2 produced by polyamine catabolism may play a role as a signaling molecule (10).
Inflammatory cytokines and SMO
Approximately 20-30% of epithelial cancers are thought to have an inflammatory component. The above results suggest that in specific cases, such as infection, SMO can be a source of DNA-damaging ROS. Therefore, we sought to determine if other mediators of inflammation could induce SMO and result in DNA damage, thus providing a more generalized potential for this pathway to be involved in carcinogenesis. Using TNFα, a pleiotropic inflammatory cytokine, it was possible to demonstrate that this general mediator of inflammation was capable of significant induction of SMO in two non-tumorigenic human lung epithelial cell lines [136]. The increased SMO resulted in substantial production of H2O2 production accompanied by oxidative DNA damage. Both the level of ROS generated and the amount of DNA damage produced could be effectively inhibited by either MDL 72,527 treatment or by specific knockdown of SMO mRNA. Additionally, it is important to note that exposure of the lung epithelial cells to the cytokine IL-6 produced similar results with respect to SMO induction, indicating that the results are not limited to a single inflammatory cytokine.
Infection, inflammation, colon cancer: a potential link to SMO
The above results indicate that inflammatory cytokines as well as infectious agents can induce SMO to sufficient levels to induce DNA damage. Another important epithelial cancer that is associated with bacterial colonization and inflammation is colon cancer. Inflammatory bowel disease and colitis, two common inflammatory conditions are associated with an increase incidence in colorectal cancer. Enterotoxigenic Bacteroides fragilis (ETBF) colonization is associated with active inflammatory bowel disease [137, 138] and colorectal cancer (CRC) [139]. However, the molecular basis underlying the potential carcinogenic role is not known.
The only known virulence factor of ETBF is a 20 kDa zinc-dependent metalloprotease toxin termed B. fragilis toxin (BFT) that is secreted by ETBF in vitro and in vivo [140]. BFT acts by receptor-mediated triggering of E-cadherin cleavage and activation of cellular signal transduction proteins including receptor and nonreceptor tyrosine kinases, mitogen activated protein kinases (MAPKs), β-catenin and NFκB, resulting in induction of c-Myc expression and intestinal epithelial cell (IEC) proliferation [141, 142].
We have recently completed preliminary studies using BTF exposure to human colon epithelial cells to determine if this virulence factor can also induce ROS-induced DNA damage through SMO activity. The results of these studies indicate that exposure of colon epithelial cells to the toxin is sufficient to induce SMO resulting in DNA damage as measured by the formation of 8-oxodeoxyguanosine and this damage can be blocked by inhibition of SMO [143]. Importantly, Sears and colleagues have recently reported a murine colitis model that will be critical to evaluate the role of SMO oxidase in ETBF induced colitis and potentially, colon cancer in vivo [144].
Prostate cancer and SMO
Another human epithelial cancer in which inflammation has been strongly implicated in carcinogenesis is prostate cancer. DeMarzo, Nelson and colleagues have provided strong evidence that inflammation is intimately associated with prostate carcinogenesis [145-151]. A suspect inflammatory lesion, proliferative inflammatory atrophy (PIA) is thought to be a precursor lesion to prostatic intraepithelial neoplasia (PIN), the key precursor to prostatic adenocarcinoma. Polyamines, particularly spermine, have long been known to be present in high concentrations in the prostate [152-154]. Therefore, in light of the strong correlation with prostatic inflammation and carcinogenesis we sought to determine whether increased SMO expression was coincident with the early lesions thought to be precursors of prostate cancer.
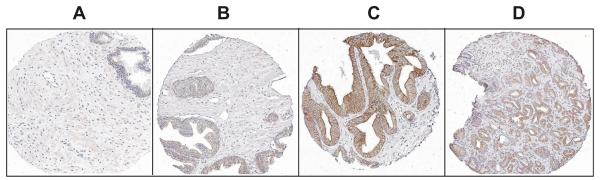
Using our highly specific SMO antisera [136] in combination with a prostate cancer tissue microarrays (TMA) we examined a total of 879 prostate tissue cores from normal individuals, and tissue cores from prostate cancer patients where individual cores represented normal appearing prostatic tissue, PIA, PIN, and prostate adenocarcinoma [155]. Staining and analysis of the TMA revealed that expression of SMO was uniformly higher in all of the tissues of the prostate cancer patients than in the normal prostate tissues (Fig. 6). However, within the tissue cores from the cancer patients, the highest staining was found in the PIN lesions > adenocarcinoma> normal appearing prostate> PIA lesions. These data suggest that expression of SMO is an early event in the development of prostate cancer.
Figure 6.
Representative SMO immunohistochemical staining observed in prostate epithelial tissue microarrays. (A) normal prostate tissue, (B) benign prostate tissue from prostate cancer patient, (C) prostatic intraepithelial neoplasia, and (D) prostate adenocarcinoma.
SMO and chemoprevention
The high expression of SMO in response to various inflammatory stimuli, its ability to produce mutagenic ROS, and its demonstrated ability to damage DNA suggest that SMO may represent a legitimate target for chemoprevention. In vitro and in vivo studies indicate that SMO activity can be inhibited or knocked down without significant deleterious results [48, 136, 156]. Therefore, the demonstration that SMO is one direct molecular link between inflammation and carcinogenesis would provide an excellent starting point for chemopreventive intervention. The development of the relevant knockout animal models will be an invaluable resource to directly test the hypothesis that SMO activity is one source of carcinogenic ROS. Hopefully, these models will be available soon.
Conclusions
The role of polyamine catabolism in maintaining polyamine homeostasis is clearly just one of many important functions in which this highly regulated pathway is involved. The response of SSAT and SMO to analogue treatment likely plays a significant role in determining tumor cell sensitivity to many of the antitumor polyamine analogues. Importantly, the dysregulation of both SSAT and SMO have the potential to lead to several pathological conditions, from the potential role of SSAT in psychiatric disorders, SSAT-dependent changes in lipid and carbohydrate metabolism, to the implication that SMO plays a direct role in the etiology of inflammation-associated carcinogenesis. Each of these functions provide a better understanding for the role of the natural polyamines in cell proliferation and function, and demonstrate the critical nature of the proper regulation of this important pathway. Importantly, these functions also suggest potential targets that may be exploited for therapeutic benefit.
Acknowledgements
Research on polyamines in the authors' laboratories is supported by NIH grants CA5108 and CA984545, the Susan G. Komen for the Cure Foundation KG 088923, and the Samuel Waxman Cancer Research Foundation, to RAC; NIH Grants CA018138 and GM26290 to AEP.
References
- 1.Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casero R, Jr., Marton L. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6:373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 3.Seiler N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 1. Selective enzyme inhibitors. Curr Drug Targets. 2003;4:537–564. doi: 10.2174/1389450033490885. [DOI] [PubMed] [Google Scholar]
- 4.Heby O, Persson L, Rentala M. Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas' disease, and leishmaniasis. Amino Acids. 2007;33:359–366. doi: 10.1007/s00726-007-0537-9. [DOI] [PubMed] [Google Scholar]
- 5.Gerner EW, Meyskens FL., Jr. Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res. 2009;15:758–761. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerner EW, Meyskens FL, Jr., Goldschmid S, Lance P, Pelot D. Rationale for, and design of, a clinical trial targeting polyamine metabolism for colon cancer chemoprevention. Amino Acids. 2007;33:189–195. doi: 10.1007/s00726-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 7.Casero RA, Jr., Pegg AE. Spermidine/spermine N1-acetyltransferase--the turning point in polyamine metabolism. Faseb J. 1993;7:653–661. [PubMed] [Google Scholar]
- 8.Bergeron RJ, Neims AH, McManis JS, Hawthorne TR, Vinson JR, Bortell R, Ingeno MJ. Synthetic polyamine analogues as antineoplastics. J Med Chem. 1988;31:1183–1190. doi: 10.1021/jm00401a019. [DOI] [PubMed] [Google Scholar]
- 9.Casero RA, Jr., Celano P, Ervin SJ, Porter CW, Bergeron RJ, Libby PR. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989;49:3829–3833. [PubMed] [Google Scholar]
- 10.Casero RA, Jr., Celano P, Ervin SJ, Wiest L, Pegg AE. High specific induction of spermidine/spermine N1-acetyltransferase in a human large cell lung carcinoma. Biochem J. 1990;270:615–620. doi: 10.1042/bj2700615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J. 2002;367:665–675. doi: 10.1042/BJ20020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Devereux W, Woster P, Stewart T, Hacker A, Casero R., Jr. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61:5370–5373. [PubMed] [Google Scholar]
- 13.Pegg AE. Spermidine/spermine N1-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab. 2008;294:E995–1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- 14.Holst CM, Nevsten P, Johansson F, Carlemalm E, Oredsson SM. Subcellular distribution of spermidine/spermine N1-acetyltransferase. Cell Biol. Int. 2008;32:39–47. doi: 10.1016/j.cellbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Bewley MC, Graziano V, Jiang J, Matz E, Studier FW, Pegg AE, Coleman CS, Flanagan JM. Structures of wild-type and mutant human spermidine/spermine N1-acetyltransferase, a potential therapeutic drug target. Proc Natl Acad Sci U S A. 2006;103:2063–2068. doi: 10.1073/pnas.0511008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hegde SS, Chandler J, Vetting MW, Yu M, Blanchard JS. Mechanistic and structural analysis of human spermidine/spermine N1-acetyltransferase. Biochemistry. 2007;46:7187–7195. doi: 10.1021/bi700256z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montemayor EJ, Hoffman DW. The crystal structure of spermidine/spermine N1-acetyltransferase in complex with spermine provides insights into substrate binding and catalysis. Biochemistry. 2008;47:9145–9153. doi: 10.1021/bi8009357. [DOI] [PubMed] [Google Scholar]
- 18.Han BW, Bingman CA, Wesenberg GE, Phillips GN., Jr. Crystal structure of Homo sapiens thialysine Nε-acetyltransferase (HsSSAT2) in complex with acetyl coenzyme A. Proteins. 2006;64:288–293. doi: 10.1002/prot.20967. [DOI] [PubMed] [Google Scholar]
- 19.Coleman CS, Stanley BA, Jones AD, Pegg AE. Spermidine/spermine N1-acetyltransferase-2 (SSAT2) acetylates thialysine and is not involved in polyamine metabolism. Biochem J. 2004;384:139–148. doi: 10.1042/BJ20040790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lüersen K. Leishmania major thialysine Nε-acetyltransferase: identification of amino acid residues crucial for substrate binding. FEBS Lett. 2005;579:5347–5352. doi: 10.1016/j.febslet.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 21.Uemura T, Yerushalmi HF, Tsaprailis G, Stringer DE, Pastorian KE, Hawel L, 3rd, Byus CV, Gerner EW. Identification and characterization of a diamine exporter in colon epithelial cells. J. Biol. Chem. 2008;283:26428–26435. doi: 10.1074/jbc.M804714200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Young BA, Coleman CS, Pegg AE, Sheppard D. Spermidine/spermine N1-acetyltransferase specifically binds to the integrin alpha9 subunit cytoplasmic domain and enhances cell migration. J Cell Biol. 2004;167:161–170. doi: 10.1083/jcb.200312166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dehart GW, Jin T, McCloskey DE, Pegg AE, Sheppard D. The α9β1 integrin enhances cell migration by polyamine-mediated modulation of an inward-rectifier potassium channel. Proc. Natl. Acad. Sci. USA. 2008;105:7188–7193. doi: 10.1073/pnas.0708044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurata HT, Marton LJ, Nichols CG. The polyamine binding site in inward rectifier k+ channels. J Gen Physiol. 2006;127:467–480. doi: 10.1085/jgp.200509467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baek JH, Liu YV, McDonald KR, Wesley JB, Zhang H, Semenza GL. Spermidine/spermine N1-acetyltransferase-1 binds to HIF-1alpha and RACK1 and promotes ubiquitination and degradation of HIF-1alpha. J. Biol. Chem. 2007;282:33358–33366. doi: 10.1074/jbc.M705627200. [DOI] [PubMed] [Google Scholar]
- 26.Baek JH, Liu YV, McDonald KR, Wesley JB, Hubbi ME, Byun H, Semenza GL. Spermidine/spermine N1-acetyltransferase 2 is an essential component of the ubiquitin ligase complex that regulates hypoxia-inducible factor 1alpha. J Biol Chem. 2007;282:23572–23580. doi: 10.1074/jbc.M703504200. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Casero RA. Mammalian Polyamine Catabolism: A therapeutic target, a pathological problem, or both? J. Biochem. 2006;139:17–25. doi: 10.1093/jb/mvj021. [DOI] [PubMed] [Google Scholar]
- 28.Babbar N, Gerner EW, Casero RA., Jr. Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem J. 2006;394:317–324. doi: 10.1042/BJ20051298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Devereux W, Stewart TM, Casero RA., Jr. Cloning and characterization of human polyamine-modulated factor-1, a transcriptional cofactor that regulates the transcription of the spermidine/spermine N1-acetyltransferase gene. J Biol Chem. 1999;274:22095–22101. doi: 10.1074/jbc.274.31.22095. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Devereux W, Stewart TM, Casero RA., Jr. Characterization of the interaction between the transcription factors human polyamine modulated factor (PMF-1) and NF-E2-related factor 2 (Nrf-2) in the transcriptional regulation of the spermidine/spermine N1-acetyltransferase (SSAT) gene. Biochem J. 2001;355:45–49. doi: 10.1042/0264-6021:3550045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husbeck B, Stringer DE, Gerner EW, Powis G. Increased thioredoxin-1 inhibits SSAT expression in MCF-7 human breast cancer cells. Biochem Biophys Res Commun. 2003;306:469–475. doi: 10.1016/s0006-291x(03)00993-8. [DOI] [PubMed] [Google Scholar]
- 32.Choi W, Proctor L, Xia Q, Feng Y, Gerner EW, Chiao PJ, Hamilton SR, Zhang W. Inactivation of IkappaB contributes to transcriptional activation of spermidine/spermine N1-acetyltransferase. Mol Carcinog. 2006;45:685–693. doi: 10.1002/mc.20239. [DOI] [PubMed] [Google Scholar]
- 33.Babbar N, Hacker A, Huang Y, Casero RA., Jr. Tumor Necrosis Factor α Induces Spermidine/Spermine N1-Acetyltransferase through Nuclear Factor κB in Non-small Cell Lung Cancer Cells. J Biol Chem. 2006;281:24182–24192. doi: 10.1074/jbc.M601871200. [DOI] [PubMed] [Google Scholar]
- 34.Babbar N, Ignatenko NA, Casero RA, Jr., Gerner EW. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem. 2003;278:47762–47775. doi: 10.1074/jbc.M307265200. [DOI] [PubMed] [Google Scholar]
- 35.Ulrich S, Loitsch SM, Rau O, von Knethen A, Brune B, Schubert-Zsilavecz M, Stein JM. Peroxisome proliferator-activated receptor γ as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res. 2006;66:7348–7354. doi: 10.1158/0008-5472.CAN-05-2777. [DOI] [PubMed] [Google Scholar]
- 36.Gerner EW, Meyskens FL., Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 37.Ignatenko NA, Babbar N, Mehta D, Casero RA, Jr., Gerner EW. Suppression of polyamine catabolism by activated Ki-ras in human colon cancer cells. Mol Carcinog. 2004;39:91–102. doi: 10.1002/mc.10166. [DOI] [PubMed] [Google Scholar]
- 38.Hyvonen MT, Uimari A, Keinanen TA, Heikkinen S, Pellinen R, Wahlfors T, Korhonen A, Narvanen A, Wahlfors J, Alhonen L, Janne J. Polyamine-regulated unproductive splicing and translation of spermidine/spermine N1-acetyltransferase. RNA. 2006;12:1569–1582. doi: 10.1261/rna.39806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butcher NJ, Broadhurst GM, Minchin RF. Polyamine-dependent regulation of spermidine-spermine N1-acetyltransferase mRNA translation. J. Biol. Chem. 2007;282:28530–28539. doi: 10.1074/jbc.M701265200. [DOI] [PubMed] [Google Scholar]
- 40.McCloskey DE, Coleman CS, Pegg AE. Properties and regulation of human spermidine/spermine N1-acetyltransferase stably expressed in Chinese hamster ovary cells. J Biol Chem. 1999;274:6175–6182. doi: 10.1074/jbc.274.10.6175. [DOI] [PubMed] [Google Scholar]
- 41.Coleman CS, Pegg AE. Polyamine analogues inhibit the ubiquitination of spermidine/spermine N1-acetyltransferase and prevent its targeting to the proteasome for degradation. Biochem J. 2001;358:137–145. doi: 10.1042/0264-6021:3580137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCloskey DE, Pegg AE. Properties of the spermidine/spermine N1-acetyltransferase mutant L156F that decreases cellular sensitivity to the polyamine analogue N1, N11-bis(ethyl)norspermine. J Biol Chem. 2003;278:13881–13887. doi: 10.1074/jbc.M205689200. [DOI] [PubMed] [Google Scholar]
- 43.Varma R, Hector S, Greco WR, Clark K, Hawthorn L, Porter C, Pendyala L. Platinum drug effects on the expression of genes in the polyamine pathway: time-course and concentration-effect analysis based on Affymetrix gene expression profiling of A2780 ovarian carcinoma cells. Cancer Chemother Pharmacol. 2007;59:711–723. doi: 10.1007/s00280-006-0325-3. [DOI] [PubMed] [Google Scholar]
- 44.Hector S, Tummala R, Kisiel ND, Diegelman P, Vujcic S, Clark K, Fakih M, Kramer DL, Porter CW, Pendyala L. Polyamine catabolism in colorectal cancer cells following treatment with oxaliplatin, 5-fluorouracil and N1, N11 diethylnorspermine. Cancer Chemother Pharmacol. 2008;62:517–527. doi: 10.1007/s00280-007-0633-2. [DOI] [PubMed] [Google Scholar]
- 45.Marverti G, Monti MG, Bettuzzi S, Caporali A, Astancolle S, Moruzzi MS. Cisplatin-resistance modulates the effect of protein synthesis inhibitors on spermidine/spermine N1-acetyltransferase expression. Int. J. Biochem. Cell Biol. 2004;36:123–137. doi: 10.1016/s1357-2725(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 46.McCloskey DE, Pegg AE. Altered spermidine/spermine N1-acetyltransferase activity as a mechanism of cellular resistance to bis(ethyl)polyamine analogues. J Biol Chem. 2000;275:28708–28714. doi: 10.1074/jbc.M004120200. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell JL, Simkus CL, Thane TK, Tokarz P, Bonar MM, Frydman B, Valasinas AL, Reddy VK, Marton LJ. Antizyme induction mediates feedback limitation of the incorporation of specific polyamine analogues in tissue culture. Biochem J. 2004;384:271–279. doi: 10.1042/BJ20040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pledgie A, Huang Y, Hacker A, Zhang Z, Woster PM, Davidson NE, Casero RA., Jr. Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J Biol Chem. 2005;280:39843–39851. doi: 10.1074/jbc.M508177200. [DOI] [PubMed] [Google Scholar]
- 49.Jiang R, Choi W, Hu L, Gerner EW, Hamilton SR, Zhang W. Activation of polyamine catabolism by N1, N11-diethylnorspermine alters the cellular localization of mTOR and downregulates mTOR protein level in glioblastoma cells. Cancer Biol Ther. 2007;6:1644–1648. doi: 10.4161/cbt.6.10.4800. [DOI] [PubMed] [Google Scholar]
- 50.Beckers A, Organe S, Timmermans L, Scheys K, Peeters A, Brusselmans K, Verhoeven G, Swinnen JV. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67:8180–8187. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- 51.Celik A, Kano Y, Tsujinaka S, Okada S, Takao K, Takagi M, Chohnan S, Soda K, Kawakami M, Konishi F. Decrease in malonyl-CoA and its background metabolic alterations in murine model of cancer cachexia. Oncol. Rep. 2009;21:1105–1111. doi: 10.3892/or_00000330. [DOI] [PubMed] [Google Scholar]
- 52.Kee K, Vujcic S, Merali S, Diegelman P, Kisiel N, Powell CT, Kramer DL, Porter CW. Metabolic and Antiproliferative Consequences of Activated Polyamine Catabolism in LNCaP Prostate Carcinoma Cells. J Biol Chem. 2004;279:27050–27058. doi: 10.1074/jbc.M403323200. [DOI] [PubMed] [Google Scholar]
- 53.Sun H, Liu B, Wang W, Jiang GS, Li W, Yang YP, Xu CX, Yan YF, Liu XX. Adenovirus-mediated expression of spermidine/spermine N1-acetyltransferase gene induces S-phase arrest in human colorectal cancer cells. Oncol. Rep. 2008;20:1229–1235. [PubMed] [Google Scholar]
- 54.Niiranen K, Keinanen TA, Pirinen E, Heikkinen S, Tusa M, Fatrai S, Suppola S, Pietila M, Uimari A, Laakso M, Alhonen L, Janne J. Mice with targeted disruption of spermidine/spermine N1-acetyltransferase gene maintain nearly normal tissue polyamine homeostasis but show signs of insulin resistance upon aging. J Cell Mol Med. 2006;10:933–945. doi: 10.1111/j.1582-4934.2006.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 55.Stanic I, Facchini A, Borzi RM, Stefanelli C, Flamigni F. The polyamine analogue N1,N11-diethylnorspermine can induce chondrocyte apoptosis independently of its ability to alter metabolism and levels of natural polyamines. J. Cell Physiol. 2009;219:109–116. doi: 10.1002/jcp.21655. [DOI] [PubMed] [Google Scholar]
- 56.Casero RA, Frydman B, Murray Stewart T, Woster PA. Significance of targeting polyamine metabolism as an antineoplastic strategy: Unique targets for polyamine analogues. Western Pharm Society. 2005;289:C826–835. [PubMed] [Google Scholar]
- 57.Boncher T, Bi X, Varghese S, Casero RA, Jr., Woster PM. Polyamine-based analogues as biochemical probes and potential therapeutics. Biochem Soc Trans. 2007;35:356–363. doi: 10.1042/BST0350356. [DOI] [PubMed] [Google Scholar]
- 58.Huang Y, Pledgie A, Casero RA, Jr., Davidson NE. Molecular mechanisms of polyamine analogs in cancer cells. Anticancer Drugs. 2005;16:229–241. doi: 10.1097/00001813-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Wallace HM, Niiranen K. Polyamine analogues - an update. Amino Acids. 2007:261–265. doi: 10.1007/s00726-007-0534-z. [DOI] [PubMed] [Google Scholar]
- 60.Hacker A, Marton LJ, Sobolewski M, Casero RA., Jr. In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;63:45–53. doi: 10.1007/s00280-008-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carew JS, Nawrocki ST, Reddy VK, Bush D, Rehg JE, Goodwin A, Houghton JA, Casero RA, Jr., Marton LJ, Cleveland JL. The novel polyamine analogue CGC-11093 enhances the antimyeloma activity of bortezomib. Cancer Res. 2008;68:4783–4790. doi: 10.1158/0008-5472.CAN-07-6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jell J, Merali S, Hensen ML, Mazurchuk R, Spernyak JA, Diegelman P, Kisiel ND, Barrero C, Deeb KK, Alhonen L, Patel MS, Porter CW. Genetically Altered Expression of Spermidine/Spermine N1-Acetyltransferase Affects Fat Metabolism in Mice via Acetyl-CoA. J Biol Chem. 2007;282:8404–8413. doi: 10.1074/jbc.M610265200. [DOI] [PubMed] [Google Scholar]
- 63.Pegg AE, Feith DJ, Fong LY, Coleman CS, O'Brien TG, Shantz LM. Transgenic mouse models for studies of the role of polyamines in normal, hypertrophic and neoplastic growth. Biochem Soc Trans. 2003;31:356–360. doi: 10.1042/bst0310356. [DOI] [PubMed] [Google Scholar]
- 64.Jänne J, Alhonen L, Pietilä M, Keinänen T. Genetic approaches to the cellular functions of polyamines in mammals. Eur. J. Biochem. 2004;271:877–894. doi: 10.1111/j.1432-1033.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- 65.Janne J, Alhonen L, Pietila M, Keinanen TA, Uimari A, Hyvonen MT, Pirinen E, Jarvinen A. Genetic manipulation of polyamine catabolism in rodents. J Biochem (Tokyo) 2006;139:155–160. doi: 10.1093/jb/mvj035. [DOI] [PubMed] [Google Scholar]
- 66.Pirinen E, Kuulasmaa T, Pietila M, Heikkinen S, Tusa M, Itkonen P, Boman S, Skommer J, Virkamaki A, Hohtola E, Kettunen M, Fatrai S, Kansanen E, Koota S, Niiranen K, Parkkinen J, Levonen AL, Yla-Herttuala S, Hiltunen JK, Alhonen L, Smith U, Janne J, Laakso M. Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol Cell Biol. 2007;27:4953–4967. doi: 10.1128/MCB.02034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kramer DL, Diegelman P, Jell J, Vujcic S, Merali S, Porter CW. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J Biol Chem. 2008;283:4241–4251. doi: 10.1074/jbc.M706806200. [DOI] [PubMed] [Google Scholar]
- 68.Soler AP, Gilliard G, M. L. C, O'Brien LG. Modulations of murine hair follicle function by alterations in ornithine decarboxylase activity. J. Invest. Dermatol. 1996;106:1108–1113. doi: 10.1111/1523-1747.ep12340155. [DOI] [PubMed] [Google Scholar]
- 69.Hyvonen MT, Merentie M, Uimari A, Keinanen TA, Janne J, Alhonen L. Mechanisms of polyamine catabolism-induced acute pancreatitis. Biochem Soc Trans. 2007;35:326–330. doi: 10.1042/BST0350326. [DOI] [PubMed] [Google Scholar]
- 70.Merentie M, Uimari A, Pietila M, Sinervirta R, Keinanen TA, Vepsalainen J, Khomutov A, Grigorenko N, Herzig KH, Janne J, Alhonen L. Oxidative stress and inflammation in the pathogenesis of activated polyamine catabolism-induced acute pancreatitis. Amino Acids. 2007;33:323–330. doi: 10.1007/s00726-007-0522-3. [DOI] [PubMed] [Google Scholar]
- 71.Hyvonen MT, Keinanen TA, Cerrada-Gimenez M, Sinervirta R, Grigorenko N, Khomutov AR, Vepsalainen J, Alhonen L, Janne J. Role of hypusinated eukaryotic translation initiation factor 5A in polyamine depletion-induced cytostasis. J Biol Chem. 2007;282:34700–34706. doi: 10.1074/jbc.M704282200. [DOI] [PubMed] [Google Scholar]
- 72.Zahedi K, Bissler JJ, Wang Z, Josyula A, Lu L, Diegelman P, Kisiel N, Porter CW, Soleimani M. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am J Physiol Cell Physiol. 2007;292:C1204–1215. doi: 10.1152/ajpcell.00451.2006. [DOI] [PubMed] [Google Scholar]
- 73.Han L, Xu C, Guo Y, Li H, Jiang C, Zhao Y. Polyamine metabolism in rat myocardial ischemia-reperfusion injury. Int. J. Cardiol. 2009;132:142–144. doi: 10.1016/j.ijcard.2007.07.163. [DOI] [PubMed] [Google Scholar]
- 74.Ryu JH, Cho YS, Chun YS, Park JW. Myocardial SSAT induction via AMPK signaling and its implication for ischemic injury. Biochem. Biophys. Res. Commun. 2008;366:438–444. doi: 10.1016/j.bbrc.2007.11.134. [DOI] [PubMed] [Google Scholar]
- 75.Zahedi K, Lentsch AB, Okaya T, Barone SL, Sakai N, Witte DP, Arend LJ, Alhonen L, Jell J, Janne J, Porter CW, Soleimani M. Spermidine/Spermine N1-acetyltransferase Ablation Protects Against Liver and Kidney Ischemia Reperfusion Injury in Mice+ Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.90507.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kee K, Foster BA, Merali S, Kramer DL, Hensen ML, Diegelman P, Kisiel N, Vujcic S, Mazurchuk RV, Porter CW. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. J Biol Chem. 2004;279:40076–40083. doi: 10.1074/jbc.M406002200. [DOI] [PubMed] [Google Scholar]
- 77.Pietila M, Parkkinen JJ, Alhonen L, Janne J. Relation of skin polyamines to the hairless phenotype in transgenic mice overexpressing spermidine/spermine N-acetyltransferase. J Invest Dermatol. 2001;116:801–805. doi: 10.1046/j.1523-1747.2001.01330.x. [DOI] [PubMed] [Google Scholar]
- 78.Tucker JM, Murphy JT, Kisiel N, Diegelman P, Barbour KW, Davis C, Medda M, Alhonen L, Janne J, Kramer DL, Porter CW, Berger FG. Potent modulation of intestinal tumorigenesis in Apcmin/+ mice by the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase. Cancer Res. 2005;65:5390–5398. doi: 10.1158/0008-5472.CAN-05-0229. [DOI] [PubMed] [Google Scholar]
- 79.Coleman CS, Pegg AE, Megosh LC, Guo Y, Sawicki JA, O'Brien TG. Targeted expression of spermidine/spermine N1-acetyltransferase increases susceptibility to chemically induced skin carcinogenesis. Carcinogenesis. 2002;23:359–364. doi: 10.1093/carcin/23.2.359. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Feith DJ, Welsh P, Coleman CS, Lopez C, Woster P, O'Brien TG, Pegg AE. Studies of the mechanism by which increased spermidine/spermine N1-acetyltransferase activity increases susceptibility to skin carcinogenesis. Carcinogenesis. 2007;287:2404–2411. doi: 10.1093/carcin/bgm162. [DOI] [PubMed] [Google Scholar]
- 81.Sequeira A, Gwadry FG, Ffrench-Mullen JM, Canetti L, Gingras Y, Casero RA, Jr., Rouleau G, Benkelfat C, Turecki G. Implication of SSAT by Gene Expression and Genetic Variation in Suicide and Major Depression. Arch Gen Psychiatry. 2006;63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 82.Guipponi M, Deutsch S, Kohler K, Perroud N, Le Gal F, Vessaz M, Laforge T, Petit B, Jollant F, Guillaume S, Baud P, Courtet P, La Harpe R, Malafosse A. Genetic and epigenetic analysis of SSAT gene dysregulation in suicidal behavior. Am. J. Med. Genet. B. 2009 doi: 10.1002/ajmg.b.30901. in press. [DOI] [PubMed] [Google Scholar]
- 83.Vaquero-Lorenzo C, Bermudo-Soriano CR, Perez-Rodriguez MM, Diaz-Hernandez M, López-Castroman J, Fernandez-Piqueras J, Saiz-Ruiz J, Baca-Garcia E. Positive association between SAT-1 -1415T/C polymorphism and anxiety. Am. J. Med. Genet. Pt. B: Neuropsych. Genetics. 2008 doi: 10.1002/ajmg.b.30850. [DOI] [PubMed] [Google Scholar]
- 84.Klempan TA, Rujescu D, Merette C, Himmelman C, Sequeira A, Canetti L, Fiori LM, Schneider B, Bureau A, Turecki G. Profiling brain expression of the spermidine/spermine N1-acetyltransferase 1 (SAT1) gene in suicide. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009 doi: 10.1002/ajmg.b.30920. in press. [DOI] [PubMed] [Google Scholar]
- 85.Emanuele E, Martinelli V, Politi P. Potential usefulness of N1,N11-diethylnorspermine to prevent suicidal behaviour. Med. Hypotheses. 2006;67:192. doi: 10.1016/j.mehy.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 86.Gabrielson E, Tully E, Hacker A, Pegg AE, Davidson NE, Casero RA., Jr. Induction of spermidine/spermine N1-acetyltransferase in breast cancer tissues treated with the polyamine analogue N1,N11-diethylnorspermine. Cancer Chemother. Pharmacol. 2004;54:122–126. doi: 10.1007/s00280-004-0786-1. [DOI] [PubMed] [Google Scholar]
- 87.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 88.Becerra-Solano LE, Butler J, Castañeda-Cisneros G, McCloskey DE, Wang X, Pegg AE, Schwartz CE, Sánchez-Corona J, Garcia-Ortiz JE. A missense mutation, p.V132G, in the X-linked spermine synthase gene (SMS) causes Snyder-Robinson syndrome. Am. J. Med. Genet. A. 2009;149A:328–335. doi: 10.1002/ajmg.a.32641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Alencastro G, McCloskey DE, Kliemann SE, Maranduba CM, Pegg AE, Wang X, Bertola DR, Schwartz CE, Passos-Bueno MR, Sertie AL. New SMS mutation leads to a striking reduction in spermine synthase protein function and a severe form of Snyder-Robinson X-linked recessive mental retardation syndrome. J. Med. Genet. 2008;45:539–543. doi: 10.1136/jmg.2007.056713. [DOI] [PubMed] [Google Scholar]
- 90.Kaasinen SK, Grohn OH, Keinanen TA, Alhonen L, Janne J. Overexpression of spermidine/spermine N1-acetyltransferase elevates the threshold to pentylenetetrazol-induced seizure activity in transgenic mice. Exp Neurol. 2003;183:645–652. doi: 10.1016/s0014-4886(03)00186-9. [DOI] [PubMed] [Google Scholar]
- 91.Kaasinen SK, Oksman M, Alhonen L, Tanila H, Janne J. Spermidine/spermine N1-acetyltransferase overexpression in mice induces hypoactivity and spatial learning impairment. Pharmacol Biochem Behav. 2004;78:35–45. doi: 10.1016/j.pbb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 92.Gimelli G, Giglio S, Zuffardi O, Alhonen L, Suppola S, Cusano R, Lo Nigro C, Gatti R, Ravazzolo R, Seri M. Gene dosage of the spermidine/spermine N1-acetyltransferase (SSAT) gene with putrescine accumulation in a patient with a Xp21.1p22.12 duplication and keratosis follicularis spinulosa decalvans (KFSD) Hum Genet. 2002;111:235–241. doi: 10.1007/s00439-002-0791-6. [DOI] [PubMed] [Google Scholar]
- 93.Rizzi F, Belloni L, Crafa P, Lazzaretti M, Remondini D, Ferretti S, Cortellini P, Corti A, Bettuzzi S. A novel gene signature for molecular diagnosis of human prostate cancer by RT-qPCR. PLoS ONE. 2008;3:e3617. doi: 10.1371/journal.pone.0003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shantz LM, Levin VA. Regulation of ornithine decarboxylase during oncogenic transformation: mechanisms and therapeutic potential. Amino Acids. 2007;33:213–223. doi: 10.1007/s00726-007-0531-2. [DOI] [PubMed] [Google Scholar]
- 95.Roy UK, Rial NS, Kachel KL, Gerner EW. Activated K-RAS increases polyamine uptake in human colon cancer cells through modulation of caveolar endocytosis. Mol Carcinog. 2008;47:538–553. doi: 10.1002/mc.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holtta E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977;16:91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- 97.Holtta E. Polyamine oxidase (rat liver) Methods Enzymol. 1983;94:306–311. doi: 10.1016/s0076-6879(83)94054-5. [DOI] [PubMed] [Google Scholar]
- 98.Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J. 2003;370:19–28. doi: 10.1042/BJ20021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu T, Yankovskaya V, McIntire WS. Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J Biol Chem. 2003;278:20514–20525. doi: 10.1074/jbc.M302149200. [DOI] [PubMed] [Google Scholar]
- 100.Wallace HM, Duthie J, Evans DM, Lamond S, Nicoll KM, Heys SD. Alterations in polyamine catabolic enzymes in human breast cancer tissue. Clin Cancer Res. 2000;6:3657–3661. [PubMed] [Google Scholar]
- 101.Ha HC, Woster PM, Yager JD, Casero RA., Jr. The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci U S A. 1997;94:11557–11562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Y, Kramer DL, Diegelman P, Vujcic S, Porter CW. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res. 2001;61:6437–6444. [PubMed] [Google Scholar]
- 103.Seiler N, Duranton B, Vincent F, Gosse F, Renault J, Raul F. Inhibition of polyamine oxidase enhances the cytotoxicity of polyamine oxidase substrates. A model study with N(1)-(n-octanesulfonyl)spermine and human colon cancer cells. Int J Biochem Cell Biol. 2000;32:703–716. doi: 10.1016/s1357-2725(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 104.Bellelli A, Cavallo S, Nicolini L, Cervelli M, Bianchi M, Mariottini P, Zelli M, Federico R. Mouse spermine oxidase: a model of the catalytic cycle and its inhibition by N,N1-bis(2,3-butadienyl)-1,4-butanediamine. Biochem Biophys Res Commun. 2004;322:1–8. doi: 10.1016/j.bbrc.2004.07.074. [DOI] [PubMed] [Google Scholar]
- 105.Bianchi M, Polticelli F, Ascenzi P, Botta M, Federico R, Mariottini P, Cona A. Inhibition of polyamine and spermine oxidases by polyamine analogues. Febs J. 2006;273:1115–1123. doi: 10.1111/j.1742-4658.2006.05137.x. [DOI] [PubMed] [Google Scholar]
- 106.Bitonti AJ, Dumont JA, Bush TL, Stemerick DM, Edwards ML, McCann PP. Bis(benzyl)polyamine analogs as novel substrates for polyamine oxidase. J Biol Chem. 1990;265:382–388. [PubMed] [Google Scholar]
- 107.Lawson KR, Marek S, Linehan JA, Woster PM, Casero RA, Jr., Payne CM, Gerner EW. Detoxification of the polyamine analogue N1-ethyl-N11-[(cycloheptyl)methy]-4,8-diazaundecane (CHENSpm) by polyamine oxidase. Clin Cancer Res. 2002;8:1241–1247. [PubMed] [Google Scholar]
- 108.Wang Y, Hacker A, Murray-Stewart T, Frydman B, Valasinas A, Fraser AV, Woster PM, Casero RA., Jr. Properties of recombinant human N1-acetylpolyamine oxidase (hPAO): potential role in determining drug sensitivity. Cancer Chemother Pharmacol. 2005;56:83–90. doi: 10.1007/s00280-004-0936-5. [DOI] [PubMed] [Google Scholar]
- 109.Devereux W, Wang Y, Stewart TM, Hacker A, Smith R, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Ward TD, Woster PM, Casero RA. Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother Pharmacol. 2003;52:383–390. doi: 10.1007/s00280-003-0662-4. [DOI] [PubMed] [Google Scholar]
- 110.Murray-Stewart T, Wang Y, Devereux W, Casero RA., Jr. Cloning and characterization of multiple human polyamine oxidase splice variants that code for isoenzymes with different biochemical characteristics. Biochem J. 2002;368:673–677. doi: 10.1042/BJ20021587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murray-Stewart T, Wang Y, Goodwin A, Hacker A, Meeker A, Casero RA., Jr. Nuclear localization of human spermine oxidase isoforms - possible implications in drug response and disease etiology. Febs J. 2008;275:2795–2806. doi: 10.1111/j.1742-4658.2008.06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bianchi M, Amendola R, Federico R, Polticelli F, Mariottini P. Two short protein domains are responsible for the nuclear localization of the mouse spermine oxidase mu isoform. Febs J. 2005;272:3052–3059. doi: 10.1111/j.1742-4658.2005.04718.x. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster P, Casero R., Jr. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun. 2003;304:605–611. doi: 10.1016/s0006-291x(03)00636-3. [DOI] [PubMed] [Google Scholar]
- 114.Coleman CS, Huang H, Pegg AE. Role of the carboxyl terminal MATEE sequence of spermidine/spermine N1-acetyltransferase in the activity and stabilization by the polyamine analog N1,N12-bis(ethyl)spermine. Biochemistry. 1995;34:13423–13430. doi: 10.1021/bi00041a020. [DOI] [PubMed] [Google Scholar]
- 115.Coleman CS, Pegg AE. Proteasomal degradation of spermidine/spermine N1-acetyltransferase requires the carboxyl-terminal glutamic acid residues. J Biol Chem. 1997;272:12164–12169. doi: 10.1074/jbc.272.18.12164. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y, Hacker A, Murray-Stewart T, Fleischer JG, Woster PM, Casero RA., Jr. Induction of human spermine oxidase SMO(PAOh1) is regulated at the levels of new mRNA synthesis, mRNA stabilization and newly synthesized protein. Biochem J. 2005;386:543–547. doi: 10.1042/BJ20041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bey P, Bolkenius FN, Seiler N, Casara P. N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem. 1985;28:1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- 118.Bolkenius FN, Bey P, Seiler N. Specific inhibition of polyamine oxidase in vivo is a method for the elucidation of its physiological role. Biochim Biophys Acta. 1985;838:69–76. doi: 10.1016/0304-4165(85)90251-x. [DOI] [PubMed] [Google Scholar]
- 119.Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA., Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci U S A. 1998;95:11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ha HC, Yager JD, Woster PA, Casero RA., Jr. Structural specificity of polyamines and polyamine analogues in the protection of DNA from strand breaks induced by reactive oxygen species. Biochem Biophys Res Commun. 1998;244:298–303. doi: 10.1006/bbrc.1998.8258. [DOI] [PubMed] [Google Scholar]
- 121.Rider JE, Hacker A, Mackintosh CA, Pegg AE, Woster PM, Casero RA., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids. 2007;33:231–240. doi: 10.1007/s00726-007-0513-4. [DOI] [PubMed] [Google Scholar]
- 122.Parchment RE. The implications of a unified theory of programmed cell death, polyamines, oxyradicals and histogenesis in the embryo. Int J Dev Biol. 1993;37:75–83. [PubMed] [Google Scholar]
- 123.Parchment RE, Pierce GB. Polyamine oxidation, programmed cell death, and regulation of melanoma in the murine embryonic limb. Cancer Res. 1989;49:6680–6686. [PubMed] [Google Scholar]
- 124.Zhao ZQ. Oxidative stress-elicited myocardial apoptosis during reperfusion. Curr Opin Pharmacol. 2004;4:159–165. doi: 10.1016/j.coph.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 125.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–678. doi: 10.1016/s0002-9343(00)00612-4. [DOI] [PubMed] [Google Scholar]
- 126.Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. 2006;3:327–337. doi: 10.2174/156720506778249515. [DOI] [PubMed] [Google Scholar]
- 127.Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- 128.Zahedi K, Wang Z, Barone S, Prada AE, Kelly CN, Casero RA, Yokota N, Porter CW, Rabb H, Soleimani M. Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2003;284:F1046–1055. doi: 10.1152/ajprenal.00318.2002. [DOI] [PubMed] [Google Scholar]
- 129.Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2005;289:C826–835. doi: 10.1152/ajpcell.00629.2004. [DOI] [PubMed] [Google Scholar]
- 130.Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, Igarashi K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun. 2003;305:143–149. doi: 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- 131.Tomitori H, Usui T, Saeki N, Ueda S, Kase H, Nishimura K, Kashiwagi K, Igarashi K. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36:2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 132.Yoshida M, Tomitori H, Machi Y, Katagiri D, Ueda S, Horiguchi K, Kobayashi E, Saeki N, Nishimura K, Ishii I, Kashiwagi K, Igarashi K. Acrolein, IL-6 and CRP as markers of silent brain infarction. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 133.Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, Hacker A, Casero RA, Jr., Wilson KT. Induction of Polyamine Oxidase 1 by Helicobacter pylori Causes Macrophage Apoptosis by Hydrogen Peroxide Release and Mitochondrial Membrane Depolarization. J Biol Chem. 2004;279:40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- 134.Cheng Y, Chaturvedi R, Asim M, Bussiere FI, Xu H, Casero RA, Jr., Wilson KT. Helicobacter pylori-induced macrophage apoptosis requires activation of ornithine decarboxylase by c-Myc. J Biol Chem. 2005;280:22492–22496. doi: 10.1074/jbc.C500122200. [DOI] [PubMed] [Google Scholar]
- 135.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, Moss SF, Hacker A, Wang Y, Casero RA, Jr., Wilson KT. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 136.Babbar N, Casero RA., Jr. Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66:11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- 137.Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J., Jr. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000;6:171–174. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Basset C, Holton J, Bazeos A, Vaira D, Bloom S. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 2004;49:1425–1432. doi: 10.1023/b:ddas.0000042241.13489.88. [DOI] [PubMed] [Google Scholar]
- 139.Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12:782–786. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 140.Sears CL. The toxins of Bacteroides fragilis. Academic Press; New York: 2005. [Google Scholar]
- 141.Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124:392–400. doi: 10.1053/gast.2003.50047. [DOI] [PubMed] [Google Scholar]
- 142.Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect Immun. 2004;72:5832–5839. doi: 10.1128/IAI.72.10.5832-5839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goodwin A, Wu S, Zhang Y, DeWeese TL, Sears CL, Casero RA. Induction of spermine oxidase by enterotoxigenic Bacteroides fragilis in colon models results in DNA damage. Proc. Amer. Assoc. Can. Res. 2008;49 #3131. [Google Scholar]
- 144.Rhee KJ, Wu S, Wu X, Huso DL, Karim B, Franco AA, Rabizadeh S, Golub J, Mathews LE, Shin J, Sartor RB, Golenbock D, Hamad A, Gan CM, Housseau F, Sears CL. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009 doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955–964. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 146.Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, De Marzo AM, Groopman JD, Nelson WG, Kensler TW. Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase P1. Cancer Res. 2001;61:103–109. [PubMed] [Google Scholar]
- 147.Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172:S6–11. doi: 10.1097/01.ju.0000142058.99614.ff. discussion S11-12. [DOI] [PubMed] [Google Scholar]
- 148.Nelson WG, De Marzo AM, Deweese TL, Lin X, Brooks JD, Putzi MJ, Nelson CP, Groopman JD, Kensler TW. Preneoplastic prostate lesions: an opportunity for prostate cancer prevention. Ann N Y Acad Sci. 2001;952:135–144. doi: 10.1111/j.1749-6632.2001.tb02734.x. [DOI] [PubMed] [Google Scholar]
- 149.Nelson WG, DeMarzo AM, DeWeese TL. The molecular pathogenesis of prostate cancer: focus on the earliest steps. Eur Urol. 2001;39(Suppl 4):8–11. doi: 10.1159/000052574. [DOI] [PubMed] [Google Scholar]
- 150.De Marzo AM, Nakai Y, Nelson WG. Inflammation, atrophy, and prostate carcinogenesis. Urol Oncol. 2007;25:398–400. doi: 10.1016/j.urolonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 151.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, Nelson WG. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–1181. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 152.Pegg AE, Lockwood DH, Williams-Ashman HG. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem J. 1970;117:17–31. doi: 10.1042/bj1170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Williams-Ashman HG, Pegg AE, Lockwood DH. Mechanisms and regulation of polyamine and putrescine biosynthesis in male genital glands and other tissues of mammals. Adv Enzyme Regul. 1969;7:291–323. doi: 10.1016/0065-2571(69)90024-7. [DOI] [PubMed] [Google Scholar]
- 154.Pegg AE, Williams-Ashman HG. Rapid effects of testosterone in prostatic polyamine-synthesizing enzyme systems. Biochem J. 1968;109:32P–33P. doi: 10.1042/bj1090032pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Goodwin AC, Jadallah S, Toubaji A, Lecksell K, Hicks JL, Kowalski J, Bova GS, De Marzo AM, Netto GJ, Casero RA., Jr. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate. 2008;68:766–772. doi: 10.1002/pros.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seiler N, Duranton B, Raul F. The polyamine oxidase inactivator MDL 72527. Prog Drug Res. 2002;59:1–40. doi: 10.1007/978-3-0348-8171-5_1. [DOI] [PubMed] [Google Scholar]