Abstract
Glutathione (GSH) plays an important role in a multitude of cellular processes, including cell differentiation, proliferation, and apoptosis, and as a result, disturbances in GSH homeostasis are implicated in the etiology and/or progression of a number of human diseases, including cancer, diseases of aging, cystic fibrosis, and cardiovascular, inflammatory, immune, metabolic, and neurodegenerative diseases. Because of GSH’s pleiotropic effects on cell functions, it has been quite difficult to define the role of GSH in the onset and/or the expression of human diseases, although significant progress is being made. GSH levels, turnover rates and/or oxidation state can be compromised by inherited or aquired defects in the enzymes, transporters, signaling molecules, or transcription factors that are involved in its homeostasis, or from exposure to reactive chemicals or metabolic intermediates. GSH deficiency or a decrease in the GSH/glutathione disulfide (GSSG) ratio manifests itself largely through an increased susceptibility to oxidative stress, and the resulting damage is thought to be involved in diseases such as cancer, Parkinson’s disease, and Alzheimer’s disease. In addition, imbalances in GSH levels affect immune system function, and are thought to play a role in the aging process. Just as low intracellular GSH levels decrease cellular antioxidant capacity, elevated GSH levels generally increase antioxidant capacity and resistance to oxidative stress, and this is observed in many cancer cells. The higher GSH levels in some tumor cells are also typically associated with higher levels of GSH-related enzymes and transporters. Although neither the mechanism nor the implications of these changes are well defined, the high GSH content makes cancer cells chemoresistant, which is a major factor that limits drug treatment. The present report highlights and integrates the growing connections between imbalances in GSH homeostasis and a multitude of human diseases.
Keywords: Glutathione, neurodegenerative diseases, aging, cancer, cardiovascular diseases, metabolic diseases
Introduction
GSH is required for many critical cell processes, buy plays a particularly important role in the maintenance and regulation of the thiol-redox status of the cell (Meister and Anderson, 1983; Meister, 1984; Deleve and Kaplowitz, 1990; Wang and Ballatori, 1998; Sies, 1999; Hammond et al., 2001; Ballatori et al., 2005; Schafer and Buettner, 2001). The redox state of the GSH/glutathione disulfide couple (GSH/GSSG) can serve as an important indicator of redox environment (Jones, 2006; Kemp et al., 2008; Schafer and Buettner, 2001), and changes in this couple appear to correlate with cell proliferation (Shaw and Chou, 1986; Suthanthiran et al., 1990), differentiation (Nkabyo et al., 2002; Esposito et al., 1994; Hansen et al., 2001; Kim et al., 2004; Huh et al., 2005), or apoptosis (Hammond et al., 2001; Ballatori et al., 2005; Won and Singh, 2006; Garcia-Ruiz and Fernandez-Checa, 2007; Sykes et al., 2007). Of significance, there is growing evidence that the reversible formation of mixed disulfides between GSH and low-pKa cysteinyl residues of proteins (S-glutathionylation) is an important mechanism for dynamic, posttranslational regulation of a variety of regulatory, structural, and metabolic proteins, and for the regulation of signaling and metabolic pathways in intact cell systems (Dalle-Donne et al., 2007; Ghezzi and Di Simplicio, 2007; Mieyal et al., 2008).
GSH also plays a central role in cell death, including apoptotic cell death. GSH levels have been shown to influence caspase activity, transcription factor activation, Bcl-2 expression and function, ceramide production, thiol-redox signaling, and phosphatidylserine (PS) externalization. Another remarkable feature of cells undergoing apoptosis is that they rapidly and selectively release a large fraction of their intracellular GSH into the extracellular space (van den Dobbelsteen et al., 1996; Ghibelli et al., 1998; Oda et al., 1999; Hammond et al., 2004, 2007). Although GSH extrusion may provide a simple mechanism to circumvent the normally protective functions of GSH, there is increasing evidence that GSH export is required for activation of specific apoptotic signaling pathways and/or for proper dismantling of cellular components (Coppola and Ghibelli, 2000; Hammond et al., 2001). GSH export may also be required for the co-transport of apoptotic signaling molecules, although there is little evidence for this hypothesis.
Given the many important cellular processes that are influenced by GSH levels, it is not surprising that alterations in GSH homeostasis have been implicated in the etiology and/or progression of a number of human diseases (Cerutti, 1985; Ballatori et al., 2005; Estrela et al., 2006; Franco et al., 2007; Kinnula et al., 2007; Liu et al., 2004; Reynolds et al., 2007; Seifried et al., 2007; Townsend et al., 2003; Vali et al., 2007; Valko et al., 2007). However, given the many roles played by GSH, it has been difficult to ascribe causal relationships between changes in GSH levels or redox state and development of disease. The present report summarizes advances in the field, and integrates findings from diverse disciplines to elucidate possible common mechanisms and pathways that contribute to human disease.
GSH homeostasis
Under normal conditions, cellular GSH levels are regulated by two major mechanisms: by controlling the rates of its synthesis and of its export from cells; however, GSH levels are also influenced by agents or conditions that alter the thiol redox state, that lead to the formation of glutathione S-conjugates or complexes, and/or that disrupt the distribution of GSH among various intracellular organelles. In addition, GSH levels are affected by the nutritional status and hormonal/stress levels, they exhibit developmental and diurnal variations, and are affected by certain physiological states, including pregnancy and exercise (DeLeve and Kaplowitz, 1990; Hahn et al., 1978; Isaacs and Binkley, 1977; Kemp et al., 2008; Lauterburg et al., 1984; Meister and Tate, 1976; Meister and Anderson, 1983; Uhlig and Wendel, 1992).
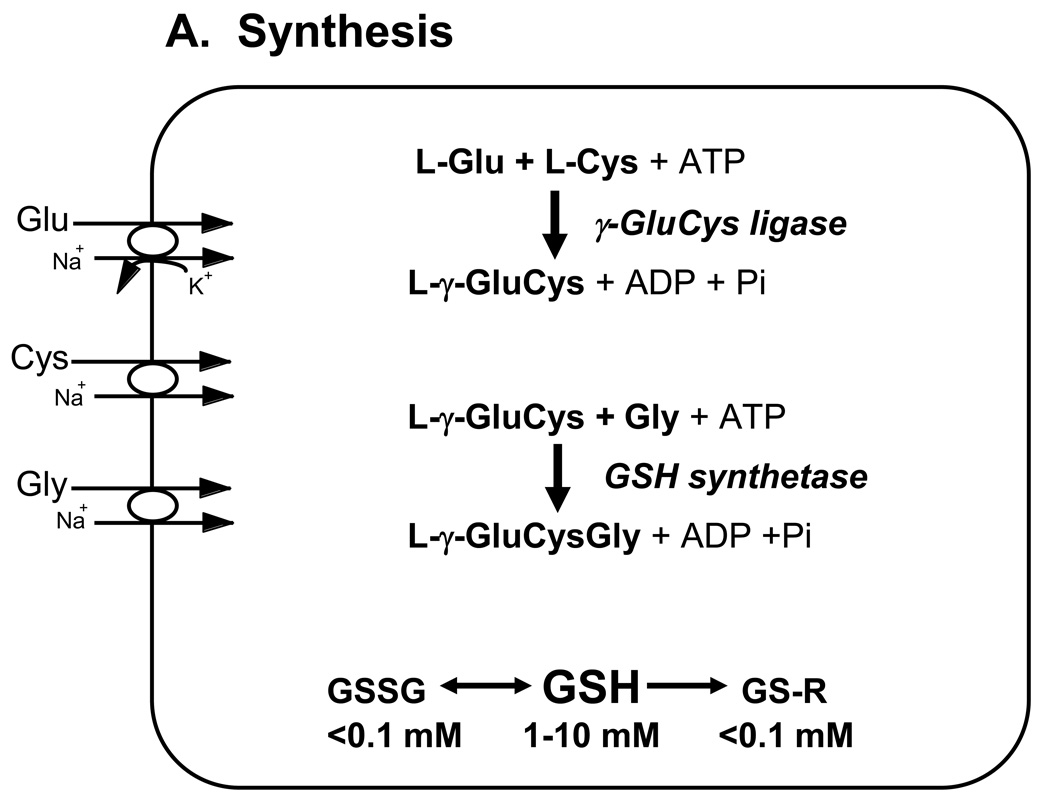
The synthesis and catabolism of GSH and its adducts occurs by a regulated series of enzymatic and plasma membrane transport steps that are collectively referred to as the γ-glutamyl cycle (Figure 1) (Meister and Tate, 1976; Meister and Anderson, 1983). GSH is synthesized in every cell of higher eukaryotes, although GSH synthesis and turnover rates, and intracellular concentrations differ among cells and tissues (Hahn et al., 1978; Lauterburg et al., 1984; Meister and Tate, 1976; Meister and Anderson, 1983; Uhlig and Wendel, 1992). GSH is synthesized in the cell cytosol from its precursor amino acids by the ATP-requiring enzymes γ-glutamylcysteine ligase and GSH synthetase (Figure 1A). The rate of GSH synthesis is controlled largely by the expression and catalytic activity of the first enzyme in its biosynthesis, γ-glutamylcysteine ligase, and by the availability of cysteine (Dalton et al., 2004; Dickinson et al., 2004; Meister and Tate, 1976; Meister and Anderson, 1983). γ-Glutamylcysteine ligase expression is subject to regulation by Nrf2, a transcription factor that regulates a wide array of antioxidant responsive element (ARE)-driven genes in various cell types, and thus plays a central role in cellular antioxidant defenses (Jaiswal, 2004; Lee and Johnson, 2004; Wild and Mulcahy, 2000).
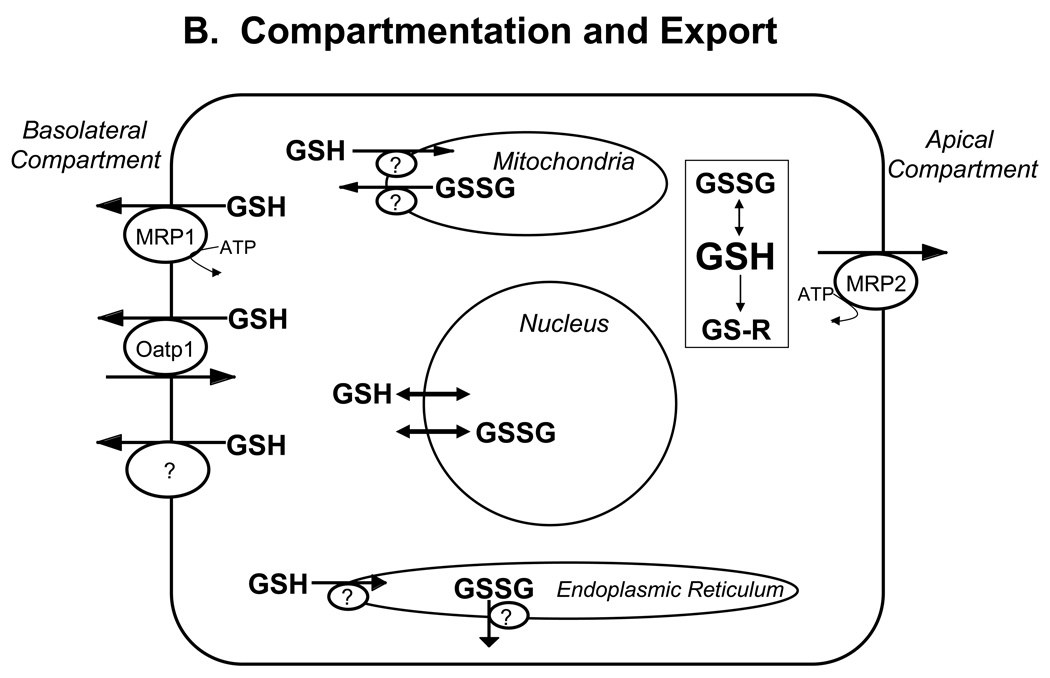
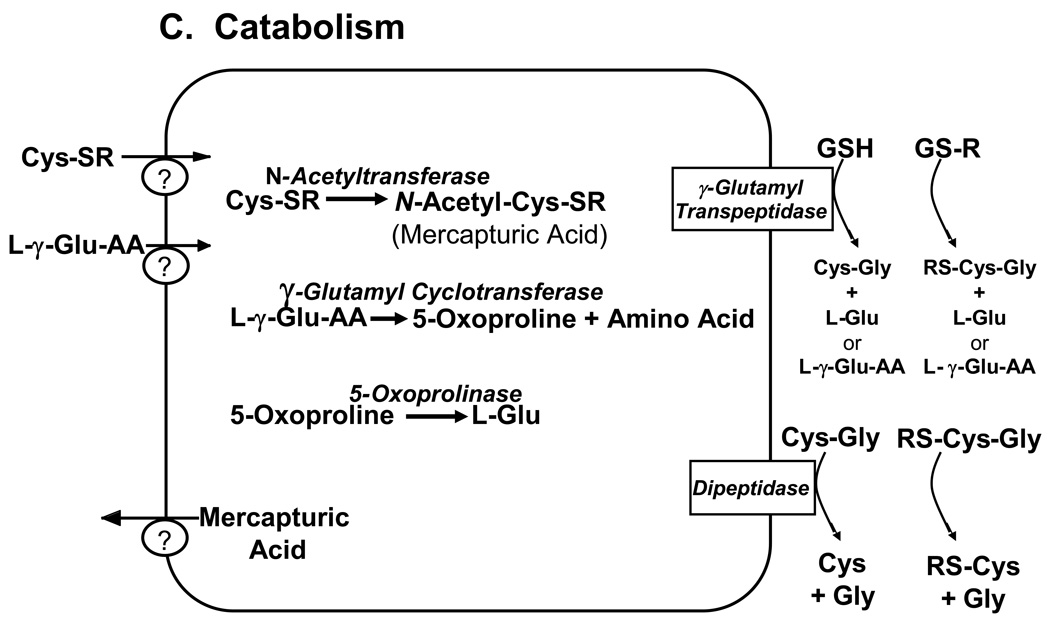
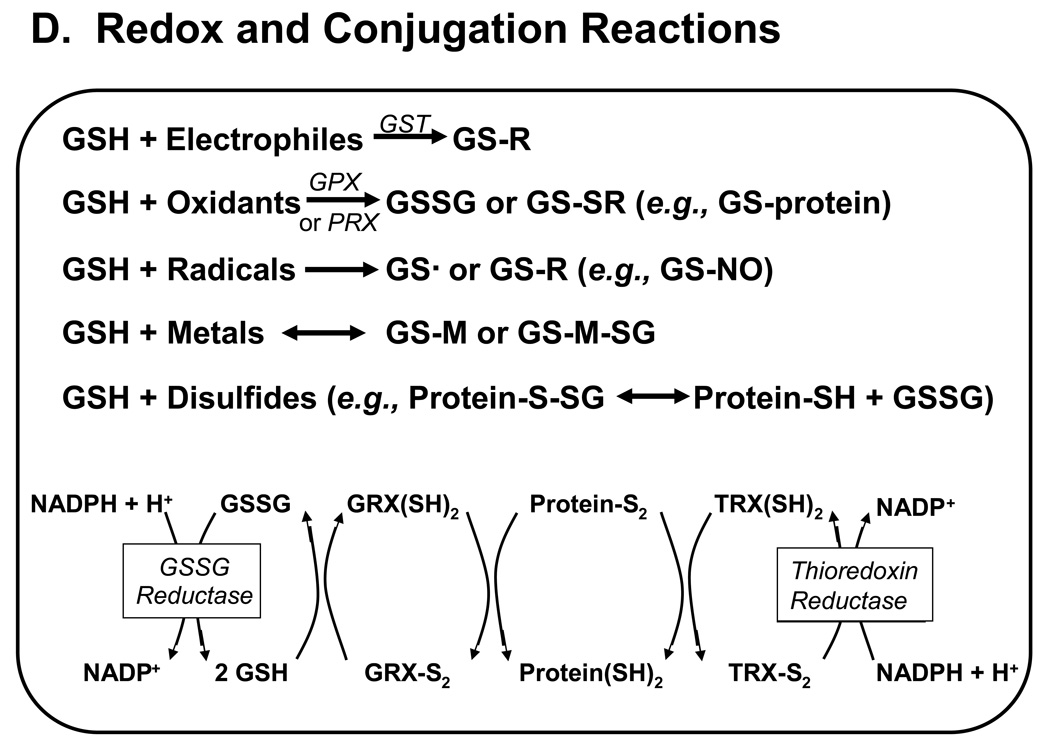
Figure 1. Major pathways of glutathione homeostasis in mammalian cells.
(A) GSH is synthesized in the cell cytosol from its precursor amino acids, glutamate, cysteine and glycine. Within the cell, it exists mainly (>98%) in the thiol-reduced form (GSH), but some is also present as glutathione disulfide (GSSG), and as glutathione conjugates (GS-R). (B) After its synthesis, some of the GSH is delivered into specific intracellular compartments, including mitochondria and endoplasmic reticulum, but much of the GSH is delivered to extracellular spaces, including blood plasma, exocrine secretions, lung lining fluid, and cerebrospinal fluid. In polarized cells, transport of GSH and its conjugates across the apical membrane is mediated largely by MRP2, whereas MRP1 and rat Oatp1 may contribute to GSH efflux across the basolateral plasma membrane into blood plasma, although the specific proteins that mediate GSH export and intracellular compartmentation remain poorly defined. (C) In contrast to GSH synthesis, which occurs intracellularly, GSH degradation occurs exclusively in the extracellular space, and only on the surface of cells that express the ectoenzyme γ-glutamyl transpeptidase. This enzyme is found on the apical membrane of many epithelial cells, but is also abundant at other key sites, including the kidney basolateral compartment. Once GSH and GSH-containing compounds are exported from cells, there are efficient intra- and inter-organ cycles of glutathione degradation and utilization consisting of: (a) extensive catabolism within apical spaces (e.g., bile and renal tubular fluid), as well as within sinusoidal compartments of some species; (b) cellular re-uptake of some of the breakdown products; and (c) intracellular utilization of these breakdown products, or conversion of cysteine S-conjugates (Cys-SR) to mercapturic acids, i.e., N-acetylcysteine S-conjugates (N-acetyl-Cys-SR). As illustrated in this panel, catabolism of glutathione S-conjugates (GS-R) leads to the formation of cysteine S-conjugates (Cys-SR). Cys-SRs are transported back into cells, where they may be substrates for the N-acetyltransferases, to generate N-acetyl-Cys-SRs, or mercapturic acids. Mercapturic acids are exported from cells for eventual elimination in urine or feces, but the transport mechanisms are not clearly defined. Note that γ-glutamyl transpeptidase can catalyze either hydrolyses reactions, to release free L-Glu, or transpeptidation reactions, to lead to the formation of γ-Glu bound to either amino acids or peptides (γ-Glu-AA). These γ-Glu-AAs can be transported back into cells, where they are substrates for the enzyme γ-glutamyl cyclotransferase: this enzyme generates 5-oxoproline and releases the amino acid or peptide that is bound to L-Glu. 5-Oxoproline is converted to L-Glu by the ATP-requiring enzyme 5-oxoprolinase. (D) GSH is a cofactor, coenzyme, and/or substrate for a number of enzymes, and can participate in a number of redox and conjugation reactions. Notably GSH can react with many electrophilic chemicals to generate glutathione S-conjugates (GS-R). Although conjugation reactions can occur spontaneously, these reactions are often catalyzed by the glutathione S-transferases (GST). GSH also facilitates the reduction of oxidants via glutathione peroxidases (GPX), and is involved in maintaining the redox state of protein thiols via the enzymes glutaredoxins (GRX), thioredoxins (TRX), and peroxiredoxins (PRX).
Within the cell the tripeptide exists mainly (>98%) in the thiol-reduced form (GSH), but some is also present as glutathione disulfide (GSSG), as well as a variety of thioether, mercaptide or other thioester forms (glutathione S-conjugates) (Table 1 and Table 2). After its synthesis, GSH is delivered to other intracellular compartments, including mitochondria, endoplasmic reticulum, nucleus, and to the extracellular space (e.g., blood plasma and bile) for utilization by other cells and tissues (Figure 1B). GSH transport into mitochondria appears to be mediated in part by the dicarboxylate carrier (DIC, Slc25a10) and the oxoglutarate carrier (OGC, Slc25a11) (Lash, 2006), whereas transport into the nucleus probably occurs by passive diffusion through the nuclear pores. The mechanism of GSH transport into the endoplasmic reticulum remains undefined, although a role for the ryanodine receptor channel in skeletal muscle sarcoplasmic reticulum membranes has been suggested (Csala et al., 2003).
Table 1.
Endogenous glutathione S-conjugates and complexes
| Thioethers: |
| Acetoacetyl |
| Acetyl |
| Acyl-adenylated bile acids* |
| Acyl-CoA thioesters of bile acids* |
| β-Alanyl-dopa |
| Anthocyanins* |
| Auxins* |
| Catechol estrogen quinones* |
| Cholesterol-5,6-oxide |
| Cytokinins* |
| Dicarboxyethyl |
| Dopa |
| Dopamine |
| 17-β-Estradiol |
| Ethyl |
| Flavonoids* |
| Hepoxilin A3 |
| Hydroxyethyl |
| 4-Hydroxyhexenal |
| 4-Hydroxynonenal |
| 5-Hydroxytryptamine |
| 5-Hydroxytryptophan |
| Indoles* |
| Leukotriene C4 |
| Linoleic acid oxidation products* |
| Menadione |
| Methyl |
| α-Methyldopamine |
| Methylglyoxal |
| Nitric oxide |
| Nitrolinoleic acid |
| 13-Oxooctadecadienoic acid |
| Palmityl |
| Porphyrins* |
| PGJ2, PGD2, PGA1, PGA2 |
| Quinones* |
| Tetrapyrroles* |
| Trans-urocanic acid |
| Thioesters: |
| Coenzyme A |
| Cysteine |
| Cysteinylglycine |
| GSH |
| Sulphate |
| Mercaptides: |
| Cr |
| Cu(I) |
| Cu(II) |
| Se |
| Zn |
This table identifies some of the endogenous compounds that are known to exist as glutathione S-conjugates in mammals.
Only certain members of these classes of chemicals can form glutathione conjugates. Please see Wang and Ballatori (1998) for a previous review of this area.
Table 2.
Glutathionylated proteins
| Enzymes with active-site thiols: |
| Aldose reductase |
| Carbonic anhydrase III |
| Caspase 3 |
| Creatine kinase |
| GAPDH |
| HIV-1 protease |
| α-Ketoglutarate dehydrogenase |
| Paraoxonase 1 |
| Tyrosine hydroxylase |
| Transcription factors: |
| AP-1 |
| c-Jun |
| NF-kB |
| IKK β subunit |
| Interferon regulatory factor 3 (IRF3) |
| p53 |
| Pax-8 |
| Signaling proteins: |
| Akt |
| Hsp60 |
| Hsp70 |
| Insulin receptor PTP-1B |
| Keap1 |
| Protein kinase A |
| Protein kinase C |
| MEKK1 (MAPK/ERK kinase kinase 1; MAP3K) |
| STAT3 |
| T cell p59fyn kinase |
| Protein tyrosine phosphatase-1B |
| Ras |
| Ion channels and Ca2+ pumps: |
| RyR1 |
| CFTR |
| SERCA |
| Other proteins: |
| Actin |
| Aconitase |
| Cyclophilin A |
| Hemoglobin |
| Complex I (NADH-ubiquinone oxidoreductase) |
| Isocitrate dehydrogenase |
| Myosin |
| Neuronal Tau protein |
| Thioredoxin 1 |
| Tubulin |
This table identifies some of the proteins that can form glutathione S-conjugates in mammals. Please see Dalle-Donne et al., 2007, Ghezzi and Di Simplicio, 2007, and Mieyal et al., 2008 for reviews of this area.
In contrast to GSH synthesis, which occurs intracellularly, GSH degradation occurs exclusively in the extracellular space, and in particular, on the surface of cells that express the ectoenzyme γ-glutamyl transpeptidase (also called γ-glutamyl transferase or GGT) (Figure 1C). γ-Glutamyl transpeptidase, which is abundant on the apical surface of most transporting epithelia, including liver canalicular and bile ductular membranes, is the only enzyme that can initiate catabolism of GSH and GSH-containing molecules (e.g., GSSG, glutathione S-conjugates, and glutathione-complexes) under physiological conditions. γ-Glutamyl transpeptidase is a heterodimeric glycoprotein that catalyzes the hydrolysis and transpeptidation of the γ-glutamyl group of GSH and related compounds. In the adult animal, high levels of γ-glutamyl transpeptidase are constitutively expressed in the kidney, intestine, and epididymis. This enzyme is expressed in a tissue-specific and developmental stage-specific pattern and is induced by various xenobiotics (Ikeda and Taniguchi, 2005). This differential expression of γ-glutamyl transpeptidase is conferred by different promoters in the 5'-untranslated regions of mRNAs generated in different tissues and at different stages of development (Ikeda and Taniguchi, 2005).
Because γ-glutamyl transpeptidase is a plasma membrane-bound enzyme with its active site on the extracellular surface of the membrane, export of GSH and its adducts into the extracellular space is the initial, and presumably regulated step in their turnover in all mammalian cells. Despite the importance of this transport step, relatively little is known at the molecular level about plasma membrane GSH transporters (Ballatori et al., 2005, 2008). The paucity of information is explained in large part by a number of practical and theoretical limitations that have hampered the functional and molecular characterization of GSH transporters (Ballatori et al., 2005, 2008).
Once GSH and GSH-containing compounds are released from cells there are efficient intra-and inter-organ cycles of glutathione degradation and utilization (Figure 1D) (Ballatori et al., 2005). In the liver, a major site of GSH metabolism, this consists of: a) extensive catabolism of GSH within biliary spaces (Ballatori et al., 1986b, 1988, 1989), as well as within sinusoidal compartments of some species (Hinchman and Ballatori, 1990, 1994), b) direct hepatic reabsorption of some of the breakdown products (Ballatori et al., 1986a, 1988; Simmons et al., 1991, 1992), and c) intracellular utilization of the amino acids, or conversion of cysteine S-conjugates to mercapturic acids, i.e., N-acetylcysteine S-conjugates (Hinchman et al., 1991, 1993, 1998). As noted above, this complex series of GSH synthetic, catabolic, and transport steps is referred to as the γ-glutamyl cycle, and is intimately associated with the functions of the tripeptide. Some of the functions of this cycle include regulation of GSH turnover, thiol-redox status of the cell, mercapturic acid biosynthesis, metal transport and excretion.
An additional function of GSH transport into hepatic bile is to serve as a primary osmotic driving force in bile formation (Ballatori and Truong, 1989, 1992). GSH is the most abundant organic molecule in bile at a concentration of 8–10 mM. The concentration of free (non-micelle associated) bile acids in rat hepatic bile is lower (only 1–3 mM), and bile pigments are ~1 mM. The high GSH concentration in canalicular bile coupled with its hydrophilic character (high osmotic reflection coefficient) generates a potent osmotic driving force for bile secretion (Ballatori and Truong, 1992). The hydrolysis of GSH into its constituent three amino acids within biliary spaces effectively delivers three osmolar equivalents to bile, which drives the uptake of water through the paracellular pathway, or across the hepatocyte via a non-channel-mediated pathway, and consequently fuels bile flow.
When exposed to oxidant stress or electrophilic chemicals, GSSG and glutathione S-conjugates are generated within the cell. These chemical reactions have been extensively characterized for a multitude of foreign chemicals, but they are also critical for the detoxification of endogenous reactive intermediates and, more importantly, for the formation of specific biological mediators (Wang and Ballatori, 1998). For example, GSH forms thioether conjugates with leukotrienes, prostaglandins, hepoxilin, nitric oxide, hydroxyalkenals, ascorbic acid, dopa, dopamine, and maleic acid, and it forms thioesters with cysteine, coenzyme A, proteins, and other cellular thiols (Table 1 and Table 2). GSH also binds endogenous metals such as Cu, Se, Cr and Zn via nonenzymatic reactions (Table 1). Of significance, the reversible glutathionylation of cellular peptides and proteins is increasingly recognized as major cell signaling and regulatory mechanism (Dalle-Donne et al., 2007; Ghezzi and Di Simplicio, 2007; and Mieyal et al., 2008). A variety of enzymes, transcription factors, signaling proteins, growth factors, ion channels, and cytokines are reported to undergo reversible glutathionylation (Table 2). Currently, no enzyme has been shown to serve as a catalyst of S-glutathionylation in situ, although roles for human glutaredoxin-1 (GRX1; Figure 1D), and for the pi isoform of glutathione-S-transferase (GSTpi) have been proposed (Mieyal et al., 2008). De-glutathionylation appears to be catalyzed by the glutaredoxins (GRX; Figure 1D), with perhaps a contribution by the thioredoxins (TRX). Recent studies provide strong evidence that protein S-glutathionylation is an important post-translational modification, providing protection of protein cysteines from irreversible oxidation and serving to transduce redox signals (Dalle-Donne et al., 2007; Ghezzi and Di Simplicio, 2007; Kemp et al., 2008; and Mieyal et al., 2008).
After their synthesis within the cell, GSSG and glutathione S-conjugates may be exported from cells, a process that is mediated mainly by the multidrug resistance-associated proteins (MRP/ABCC). A total of nine functional MRP genes have been identified (MRP1/ABCC1 to MRP9/ABCC9), although the physiological functions of many MRPs remain undefined (Borst and Oude Elferink, 2002; Kruh et al., 2007). In general, the MRPs function as organic anion export pumps, and they appear to have broad and partially overlapping substrate specificity. Many of the MRPs accept glutathione S-conjugates as substrates (Ballatori et al., 2008).
Recent studies have provided evidence for the existence of at least two classes of plasma membrane GSH exporters (Ballatori et al., 2005, 2008). First, a role for the MRP proteins in GSH transport was indicated by studies in the yeast S. cerevisiae (Rebbeor et al., 1998a, 1998b, 2002), by studies in hepatocyte membranes from the liver of the little skate (Rebbeor et al., 2000), and by studies utilizing membrane vesicles isolated from yeast and from rat liver (Rebbeor et al., 2002). The studies in yeast provided the first direct evidence for ATP-dependent, low-affinity transport of GSH in any cell type (Rebbeor et al., 1998a), and demonstrated that this ATP-dependent GSH transport in yeast is mediated by Ycf1p, the yeast orthologue of mammalian MRP1 and MRP2 (Rebbeor et al., 1998b). Because Ycf1p is structurally and functionally homologous to MRP1 and MRP2, these data indicated that GSH efflux from mammalian cells could be mediated in part by these proteins. Second, rat Oatp1, the sinusoidal organic solute uptake transporter, was shown to function as a GSH/organic solute exchanger (Li et al., 1998), and thus could potentially contribute to GSH release from cells. However, studies with other members of the OATP family of proteins have failed to identify a comparable GSH requirement for transport. For example, recent studies with two human OATPs demonstrated no role of GSH in their transport mechanism (Mahagita et al., 2007).
Studies in rat liver canalicular membrane vesicles provided the first direct evidence for GSH transport on Mrp2 (Rebbeor et al., 2002). Rebbeor et al. (2002) demonstrated that the inability to detect ATP-dependent GSH transport in previous studies with mammalian plasma membrane vesicles was due in part to the inhibitory effect of dithiothreitol (DTT) on Mrp2-mediated transport. DTT is a reducing agent that is normally added to prevent GSH oxidation in membrane vesicle studies. Because all previous studies of GSH transport utilized high concentrations of DTT or other reducing agents, they probably underestimated GSH transport rates. Thus, these results demonstrate that both rat Mrp2 and its yeast orthologue, Ycf1p, are able to transport GSH by an ATP-dependent, low-affinity mechanism (Rebbeor et al., 2002).
Support for a role of Mrp1 and Mrp2 in GSH export is provided by studies in knockout mouse models. Measurements of GSH levels in tissues of mice deficient either in Mrp1, Mrp2, or Cftr, reveal that these mice have altered GSH levels (Lorico et al., 1997; Velsor et al., 2001; Chu et al., 2006; Kruh et al., 2007). Lorico et al. (1997) reported that GSH levels are about 20–40% higher in tissues of Mrp1−/− mice that normally express relatively high levels of this protein. In Mrp2-deficient rats and mice, hepatic GSH levels are increased about 2-fold (e.g., Ballatori et al., 1995; Chu et al., 2006). In Cftr−/− mice, the epithelial lining fluid GSH concentration is slightly lower than that of wild type mice, but the GSH concentration in the lung tissue is not affected (Velsor et al., 2001). Because Mrp1 is expressed in all tissues, it may play a ubiquitous role in GSH export from all cells, whereas Mrp2 is expressed in only a few cell types, and thus can only contribute to GSH export in those cells (Ballatori et al., 2008).
GSH and human diseases
As noted above, maintaining proper GSH levels, turnover rates, and oxidation state are important for a number of critical cell functions, and disruptions in these processes are observed in many human pathologies. GSH deficiency manifests itself largely through an increased susceptibility to oxidative stress, and the resulting damage is thought to be a key step in the onset and progression of many disease states. Conversely, elevated GSH levels generally increase antioxidant capacity and resistance to oxidative stress, and this is observed in many types of cancer cells.
The importance of GSH to human diseases is perhaps best illustrated by the multitude of diseases that are observed in patients with inborn errors in GSH metabolism (Table 3). As summarized by Njalsson and Norgren (2005) and Ristoff and Larsson (2007), of the six enzymes in the γ-glutamyl cycle, diseases have been associated with defects in five of the enzymes, namely γ-glutamylcysteine ligase, glutathione synthetase, γ-glutamyl transpeptidase, dipeptidase, and 5-oxoprolinase. The only enzyme that has not yet been associated with disease is γ-glutamyl cyclotranferase. These inborn errors of GSH metabolism are relatively rare, but when they do occur the resulting phenotype can be quite dramatic (Table 3). Indeed, patients with severely compromised GSH levels die early in life, usually before birth (Njalsson and Norgren, 2005; Ristoff and Larsson, 2007).
Table 3.
GSH-related proteins and their possible disease associations
| GSH-related proteins | Disease association |
|---|---|
| Enzymes: | |
| γ-Glutamylcysteine ligase | Hemolytic anemia, myocardial infarction, schizophrenia, neurological symptoms, COPD |
| Glutathione synthetase | Hemolytic anemia, metabolic acidosis, CNS dysfunction |
| γ-Glutamyl transpeptidase | Glutathionuria, atherosclerosis |
| Dipeptidase | Neurological symptoms |
| γ-Glutamyl cyclotransferase | (none) |
| 5-Oxoprolinase | Neurological symptoms, hypoglycemia, kidney stones |
| N-Acetyltransferases | Drug sensitivity |
| Glutathione S-transferases | Cancer, COPD, cardiovascular disease, age-related hearing loss |
| Glutathione reductase | Hemolytic anemia, cardiovascular disease |
| Glutathione peroxidases | Osteoporosis, emphysema, cardiovascular disease |
| Peroxiredoxins | Hemolytic anemia, cancer, cardiovascular disease, CNS dysfunction |
| Glutaredoxin | Cardiovascular disease |
| Thioredoxin | Cardiovascular disease |
| Transporters: | |
| MRP1/ABCC1 | Reduced inflammatory response, multidrug resistance |
| MRP2/ABCC2 | Dubin-Johnson syndrome |
| CFTR/ABCC7 | Cystic fibrosis |
A deficiency in the first and rate-limiting enzyme in GSH synthesis, namely γ-glutamylcysteine ligase, is a very rare autosomal recessive disease characterized by hemolytic anemia, and in some cases, by neurological symptoms. Some patients exhibit spinocerebellar degeneration, peripheral neuropathy, myopathy, and aminoaciduria (Njalsson and Norgren, 2005; Ristoff and Larsson, 2007). γ-Glutamylcysteine ligase is a dimer consisting of a heavy (catalytic) and a light (modifier) subunit. Although the heavy subunit by itself can catalyze the formation of L-γ-glutamyl-L-cysteine, binding with the modifier subunit enhances the enzyme activity by lowering the Km for glutamate and ATP, and increasing the Ki for GSH inhibition. Knockout mice have been created for both the catalytic and modifier subunits (Dalton et al., 2000; Shi et al., 2000; Yang et al., 2002). Mice lacking the modifier subunit (Gclm−/− mice) are viable and fertile and have no overt phenotype under basal conditions; however, GSH levels in liver, lung, pancreas, erythrocytes, and plasma of these animals are 9–16% of that wild type littermates, and cysteine levels are 9, 35, and 40% of that of wild type mice in kidney, pancreas, and plasma, respectively, but are unchanged in the liver and erythrocytes (Yang et al., 2002). Of significance, the decrease in GSH combined with diminished γ-glutamylcysteine ligase activity in these animals, renders Gclm−/− fetal fibroblasts strikingly more sensitive to chemical oxidants (Yang et al., 2002). In contrast to mice lacking the regulatory subunit of γ-glutamylcysteine ligase, mouse embryos lacking the catalytic subunit fail to gastrulate and die before day 8.5 of gestation (Dalton et al., 2000; Shi et al., 2000).
The second step of GSH biosynthesis is catalyzed by glutathione synthetase, which adds glycine to the preformed γ-glutamylcysteine dipeptide. Glutathione synthetase deficiency is the most frequently recognized disorder of GSH metabolism, although it is also a comparatively rare disease (Njalsson and Norgren, 2005; Ristoff and Larsson, 2007). Patients with mild glutathione synthetase deficiency often have mutations that affect the stability of the enzyme, and these patients display a compensated hemolytic anemia. Patients with moderate to severe glutathione synthetase deficiency typically have mutations that affect the catalytic properties of the enzyme. Moderate glutathione synthetase deficiency leads to hemolytic anemia and metabolic acidosis, whereas patients with a more severe deficiency also develop 5-oxoprolinuria, recurrent bacterial infections, and progressive dysfunction of the central nervous system, including mental retardation and motor functional disturbances. Some individuals also have retinal pigmentations and pathological electroretinograms. There is no cure for this disease. Patients are given vitamins C and E to boost antioxidant levels and bicarbonate to correct the metabolic acidosis. The metabolic acidosis and the 5-oxoprolinuria in these patients are explained by the decreased feedback inhibition of γ-glutamylcysteine ligase resulting from the lower GSH levels. Under these conditions, the enzyme γ-glutamylcysteine ligase continues to produce large quantities of γ-glutamylcysteine, which is converted by γ-glutamyl cyclotransferase into 5-oxoproline (Figure 1C). The overproduction of 5-oxoproline exceeds the capacity of its degradative enzyme, 5-oxoprolinase, and 5-oxoproline therefore accumulates in body fluids and is excreted in the urine.
The third enzyme of the γ-glutamyl cycle is the ectoenzyme γ-glutamyl transpeptidase, which cleaves the glutamate residue from GSH and GSH-containing molecules (Figure 1C). Humans with γ-glutamyl transpeptidase deficiency have been reported, and these individuals display increased glutathione concentration in urine (glutathionuria) and in blood plasma, and some display central nervous system abnormalities. The glutathionuria is due to their inability to break down the GSH that is both filtered and secreted in renal tubular fluid. Mice deficient in γ-glutamyl transpeptidase have been generated, and these animals exhibit glutathionuria, glutathionemia, growth failure, cataracts, lethargy, shortened life span, and infertility (Lieberman et al., 1996; Kumar et al., 2000). No therapy has been approved for human use, although continuous supplementation with N-acetylcysteine restores fertility and extends the life span of the knockout mice (Lieberman et al., 1996; Kumar et al., 2000).
Hydrolysis of cysteinylglycine and of cysteinylglycine S-conjugates formed from the γ-glutamyl transpeptidase-mediated degradation of GSH and GS-conjugates, respectively, is mediated by various dipeptidase activities, including the plasma membrane-bound ectoenzyme aminopeptidase N (Figure 1C), and cytosolic leucyl aminopeptidase (EC 3.4.11.1; Jösch et al., 2003). To date, only a single patient has been identified with a suspected deficiency in dipeptidase activity (Njalsson and Norgren, 2005; Ristoff and Larsson, 2007). This was a 15-year-old boy who presented with mental retardation, mild motor impairment, and partial deafness (Mayatepek et al., 2005). Biochemical investigations showed a normal level of cysteinylglycine in plasma, an abnormal urinary profile with markedly increased excretion of cysteinylglycine and leukotriene D4 (LTD4). The urinary concentration of LTD4, which is usually not detectable in urine, was highly increased, whereas LTE4, the major urinary leukotriene metabolite in humans, was absent in this patient.
5-Oxoprolinase catalyses the ring opening of 5-oxoproline to yield glutamate (Figure 1D). 5-Oxoprolinase deficiency is a very rare autosomal recessive disease characterized by 5-oxoprolinuria, and a very heterogeneous clinical presentation, including renal stone formation, enterocolitis, mental retardation, neonatal hypoglycemia, microcytic anemia and microcephaly (Njalsson and Norgren, 2005; Ristoff and Larsson, 2007).
Neurodegenerative diseases
As noted above, central nervous system dysfunction has been observed in all diseases related to inborn errors of GSH metabolism, suggesting that the brain is particularly susceptible to alterations in GSH homeostasis. The reason for this sensitivity is unknown, although two general possibilities may be considered, namely the high susceptibility of the brain to oxidative stress due to its high oxygen consumption, and the possibility that GSH may be a neuromodulator or neurotransmitter and may thus be essential for central nervous system activities.
Note that although the brain represents only about 2% of the body weight in humans, it consumes about 20% of the total oxygen, and about 90% of this oxygen is utilized by mitochondria to produce ATP. Unfortunately, some of the oxygen consumed in mitochondria is converted to reactive oxygen species rather than to water (Richter, 1992). Under certain pathological states of mitochondrial dysfunction, this rate of conversion to reactive oxygen species may increase as a result of leakage of electrons from the electron transport chain and the subsequent reduction of oxygen to superoxide. Consistent with this general mechanism, oxidative stress is a major proposed pathogenic mechanism for neurodegenerative disorders in which mitochondrial defects have been reported, including Parkinson’s, Huntington’s, and Alzheimer’s diseases. Lipid peroxidation and damage to DNA and other proteins have been detected in samples from such patients.
As in other tissues, the brain is equipped with defense mechanisms against reactive oxygen species, including the antioxidant enzymes superoxide dismutase, glutathione peroxidase and catalase, along with GSH: however, GSH levels in the brain are low when compared with other tissues and are cell-type dependent. For example, GSH concentrations in neurons (~2.5 mM) are relatively low when compared with astrocytes (~ 3.8 mM) or some other cell types (Bolanos et al., 1995; Rice et al., 1998), probably as a result of the lower specific activity for γ-glutamylcysteine ligase in neurons (Gegg et al., 2003). This difference may account for part of the greater inherent sensitivity of neurons to many toxic insults. Interestingly, astrocytes are able to share GSH with neighboring neurons by releasing GSH into the extracellular space (Hirrlinger et al., 2002c). This export step appears to be mediated in part by MRP1. From this shared extracellular microenvironment, GSH is cleaved by γ-glutamyl transpeptidase on the astrocytic plasma membrane to generate precursors for neuronal GSH synthesis. In contrast with astrocytes, neurons, microglia, and oligodendrocytes do not readily release GSH (Hirrlinger et al., 2002c), and this is likely explained by the low level expression of MRP1 in these cell types (Hirrlinger et al., 2002a). Thus, astrocytes are major contributors to the extracellular levels of GSH in the brain. Using in vivo microdialysis, extracellular GSH levels of about 2 µM were reported for rat brain, which are low when compared with blood plasma levels of 5–20 µM (Yang et al., 1994; Han et al., 1999). Consistent with a neuroprotective role, reductions in GSH levels are reported in patients and animal models of various neurodegenerative disorders. As detailed below, there is growing evidence for a role of GSH in the pathogenesis of Parkinson’s disease, whereas conflicting results have been reported for Alzheimer’s disease.
Alzheimer’s disease, characterized by impairments in memory and cognition, is a progressive neurodegenerative disorder that represents the most common form of dementia in the elderly. Increasing age and genetic mutations are both risk factors for Alzheimer’s disease, and only about 5% of Alzheimer’s disease cases are inherited in an autosomal dominant manner. Mutations in genes encoding amyloid precursor protein or amyloid precursor protein processing proteins presenilin-1 or presenilin-2 constitute the familial forms of Alzheimer’s disease. The remaining cases of Alzheimer’s disease are sporadic with unknown etiology. In both familial and sporadic forms of Alzheimer’s disease, the neuropathology is characterized by the loss of pyramidal neurons in the hippocampus and cortex as well as cholinergic neurons in the basal forebrain. Depositions of extracellular amyloid plaques and intracellular neurofibrillary tangles in the hippocampus and cortex are striking features of Alzheimer’s disease. Oxidative stress is proposed as a major pathogenic mechanism in Alzheimer’s disease (Liu et al., 2004; Viña et al. 2004). Alzheimer's patients show increased blood markers of oxidative stress, including increased oxidation of red blood cells glutathione, and this is correlated with the cognitive status of the patients (Viña et al. 2004). On the other hand, measurements of GSH levels in brains of Alzheimer’s patients have yielded contradictory results. GSH levels were reported to be lower in the substantia innominata and the cingulated cortex in brain samples of Alzheimer’s disease patients by Gu et al. (1998), whereas Perry et al. (1987) failed to detect changes in GSH levels in these same brain regions, and Adams, Jr. et al. (1991) found higher GSH levels in the hippocampus of post-mortem samples from Alzheimer’s disease patients. Variables such as differences in techniques and quality of post-mortem samples between laboratories may account for these discrepancies. In an attempt to avoid using post-mortem samples, Liu et al. (2005) collected fresh red blood cells and assessed their GSH content. Interestingly, they detect significantly lower GSH levels in male patients, but not female patients with Alzheimer’s disease (Liu et al., 2005), complicating the interpretation of the involvement of GSH in the pathogenesis of Alzheimer’s disease.
Parkinson’s disease is the second most common neurodegenerative disorder. Parkinson’s disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta, leading to a loss of dopamine in the striatum (Dauer et al., 2003). Although Parkinson’s disease has been described for more than 200 years, the basis for the disease remains largely unknown. Five genes have recently been identified as possible contributors (namely SNCA, Parkin, DJ1, PINK1, LRRK2); however, these genetic mutations represent only a small fraction (<10%) of patients with Parkinson’s disease.
Environmental toxicants have long been hypothesized to play a dominant role the sporadic cases of Parkinson’s disease and oxidative stress has been proposed as a major mechanism of cell death in Parkinson’ s disease (Beal, 2002; Jenner, 2003). In experimental animal models of the disease, the sources of oxidative stress are from both the intracellular and extracellular compartments (Tieu et al., 2003). Extracellularly, superoxide can be released by activated NADPH-oxidase in microglia, and this extracellular superoxide may react with nitric oxide generated by neuronal nitric oxide synthase (nNOS) and microgial inducible nitric oxide synthase (iNOS) to produce the more stable and reactive peroxynitrite. Intracellularly, superoxide can be generated via the disruptions of mitochondrial respiration. Relevant to this latter pathogenic mechanism, reduction in the mitochondrial complex I activity has been reported in samples from Parkinson’s disease patients (Parker, Jr. et al., 1989; Schapira et al., 1990).
The role of oxidative stress in Parkinson’s disease is further highlighted by the observation that GSH content is significantly lower (~40%) in the substantia nigra of Parkinson’s disease patients (Sian et al., 1994). At the cellular level, surviving nigral dopaminergic neurons display a significant loss of GSH (Pearce et al., 1997). Although the mechanism of this decrease has not been established, it is unlikely secondary to neuronal loss or drug treatment. Because the nigrostriatal region is rich in dopamine, it is possible that oxidized dopamine causes the depletion of GSH, and indeed, intrastriatal injection of dopamine causes a significant decrease in GSH levels (Rabinovic and Hastings, 1998), probably through the interactions of dopamine quinone with GSH and/or cysteine (Spencer et al., 1998; Hirrlinger et al., 2002b). In genetic models of Parkinson’s disease, astrocyte cultures from Parkin knockout mice have lower levels of GSH than those from wild type animals (Solano et al., 2008). The significance of GSH depletion is further demonstrated by the death of dopaminergic cells in Drosophila models with Parkin mutation (Whitworth et al., 2005) or with overexpression of α-synuclein (Trinh et al., 2008)
Interestingly, GSH depletion has also been reported in recent studies aimed at inhibiting mitochondrial complex I activity through S-nitrosylation of this electron transport chain subunit (Burwell et al., 2006; Chinta et al., 2007). This effect of GSH depletion, however, is unlikely the primary cause of mitochondrial defect in patients with Parkinson’s disease since complex I deficiency is widespread in various tissues (Parker, Jr. et al., 1989; Schapira et al., 1990), whereas GSH loss is only detected in the substantia nigra (Sian et al., 1994). Furthermore, depletion of GSH alone may not be sufficient to induce cell death because buthionine sulfoximine, an inhibitor of γ-glutamylcysteine ligase, does not kill dopaminergic cells (Toffa et al., 1997). However, GSH depletion enhances cell death when mitochondrial function is inhibited (Zeevalk et al., 1998). Clearly, the effects are quite complex, and likely involve both oxidative stress-induced macromolecular damage and disruption of redox signaling. Nevertheless, the loss of GSH in the substania nigra may explain, at least in part, the apparent discrepancy between the widespread complex I deficiency in different tissues versus the selective cell death in the nigral dopaminergic cells.
Another more speculative possibility for the sensitivity of the central nervous system to GSH depletion is that GSH may function as a neuromodulator or neurotransmitter, and thus any change in either GSH levels, turnover rates, or oxidation state would adversely affect central nervous system activity (Cobb et al., 1982; Guo et al., 1992; Janaky et al., 1999, 2000, 2007; Oja et al., 2000). Note that GSH is made up of three neuroactive amino acids (glutamate, cysteine, and glycine), and thus this peptide may be viewed as a storage or precursor form for these biologically active molecules. Glutamate and glycine are known excitatory and inhibitory neurotransmitters, respectively, but cysteine also has excitatory effect at the N-methyl-D-aspartate (NMDA) class of glutamate receptors. Degradation of extracellular GSH by γ-glutamyl transpeptidase liberates glutamate (and cysteinylglycine) at specific sites within the brain, and the subsequent peptidasemediated hydrolysis of cysteinylglycine liberates cysteine and glycine. In addition to functioning as a source of neuroactive amino acids, there is growing evidence that GSH itself is a neurotransmitter or neuromodulator (Cobb et al., 1982; Guo et al., 1992; Janaky et al., 1999, 2000, 2007; Leslie et al., 1992; Ogita et al., 1995; Oja et al., 2000). Janaky et al. (2000) identified putative GSH binding sites in pig cerebral cortical synaptic membranes, and suggested the presence of a glutathione receptor, although this has not yet been firmly established.
However, there is strong evidence that GSH is both a ligand and modulator of the NMDA receptor (Janáky et al., 1999; Leslie et al., 1992; Ogita et al., 1995; Oja et al., 2000), and thus may be involved in diseases that relate to this receptor, including schizophrenia (Do et al., 2000; Jacobsen et al., 2005). Interestingly, Matsuzawa et al. (2008) recently reported that although overall levels of GSH in the posterior medial frontal cortex of schizophrenia patients are not different from those of normal controls, there was a significant correlation between GSH levels and the severity of symptoms in patients. Matsuzawa et al., (2008) suggested that therapies aimed at increasing GSH levels in the affected brain regions may benefit schizophrenic patients. In this regard, Lavoie et al. (2008) recently demonstrated that administration of N-acetylcysteine improves mismatch negativity in schizophrenia patients, although the mechanism by which N-acetylcysteine exerted its effects remain to be identified.
The critical role played by GSH in neuronal survival provides a strong rationale for the development of therapies aimed at restoring brain GSH levels (Zeevalk et al., 2008). However, to date these approaches have generally met with little success, as there are significant obstacles to the delivery of GSH to the target neurons, including the absence of selective GSH uptake transporters, the relative impermeability of the blood-brain barrier, and the fact that cysteine, which is rate-limiting for GSH synthesis, may be cytotoxic when administered at high doses. For example, attempts to deliver GSH systemically through mini-osmotic pumps (50mg/kg/day) failed to elevate GSH levels in the rat brain (Zeevalk et al., 2007). Pinnen et al. (2007) attempted to deliver GSH to dopaminergic neurons by covalently linking GSH to L-dopa, a precursor of dopamine and a cornerstone treatment of Parkinson’s disease (Pinnen et al., 2007); however, because these derivatives are rapidly cleared from blood plasma, they are unable to increase neuronal GSH. Because cysteine can cross the blood-brain barrier, this amino acid represents an alternative approach to increasing GSH; however, the potential excitotoxicity induced by high cysteine concentrations poses a significant limitation to this strategy. In a recent study, Trinh et al. (2008) took a different approach: using Drosophila genetic models of Parkinson’s disease they were able to attenuate neuronal loss by using the chemicals sulforaphane and allyl disulfide to induce GSH biosynthetic enzymes. However, it is not known whether these chemicals actually induced these enzymes and increased GSH levels in the brain, nor whether they would produce the same effects in vertebrate animal models.
Overall, growing evidence supports the protective effect of GSH in neurodegenative disorders, and especially in Parkinson’s disease; however, no effective therapies have yet been identified that can enhance GSH levels in affected brain regions. Additional strategies that could be explored to enhance brain GSH levels include the administration of lipophilic GSH-derivatives or precursors, the administration of drugs to induce GSH biosynthetic enzymes or to inhibit γ-glutamyl transpeptidase (and thus inhibit GSH degradation), and/or the administration of GSH export inhibitors. However, given that GSH plays multiple, critical roles in all cells of the body, each of these potential approaches has many limitations and is likely to have many unintended consequences unless selectively targeted and rigorously controlled.
Other diseases of aging
In addition to the neurologic diseases discussed above, GSH is strongly associated with other age-related pathologies (Samiec et al., 1998). As individuals age, there is a gradual lowering of GSH levels and of general antioxidant defenses, as well as a decline in the ability of these systems to be induced by exogenous stimuli (Knight, 2000; Lang et al., 1992; Rikans et al., 1997; Viveros et al., 2007), and this decline is associated with a higher incidence of age-related chronic illnesses (Lang et al., 2000). Although the exact connection and mechanisms for these events are unknown, decreased activity of γ-glutamylcysteine ligase and glutathione syntethase (Sethna et al., 1982), γ–cystathionase (Sastre et al., 2005), and glutathione reductase (Holleschau et al., 1994; Katakura et al., 2004) are thought to be contributing factors.
GSH is normally present in high levels in the ocular lens, cornea, aqueous humor, and retina, where it performs multiple roles, including maintaining normal tissue hydration and lens transparency, and protecting against oxidative damage. GSH levels decline in human eyes with age (Harding et al., 1970), and the decreased GSH levels have been associated with several age-related eye disorders, including age-related nuclear cataracts (Pau et al., 1982; Bhat et al., 1991), glaucoma (Moreno et al., 2004), and macular degeneration (Sternberg et al., 1993).
Oxidation is the hallmark of age-related nuclear cataracts. Oxidation of methionine and cysteine residues, and the loss of thiol groups in structural proteins increase progressively as cataracts worsen. Because the lens depends on a balanced thiol redox state for maintaining complete transparency, high GSH levels are important for protecting protein thiol groups against reactive oxygen species. In the central region of cataractous lenses, an increase in GSSG leads to an imbalanced GSH/GSSG ratio and extensive nuclear protein modifications, including oxidation, insolubilization, and cross-linking (David et al., 1984), along with a loss of GSH (Calvin et al., 1986). The retina is especially susceptible to oxidative stress because of its high consumption of oxygen and exposure to light. Likewise, GSH is a major antioxidant in the retina, and depletion of GSH has been associated with the etiology of two major retinopathies, age-related macular degeneration and glaucoma. Age-related macular degeneration is a complex multi-factorial disease that affects the central region of the retina. Although the exact etiology is not known, oxidative damage to the retinal pigment epithelium has been implicated in the pathogenesis of this disease (Young et al. 1987, 1988). In patients with exudative age-related macular degeneration, plasma GSH and total thiol content decrease significantly (Coral et al., 2006). Deficiency of GSH synthesis (Sternberg et al., 1993) and GSH recycling (Cohen et al., 1994) in the retinal epithelial cells may be responsible for the decline. Although increased intraocular pressure is a major risk factor for glaucoma, progressive apoptotic loss of retinal ganglion cells is the ultimate reason for vision loss in all cases of glaucoma (Kerrigan-Baumrind et al., 2000). Apoptosis of retinal ganglion cells can be induced by GSH depletion, involving the production of endogenous oxygen reactive species (Maher et al., 2005). Retinal GSH levels significantly decrease in the experimental rat glaucoma model (Moreno et al., 2004). As illustrated above, compromised antioxidant defense systems are commonly associated with age-related eye diseases, and thus antioxidant supplements have become one of the therapeutic strategies for preventing or delaying the onset of ocular disorders (Head et al., 2001). For example, patients with age-related macular degeneration benefit from antioxidant adminstration (Moriarty-Craige et al., 2005).
Hearing impairment is another common age-related chronic condition associated with the production of reactive oxygen species (Knight, 2000; Seidman et al., 2002; Jiang et al., 2007). Age-related hearing loss develops earlier and is more severe in mice deficient in superoxide dismutase (McFadden et al., 1999a, 1999b, 2001). Likewise, targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice (Ohlemiller et al., 2000).
Oxidative stress is also thought to be involved in the pathogenesis of osteoporosis (Isomura et al., 2004; Sanchez-Rodriguez et al., 2007). Post-menopausal osteoporosis is characterized by bone mass loss and micro-architectural deterioration, resulting in decreased biomechanical competence and, consequently, increased risk of fracture. In post-menopausal osteoporotic women, osteoblastic activity is consistently depressed as osteoclastic activity is enhanced, indicating that reactive oxygen species play a major role in bone metabolism (Sontakke et al., 2002). Glutathione peroxidase and superoxide dismutase activity is significantly lower in osteoporotic women, suggesting that compromised antioxidant defenses play an important role in the development of osteoporosis (Maggio et al., 2003; Ozgocmen et al., 2007a, 2007b). In mice, N-acetylcysteine stimulates osteoblast differentiation in part by increasing GSH synthesis (Jun et al., 2008). Dietary supplement with N-acetylcysteine is able to completely block bone loss associated with ovariectomy in rodents (Lean et al., 2003; Lee et al., 2005), and also shows some beneficial effects in slowing bone resorption in early post-menopausal women (Sanders et al., 2007). Given these findings, it would be of interest to examine whether clinical therapy with GSH precursors or derivatives or antioxidant supplements in early postmenopausal women would have significant health and economic benefits.
Cancer
GSH also plays an important role in cancer development and treatment, and several recent reviews have addressed this topic (e.g., Balendiran et al., 2004; Estrela et al., 2006). Thus, only a brief discussion will be presented here. It is now well established that reactive oxygen species and electrophilic chemicals can damage DNA, and that GSH can protect against this type of damage (Valko et al., 2007). GSH can also directly detoxify carcinogens through phase II metabolism and subsequent export of these chemicals from the cell. On the other hand, elevated GSH levels are observed in various types of cancerous cells and solid tumors, and this tends to make these cells and tissues more resistant to chemotherapy (Calvert et al., 1998; Balendiran et al., 2004; Estrela et al., 2006). Some cancer cells also exhibit higher γ–glutamylcysteine ligase and γ–glutamyl transpeptidase activities, and higher expression of the GSH-transporting MRP/ABCC export pumps (O’Brien and Tew, 1996; Estrela et al., 2006).
Cancer cells also tend to be more resistant to apoptosis, and although the mechanism is not well understood, a role for GSH has been proposed (Table 4) (Estrela et al., 2006; Ballatori et al., 2008). A more reduced intracellular environment is thought to facilitate proliferation and reduce apoptosis of the tumor cells (Schafer and Buettner, 2001; Estrela et al., 2006). GSH levels are able to influence the apoptotic process by affecting caspase and transcription factor activation, ceramide production, thiol-redox signaling, and phosphatidylserine externalization (Hammond et al., 2001; Ballatori et al., 2005). GSH levels also appear to be influenced by Bcl-2 family anti-apoptotic proteins. The oncogene Bcl-2 was initially reported to have antioxidant functions based on the phenotype of the Bcl-2-deficient mouse and reduced lipid peroxidation in cells overexpressing Bcl-2 (Hockenbery et al., 1993; Veis et al., 1993; Hochman et al., 1998). These antioxidant properties may be related to the correlation between high Bcl-2 or Bcl-XL levels and elevated intracellular GSH levels (Celli et al., 1998; McCullough et al., 2001; Ortega et al., 2003; Benlloch et al., 2005). The mechanism by which Bcl-2 expression alters intracellular GSH levels is unknown and may be cell type dependent, as not all cells overexpressing Bcl-2 have elevated GSH levels (Schor et al., 2000). High intracellular GSH levels have been reported to be caused by an increase in γ-glutamylcysteine ligase due to NFkB activation (Jang and Surh, 2004) and by a decrease in GSH efflux (Bojes et al., 1997; Meredith et al., 1998; Schor et al., 2000; Ortega et al., 2003; Benlloch et al., 2005). In addition, it has also been suggested that Bcl-2 can act as a pro-oxidant and increase reactive oxygen species generated by mitochondria, and thus the increase in GSH is in response to the oxidative stress conditions (Kowaltowski and Fiskum, 2005)
Table 4.
Correlation between GSH levels and rate of apoptosis in human diseases
| Too much apoptosis: | GSH Levels |
|---|---|
| Neurodegenerative disorders | |
| Parkinson’s Disease | Low |
| Alzheimer’s Disease | Low |
| Ischemic | |
| Myocardial Infarction | Low |
| Immune | |
| AIDS | Low |
| Rheumatoid Arthritis | Low |
| Insulin-dependent diabetes mellitus | Low |
| Multiple Sclerosis | Low |
| Too little apoptosis: | GSH Levels |
| Cancer | High |
From Fadeel et al., 1999 and Pastore et al., 2003.
As noted above, a higher GSH content in some cancer cells makes these cells chemoresistant, which is a major factor that limits the effectiveness of drug treatment (Calvert et al., 1998). Overexpression of specific glutathione S-transferases can also affect chemoresistance, whereas polymorphisms that decrease glutathione S-transferase activity are associated with elevated risk of developing certain cancers (Balendiran et al., 2004; Masella et al., 2005). Elevated expression of glutathione S-transferases combined with high GSH levels can increase the rate of conjugation and detoxification of chemotherapy agents, thus reducing their effectiveness. In addition, increased expression levels of the MRP/ABCC transporters can contribute to chemoresistance. Nearly all members of the MRP/ABCC family of transporters are known to transport GSH conjugates and/or GSH itself, and thus are likely involved in chemoresistance (Ballatori et al., 2005, 2008; Conseil et al., 2005; Deeley et al., 2006). Elevated expression of MRP1 has been observed in a variety of hematological and solid tumors, and increased expression levels of other MRP transporters are found in a few cancerous tissues (Leslie et al., 2005; Deeley et al., 2006). Furthermore, mice deficient in Mrp1, Mrp2, and Mrp4 are more sensitive to certain chemotherapeutic agents, indicating that the MRP/ABCC family of transporters is important in chemoresistance (Lorico et al., 1997; Wijnholds et al., 1998; Conseil et al., 2005; Vlaming et al., 2006).
To circumvent the protective effects of GSH in cancer chemotherapy, GSH depletion strategies, such as L-buthionine sulfoximine inhibition of GSH synthesis have been employed (Calvert et al., 1998; Balendiran et al., 2004); however, there are potential drawbacks to this approach, including deleterious effects on non-cancerous tissues (Estrela et al., 2006).
Cardiovascular diseases
The major cardiovascular diseases associated with redox imbalances are hypertension and atherosclerosis. As reviewed by Leopold and Loscalzo (2005), polymorphisms in antioxidant enzymes including glutathione peroxidases and glutathione S-transferases are associated with an increased risk of vascular disease due to increases in reactive oxygen species accumulation (Table 4). In particular, glutathione peroxidase polymorphisms appear to increase the risk of developing coronary heart disease, stroke, and cerebral venous thrombosis and glutathione S-transferase polymorphisms are associated with elevated inflammatory markers and an increased risk of coronary heart disease in smokers (Leopold and Loscalzo, 2005). Thioredoxin and glutaredoxin also play an important role in protection against cardiovascular disease through ameliorating the effects of reactive oxygen species and inflammation (Berndt et al., 2007).
Additional studies have also strengthened the connection between GSH and cardiovascular conditions. The development of hypertension is associated with increases in reactive oxygen species in endothelial cells, and angiotensin II can induce hypertension and is an important stimulus for the production of reactive oxygen species. Widder et al. (2007) demonstrated that angiotensin II-induced hypertension is prevented in Mrp1−/− mice, and this was attributed to the increase in GSH levels in the vascular endothelial cells of these mice owing to decreased export of GSH. In addition, GSH hydrolysis via γ-glutamyl transpeptidase may be associated with the progression of atherosclerosis (Emdin et al., 2005). It has been suggested that the presence of γ-glutamyl transpeptidase in atherosclerotic plaques may lead to increases in reactive oxygen species and LDL oxidation through the reactions of cysteinylglycine and iron (Emdin et al., 2005). It has been speculated that these oxidative events can lead to the release of the atherosclerotic plaques, thus increasing the risk of myocardial infarction and stroke (Emdin et al., 2005). One strategy for attenuating atherosclerosis and overcoming LDL and HDL oxidation may be to administer GSH entrapped in liposomes, as suggested by Rosenblat et al. (2007). Liposomal GSH administered to a mouse model of atherosclerosis diminished blood plasma and macrophage oxidative stress levels and macrophage cholesterol mass (Rosenblat et al., 2007).
Pulmonary diseases
An imbalance in GSH homeostasis and in thiol-redox state is also thought to contribute to the etiology and/or progression of both chronic obstructive pulmonary diseases (COPD) such as emphysema and asthma, and to more acute lung diseases such as acute respiratory distress syndrome (ARDS). As in other tissues, the regulation of GSH levels in the lung is quite complex (Rahman, 2005; Kinnula et al., 2007). The redox sensitive transcription factor Nrf2 plays a key role in regulating the expression of numerous antioxidant genes, including γ-glutamylcysteine ligase, the glutathione S-transferases, and glutathione peroxidases (Motohashi and Yamamoto, 2004; Rahman, 2005; Singh et al., 2006; Kinnula et al., 2007; Sibhatu et al., 2008), and a number of environmental and occupational factors are thought to disrupt this regulation and contribute to lung disease. Nrf2 and glutathione peroxidase 2 levels are reduced in lung tissue from emphysema patients, whereas Keap1 is increased (Goven et al., 2008). In COPD, Nrf2 regulated antioxidants including GSH are decreased due to the loss of DJ-1, a protein that stabilizes Nrf2 protein by impairing its disassociation from Keap1 (Malhotra et al., 2008). Nrf2 also appears to control the GSH content through regulating the expression of Mrp1, a GSH exporter whose levels are greatly diminished in the absence of Nrf2 (Hayashi et al., 2003; Sibhatu et al., 2008). In addition to the regulation of GSH-related genes, some conditions lead to the formation of nonreducible glutathione-aldehyde derivatives (e.g., GSH adducts with acrolein and crotonaldehyde from cigarette smoke), thereby depleting the total available GSH pool (van der Toorn et al., 2007).
Polymorphisms of glutathione related enzymes are also associated with increased risk for some lung diseases. As summarized by Bentley et al. (2008), polymorphisms in the enzymes γ-glutamylcysteine ligase (catalytic and modulatory subunits) and glutathione S-transferases (GST) M1 and P1 may contribute to increased risk of COPD. These polymorphisms in combination with other risk factors such as smoking and low vitamin C intake likely contribute to decreased lung function (Siedlinski et al., 2008). GSTM1 and GSTT1 deficiencies also appear to increase the risk of childhood asthma and other adverse health effects from current and in utero passive smoking (Kabesch et al., 2004).
Because of the importance of GSH to lung diseases, many studies have evaluated the beneficial effects of antioxidants, including N-acetylcysteine and the polyphenol resveratrol, in various drug pathologies (Rahman, 2005; Kode et al., 2008). In addition, GSH itself may also be a therapeutic option. Recent studies suggest that direct administration of GSH or inhibition of its catabolic enzyme γ-glutamyl transpeptidase may be protective against epithelial cell induction of asthma (Lowry et al., 2008).
Cystic fibrosis
GSH appears to play a major role in the etiology, progression, and potential treatment of cystic fibrosis, the most common hereditary disorder in Caucasian populations (Davies et al., 2007). In 1989, Riordan and colleagues identified a chloride channel, the cystic fibrosis Transmembrane Conductance Regulator (CFTR/ABCC7), which is mutated and non-functional in cystic fibrosis. CFTR contributes to anion flow in epithelial cells of the airways, pancreas, intestines, and sweat glands, which when absent leads to pancreatic insufficiency, male infertility, chronic inflammation, and the collection of mucus in airways that leads to increased bacterial infections in the lung. The recurrent lung infections cause the majority of morbidity and mortality in cystic fibrosis. Of significance, patients with cystic fibrosis have decreased GSH levels in lung epithelial lining fluid and blood plasma; however, GSH levels in the lung itself appear to be unaffected (Roum et al., 1993). In normal individuals, GSH is elevated 140-fold in epithelial lining fluid compared with blood plasma levels and has several functions, including breaking disulfide bonds to reduce the viscosity of mucus, affecting mucus hydration, and regulating inflammation and the immune response (Cantin et al., 1987, 2007; Hudson, 2001). Given that CFTR is present on the apical side of lung epithelial cells and that it may transport GSH, it may be responsible for delivery of GSH into epithelial lining fluid; however, this has not yet been established (see below).
Neutrophil GSH levels may also be decreased in cystic fibrosis patients, and this could contribute to the excessive recruitment to airways, abnormal function, and increased necrosis of neutrophils observed in these patients (Tirouvanziam et al., 2006). Similarly to cystic fibrosis patients, the CFTR-deficient mouse has decreased GSH content in the epithelial lining fluid; whereas lung tissue GSH levels remain the same as wild type mice (Velsor et al., 2001). GSH increases in the epithelial lining fluid after Pseudomonas aeruginosa infection in wild type mice; however, this increase after infection is absent in CFTR−/− mice (Day et al., 2004). The inability to increase GSH in the epithelial lining fluid may contribute to the poor response to infection seen in cystic fibrosis patients.
Exactly how CFTR influences GSH transport is still under debate. CFTR is a member of the MRP/ABCC family of proteins which include the GSH transporting MRPs. CFTR was originally shown to allow passage of GSH using the patch clamp technique (Linsdell and Hanrahan, 1998) and more recently, direct transport of GSH on CFTR has been demonstrated in membrane vesicle and proteoliposome experiments (Kogan et al., 2003). Interestingly, GSH appears to inhibit CFTR ATPase activity (Kogan et al., 2001), and this inhibition may alter the properties of CFTR such that it now favors GSH flux over chloride flux (Kogan et al., 2003). Although CFTR has been shown to transport GSH in artificial systems, whether or not this occurs physiologically is still controversial. Cells from cystic fibrosis patients lacking functional CFTR still transport GSH (Gao et al., 1999), albeit at a significantly slower rate. When chloride transport is restored to these cells by an artificial peptide channel that cannot transport or conduct GSH itself, GSH transport increases to a normal rate (Gao et al., 2001). In addition, mRNA for MRP1, a known GSH transporter, is decreased in nasal ciliated cells with mutated CFTR, and further decreased in cells more severely compromised in basal chloride conductance (Hurbain et al., 2003). Thus, CFTR may not actually conduct GSH, but may regulate its transport indirectly through chloride transport. Furthermore, CFTR interacts with the outwardly rectifying chloride channel, the renal outer medullary potassium channel, and the epithelial sodium channel and is necessary for their proper functioning (Schwiebert et al., 1999), and it is possible that CFTR also interacts with MRP transporters or other GSH transport proteins. Along these lines, CFTR has been shown to functionally and physically associate with MRP4, a cAMP transporter that can modulate CFTR activity by altering cAMP levels (Li et al., 2007). MRP4 is also a putative GSH transporter, and although not examined by Li et al., (2007), it is possible that GSH transport could be affected by this interaction.
Cystic fibrosis airways are not only exposed to normal environmental oxidant burden from inhalation, but are also exposed to oxidants from increased inflammation and infections present in the lungs. Combined with low GSH levels in epithelial lining fluid and neutrophils/blood plasma, the oxidant stress in cystic fibrosis is magnified. Increasing or restoring normal GSH levels in cystic fibrosis patients has the potential to counteract these oxidative stress conditions. Inhaled GSH increases epithelial lining fluid GSH levels modestly without causing a systemic increase in GSH (Roum et al., 1999; Buhl et al., 1990). A few clinical studies have assessed lung function improvements due to inhaled GSH in cystic fibrosis patients with modest success (Griese et al., 2004; Bishop et al., 2005). The treatments are well tolerated by patients; however, one concern is that GSSG increases after treatment and may have negative effects on the redox status (Roum et al., 1999). Additionally, inhaled GSH may cause life-threatening bronchoconstriction in sensitive patients and those who have asthma, particularly if the pH of the GSH solutions is not adjusted prior to their administration (Marrades et al., 1997; Prousky, 2008).
N-Acetylcysteine has been used as a mucolytic agent in cystic fibrosis for decades, although the actual benefits of N-acetylcysteine for improving lung function in cystic fibrosis are still being debated (Duijvestijn and Brand 1999; Aitio 2005). Inhaled N-acetylcysteine is thought to increase clearance of sputum in the airways by reducing disulfide bonds and lowering mucus viscosity, but does not increase systemic GSH levels (Atkuri et al., 2007). Oral N-acetylcysteine administration, on the other hand, increases GSH levels by providing the liver with an increased supply of cysteine promoting an increase in GSH synthesis. Oral N-acetylcysteine treatment has been shown to increase neutrophil and whole blood concentrations of GSH, diminish neutrophil recruitment to airways, and decrease elastase activity, a measurement of cystic fibrosis lung dysfunction, although other measurements of lung function did not improve (Tirouvanziam et al., 2006). Analysis of five additional studies on lung function after N-acetylcysteine treatment show small improvements in lung function (Duijvestijn and Brand 1999); however, each of these studies are short term studies, and long term evaluation of N-acetylcysteine benefits to lung function are lacking.
Therapeutic intervention with S-nitrosoglutathione may also benefit cystic fibrosis patients. S-Nitrosoglutathione is an endogenous molecule that relaxes airway smooth muscle, improves motility of airway cilia, and has antimicrobial effects (Hudson, 2001). Lung S-nitrosoglutathione levels are decreased in cystic fibrosis patients (Grasemann et al., 1999). Inhalation of S-nitrosoglutathione has been shown to be well tolerated by a small group of cystic fibrosis patients and led to a modest increase in oxygenation (Snyder et al., 2002). Adding physiological levels of S-nitrosoglutathione to cell lines expressing ΔF508-CFTR and primary nasal epithelia cells of cystic fibrosis patients improves maturation of CFTR, expression on the plasma membrane, and chloride flux (Zaman et al., 2001; Andersson et al., 2002; Howard et al., 2003; Chen et al., 2006; Servetnyk et al., 2006). However, S-nitrosoglutathione may need to be used in conjunction with an additional treatment that would aid protein stability, because ΔF508-CFTR appears to be unstable at the plasma membrane after S-nitrosoglutathione treatment (Howard et al., 2003).
Inflammatory and immune system diseases
Mounting attention is being given to the role thiol status plays during the onset and progression of inflammatory and autoimmune states, as well as the effectiveness of thiol repletion therapies in the treatment of immune diseases. Cellular GSH levels affect T helper cell maturation (Peterson et al., 1998), T cell proliferation (Messina and Lawrence, 1989), viral replication (Staal et al., 1990; Palamara et al., 1995; Cai et al., 2003), as well as susceptibility to reactive species secreted by inflammatory cells. Maintaining proper T cell function is critical for eliciting an appropriate immune response, as abnormalities often result in disease. Additionally, many correlations exist between immune system dysfunction and alterations in GSH levels, as summarized below. In most cases, the mechanism for the change in GSH levels has not been identified.
GSH and Th1/Th2 balance
T helper cells play an important role in directing appropriate immune responses. T helper 1 cells (Th1) drive cellular immunity and combat viruses, intracellular pathogens, as well as cancer cells; whereas T helper 2 cells (Th2) elicit humoral immunity, and increase antibody production to battle multicellular organisms. Th1/Th2 imbalance is a hallmark in many immune diseases (Romagnani, 1996). The Th1 response is overactive in a number of organ-specific autoimmune diseases, including Rheumatoid Arthritis, type 1 diabetes, and Multiple Sclerosis, whereas the Th2 pathway activity is associated with allergy and IgE-based disease, and systemic autoimmunity (Kidd, 2003). A number of factors influence Th1/Th2 maturation, including GSH levels. Peterson et al. (1998) reported that GSH depletion in antigen presenting cells inhibit Th1-related cytokine production (interferon gamma, and interleukin 12), and supports the Th2-mediated humoral immune response. Furthermore, when antigen presenting cells have high intracellular GSH levels they secret cytokines that favor the development of Th1 cells (Murata et al., 2002). In addition, Murata et al. (2002) found that specific cytokines can alter GSH levels in antigen presenting cells. Exposure to IFN-gamma, a Th1 cytokine, resulted in increased GSH levels, whereas exposure to IL-4, a Th2 cytokine, resulted in decreased intracellular GSH. Because GSH has a significant impact on the immune system’s ability to activate the appropriate Th response, altering its levels may have significant implications in Th1/Th2-related diseases.
Interestingly, low GSH levels are associated with many autoimmune, and inflammatory diseases, including Rheumatoid Arthritis (Hassan et al., 2001), systemic lupus erythematous (Perl et al., 2004), Crohn’s disease (Sido et al., 1998), multiple sclerosis (Zargari et al., 2007), psoriasis (Kokcam and Naziroglu, 1999), and contact dermatitis (Eisen et al., 2004). However, because this list of diseases does not fall exclusively into the category of a Th1-dominant or Th2-dominant disorder, it suggests that compromised GSH levels may be a more critical factor in the initial onset of these conditions. This hypothesis is supported by the observation that in systemic lupus erythematous Th-2-dominance occurs in early disease stages (Horwitz et al., 1998), and that Th-1 commitment then prevails in later disease progression (Akahoshi et al., 1999).
In general, high oxidant burdens are a common feature in many immune dysfunctions and GSH plays a major role in quenching these oxidant species, and hence protecting the cell from damage. Therefore, any decrease in GSH may influence the immune system through perpetuating reactive species signaling events and increasing ROS-related damage. Interestingly, a number of stimuli in concentrations that deplete GSH levels, such as ethanol, irradiation, cyclophosphamide, and stress, are known to alter the immune response (Peterson et al., 1998). Recently, there has been growing interest in reports that associate acetaminophen use (a well known GSH-depleting agent), with an increased risk of asthma (Shaheen et al., 2000; Barr et al., 2004; Eneli et al., 2005; McKeever et al., 2005).
GSH and viral infection
In addition to affecting Th1/Th2 balance, intracellular thiols have been implicated in T cell proliferation that occurs during viral infection (Hamilos et al., 1989; Messina and Lawrence, 1989; Hadzic et al., 2005). In particular, low intracellular GSH levels are observed in HIV-infected individuals, and are associated with decreased survivability of affected patients (Herzenberg et al., 1997). GSH levels are lower in blood plasma, epithelial lining fluid, peripheral blood mononuclear cells, and monocytes of HIV-infected individuals (Buhl et al., 1989). Neither the mechanism for the GSH depletion, nor the functional significance of the lower GSH levels is as yet known. Interestingly, the lower GSH levels tend to favor HIV viral replication, and thus may facilitate progression of the disease. How this occurs is not clear, although there is evidence that GSH deficiency enhances the signal transduction pathways associated with HIV expression (Duh et al., 1989). Decreased GSH levels are known to activate NFκB, which has been shown to bind and activate genes controlled by the HIV long terminal repeat (Duh et al., 1989), and thus may affect viral replication. Supplementation with N-acetylcysteine has been demonstrated to block HIV long terminal repeat gene expression (Staal et al., 1990). Additionally, CD4+ lymphocyte depletion accompanies HIV progression and decreases in GSH levels contributes to apoptosis in these CD4+ cells (Suthanthiran et al., 1990).
In vitro and in vivo experiments show that enhanced GSH levels also inhibit replication of both the influenza virus (Nencioni et al., 2003), and the herpes simplex virus (Vogel et al., 2005). Not only do GSH levels appear to affect the activity of invading viruses, but they also affect the activation of important cell signaling pathways associated with the host immune system response. There are many ways that GSH affects cell signaling, including modulating intracellular free radicals, maintaining the thiol status of proteins, and covalently binding cysteine moieties in cytosolic proteins (S-glutathionylation; Table 2). One example that is especially important to the regulation of immune function is the sensitivity of tyrosine phosphatase activity to intracellular thiol status (Zipser et al., 1997; Mustelin et al., 2005). Lower GSH levels accentuate H2O2-induced tyrosine phosphorylation (Iantomasi et al., 2001).
Not surprisingly, there have been interesting connections between GSH levels and cell signaling pathways involved in immune responses. Bruchhausen et al. (2003) reported that thiols, such as N-acetylcysteine, are specifically able to inhibit contact sensitization reactions in antigen-presenting cells via inhibition of tyrosine phosphorylation, whereas other antioxidants, such as absorbic acid, and alpha-tocopherol were ineffective. Additionally, incubation with thiols was able to improve defects in tyrosine phosphatase activity and proliferative responses in HIV-infected CD4+ lymphocytes (Cayota et al., 1996).
Diabetes Mellitus
GSH synthesis and metabolism are also altered during hyperglycemia and diabetes. Diabetes mellitus is characterized by hyperglycemia, the chronic prolonged elevation of glucose, and insulin deficiency. Type 1 diabetes is characterized by an autoimmune destruction of the insulin producing β-cells in the pancreas. Increases in reactive oxygen species from macrophages and inflammation are thought to contribute to the initial destruction of β-cells (Kaneto et al., 2007). Type 2 diabetes occurs through a variety of polygenic and environmental origins, such as obesity, that result from a combination of insulin insensitivity in peripheral tissues and/or a pancreatic β-cell insulin producing deficiency. Complications of diabetes include: pathology of the retina, renal glomerulus, peripheral nerve damage, and acceleration of atherosclerotic disease affecting arteries to the heart, brain, and lower extremities, all of which can lead to amputations, blindness, kidney failure, and death.
Interestingly, GSH concentrations are lower in erythrocytes and blood plasma of diabetic patients and patients with metabolic syndrome, who are at high risk for developing diabetes (Yoshida et al., 1995; Sharma et al., 2000; Giral et al., 2008). Lower levels of GSH in diabetics potentiate the effects of the increased reactive oxygen species. GSH levels appear to be diminished by at least two different mechanisms: direct effects of glucose and insulin on GSH synthesis, and by consumption of a cofactor required for GSH regeneration from GSSG (i.e., NAPDH) via the polyol pathway. The first enzyme of the polyol pathway is aldose reductase, which converts glucose to sorbitol through an NADPH-dependent reaction. Interestingly, aldose reductase activity appears to be regulated by S-glutathionylation (Table 2; Cappiello et al., 2001), providing an additional link between GSH and these disease states. Changes in GSH-dependent enzyme activities, such as glutathione peroxidase, γ-glutamyl transpeptidase, and glutathione S-transferase are also noted in diabetes.
Effects of insulin and glucose on GSH synthesis
Hyperglycemia and insulin appear to regulate GSH levels through γ-glutamylcysteine ligase, the rate-limiting enzyme for GSH synthesis. Prolonged exposure to high glucose conditions leads to a decrease in cellular GSH levels and decreases in mRNA for both the catalytic and regulatory subunits of γ-glutamylcysteine ligase (Urata et al., 1996; Lu et al., 1999; Catherwood et al., 2002). Decreases in γ-glutamylcysteine ligase activity have also been observed in erythrocytes of diabetic patients (Yoshida et al., 1995). Activity of glutathione synthetase, the other enzyme involved in GSH synthesis, does not appear to be effected in diabetes (Lu et al., 1992). In contrast with the effect of glucose on GSH levels, high and physiological levels of insulin increase GSH content, γ-glutamylcysteine ligase catalytic subunit mRNA and protein, and γ-glutamylcysteine ligase activity levels in primary rat hepatocytes (Lu et al., 1992; Cai et al., 1995; Kim et al., 2004). Insulin also increases GSH content in cultured erythrocytes from diabetic patients (Bravi et al., 2006). Protein and mRNA levels of the catalytic subunit of γ-glutamylcysteine ligase appear to increase to a greater extent than GSH content in response to insulin treatment (Kim et al., 2004). Insulin does not affect the regulatory subunit of γ-glutamylcysteine ligase and this may be a limiting factor in the increase in GSH levels (Cai et al., 1997; Kim et al., 2004). Therefore, as high glucose/low insulin levels are known to increase reactive oxygen species, the concomitant decrease in GSH synthesis and GSH levels will weaken cellular defenses against oxidative stress.
The polyol pathway
The polyol pathway may lead to a reduction in GSH due the consumption of NADPH in converting glucose to sorbitol by the enzyme aldose reductase (Brownlee, 2001). Under normal glucose conditions, little glucose is reduced by this pathway; however, during hyperglycemic conditions, glucose reduction by the polyol pathway increases along with a decrease in NADPH levels. The consumption of NADPH in this pathway may compromise the regeneration of reduced GSH, because glutathione reductase uses NADPH to convert GSSG back to GSH. Under oxidative stress conditions, this may lead to GSH depletion.
Lower glutathione peroxidase activity in pancreatic β-cells
Pancreatic β-cells are at high risk for oxidative damage due to comparably lower levels of antioxidant enzymes than other tissues, including glutathione peroxidase, superoxide dismutase, and catalase (Tiedge et al., 1997). Exposure to high glucose concentrations does not increase expression of glutathione peroxidase in islet cells, as seen in other tissues (Tiedge et al., 1997). The low initial levels of glutathione peroxidase and the inability of islet cells to increase glutathione peroxidase expression due to oxidant stress, leaves these insulin producing cells vulnerable. In addition, the low GSH levels in diabetes decrease the substrate available for glutathione peroxidase activity, contributing to the low activity observed in the periphery, and leaving these cells less able to detoxify hydrogen peroxides. For example, prolonged exposure to elevated glucose decreases glutathione peroxidase activity in vascular endothelial cells, kidney cells and brain mitochondria (Urata et al., 1996; Catherwood et al., 2002; Mastrocola et al., 2005). Furthermore, GSH forms adducts with monosaccharides, which may be elevated in cells during diabetes, and these adducts cannot be used as proper substrates for glutathione peroxidase, which may also contribute to decreased enzyme activity (Januel et al., 2003).
γ-Glutamyl transpeptidase and glutathione S-transferases
Other glutathione related enzymes, such as γ-glutamyl transpeptidase and glutathione S-transferase appear to be effected by changes in glucose and/or insulin levels, although these enzymes are also altered by reactive oxygen species. Hepatic γ-glutamyl transpeptidase activity is increased in a diabetic rat model and insulin treatment restores the γ-glutamyl transpeptidase activity to normal (McLennan et al., 1991; Lu et al., 1997). Bile GSH levels are lower in these models and inhibiting γ-glutamyl transpeptidase with acivicin partially restores the GSH levels (Lu et al., 1997). The increased breakdown of GSH in bile may contribute to the impaired bile flow that is observed in diabetes models. In addition, glutathione S-transferases appear to be differentially altered by insulin or glucose levels. Alpha-class glutathione S-transferase is increased by insulin and alpha and piclass glutathione S-transferase are decreased by glucagon; whereas mu-class glutathione S-transferase is not sensitive to these compounds (Kim et al., 2003). Transcription of glutathione S-transferase enzymes may also be effected by the redox state and oxidative stress through the antioxidant response element present in the promoter region (Wasserman and Fahl, 1997). Although the exact ramifications of these changes in γ-glutamyl transpeptidase and glutathione S-transferase to the diabetic state are not clear, they further substantiate the importance of GSH metabolism and GSH dependent enzymes in diabetes.
GSH-based therapies in diabetes
GSH infusion in non-insulin dependent diabetes patients improves insulin-mediated glucose uptake and erythrocyte GSH levels, although the effects are modest and require a one hour infusion (De Mattia et al., 1998). N-Acetylcysteine has also been used to improve the redox state of cells. Treating pancreatic islet cells with N-acetylcysteine prevents the decrease in insulin mRNA and protein content after ribose exposure, and co-treatment with both N-acetylcysteine and a GSH synthesis inhibitor abrogate this effect, suggesting that increases in GSH synthesis protects the islets from toxicity (Tanaka et al., 2002). Treatment of rat or mouse models that develop characteristics of type 2 diabetes as they age with N-acetylcysteine partially prevents the development of hyperglycemia and the decrease in insulin mRNA that would otherwise occur (Kaneto et al., 1999; Tanaka et al., 1999). N-acetylcysteine also reduces apoptosis in pancreatic β-cells of a diabetic mouse model, as the β-cell mass was larger in N-acetylcysteine-treated diabetic mice than untreated diabetic mice (Kaneto et al., 1999). In addition, N-acetylcysteine administration to streptozotocin induced diabetic rats prevents apoptosis and other damage to the glomeruli (Odetti et al., 2003). These results suggest that N-acetylcysteine may attenuate the transition from chronic hyperglycemia to the development of diabetes and could prevent damage in peripheral tissues.
GSH-based therapies may also be beneficial in diabetic pregnancies. Rat embryos incubated in high glucose media or from diabetic pregnant rats have less GSH, γ-glutamylcysteine ligase activity, and γ-glutamylcysteine ligase mRNA expression (Trocino et al., 1995; Sakamaki et al., 1999). After GSH ester supplementation, partial recovery of GSH levels and γ-glutamylcysteine ligase activity is observed during hyperglycemic conditions, improving growth and decreasing malformations in embryos incubated in elevated glucose conditions (Trocino et al., 1995). GSH ester or insulin treatment of diabetic mothers improved GSH concentrations, neural lesions, and growth in the rat embryos (Sakamaki et al., 1999). Thus, improving GSH status in diabetic mothers may be beneficial for the growth and development of the infant.
Summary
Changes in GSH levels and/or oxidation state have now been reported in nearly all major human diseases. Although in many cases these changes likely occur as a result of the underlying disease progression, in other cases these changes are closely linked to the onset and/or development of the disease. The growing recognition that GSH is involved in critical cell signaling pathways that are regulated by S-glutathionylation and/or by the thiol redox status, and that GSH may function as a neurotransmitter or neuromodulator, provides considerable new insight into possible links between GSH and disease progression, and raises additional questions that can be addressed experimentally. In addition, the recognition that GSH is intimately involved in so many disease states has generated considerable interest in identifying therapies aimed at modulating GSH levels so as to modulate disease risk or progression. Although such therapies have significant hurdles to overcome, they offer significant promise for many human diseases.
Acknowledgements
Our apologies for the many relevant reports that we were unable to cite due to space limitations or to oversight. This work was supported in part by the National Institute of Health Grant DK48823, NIEHS Center Grants ES01247 and ES03828, and Toxicology Training Program Grants ES07026 and ES16254.
References
- Adams JD, Jr, Klaidman LK, Odunze IN, Shen HC, Miller CA. Alzheimer's and Parkinson's disease. Brain levels of glutathione, glutathione disulfide, and vitamin E. Mol. Chem. Neuropathol. 1991;14:213–226. doi: 10.1007/BF03159937. [DOI] [PubMed] [Google Scholar]
- Aitio M. N-acetylcysteine " passé-partout or much ado about nothing? Br. J. Clin. Pharmacol. 2005;61:5–15. doi: 10.1111/j.1365-2125.2005.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahoshi M, Nakashima H, Tanaka Y, Kohsaka T, Nagano S, Ohgami E, Arinobu Y, Yamaoka K, Niiro H, Shinozaki M, Hirakata H, Horiuchi T, Otsuka T, Niho Y. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis. Rheum. 1999;42:1644–1648. doi: 10.1002/1529-0131(199908)42:8<1644::AID-ANR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Andersson C, Gaston B, Roomans GM. S-Nitrosoglutathione induces functional DeltaF508-CFTR in airway epithelial cells. Biochem Biophys Res Commun. 2002;297:552–557. doi: 10.1016/s0006-291x(02)02245-3. [DOI] [PubMed] [Google Scholar]
- Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-Acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007;7:355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell. Biochem. Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Truong AT. Relation between biliary glutathione excretion and bile acid-independent bile flow. Am. J. Physiol. 1989;256:G22–G30. doi: 10.1152/ajpgi.1989.256.1.G22. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Truong AT. Glutathione as a primary osmotic driving force in hepatic bile formation. Am. J. Physiol. 1992;263:G617–G624. doi: 10.1152/ajpgi.1992.263.5.G617. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Gatmaitan Z, Truong AT. Impaired biliary excretion and whole body elimination of methylmercury in rats with congenital defect in biliary glutathione excretion. Hepatology. 1995;22:1469–1473. [PubMed] [Google Scholar]
- Ballatori N, Moseley RH, Boyer JL. Sodium gradient-dependent L-glutamate transport is localized to the canalicular domain of liver plasma membranes. Studies in rat liver sinusoidal and canalicular membrane vesicles. J. Biol. Chem. 1986a;261:6216–6221. [PubMed] [Google Scholar]
- Ballatori N, Jacob R, Boyer JL. Intrabiliary glutathione hydrolysis - a source of glutamate in bile. J. Biol. Chem. 1986b;261:7860–7865. [PubMed] [Google Scholar]
- Ballatori N, Jacob R, Barrett C, Boyer JL. Biliary catabolism of glutathione and differential reabsorption of its amino acid constituents. Am. J. Physiol. 1988;254:G1–G7. doi: 10.1152/ajpgi.1988.254.1.G1. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Truong AT, Ma AK, Boyer JL. Determinants of glutathione efflux and biliary GSH/GSSG ratio in perfused rat liver. Am. J. Physiol. 1989;256:G482–G490. doi: 10.1152/ajpgi.1989.256.3.G482. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: Role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol. Appl. Pharmacol. 2005;204:238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Krance SM, Marchan R, Hammond CL. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Aspects. Med. 2008 doi: 10.1016/j.mam.2008.08.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RG, Wentowski CC, Curhan GC, Somers SC, Stampfer MJ, Schwartz J, Speizer FE, Camargo CA., Jr Prospective study of acetaminophen use and newly diagnosed asthma among women. Am. J. Respir. Crit. Care Med. 2004;169:836–841. doi: 10.1164/rccm.200304-596OC. [DOI] [PubMed] [Google Scholar]
- Beal MF. Oxidatively modified proteins in aging and disease. Free Radic. Biol. Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- Benlloch M, Ortega A, Ferrer P, Segarra R, Obrador E, Asensi M, Carretero J, Estrela JM. Acceleration of glutathione efflux and inhibition of gamma-glutamyltranspeptidase sensitize metastatic B16 melanoma cells to endothelium-induced cytotoxicity. J. Biol. Chem. 2005;280:6950–6959. doi: 10.1074/jbc.M408531200. [DOI] [PubMed] [Google Scholar]
- Bentley AR, Emrani P, Cassano PA. Genetic Variation and Gene Expression in Antioxidant-Related Enzymes and Risk of Chronic Obstructive Pulmonary Disease: A Systematic Review. Thorax. 2008 doi: 10.1136/thx.2007.086199. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- Bhat KS, John A, Reddy PR, Reddy PS, Reddy VN. Effect of pigmentation on glutathione redox cycle antioxidant defense in whole as well as different regions of human cataractous lens. Exp. Eye Res. 1991;52:715–721. doi: 10.1016/0014-4835(91)90023-8. [DOI] [PubMed] [Google Scholar]
- Bishop C, Hudson VM, Hilton SC, Wilde C. A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis. Chest. 2005;127:308–317. doi: 10.1378/chest.127.1.308. [DOI] [PubMed] [Google Scholar]
- Bojes HK, Datta K, Xu J, Chin A, Simonian P, Nunez G, Kehrer JP. Bcl-xL overexpression attenuates glutathione depletion in FL5.12 cells following interleukin-3 withdrawal. Biochem. J. 1997;325:315–319. doi: 10.1042/bj3250315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos JP, Heales SJ, Land JM, Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J. Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- Bravi MC, Armiento A, Laurenti O, Cassone-Faldetta M, De Luca O, Moretti A, De Mattia G. Insulin decreases intracellular oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2006;55:691–695. doi: 10.1016/j.metabol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Bruchhausen S, Zahn S, Valk E, Knop J, Becker D. Thiol antioxidants block the activation of antigen-presenting cells by contact sensitizers. J. Invest. Dermatol. 2003;121:1039–1044. doi: 10.1046/j.1523-1747.2003.12510.x. [DOI] [PubMed] [Google Scholar]
- Buhl R, Jaffe HA, Holroyd KJ, Wells FB, Mastrangeli A, Saltini C, Cantin AM, Crystal RG. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989;2:1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- Buhl R, Vogelmeier C, Critenden M, Hubbard RC, Hoyt RF, Jr, Wilson EM, Cantin AM, Crystal RG. Augmentation of glutathione in the fluid lining the epithelium of the lower respiratory tract by directly administering glutathione aerosol. Proc. Natl. Acad. Sci. USA. 1990;87:4063–4067. doi: 10.1073/pnas.87.11.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem. J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Sun WM, Lu SC. Hormonal and cell density regulation of hepatic gamma-glutamylcysteine synthetase gene expression. Mol. Pharmacol. 1995;48:212–218. [PubMed] [Google Scholar]
- Cai J, Huang ZZ, Lu SC. Differential regulation of gamma-glutamylcysteine synthetase heavy and light subunit gene expression. Biochem. J. 1997;326:167–172. doi: 10.1042/bj3260167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP. Inhibition of influenza infection by glutathione. Free Radic. Biol. Med. 2003;34:928–936. doi: 10.1016/s0891-5849(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Calvert P, Yao KS, Hamilton TC, O'Dwyer PJ. Clinical studies of reversal of drug resistance based on glutathione. Chem. Biol. Interact. 1998;111–112:213–224. doi: 10.1016/s0009-2797(98)00008-8. [DOI] [PubMed] [Google Scholar]
- Calvin HI, Medvedovsky C, Worgul BV. Near-total glutathione depletion and age-specific cataracts induced by buthionine sulfoximine in mice. Science. 1986;233:553–555. doi: 10.1126/science.3726547. [DOI] [PubMed] [Google Scholar]
- Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis. Conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11–12, 2003. Free Radic. Biol. Med. 2007;42:15–31. doi: 10.1016/j.freeradbiomed.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappiello M, et al. Modulation of aldose reductase activity through S-thiolation by physiological thiols. Chem. Biol. Interact. 2001;130–132:597–608. doi: 10.1016/s0009-2797(00)00286-6. [DOI] [PubMed] [Google Scholar]
- Catherwood MA, Powell LA, Anderson P, McMaster D, Sharpe PC, Trimble ER. Glucose-induced oxidative stress in mesangial cells. Kidney Int. 2002;61:599–608. doi: 10.1046/j.1523-1755.2002.00168.x. [DOI] [PubMed] [Google Scholar]
- Cayota A, Vuillier F, Gonzalez G, Dighiero G. CD4+ lymphocytes from HIV-infected patients display impaired CD45-associated tyrosine phosphatase activity which is enhanced by anti-oxidants. Clin. Exp. Immunol. 1996;104:11–17. doi: 10.1046/j.1365-2249.1996.d01-652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli A, Que FG, Gores GJ, LaRusso NF. Glutathione depletion is associated with decreased Bcl-2 expression and increased apoptosis in cholangiocytes. Am. J. Physiol. 1998;275:G749–G757. doi: 10.1152/ajpgi.1998.275.4.G749. [DOI] [PubMed] [Google Scholar]
- Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chen L, Patel RP, Teng X, Bosworth CA, Lancaster JR, Jr, Matalon S. Mechanisms of cystic fibrosis transmembrane conductance regulator activation by S-nitrosoglutathione. J Biol. Chem. 2006;281:9190–9199. doi: 10.1074/jbc.M513231200. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Kumar MJ, Hsu M, Rajagopalan S, Kaur D, Rane A, Nicholls DG, Choi J, Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J. Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, Evers R. Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2) J. Pharmacol. Exp. Ther. 2006;317:579–589. doi: 10.1124/jpet.105.098665. [DOI] [PubMed] [Google Scholar]
- Cobb MH, Heagy W, Danner J, Lenhoff HM, Marshall GR. Structural and conformational properties of peptides interacting with the glutathione receptor of hydra. Mol Pharmacol. 1982;21:629–636. [PubMed] [Google Scholar]
- Cohen SM, Olin KL, Feuer WJ, Hjelmeland L, Keen CL, Morse LS. Low glutathione reductase and peroxidase activity in age-related macular degeneration. Br. J. Ophthalmol. 1994;78:791–794. doi: 10.1136/bjo.78.10.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conseil GC, Deeley RG, Cole SPC. Polymorphisms of MRP1 (ABCC1) and related ATP-dependent drug transporters. Pharmacogenet. Genomics. 2005;15:523–533. doi: 10.1097/01.fpc.0000167333.38528.ec. [DOI] [PubMed] [Google Scholar]
- Coppola S, Ghibelli L. GSH extrusion and and the mitochondrial pathway of apoptotic signalling. Biochem. Soc. Trans. 2000;28:56–61. doi: 10.1042/bst0280056. [DOI] [PubMed] [Google Scholar]
- Coral K, Raman R, Rathi S, Rajesh M, Sulochana KN, Angayarkanni N, Paul PG, Ramakrishnan S. Plasma homocysteine and total thiol content in patients with exudative age-related macular degeneration. Eye. 2006;20:203–207. doi: 10.1038/sj.eye.6701853. [DOI] [PubMed] [Google Scholar]
- Csala M, Fulceri R, Mandl J, Benedetti A, Bánhegyi G. Glutathione transport in the endo/sarcoplasmic reticulum. Biofactors. 2003;17:27–35. doi: 10.1002/biof.5520170104. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-Glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Yang Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem. Biophys. Res. Commun. 2000;279:324–329. doi: 10.1006/bbrc.2000.3930. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic. Biol. Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- David LL, Shearer TR. State of sulfhydryl in selenite cataract. Toxicol Appl. Pharmacol. 1984;74:109–115. doi: 10.1016/0041-008x(84)90276-x. [DOI] [PubMed] [Google Scholar]
- Davies JC, Alton EW, Bush A. Cystic fibrosis. BMJ. 2007;335:1255–1259. doi: 10.1136/bmj.39391.713229.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BJ, van Heeckeren AM, Min E, Velsor LW. Role for cystic fibrosis transmembrane conductance regulator protein in a glutathione response to bronchopulmonary pseudomonas infection. Infect. Immun. 2004;72:2045–2051. doi: 10.1128/IAI.72.4.2045-2051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SPC. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol. Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Kaplowitz N. Importance and regulation of hepatic glutathione. Semin. Liver Dis. 1990;10:251–266. doi: 10.1055/s-2008-1040481. [DOI] [PubMed] [Google Scholar]
- De Mattia G, Bravi MC, Laurenti O, Cassone-Faldetta M, Armiento A, Ferri C, Balsano F. Influence of reduced glutathione infusion on glucose metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1998;47:993–997. doi: 10.1016/s0026-0495(98)90357-2. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Levonen AL, Moellering DR, Arnold EK, Zhang H, Darley-Usmar VM, Forman HJ. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic. Biol. Med. 2004;37:1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Krüger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuénod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn YC, Brand PL. Systematic review of N-acetylcysteine in cystic fibrosis. Acta Paediatr. 1999;88:38–41. doi: 10.1080/08035259950170574. [DOI] [PubMed] [Google Scholar]
- Eisen M, Kaur S, Rehema A, Kullisaar T, Vihalemm T, Zilmer K, Kairane C, Zilmer M. Allergic contact dermatitis is accompanied by severe abnormal changes in antioxidativity of blood. Biomed. Pharmacother. 2004;58:260–263. doi: 10.1016/j.biopha.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Ellerby LM, Ellerby HM, Park SM, Holleran AL, Murphy AN, Fiskum G, Kane DJ, Testa MP, Kayalar C, Bredesen DE. Shift of the cellular oxidation-reduction potential in neural cells expressing Bcl-2. J. Neurochem. 1996;67:1259–1267. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- Emdin M, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation. 2005;112:2078–2080. doi: 10.1161/CIRCULATIONAHA.105.571919. [DOI] [PubMed] [Google Scholar]
- Eneli I, Sadri K, Camargo C, Jr, Barr RG. Acetaminophen and the risk of asthma: the epidemiologic and pathophysiologic evidence. Chest. 2005;127:604–612. doi: 10.1378/chest.127.2.604. [DOI] [PubMed] [Google Scholar]
- Esposito F, Agosti V, Morrone G, Morra F, Cuomo C, Russo T, Venuta S, Cimino F. Inhibition of the differentiation of human myeloid cell lines by redox changes induced through glutathione depletion. Biochem. J. 1994;301:649–653. doi: 10.1042/bj3010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- Fadeel B, Orrenius S, Zhivotovsky B. Apoptosis in human disease: a new skin for the old ceremony? Biochem. Biophys. Res. Commun. 1999;6:699–717. doi: 10.1006/bbrc.1999.1888. [DOI] [PubMed] [Google Scholar]
- Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- Gao L, Broughman JR, Iwamoto T, Tomich JM, Venglarik CJ, Forman HJ. Synthetic chloride channel restores glutathione secretion in cystic fibrosis airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L24–L30. doi: 10.1152/ajplung.2001.281.1.L24. [DOI] [PubMed] [Google Scholar]
- Gao L, Broughman JR, Iwamoto T, Tomich JM, Venglarik CJ, Forman HJ. Synthetic chloride channel restores glutathione secretion in cystic fibrosis airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L24–L30. doi: 10.1152/ajplung.2001.281.1.L24. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Fernandez-Checa JC. Redox regulation of hepatocyte apoptosis. J. Gastroenterol. Hepatol. 2007;22:S38–S42. doi: 10.1111/j.1440-1746.2006.04644.x. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Beltran B, Salas-Pino S, Bolanos JP, Clark JB, Moncada S, Heales SJ. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/neurodegeneration? J. Neurochem. 2003;86:228–237. doi: 10.1046/j.1471-4159.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Di Simplicio P. Glutathionylation pathways in drug response. Curr Opin Pharmacol. 2007;7:398–403. doi: 10.1016/j.coph.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Ghibelli L, Fanelli C, Rotilio G, Lafavia E, Coppola S, Colussi C, Civitareale P, Ciriolo MR. Rescue of cells from apoptosis by inhibition of active GSH extrusion. Faseb J. 1998;12:479–486. doi: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- Giral P, Jacob N, Dourmap C, Hansel B, Carrié A, Bruckert E, Girerd X, Chapman MJ. Elevated gamma-glutamyltransferase activity and perturbed thiol profile are associated with features of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008;28:587–593. doi: 10.1161/ATVBAHA.107.157891. [DOI] [PubMed] [Google Scholar]
- Goven D, Boutten A, Le"on-Malas V, Marchal-Sommé J, Amara N, Crestani B, Fournier M, Lesèche G, Soler P, Boczkowski J, Bonay M. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63:916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- Grasemann H, Gaston B, Fang K, Paul K, Ratjen F. Decreased levels of nitrosothiols in the lower airways of patients with cystic fibrosis and normal pulmonary function. J. Pediatr. 1999;135:770–772. doi: 10.1016/s0022-3476(99)70101-0. [DOI] [PubMed] [Google Scholar]
- Griese M, Ramakers J, Krasselt A, Starosta V, Van Koningsbruggen S, Fischer R, Ratjen F, Müllinger B, Huber RM, Maier K, Rietschel E, Scheuch G. Improvement of alveolar glutathione and lung function but not oxidative state in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2004;169:822–828. doi: 10.1164/rccm.200308-1104OC. [DOI] [PubMed] [Google Scholar]
- Gu M, Owen AD, Toffa SE, Cooper JM, Dexter DT, Jenner P, Marsden CD, Schapira AH. Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J. Neurol. Sci. 1998;158:24–29. doi: 10.1016/s0022-510x(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Guo N, McIntosh C, Shaw C. Glutathione: new candidate neuropeptide in the central nervous system. Neuroscience. 1992;51:835–842. doi: 10.1016/0306-4522(92)90524-6. [DOI] [PubMed] [Google Scholar]
- Hadzic T, Li L, Cheng N, Walsh SA, Spitz DR, Knudson CM. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J. Immunol. 2005;175:7965–7972. doi: 10.4049/jimmunol.175.12.7965. [DOI] [PubMed] [Google Scholar]
- Hahn R, Wendel A, Flohe L. The fate of extracellular glutathione in the rat. Biochim. Biophys. Acta. 1978;539:324–337. doi: 10.1016/0304-4165(78)90037-5. [DOI] [PubMed] [Google Scholar]
- Hamilos DL, Zelarney P, Mascali JJ. Lymphocyte proliferation in glutathione-depleted lymphocytes: direct relationship between glutathione availability and the proliferative response. Immunopharmacology. 1989;18:223–235. doi: 10.1016/0162-3109(89)90020-9. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Lee TK, Ballatori N. Novel roles for glutathione in gene expression, cell death, and membrane transport of organic solutes. J. Hepatol. 2001;34:946–954. doi: 10.1016/s0168-8278(01)00037-x. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Madejczyk MS, Ballatori N. Activation of plasma membrane reduced glutathione transport in death receptor apoptosis of HepG2 cells. Toxicol. Appl. Pharmacol. 2004;195:12–22. doi: 10.1016/j.taap.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Hammond CL, Marchan R, Krance SM, Ballatori N. Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J. Biol. Chem. 2007;282:14337–14347. doi: 10.1074/jbc.M611019200. [DOI] [PubMed] [Google Scholar]
- Han J, Cheng FC, Yang Z, Dryhurst G. Inhibitors of mitochondrial respiration, iron (II), and hydroxyl radical evoke release and extracellular hydrolysis of glutathione in rat striatum and substantia nigra: potential implications to Parkinson's disease. J. Neurochem. 1999;73:1683–1695. doi: 10.1046/j.1471-4159.1999.731683.x. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Carney EW, Harris C. Altered differentiation in rat and rabbit limb bud micromass cultures by glutathione modulating agents. Free Radic. Biol. Med. 2001;31:1582–1592. doi: 10.1016/s0891-5849(01)00751-1. [DOI] [PubMed] [Google Scholar]
- Harding JJ. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem. J. 1970;117:957–960. doi: 10.1042/bj1170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MQ, Hadi RA, Al-Rawi ZS, Padron VA, Stohs SJ. The glutathione defense system in the pathogenesis of rheumatoid arthritis. J. Appl. Toxicol. 2001;21:69–73. doi: 10.1002/jat.736. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem. Biophys. Res. Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Head KA. Natural therapies for ocular disorders, part two: Cataracts and glaucoma. Altern. Med. Rev. 2001;6:141–166. [PubMed] [Google Scholar]
- Herzenberg LA, De Rosa SC, Dubs JG, Roederer M, Anderson MT, Ela SW, Deresinski SC, Herzenberg LA. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. USA. 1997;94:1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchman CA, Ballatori N. Glutathione-degrading capacities of liver and kidney in different species. Biochem. Pharmacol. 1990;40:1131–1135. doi: 10.1016/0006-2952(90)90503-d. [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Ballatori N. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J. Toxicol. Environ. Health. 1994;41:387–409. doi: 10.1080/15287399409531852. [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Matsumoto H, Simmons TW, Ballatori N. Intrahepatic conversion of a glutathione conjugate to its mercapturic acid. J. Biol. Chem. 1991;266:22179–22185. [PubMed] [Google Scholar]
- Hinchman CA, Truong AT, Ballatori N. Hepatic uptake of intact glutathione S-conjugate, inhibition by organic anions, and sinusoidal catabolism. Am. J. Physiol. 1993;265:G547–G554. doi: 10.1152/ajpgi.1993.265.3.G547. [DOI] [PubMed] [Google Scholar]
- Hinchman CA, Rebbeor JF, Ballatori N. Efficient hepatic uptake and concentrative biliary excretion of a mercapturic acid. Am. J. Physiol. 1998;275:G612–G619. doi: 10.1152/ajpgi.1998.275.4.G612. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Konig J, Dringen R. Expression of mRNAs of multidrug resistance proteins (Mrps) in cultured rat astrocytes, oligodendrocytes, microglial cells and neurones. J. Neurochem. 2002a;82:716–719. doi: 10.1046/j.1471-4159.2002.01082.x. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Schulz JB, Dringen R. Effects of dopamine on the glutathione metabolism of cultured astroglial cells: implications for Parkinson's disease. J. Neurochem. 2002b;82:458–467. doi: 10.1046/j.1471-4159.2002.01013.x. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Schulz JB, Dringen R. Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J. Neurosci. Res. 2002c;69:318–326. doi: 10.1002/jnr.10308. [DOI] [PubMed] [Google Scholar]
- Hochman A, Sternin H, Gorodin S, Korsmeyer S, Ziv I, Melamed E, Offen D. Enhanced oxidative stress and altered antioxidants in brains of Bcl-2-deficient mice. J. Neurochem. 1998;71:741–748. doi: 10.1046/j.1471-4159.1998.71020741.x. [DOI] [PubMed] [Google Scholar]
- Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Holleschau AM, Rathbun WB. The effects of age on glutathione peroxidase and glutathione reductase activities in lenses of old world simians and prosimians. Curr. Eye Res. 1994;13:331–336. doi: 10.3109/02713689409167296. [DOI] [PubMed] [Google Scholar]
- Horwitz DA, Gray JD, Behrendsen SC, Kubin M, Rengaraju M, Ohtsuka K, Trinchieri G. Decreased production of interleukin-12 and other Th1-type cytokines in patients with recent-onset systemic lupus erythematosus. Arthritis Rheum. 1998;41:838–844. doi: 10.1002/1529-0131(199805)41:5<838::AID-ART10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Howard M, Fischer H, Roux J, Santos BC, Gullans SR, Yancey PH, Welch WJ. Mammalian osmolytes and S-nitrosoglutathione promote Delta F508 cystic fibrosis transmembrane conductance regulator (CFTR) protein maturation and function. J. Biol. Chem. 2003;278:35159–35167. doi: 10.1074/jbc.M301924200. [DOI] [PubMed] [Google Scholar]
- Hudson VM. Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radic. Biol. Med. 2001;30:1440–1461. doi: 10.1016/s0891-5849(01)00530-5. [DOI] [PubMed] [Google Scholar]
- Huh YJ, Kim JM, Kim H, Song H, So H, Lee SY, Kwon SB, Kim HJ, Kim HH, Lee SH, Choi Y, Chung SC, Jeong DW, Min BM. Regulation of osteoclast differentiation by the redox-dependent modulation of nuclear import of transcription factors. Cell Death Differ. 2005;13:1138–1146. doi: 10.1038/sj.cdd.4401793. [DOI] [PubMed] [Google Scholar]
- Hurbain I, Sermet-Gaudelus I, Vallee B, Feuillet MN, Lenoir G, Bernaudin JF, Edelman A, Fajac A. Evaluation of MRP1-5 gene expression in cystic fibrosis patients homozygous for the delta F508 mutation. Pediatr. Res. 2003;54:627–634. doi: 10.1203/01.PDR.0000090926.16166.3F. [DOI] [PubMed] [Google Scholar]
- Iantomasi T, Favilli F, Catarzi S, Vincenzini MT. GSH role on platelet-derived growth factor receptor tyrosine phosphorylation induced by H2O2. Biochem. Biophys. Res. Commun. 2001;280:1279–1285. doi: 10.1006/bbrc.2001.4274. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Taniguchi N. Gene expression of gamma-glutamyltranspeptidase. Methods Enzymol. 2005;401:408–425. doi: 10.1016/S0076-6879(05)01025-6. [DOI] [PubMed] [Google Scholar]
- Isaacs J, Binkley F. Glutathione dependent control of protein disulfide-sulfhydryl content by subcellular fractions of hepatic tissue. Biochim. Biophys. Acta. 1977;497:192–204. doi: 10.1016/0304-4165(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Isomura H, Fujie K, Shibata K, Inoue N, Iizuka T, Takebe G, Takahashi K, Nishihira J, Izumi H, Sakamoto W. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004;197:93–100. doi: 10.1016/j.tox.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP, Rodriguiz RM, Mørk A, Wetsel WC. Monoaminergic dysregulation in glutathione-deficient mice: possible relevance to schizophrenia? Neuroscience. 2005;132:1055–1072. doi: 10.1016/j.neuroscience.2005.01.059. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Janáky R, Ogita K, Pasqualotto BA, Bains JS, Oja SS, Yoneda Y, Shaw CA. Glutathione and signal transduction in the mammalian CNS. J. Neurochem. 1999;73:889–902. doi: 10.1046/j.1471-4159.1999.0730889.x. [DOI] [PubMed] [Google Scholar]
- Janáky R, Shaw CA, Varga V, Hermann A, Dohovics R, Saransaari P, Oja SS. Specific glutathione binding sites in pig cerebral cortical synaptic membranes. Neuroscience. 2000;95:617–624. doi: 10.1016/s0306-4522(99)00442-x. [DOI] [PubMed] [Google Scholar]
- Janáky R, Dohovics R, Saransaari P, Oja SS. Modulation of [3H]dopamine release by glutathione in mouse striatal slices. Neurochem. Res. 2007;32:1357–1364. doi: 10.1007/s11064-007-9315-z. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Bcl-2 attenuation of oxidative cell death is associated with up-regulation of gamma-glutamylcysteine ligase via constitutive NF-kappaB activation. J. Biol. Chem. 2004;279:38779–38786. doi: 10.1074/jbc.M406371200. [DOI] [PubMed] [Google Scholar]
- Januel C, Fay LB, Ruggiero D, Lagarde M, Véricel E. Covalent coupling of reduced glutathione with ribose: loss of cosubstrate ability to glutathione peroxidase. Biochim. Biophys. Acta. 2003;1620:125–132. doi: 10.1016/s0304-4165(02)00525-1. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann. Neurol. 2003;53:S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol. Aging. 2007;28:1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid. Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Jösch C, Klotz LO, Sies H. Identification of cytosolic leucyl aminopeptidase (EC 3.4.11.1) as the major cysteinylglycine-hydrolysing activity in rat liver. Biol. Chem. 2003;384:213–218. doi: 10.1515/BC.2003.023. [DOI] [PubMed] [Google Scholar]
- Jun JH, Lee SH, Kwak HB, Lee ZH, Seo SB, Woo KM, Ryoo HM, Kim GS, Baek JH. N-acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. J. Cell Biochem. 2008;103:1246–1255. doi: 10.1002/jcb.21508. [DOI] [PubMed] [Google Scholar]
- Kabesch M, Hoefler C, Carr D, Leupold W, Weiland SK, von Mutius E. Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax. 2004;59:569–573. doi: 10.1136/thx.2003.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Katakami N, Kawamori D, Miyatsuka T, Sakamoto K, Matsuoka TA, Matsuhisa M, Yamasaki Y. Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid. Redox Signal. 2007;9:355–366. doi: 10.1089/ars.2006.1465. [DOI] [PubMed] [Google Scholar]
- Katakura K, Kishida K, Hirano H. Changes in rat lens proteins and glutathione reductase activity with advancing age. Int. J. Vitam. Nutr. Res. 2004;74:329–333. doi: 10.1024/0300-9831.74.5.329. [DOI] [PubMed] [Google Scholar]
- Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic. Biol. Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest. Ophthalmol. Vis. Sci. 2000;41:741–748. [PubMed] [Google Scholar]
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- Kim JM, Kim H, Kwon SB, Lee SY, Chung SC, Jeong DW, Min BM. Intracellular glutathione status regulates mouse bone marrow monocyte-derived macrophage differentiation and phagocytic activity. Biochem. Biophys. Res. Commun. 2004;325:101–108. doi: 10.1016/j.bbrc.2004.09.220. [DOI] [PubMed] [Google Scholar]
- Kim SK, Woodcroft KJ, Novak RF. Insulin and glucagon regulation of glutathione S-transferase expression in primary cultured rat hepatocytes. J. Pharmacol. Exp. Ther. 2003;305:353–361. doi: 10.1124/jpet.102.045153. [DOI] [PubMed] [Google Scholar]
- Kim SK, Woodcroft KJ, Khodadadeh SS, Novak RF. Insulin signaling regulates gamma-glutamylcysteine ligase catalytic subunit expression in primary cultured rat hepatocytes. J. Pharmacol. Exp. Ther. 2004;311:99–108. doi: 10.1124/jpet.104.070375. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Vuorinen K, Ilumets H, Rytilä P, Myllärniemi M. Thiol proteins, redox modulation and parenchymal lung disease. Curr. Med. Chem. 2007;14:213–222. doi: 10.2174/092986707779313345. [DOI] [PubMed] [Google Scholar]
- Knight JA. The biochemistry of aging. Adv. Clin. Chem. 2000;35:1–62. doi: 10.1016/s0065-2423(01)35014-x. [DOI] [PubMed] [Google Scholar]
- Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- Kogan I, Ramjeesingh M, Huan LJ, Wang Y, Bear CE. Perturbation of the pore of the cystic fibrosis transmembrane conductance regulator (CFTR) inhibits its atpase activity. J Biol. Chem. 2001;276:11575–11581. doi: 10.1074/jbc.M010403200. [DOI] [PubMed] [Google Scholar]
- Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SP, Bear CE. CFTR directly mediates nucleotide-regulated glutathione flux. Embo J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokcam I, Naziroglu M. Antioxidants and lipid peroxidation status in the blood of patients with psoriasis. Clin. Chim. Acta. 1999;289:23–31. doi: 10.1016/s0009-8981(99)00150-3. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Fiskum G. Redox mechanisms of cytoprotection by Bcl-2. Antioxid Redox Signal. 2005;7:508–514. doi: 10.1089/ars.2005.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh GD, Belinsky MG, Gallo JM, Lee K. Physiological and pharmacological functions of Mrp2, Mrp3 and Mrp4 as determined from recent studies on gene-disrupted mice. Cancer Metastasis. Rev. 2007;26:5–14. doi: 10.1007/s10555-007-9039-1. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wiseman AL, Kala G, Kala SV, Matzuk MM, Lieberman MW. Reproductive defects in gamma-glutamyl transpeptidase-deficient mice. Endocrinology. 2000;141:4270–4277. doi: 10.1210/endo.141.11.7760. [DOI] [PubMed] [Google Scholar]
- Lang CA, Mills BJ, Mastropaolo W, Liu MC. Blood glutathione decreases in chronic diseases. J. Lab. Clin. Med. 2000;135:402–405. doi: 10.1067/mlc.2000.105977. [DOI] [PubMed] [Google Scholar]
- Lang CA, Naryshkin S, Schneider DL, Mills BJ, Lindeman RD. Low blood glutathione levels in healthy aging adults. J. Lab. Clin. Med. 1992;120:720–725. [PubMed] [Google Scholar]
- Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterburg BH, Adams JD, Mitchell JR. Hepatic glutathione homeostasis in the rat: efflux accounts for glutathione turnover. Hepatology. 1984;4:586–590. doi: 10.1002/hep.1840040402. [DOI] [PubMed] [Google Scholar]
- Lavoie S, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Invest. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- Leopold JA, Loscalzo J. Oxidative enzymopathies and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005;25:1332–1340. doi: 10.1161/01.ATV.0000163846.51473.09. [DOI] [PubMed] [Google Scholar]
- Leslie SW, Brown LM, Trent RD, Lee YH, Morris JL, Jones TW, Randall PK, Lau SS, Monks TJ. Stimulation of N-methyl-D-aspartate receptor-mediated calcium entry into dissociated neurons by reduced and oxidized glutathione. Mol. Pharmacol. 1992;41:308–314. [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SPC. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Tox. Appl. Pharm. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Li L, Lee TK, Meier PJ, Ballatori N. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J. Biol. Chem. 1998;273:16184–16191. doi: 10.1074/jbc.273.26.16184. [DOI] [PubMed] [Google Scholar]
- Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, Jalink K, Li M, Nelson DJ, Schuetz JD, Naren AP. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131:940–951. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MW, Wiseman AL, Shi ZZ, Carter BZ, Barrios R, Ou CN, Chévez-Barrios P, Wang Y, Habib GM, Goodman JC, Huang SL, Lebovitz RM, Matzuk MM. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93:7923–7926. doi: 10.1073/pnas.93.15.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell P, Hanrahan JW. Glutathione permeability of CFTR. Am. J. Physiol. 1998;275:C323–C326. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- Liu H, Harrell LE, Shenvi S, Hagen T, Liu RM. Gender differences in glutathione metabolism in Alzheimer's disease. J. Neurosci. Res. 2005;79:861–867. doi: 10.1002/jnr.20424. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Shenvi S, Hagen TM, Liu RM. Glutathione metabolism during aging and in Alzheimer disease. Ann. N Y Acad. Sci. 2004;1019:346–349. doi: 10.1196/annals.1297.059. [DOI] [PubMed] [Google Scholar]
- Lorico A, Rappa G, Finch RA, Yang D, Flavell RA, Sartorelli AC. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 1997;57:5238–5242. [PubMed] [Google Scholar]
- Lowry MH, McAllister BP, Jean JC, Brown LA, Hughey RP, Cruikshank WW, Amar S, Lucey EC, Braun K, Johnson P, Wight TN, Joyce-Brady M. Lung lining fluid glutathione attenuates IL-13-induced asthma. Am. J. Respir. Cell. Mol. Biol. 2008;38:509–516. doi: 10.1165/rcmb.2007-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Ge JL, Kuhlenkamp J, Kaplowitz N. Insulin and glucocorticoid dependence of hepatic gamma-glutamylcysteine synthetase and glutathione synthesis in the rat. Studies in cultured hepatocytes and in vivo. J. Clin. Invest. 1992;90:524–532. doi: 10.1172/JCI115890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Kuhlenkamp J, Wu H, Sun WM, Stone L, Kaplowitz N. Progressive defect in biliary GSH secretion in streptozotocin-induced diabetic rats. Am. J. Physiol. 1997;272:G374–G382. doi: 10.1152/ajpgi.1997.272.2.G374. [DOI] [PubMed] [Google Scholar]
- Lu SC, Bao Y, Huang ZZ, Sarthy VP, Kannan R. Regulation of gamma-glutamylcysteine synthetase subunit gene expression in retinal Müller cells by oxidative stress. Invest Ophthalmol. Vis. Sci. 1999;40:1776–1782. [PubMed] [Google Scholar]
- Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003;88:1523–1527. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- Mahagita C, Grassl SM, Piyachaturawat P, Ballatori N. Human organic anion transporter 1B1 (OATP1B1/OATP-C) and 1B3 (OATP1B3/OATP-8) function as bidirectional carriers and do not mediate GSH-bile acid co-transport. Am. J. Physiol. 2007;293:G271–G278. doi: 10.1152/ajpgi.00075.2007. [DOI] [PubMed] [Google Scholar]
- Maher P, Hanneken A. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Invest. Ophthalmol. Vis. Sci. 2005;46:4796–4803. doi: 10.1167/iovs.05-0397. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J. Respir. Crit. Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Marrades RM, Roca J, Barberà JA, de Jover L, MacNee W, Rodriguez-Roisin R. Nebulized glutathione induces bronchoconstriction in patients with mild asthma. Am. J. Respir. Crit. Care Med. 1997;156:425–430. doi: 10.1164/ajrccm.156.2.9611001. [DOI] [PubMed] [Google Scholar]
- Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Mastrocola R, Restivo F, Vercellinatto I, Danni O, Brignardello E, Aragno M, Boccuzzi G. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J. Endocrinol. 2005;187:37–44. doi: 10.1677/joe.1.06269. [DOI] [PubMed] [Google Scholar]
- Mayatepek E, Badiou S, Bellet H, Lehmann WD. A patient with neurological symptoms and abnormal leukotriene metabolism: a new defect in leukotriene biosynthesis. Ann. Neurol. 2005;58:968–970. doi: 10.1002/ana.20687. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40:313–321. [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol. Aging. 1999a;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J. Comp. Neurol. 1999b;413:101–112. [PubMed] [Google Scholar]
- McKeever TM, Lewis SA, Smit HA, Burney P, Britton JR, Cassano PA. The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. Am. J. Respir. Crit. Care Med. 2005;171:966–971. doi: 10.1164/rccm.200409-1269OC. [DOI] [PubMed] [Google Scholar]
- McLennan SV, Heffernan S, Wright L, Rae C, Fisher E, Yue DK, Turtle JR. Changes in hepatic glutathione metabolism in diabetes. Diabetes. 1991;40:344–348. doi: 10.2337/diab.40.3.344. [DOI] [PubMed] [Google Scholar]
- Meister A. New developments in glutathione metabolism and their potential application in therapy. Hepatology. 1984;4:739–742. doi: 10.1002/hep.1840040431. [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson ME. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A, Tate SS. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu. Rev. Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Meredith MJ, Cusick CL, Soltaninassab S, Sekhar KS, Lu S, Freeman ML. Expression of Bcl-2 increases intracellular glutathione by inhibiting methionine-dependent GSH efflux. Biochem. Biophys. Res. Commun. 1998;248:458–463. doi: 10.1006/bbrc.1998.8998. [DOI] [PubMed] [Google Scholar]
- Messina JP, Lawrence DA. Cell cycle progression of glutathione-depleted human peripheral blood mononuclear cells is inhibited at S phase. J. Immunol. 1989;143:1974–1981. [PubMed] [Google Scholar]
- Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic. Biol. Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Moriarty-Craige SE, Adkison J, Lynn M, Gensler G, Bressler S, Jones DP, Sternberg P., Jr Antioxidant supplements prevent oxidation of cysteine/cystine redox in patients with age-related macular degeneration. Am. J. Ophthalmol. 2005;140:1020–1026. doi: 10.1016/j.ajo.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Murata Y, Shimamura T, Hamuro J. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int. Immunol. 2002;14:201–212. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nat. Rev. Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- Nencioni L, Iuvara A, Aquilano K, Ciriolo MR, Cozzolino F, Rotilio G, Garaci E, Palamara AT. Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. Faseb J. 2003;17:758–760. doi: 10.1096/fj.02-0508fje. [DOI] [PubMed] [Google Scholar]
- Njålsson R, Norgren S. Physiological and pathological aspects of GSH metabolism. Acta Paediatr. 2005;94:132–137. doi: 10.1111/j.1651-2227.2005.tb01878.x. [DOI] [PubMed] [Google Scholar]
- Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G1352–G1359. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- O’Brien ML, Tew KD. Glutathione and Related Enzymes in Multidrug Resistance. Eur. J. Cancer. 1996;32:967–978. doi: 10.1016/0959-8049(96)00051-2. [DOI] [PubMed] [Google Scholar]
- Oda T, Sadakata N, Komatsu N, Muramatsu T. Specific efflux of glutathione from the basolateral membrane domain in polarized MDCK cells during ricin-induced apoptosis. J. Biochem. 1999;126:715–721. doi: 10.1093/oxfordjournals.jbchem.a022508. [DOI] [PubMed] [Google Scholar]
- Odetti P, Pesce C, Traverso N, Menini S, Maineri EP, Cosso L, Valentini S, Patriarca S, Cottalasso D, Marinari UM, Pronzato MA. Comparative trial of N-acetyl-cysteine, taurine, and oxerutin on skin and kidney damage in long-term experimental diabetes. Diabetes. 2003;52:499–505. doi: 10.2337/diabetes.52.2.499. [DOI] [PubMed] [Google Scholar]
- Ogita K, Enomoto R, Nakahara F, Ishitsubo N, Yoneda Y. A possible role of glutathione as an endogenous agonist at the N-methyl-D-aspartate recognition domain in rat brain. J. Neurochem. 1995;64:1088–1096. doi: 10.1046/j.1471-4159.1995.64031088.x. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J. Assoc. Res. Otolaryngol. 2000;1:243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja SS, Janáky R, Varga V, Saransaari P. Modulation of glutamate receptor functions by glutathione. Neurochem. Int. 2000;37:299–306. doi: 10.1016/s0197-0186(00)00031-0. [DOI] [PubMed] [Google Scholar]
- Ortega A, Ferrer P, Carretero J, Obrador E, Asensi M, Pellicer JA, Estrela JM. Down-regulation of glutathione and Bcl-2 synthesis in mouse B16 melanoma cells avoids their survival during interaction with the vascular endothelium. J. Biol. Chem. 2003;278:39591–39599. doi: 10.1074/jbc.M303753200. [DOI] [PubMed] [Google Scholar]
- Ozgocmen S, Kaya H, Fadillioglu E, Yilmaz Z. Effects of calcitonin, risedronate, and raloxifene on erythrocyte antioxidant enzyme activity, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Arch. Med. Res. 2007a;38:196–205. doi: 10.1016/j.arcmed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol. Cell Biochem. 2007b;295:45–52. doi: 10.1007/s11010-006-9270-z. [DOI] [PubMed] [Google Scholar]
- Palamara AT, Perno CF, Ciriolo MR, Dini L, Balestra E, D'Agostini C, Di Francesco P, Favalli C, Rotilio G, Garaci E. Evidence for antiviral activity of glutathione: in vitro inhibition of herpes simplex virus type 1 replication. Antiviral Res. 1995;27:237–253. doi: 10.1016/0166-3542(95)00008-a. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann. Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin. Chim. Acta. 2003;333:19–39. doi: 10.1016/s0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- Pau H, Graf P, Sies H. Glutathione content of the lens in various forms of cataract. Graefes Arch. Clin. Exp. Ophthalmol. 1982;219:140–142. doi: 10.1007/BF02152299. [DOI] [PubMed] [Google Scholar]
- Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. J. Neural Transm. 1997;104:661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- Perl A, Gergely P, Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TL, Yong VW, Bergeron C, Hansen S, Jones K. Amino acids, glutathione, and glutathione transferase activity in the brains of patients with Alzheimer's disease. Ann. Neurol. 1987;21:331–336. doi: 10.1002/ana.410210403. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnen F, Cacciatore I, Cornacchia C, Sozio P, Iannitelli A, Costa M, Pecci L, Nasuti C, Cantalamessa F, Di Stefano A. Synthesis and study of L-dopa-glutathione codrugs as new anti-Parkinson agents with free radical scavenging properties. J. Med. Chem. 2007;50:2506–2515. doi: 10.1021/jm070037v. [DOI] [PubMed] [Google Scholar]
- Prousky J. The treatment of pulmonary diseases and respiratory-related conditions with inhaled (nebulized or aerosolized) glutathione. Evid. Based Complement. Alternat. Med. 2008;5:27–35. doi: 10.1093/ecam/nem040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovic AD, Hastings TG. Role of endogenous glutathione in the oxidation of dopamine. J. Neurochem. 1998;71:2071–2078. doi: 10.1046/j.1471-4159.1998.71052071.x. [DOI] [PubMed] [Google Scholar]
- Rahman I. Regulation of glutathione in inflammation and chronic lung diseases. Mutat. Res. 2005;579:58–80. doi: 10.1016/j.mrfmmm.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Rebbeor JF, Connolly GC, Dumont ME, Ballatori N. ATP-dependent transport of reduced glutathione on YCF1, the yeast orthologue of mammalian multidrug resistance associated proteins. J. Biol. Chem. 1998a;273:33449–33454. doi: 10.1074/jbc.273.50.33449. [DOI] [PubMed] [Google Scholar]
- Rebbeor JF, Wang W, Clifton D, Ballatori N. Glutathione S-conjugate formation and metabolism in HepG2 cells: a cell model of mercapturic acid biosynthesis. J. Toxicol. Environ. Health. 1998b;53:651–663. doi: 10.1080/009841098159097. [DOI] [PubMed] [Google Scholar]
- Rebbeor JF, Connolly GC, Henson JH, Boyer JL, Ballatori N. ATP-dependent GSH and glutathione S-conjugate transport in skate liver: role of an Mrp functional homologue. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G417–G425. doi: 10.1152/ajpgi.2000.279.2.G417. [DOI] [PubMed] [Google Scholar]
- Rebbeor JF, Connolly GC, Ballatori N. Inhibition of Mrp2- and Ycf1p-mediated transport by reducing agents: evidence for GSH transport on rat Mrp2. Biochim. Biophys. Acta. 2002;1559:171–178. doi: 10.1016/s0005-2736(01)00454-0. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Laurie C, Lee Mosley R, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Richter C. Reactive oxygen and DNA damage in mitochondria. Mutat. Res. 1992;275:249–255. doi: 10.1016/0921-8734(92)90029-o. [DOI] [PubMed] [Google Scholar]
- Rikans LE, Hornbrook KR. Lipid peroxidation, antioxidant protection and aging. Biochim. Biophys. Acta. 1997;1362:116–127. doi: 10.1016/s0925-4439(97)00067-7. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Ristoff E, Larsson A. Inborn errors in the metabolism of glutathione. Orphanet. J. Rare Dis. 2007;2:16. doi: 10.1186/1750-1172-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Th1 and Th2 in human diseases. Clin. Immunol. Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- Rosenblat M, Volkova N, Coleman R, Aviram M. Anti-oxidant and anti-atherogenic properties of liposomal glutathione: studies in vitro, and in the atherosclerotic apolipoprotein -deficient mice. Atherosclerosis. 2007;195:e61–e68. doi: 10.1016/j.atherosclerosis.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J. Appl. Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- Roum JH, Borok Z, McElvaney NG, Grimes GJ, Bokser AD, Buhl R, Crystal RG. Glutathione aerosol suppresses lung epithelial surface inflammatory cell-derived oxidants in cystic fibrosis. J. Appl. Physiol. 1999;87:438–443. doi: 10.1152/jappl.1999.87.1.438. [DOI] [PubMed] [Google Scholar]
- Sakamaki H, Akazawa S, Ishibashi M, Izumino K, Takino H, Yamasaki H, Yamaguchi Y, Goto S, Urata Y, Kondo T, Nagataki S. Significance of glutathione-dependent antioxidant system in diabetes-induced embryonic malformations. Diabetes. 1999;48:1138–1144. doi: 10.2337/diabetes.48.5.1138. [DOI] [PubMed] [Google Scholar]
- Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Jr, Reed RL, Jones DP. Glutathione in human plasma: Decline in association with aging, age-related macular degeneration, and diabetes. Free Radic. Biol. Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rodriguez MA, Ruiz-Ramos M, Correa-Munoz E, Mendoza-Nunez VM. Oxidative stress as a risk factor for osteoporosis in elderly mexicans as characterized by antioxidant enzymes. BMC Musculoskelet. Disord. 2007;8:124. doi: 10.1186/1471-2474-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Kotowicz MA, Nicholson GC. Potential role of the antioxidant N-acetylcysteine in slowing bone resorption in early post-menopausal women: a pilot study. Transl. Res. 2007;150:215. doi: 10.1016/j.trsl.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Sastre J, Martin JA, Gomez-Cabrera MC, Pereda J, Borras C, Pallardo FV, Vina J. Age-associated oxidative damage leads to absence of gamma-cystathionase in over 50% of rat lenses: Relevance in cataractogenesis. Free Radic. Biol. Med. 2005;38:575–582. doi: 10.1016/j.freeradbiomed.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schor NF, Rudin CM, Hartman AR, Thompson CB, Tyurina YY, Kagan VE. Cell line dependence of Bcl-2-induced alteration of glutathione handling. Oncogene. 2000;19:472–476. doi: 10.1038/sj.onc.1203324. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol. Rev. 1999;79:S145–S166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Khan MJ, Tang WX, Quirk WS. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol. Head Neck Surg. 2002;127:138–144. doi: 10.1067/mhn.2002.127627. [DOI] [PubMed] [Google Scholar]
- Seifried HE. Oxidative stress and antioxidants: a link to disease and prevention? J. Nutr. Biochem. 2007;18:168–171. doi: 10.1016/j.jnutbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Servetnyk Z, Krjukova J, Gaston B, Zaman K, Hjelte L, Roomans GM, Dragomir A. Activation of chloride transport in CF airway epithelial cell lines and primary CF nasal epithelial cells by S-nitrosoglutathione. Respir. Res. 2006;7:124. doi: 10.1186/1465-9921-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna SS, Holleschau AM, Rathbun WB. Activity of glutathione synthesis enzymes in human lens related to age. Curr. Eye Res. 1982;2:735–742. doi: 10.3109/02713688209020005. [DOI] [PubMed] [Google Scholar]
- Shaheen SO, Sterne JA, Songhurst CE, Burney PG. Frequent paracetamol use and asthma in adults. Thorax. 2000;55:266–270. doi: 10.1136/thorax.55.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Kharb S, Chugh SN, Kakkar R, Singh GP. Effect of glycemic control and vitamin E supplementation on total glutathione content in non-insulin-dependent diabetes mellitus. Ann. Nutr. Metab. 2000;44:11–13. doi: 10.1159/000012815. [DOI] [PubMed] [Google Scholar]
- Shaw JP, Chou IN. Elevation of intracellular glutathione content associated with mitogenic stimulation of quiescent fibroblasts. J. Cell. Physiol. 1986;129:193–198. doi: 10.1002/jcp.1041290210. [DOI] [PubMed] [Google Scholar]
- Shi ZZ, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, Danney CM, Matzuk MM, Lieberman MW. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc. Natl. Acad. Sci. USA. 2000;97:5101–5106. doi: 10.1073/pnas.97.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Sibhatu MB, Smitherman PK, Townsend AJ, Morrow CS. Expression of MRP1 and GSTP1-1 modulate the acute cellular response to treatment with the chemopreventive isothiocyanate, sulforaphane. Carcinogenesis. 2008;29:807–815. doi: 10.1093/carcin/bgn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C, Droge W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 1998;42:485–492. doi: 10.1136/gut.42.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlinski M, Postma DS, van Diemen CC, Blokstra A, Smit HA, Boezen HM. Lung function loss, smoking, vitamin C intake, and polymorphisms of the glutamate-cysteine ligase genes. Am. J. Respir. Crit. Care Med. 2008;178:13–19. doi: 10.1164/rccm.200711-1749OC. [DOI] [PubMed] [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Simmons TW, Hinchman CA, Ballatori N. Polarity of hepatic glutathione and glutathione S-conjugate efflux, and intraorgan mercapturic acid formation in the skate. Biochem. Pharmacol. 1991;42:2221–2228. doi: 10.1016/0006-2952(91)90359-d. [DOI] [PubMed] [Google Scholar]
- Simmons TW, Anders MW, Ballatori N. Cysteine and S-(1,2-dichlorovinyl)-L-cysteine transport in rat liver canalicular membrane vesicles: Potential reabsorption mechanisms for biliary metabolites of glutathione and its S-conjugates. J. Pharmacol. Exp. Ther. 1992;262:1182–1188. [PubMed] [Google Scholar]
- Singh A, Rangasamy T, Thimmulappa RK, Lee H, Osburn WO, Brigelius-Flohé R, Kensler TW, Yamamoto M, Biswal S. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am. J. Respir. Cell. Mol. Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002;165:922–926. doi: 10.1164/ajrccm.165.7.2105032. [DOI] [PubMed] [Google Scholar]
- Solano RM, Casarejos MJ, Menendez-Cuervo J, Rodriguez-Navarro JA, Garcia dY, Mena MA. Glial dysfunction in parkin null mice: effects of aging. J. Neurosci. 2008;28:598–611. doi: 10.1523/JNEUROSCI.4609-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontakke AN, Tare RS. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin. Chim. Acta. 2002;318:145–148. doi: 10.1016/s0009-8981(01)00766-5. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Jenner P, Daniel SE, Lees AJ, Marsden DC, Halliwell B. Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: possible mechanisms of formation involving reactive oxygen species. J. Neurochem. 1998;71:2112–2122. doi: 10.1046/j.1471-4159.1998.71052112.x. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Roederer M, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg P, Jr, Davidson PC, Jones DP, Hagen TM, Reed RL, Drews-Botsch C. Protection of retinal pigment epithelium from oxidative injury by glutathione and precursors. Invest. Ophthalmol. Vis. Sci. 1993;34:3661–3668. [PubMed] [Google Scholar]
- Suthanthiran M, Anderson ME, Sharma VK, Meister v. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc. Natl. Acad. Sci. USA. 1990;87:3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes MC, Mowbray AL, Jo H. Reversible glutathiolation of caspase-3 by glutaredoxin as a novel redox signaling mechanism in tumor necrosis factor-alpha-induced cell death. Circ. Res. 2007;100:152–154. doi: 10.1161/01.RES.0000258171.08020.72. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc. Natl. Acad. Sci. USA. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl. Acad. Sci. USA. 2002;99:12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- Tieu K, Ischiropoulos H, Przedborski S. Nitric oxide and reactive oxygen species in Parkinson's disease. IUBMB. Life. 2003;55:329–335. doi: 10.1080/1521654032000114320. [DOI] [PubMed] [Google Scholar]
- Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. USA. 2006;103:4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffa S, Kunikowska GM, Zeng BY, Jenner P, Marsden CD. Glutathione depletion in rat brain does not cause nigrostriatal pathway degeneration. J. Neural Transm. 1997;104:67–75. doi: 10.1007/BF01271295. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K, Moore K, Wes PD, Muchowski PJ, Dey J, Andrews L, Pallanck LJ. Induction of the phase II detoxification pathway suppresses neuron loss in Drosophila models of Parkinson's disease. J. Neurosci. 2008;28:465–472. doi: 10.1523/JNEUROSCI.4778-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocino RA, Akazawa S, Ishibashi M, Matsumoto K, Matsuo H, Yamamoto H, Goto S, Urata Y, Kondo T, Nagataki S. Significance of glutathione depletion and oxidative stress in early embryogenesis in glucose-induced rat embryo culture. Diabetes. 1995;44:992–998. doi: 10.2337/diab.44.8.992. [DOI] [PubMed] [Google Scholar]
- Uhlig S, Wendel A. The physiological consequences of glutathione variations. Life Sci. 1992;51:1083–1094. doi: 10.1016/0024-3205(92)90509-n. [DOI] [PubMed] [Google Scholar]
- Urata Y, Yamamoto H, Goto S, Tsushima H, Akazawa S, Yamashita S, Nagataki S, Kondo T. Long exposure to high glucose concentration impairs the responsive expression of gamma-glutamylcysteine synthetase by interleukin-1beta and tumor necrosis factor-alpha in mouse endothelial cells. J. Biol. Chem. 1996;271:15146–15152. doi: 10.1074/jbc.271.25.15146. [DOI] [PubMed] [Google Scholar]
- Vali S, Mythri RB, Jagatha B, Padiadpu J, Ramanujan KS, Andersen JK, Gorin F, Bharath MM. Integrating glutathione metabolism and mitochondrial dysfunction with implications for Parkinson's disease: A dynamic model. Neuroscience. 2007;149:917–930. doi: 10.1016/j.neuroscience.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- van den Dobbelsteen DJ, Nobel CS, Schlegel J, Cotgreave IA, Orrenius S, Slater AF. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J. Biol. Chem. 1996;271:15420–15427. doi: 10.1074/jbc.271.26.15420. [DOI] [PubMed] [Google Scholar]
- van der Toorn M, Smit-de Vries MP, Slebos DJ, de Bruin HG, Abello N, van Oosterhout AJ, Bischoff R, Kauffman HF. Cigarette smoke irreversibly modifies glutathione in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L1156–L1162. doi: 10.1152/ajplung.00081.2007. [DOI] [PubMed] [Google Scholar]
- Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Velsor LW, van Heeckeren A, Day BJ. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L31–L38. doi: 10.1152/ajplung.2001.281.1.L31. [DOI] [PubMed] [Google Scholar]
- Viña J, Lloret A, Ortí R, Alonso D. Molecular bases of the treatment of Alzheimer's disease with antioxidants: prevention of oxidative stress. Mol. Aspects Med. 2004;25:117–123. doi: 10.1016/j.mam.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Arranz L, Hernanz A, Miquel J, De la Fuente M. A model of premature aging in mice based on altered stress-related behavioral response and immunosenescence. Neuroimmunomodulation. 2007;14:157–162. doi: 10.1159/000110640. [DOI] [PubMed] [Google Scholar]
- Vlaming MLH, Mohrmann K, Wagenaar E, de Waart DR, Oude Elferink RPJ, Lagas JS, van Telligen O, Vainchtein LD, Rosing H, Beijnen JH, Schellens JHM, Schinkel AH. Carcinogen and anticancer drug transport by Mrp2 in vivo: studies using Mrp2 (Abcc2) knockout mice. J. Pharmacol. Exp. Ther. 2006;318:319–327. doi: 10.1124/jpet.106.101774. [DOI] [PubMed] [Google Scholar]
- Vogel JU, Cinatl J, Dauletbaev N, Buxbaum S, Treusch G, Cinatl J, Jr, Gerein V, Doerr HW. Effects of S-acetylglutathione in cell and animal model of herpes simplex virus type 1 infection. Med. Microbiol. Immunol. 2005;194:55–59. doi: 10.1007/s00430-003-0212-z. [DOI] [PubMed] [Google Scholar]
- Wang W, Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol. Rev. 1998;50:335–356. [PubMed] [Google Scholar]
- Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc. Natl. Acad. Sci. USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth AJ, Theodore DA, Greene JC, Benes H, Wes PD, Pallanck LJ. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc. Natl. Acad. Sci. USA. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2007;27:762–768. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- Wijnholds J, Scheffer GL, van der Valk M, van der Valk P, Beijnen JH, Scheper RJ, Borst P. Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J. Exp. Med. 1998;188:797–808. doi: 10.1084/jem.188.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild AC, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic. Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- Won JS, Singh I. Sphingolipid signaling and redox regulation. Free Radic. Biol. Med. 2006;40:1875–1888. doi: 10.1016/j.freeradbiomed.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Yang CS, Chou ST, Lin NN, Liu L, Tsai PJ, Kuo JS, Lai JS. Determination of extracellular glutathione in rat brain by microdialysis and high-performance liquid chromatography with fluorescence detection. J. Chromatogr. Biomed. Appl. 1994;661:231–235. doi: 10.1016/s0378-4347(94)80050-2. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J. Biol. Chem. 2002;277:49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia. 1995;38:201–210. doi: 10.1007/BF00400095. [DOI] [PubMed] [Google Scholar]
- Young RW. Pathophysiology of age-related macular degeneration. Surv. Ophthalmol. 1987;31:291–306. doi: 10.1016/0039-6257(87)90115-9. [DOI] [PubMed] [Google Scholar]
- Young RW. Solar radiation and age-related macular degeneration. Surv. Ophthalmol. 1988;32:252–269. doi: 10.1016/0039-6257(88)90174-9. [DOI] [PubMed] [Google Scholar]
- Zaman K, McPherson M, Vaughan J, Hunt J, Mendes F, Gaston B, Palmer LA. S-nitrosoglutathione increases cystic fibrosis transmembrane regulator maturation. Biochem. Biophys. Res. Commun. 2001;284:65–70. doi: 10.1006/bbrc.2001.4935. [DOI] [PubMed] [Google Scholar]
- Zargari M, Allameh A, Sanati MH, Tiraihi T, Lavasani S, Emadyan O. Relationship between the clinical scoring and demyelination in central nervous system with total antioxidant capacity of plasma during experimental autoimmune encephalomyelitis development in mice. Neurosci. Lett. 2007;412:24–28. doi: 10.1016/j.neulet.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Sinha C, Ehrhart J, Nicklas WJ. Excitotoxicity and oxidative stress during inhibition of energy metabolism. Dev. Neurosci. 1998;20:444–453. doi: 10.1159/000017342. [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Manzino L, Sonsalla PK, Bernard LP. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson's disease. Exp. Neurol. 2007;203:512–520. doi: 10.1016/j.expneurol.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevalk GD, Razmpour R, Bernard LP. Glutathione and Parkinson's disease: is this the elephant in the room? Biomed. Pharmacother. 2008;62:236–249. doi: 10.1016/j.biopha.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Zipser Y, Piade A, Kosower NS. Erythrocyte thiol status regulates band 3 phosphotyrosine level via oxidation/reduction of band 3-associated phosphotyrosine phosphatase. FEBS Lett. 1997;406:126–130. doi: 10.1016/s0014-5793(97)00263-9. [DOI] [PubMed] [Google Scholar]