Abstract
Oxidative injury and dysfunction of the vascular endothelium is an early and causal feature of many vascular diseases and single antioxidant strategies to prevent vascular injury have met with mixed results. Here we report that induction of a metabolic stress response with AMP kinase prevents oxidative endothelial cell injury. This response is characterized by stabilization of the mitochondrion and increased mitochondrial biogenesis resulting in attenuation of oxidative c-Jun N-terminal kinase (JNK) activation. We report that peroxisome proliferator coactivator 1α (PGC-1α) is a key downstream target of AMPK that is both necessary and sufficient for the metabolic stress response and JNK attenuation. Moreover, induction of the metabolic stress response in vivo attenuates ROS-mediated JNK activation and endothelial dysfunction in response to angiotensin II in wild-type mice, but not animals lacking either the endothelial isoform of AMPK or PGC-1α. These data highlight AMPK and PGC-1α as potential therapeutic targets for the amelioration of endothelial dysfunction and, as a consequence, vascular disease.
Introduction
The vascular endothelium mediates local tissue homeostasis through the regulation of blood flow, coagulation, and the trafficking of both macromolecules and inflammatory cells.1 Dysfunction of the endothelium is an early feature of chronic diseases such as atherosclerosis and diabetes and the presence of endothelial dysfunction predicts future vascular consequences.2, 3 These chronic vascular diseases exhibit excess ambient levels of reactive oxygen species (ROS) in the vascular wall that contribute to endothelial injury and dysfunction.4 Attempts to limit endothelial injury and vascular disease via exogenous ROS scavengers have not proven effective in clinical settings,5 indicating that the mechanisms of oxidative endothelial injury are not well defined.
One means of cellular protection against injury is caloric restriction, where ample data indicate this intervention not only extends the lifespan of model organisms, but also reduces risk of chronic degenerative diseases.6 However, the exact mechanisms responsible for these effects have not been fully elucidated. Recent efforts in model systems have uncovered a number of caloric restriction “mimetics” that have proven useful in studying metabolic stress and among these compounds are 2-deoxy glucose (2DG) and metformin.7 The former is a non-metabolizable form of glucose that inhibits the phosphohexose isomerase enzyme and extends lifespan in C. elegans. 6 Metformin is a drug for improving insulin resistance that induces changes in metabolism and gene expression changes that closely parallel caloric restriction.6 The specific mechanism(s) whereby caloric restriction and its mimetics provide cellular protection and longevity are not yet clear.
Among the pathways sensitive to nutrient deprivation is the AMPK-activated protein kinase (AMPK). This ubiquitous kinase is a heterotrimeric enzyme consisting α, β, and γ-subunits that is sensitive to the cellular AMP:ATP ratio and, as a consequence, plays a pivotal role in cellular adaptation to energy stress.8 Activation of AMPK attenuates anabolic processes such as the synthesis of proteins, fatty acids, and cholesterol and it stimulates ATP-generating catabolic pathways.9 Accordingly, downstream targets of AMPK include key enzymes of glucose and lipid metabolism,10 mitochondrial enzymes,11 and transcriptional coactivators controlling mitochondrial biogenesis.12 The precise role of AMPK and its molecular targets in more generalized stress responses, however, is not well defined.8 The purpose of this study, therefore, was to examine the implications of AMPK in mediating the response to oxidative stress, a key feature of many chronic diseases.
Materials and Methods
Materials
Cell culture reagents were obtained from Invitrogen (Carlsbad, CA) and Cambrex (East Rutherford, NJ). 5-aminoimidazole-4-carboxamide riboside (AICAR) was obtained from Toronto Research Chemicals (Toronto, Canada). Tumor necrosis factor alpha (TNFα) was purchased from R&D systems (Minneapolis, MN). Compound C was a kind gift from Merck. We obtained LY294002, PP2, bisindolylmaleimide, BAPTA, PD98059, and SB202190 from Calbiochem (La Jolla, CA). Polyclonal antibodies against phospho-AMPK (Thr-172), α-AMPK, phospho-acetyl CoA carboxylase (ACC; Ser-79) were from Cell Signaling Technology (Beverly, MA). We obtained antibodies for catalase and mitochondrial Transcription Factor A (mtTFA) from Abcam (Cambridge, MA) and the heme oxygenase-1 antibody from Stressgen (Victoria, BC, Canada). Antibodies against ACC, α1-AMPK, α2-AMPK, SOD1, SOD2 were from Upstate Biotechnology (Lake Placid, NY) as well as the siRNA constructs SMARTPool® with controls. [32P]-ATP (250 μCi, 10 mCi/ml) was obtained from Perkin Elmer Life Sciences (Boston, MA). Dihydrorhodamine, JC-1, nonyl acridine orange (NAO) and MitoTracker™ Green FM were purchased from Molecular Probes (Eugene, OR). Lipopolysaccharide (LPS, Escherichia coli stereotype 0128:B12) and all other reagents were obtained from Sigma.
Adenoviruses
The adenoviral vector expressing a dominant negative α2-AMPK mutant was a kind gift of Dr. Morris J. Birnbaum (University of Pennsylvania).13 The adenoviral vector expressing a PGC-1α was a kind gift by Dr. Bruce M. Spiegelman (Dana-Farber Cancer Institute). Cells were typically infected at a MOI of 10 - 50 and control adenovirus consisted of a LacZ construct at the same MOI.
Cell Culture
Human umbilical vein endothelial cells (HUVEC) were cultured in EGM-2 medium (Clonetics) with all supplements and used between passages 3 and 6. Four hours prior to AMPK activation HUVECs were cultivated in a reduced serum medium containing 0.4% FBS with all EGM-2 supplements in a 1 to 5 dilution. COS-7 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco) supplemented with 10% FBS, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. For experiments, confluent cells were used on either 6-well or 12-well plates. Porcine aortic endothelial cells (PAEC) were cultured as described.14 Overnight AICAR (1mM) or Metformin (5mM) treatment was directly added to the cell medium and prior to H2O2 exposure, cells were washed in HEPES-buffered physiologic salt solution (PSS) as described.14
Cellular ATP content and AMPK activity assay
Measurement of ATP was performed with the bioluminescent somatic cell assay kit (Sigma) according to instructions. Determination of AMPK catalytic activity was performed by incorporation of [32P] into the specific AMPK target sequence HMRSAMSGLHLVKRR (SAMS peptide) as described previously.15
Mitochondrial ROS production
Mitochondrial ROS production was assessed by measuring dihydrorhodamine 123 (DHR) fluorescence. After treatments, PAEC were washed and incubated for 30 minutes with 10 μM DHR in PSS, washed in PSS, and treated with 200μM H2O2 for 30 minutes. Cells were then washed, scraped in ice-cold PBS, dispersed by repeated pipetting and aliquots added to either coverslips or 96 well plates. DHR fluorescence was assessed using a fluorescent plate reader (Molecular Devices) with excitation at 480 nM and emission at 535 nM.
Mitochondrial membrane potential
Mitochondrial membrane potential was estimated by fluorescence of JC-1 aggregates that are formed as a function of inner mitochondrial membrane potential.16 PAEC were washed twice with PSS, equilibrated for 30 minutes and then treated with H2O2 or vehicle for 1 hour. After treatment, cells were carefully washed 2 times and incubated with 2.5μg/ml JC-1 for 15 minutes in PSS, washed 3x in PSS, and subjected to fluorescence (excitation 550nm, emission 600nm for red fluorescence; excitation 485nm, emission 535nm for green fluorescence) ratio detection.
Transcriptional activation assays and immunoblotting
All experiments were performed using the Dual Luciferase Reporter Assay (Promega, San Luis Obispo, CA) with an internal Renillaluciferase control plasmid to normalize for transfection efficiencies. COS-7 cells were transiently transfected for forty hours with the human mitochondrial transcription factor A (mtTFA) luciferase promoter as described.17 Immunoblotting was performed as previously described.14
Cell death assays
PAEC in 12-well plates were treated with vehicle, AICAR or Metformin overnight in regular medium. Cells were then kept in HEPES-buffered PSS (30 min) and exposed to increasing concentrations of H2O2 for 2 hours. For the LDH release assay, we used the Cytotoxicity detection kit (Roche Applied Science) normalized to one well treated with 1% Triton X-100 for maximum LDH release. Cell viability was also assessed by the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) using the manufacturer's instructions.
Mitochondrial mass determination
Mitochondrial mass was estimated by fluorescence of Nonyl-Acridine Orange (NAO) or MitoTracker® Green FM, both mitochondrial specific dyes, independent of the mitochondrial membrane potential.18, 19 After various treatments, HUVEC were washed twice with PBS, then incubated in full growth medium including 100 nM of NAO or MitoTracker® Green FM for 30 minutes at 37°C, 5% CO2. After 3 washes, cells were then subjected to fluorescence detection (excitation 490nm, emission 516nm for MitoTracker® Green FM; excitation 495nm, emission 519nm for NAO) as an indicator of mitochondrial mass.
Gene Silencing by Small Interfering RNA (siRNA)
Double-stranded RNAi was transfected into cells using RNAiFect™(Qiagen, Valencia, Ca). After 72h of transfection, cells were incubated with 1mM AICAR overnight for chronic AMPK activation. 100 μM H2O2 for 2h was further applied to the cells for cell death and cell survival assays. Scrambled RNAi (Upstate) was used as control.
Experimental Animals
Animal experiments were in accordance with the Declaration of Helsinki and with NIH guidelines and performed with approval of the Ethics Committee of the University Hospital Mainz. To study in vivo AMPK activation by AICAR, we used male C57Bl6 mice. The mice were anesthetized by isoflurane inhalation and treated with a subcutaneous osmotic minipump (Alzet model 1007) containing either angiotensin II (AT II) or solvent (NaCl 0.9%) for 7 days. Angiotensin infusion rate averaged 1 mg/kg/d. Animals from both groups were randomized to receive either AICAR (200 mg/kg/d) or the vehicle (NaCl 0.9%) via subcutaneous injection once daily, starting at the time of the AT II containing minipump implantation. To probe the role of AMPK, we used 100 α1-AMPK knockout mice20 and corresponding littermate wild-type mice (C57Bl6/129Sv/FVB-N background) as controls. To probe the role of PGC-1α, we utilized animals with severely diminished PGC-1α levels as described.21 In a second approach, endothelial dysfunction was induced in wildtype mice by single intraperitoneal injection of lipopolysaccharide (LPS, 15mg/kg) and sacrificed 24 hours later. Mice were randomized to receive either AICAR (200 mg/kg/d) or the vehicle (NaCl 0.9%) via subcutaneous injection once daily, starting two days prior to LPS treatment. To dissect the role of AMPK, LPS/AICAR injections were also performed in α1-AMPK knockout mice. After all treatment protocols, animals were sacrificed and tissues removed and subjected to further analysis.
Assessment of Endothelial Function and Superoxide
Endothelial function was assayed as endothelium-dependent arterial relaxation in segments of thoracic aorta as described previously.22 Vascular superoxide was estimated using dihydroethidium staining23 and NADPH-oxidase activity was estimated in heart membrane fractions as described.22
Statistical Analysis
All immunoblots are representative of 3-4 independent experiments. Numerical data is presented as mean ± standard error of the mean (SEM). Comparisons among treatment groups were performed with one-way analysis of variance (ANOVA) and an appropriate post-hoc Dunnet's or Tukey's comparison. Statistical significance was accepted if the null hypothesis was rejected with p < 0.05.
Results
Peroxide induces AMPK activation in the endothelium
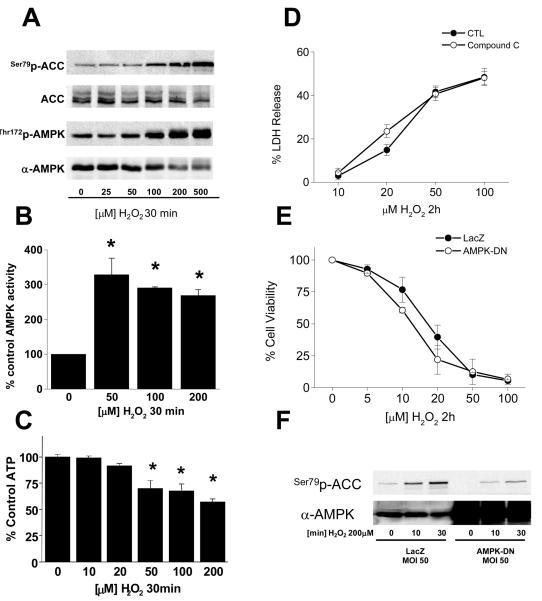
Since AMPK isoform composition is known to impact its function in various cell types,24, 25 we utilized RT-PCR and immunoblotting to determine that endothelial cells almost exclusively harbor the α1 catalytic isoform (Supplementary Figs. S1A and B). There is also a predominance of the β1 and γ2 isoforms (Fig S1A). We then treated endothelial cells with H2O2 and observed concentration-dependent AMPK activation as assessed by Western blot and AMPK activity assay (Figs. 1A and B). Inhibitors targeting phosphoinositide 3-kinase, protein kinase C, src-family kinases, general tyrosine kinases, mitogen-activated protein kinases or intracellular calcium transients did not block H2O2-induced AMPK activation (data not shown). We next examined cellular energy status and found that endothelial ATP levels decline rapidly after H2O2 exposure (Fig. 1C), suggesting AMPK activation is, at least in part, mediated by the resulting rise in cellular AMP that can allosterically activate AMPK.26
Figure 1. Peroxide induces AMPK activation in endothelium.
PAECs in 6-well plates were exposed to H2O2 as indicated, lysed, and the lysates probed for (A) phosphorylation of AMPK and ACC, (B) AMPK activity, and (C) ATP content as described in “Methods.” PAECs were then treated with H2O2 as indicated after treatment with either the AMPK inhibitor, compound C (25 uM; D) or dominant-negative AMPK adenovirus (E) and cell death or viability determined by LDH release and MTS assay, respectively as described in “Methods.” (F) PAECs were treated with dominant-negative AMPK adenovirus and AMPK activation assessed after H2O2 exposure by phosphorylation of the AMPK target, acetyl-CoA carboxylase (ACC).
To determine if AMPK activation promotes survival, we inhibited H2O2-mediated AMPK activation with compound C, but observed no impact on cell death (Fig. 1D). Similarly, overexpression of a dominant-negative AMPK mutant (AMPK-DN) had no impact on H2O2-mediated cell death (Fig. 1E) despite significant inhibition of AMPK activation (Fig. 1F). These data indicate that acute AMPK activation has limited implications for endothelial cell death in response to H2O2.
Chronic AMPK activation induces stress adaptation in endothelium
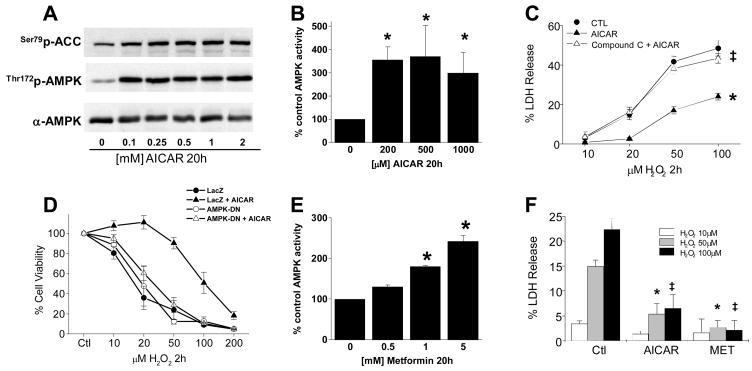
We were able to elicit sustained endothelial cell AMPK activation with AICAR treatment over 20 hours based upon AMPK activity and phosphorylation of its downstream target, ACC (Figs. 2A and B). This chronic AMPK activation prior to H2O2 challenge produced a significant attenuation in the response to H2O2 that was reversed by either pharmacologic (Fig. 2C) or molecular (Fig. 2D) inhibition of AMPK. Shorter AICAR treatment for 30 minutes or 4 hours did not mimic the effects observed with a 20 hour treatment period (Fig. S2). To determine the general nature of these findings, we chronically activated AMPK with the drug metformin (Fig. 2E)27 and also observed a significant inhibition of H2O2-induced cell death that was quantitatively similar to that observed with AICAR (Fig. 2F). Thus, chronic AMPK activation attenuates H2O2-induced endothelial cell death.
Figure 2. Chronic AMPK activation induces stress adaptation in endothelium.
PAECs in 6-well plates were exposed to AICAR as indicated, lysed, and the lysates probed for (A) phosphorylation of AMPK and ACC and (B) AMPK activity; *p<0.05 vs. 0uM by one-way ANOVA and Dunnett's test. PAECs were treated with either 20 uM compound C (C) or dominant-negative AMPK adenovirus (D) before a 20h exposure to 1mM AICAR; *p<0.05 vs. CTL, ‡p<0.05 vs. AICAR by two-way ANOVA. Cells were then treated with H2O2 and either cell death or viability determined as indicated by LDH release or MTT assay, respectively. (E) PAECs were treated with Metformin as indicated and AMPK activity determined in cell lysates as 32P incorporation into the SAMS peptide as described in “Methods,” *p<0.05 vs. 0mM by one-way ANOVA with a post hoc Dunnett's test. (F) PAECs were treated with 1mM metformin or AICAR prior to a 2h exposure to H2O2 as indicated and cell death determined by LDH release; *p<0.05 vs. CTL 50 uM H2O2, ‡p<0.05 vs. CTL 100 uM H2O2 both by one-way ANOVA with a post hoc Dunnett's test.
AMPK-mediated stress adaptation involves the mitochondrion
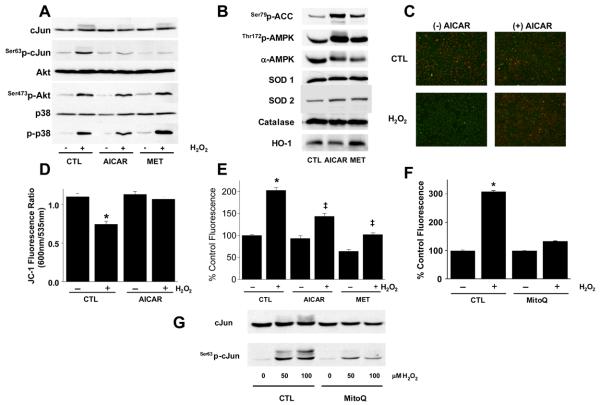
Cell viability after H2O2 exposure is, in part, dictated by the relative activity of death vs. survival pathways,28 we probed Akt and MAP kinase activation and found that AICAR and metformin had no impact on H2O2-induced Akt or P38 MAPK activation (Fig. 3A), whereas JNK activation determined as c-Jun phosphorylation was abrogated by AMPK (Fig 3A). We did not find any change in cytosolic antioxidant enzymes with AICAR or metformin, however we did detect a 30 – 40% increase in mitochondrial SOD (Fig. 3B and S3).
Figure 3. AMPK-mediated adaptation involves the mitochondrion.
(A) PAECs in 6-well plates were exposed to AICAR, metformin (MET), or buffer alone (CTL) as in Fig. 2 followed by assessment of JNK, Akt, and p38 MAP kinase activation as described.50 (B) PAECs treated as in (A) were lysed and the content of the indicated proteins determined by immunoblotting. (C) PAECs treated with AICAR as in (A) were washed and exposed to 100 μM H2O2 (60 min) before loading with 2.5 μg/ml JC-1 (final conc.) and then examined either qualitatively by microscopy (C) or quantitatively (D) in a plate reader for red (ex 550nm; em 600nm) and green (ex 485nm; em 535nm) fluorescence. Images are representative of 3 independent experiments and quantitative analysis represents mean ± S.E.M of 3 independent experiments; *p<0.05 vs. without H2O2 by two-way ANOVA and a Tukey's post hoc test. (E) PAEC were treated as in (A) and loaded with 10μM dihydrorhodamine before H2O2 treatment and fluorescence (ex 480nm, em 535nm) detection; n=5, , ‡ p<0.05 vs. control H2O2 treated by two-way ANOVA and a Tukey test. PAEC were incubated with 1μM MitoQ as described14 before assessment of H2O2-induced mitochondrial ROS (F) or H2O2-induced JNK activation (G); images are representative of n=4, *p<0.05 vs. no H2O2.
Since JNK is a downstream component of mitochondrial death signals,29 and AMPK activation increased mitochondrial SOD, we explored the implications of chronic AMPK activation for mitochondrial response(s) to toxic stimuli. Endothelial cell H2O2 treatment reduced mitochondrial membrane potential and chronic AMPK activation with AICAR (Figs. 3C and D) or metformin (not shown) prevented this effect. Similarly, the H2O2-induced mitochondrial ROS signal determined by dihydrorhodamine fluorescence was attenuated by chronic AMPK activation using either metformin or AICAR (Fig. 3E). We could also mimic the effect of AICAR to suppress the mitochondrial ROS signal and JNK activation in response to H2O2 using the mitochondrial-targeted antioxidant, mitoQ14, 30 (Figs. 3F and G). Collectively, these data indicate that chronic AMPK activation modifies mitochondrial responses to H2O2.
Chronic AMPK-Activation Induces PGC-1α-Dependent Mitochondrial Biogenesis
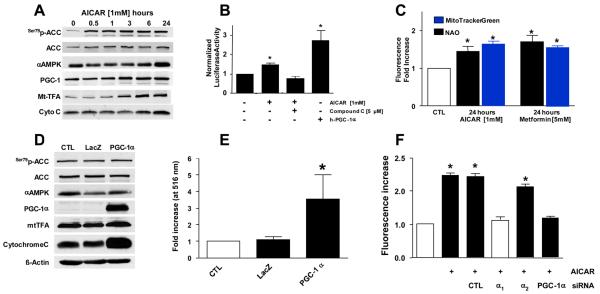
One recognized link between AMPK and the mitochondrion is mitochondrial biogenesis12 that appears dependent on PGC-1α in many tissues.31 In agreement with these data, AICAR treatment increased the abundance of markers associated with mitochondrial biogenesis such as PGC-1α, mitochondrial transcription factor A (mTFA), and cytochrome c (Figs. 4A and S4). Consistent with this observation, AICAR treatment produced PGC-1α-driven gene transcription assessed by the mitochondrial transcription factor A (mt-TFA) promoter linked to luciferase (Fig. 4B).31 Similarly, chronic AMPK activation produced an increase in endothelial cell mitochondrial mass (Fig. 4C) and this effect was recapitulated using adenoviral overexpression of PGC-1α (Figs. 4D and E). Finally, AICAR stimulation increased mitochondrial mass in an α1-AMPK- and PGC-1α-dependent manner (Fig. 4F). Collectively, these data indicate that chronic AMPK activation increases endothelial cell mitochondrial biogenesis and mass.
Figure 4. AMPK-activation in endothelial cells induces PGC-1α-dependent mitochondrial biogenesis.
(A) PAECs were exposed to AICAR as indicated, lysed, and subjected to immunoblotting for assessment of AMPK activation (p-ACC) and the levels of PGC-1α and mitochondrial transcription factor A (Mt-TFA). (A) BAECs were transfected with the Mt-TFA-promoter linked to a luciferase reporter31 prior to incubation with AICAR ± compound C followed by assessment of luciferase activity. Adenoviral transfection of human PGC-1α served as positive control. (C) PAECs were incubated with AICAR or metformin as indicated and mitochondrial mass determined fluorometrically with Mitotracker Green or nonyl-acridine orange (NAO) as indicated (*p<0.05 vs. CTL by two-way ANOVA and Dunnet's test) HUVECs were transfected with adenoviral vectors expressing either β-galactosidase (LacZ) or human PGC-1α. Cells were then lysed and assessed for the indicated proteins (D) or mitochondrial mass using Mitotracker (E; *p<0.05 vs. CTL by one-way ANOVA and a Dunnett's test). (F) HUVECs were treated with the indicated siRNA or buffer control for 72hr before a 24 hour incubation with AICAR. Mitochondrial mass was then determined using Mitotracker Green, *p<0.05 vs. no AICAR exposure. All experiments are N=5 – 7.
Stimulation of Mitochondrial Biogenesis Enhances Endothelial Cell Resistance to H2O2
The endothelial cell resistance to H2O2-induced death and dysfunction afforded by AMPK extended beyond H2O2, since TNF-α-induced cell death (measured as JNK activation; Fig. 5A) was also inhibited by AMPK. Since we believed the action of AICAR and metformin involved the mitochondrion (Fig. 3), and that AMPK facilitates mitochondrial biogenesis (Fig. 4), we examined the link between mitochondrial biogenesis and endothelial cell resistance to stress. We found that AMPK activation reduced H2O2-induced cell death, and this response was recapitulated with PGC-1α overexpression via adenoviral transfection (Figs. 5B and C). The effect of AICAR against H2O2-mediated cell death was lost by siRNA-mediated suppression of either α1-AMPK or PGC-1α (Figs. 5D and E). We also found that overexpression of PGC-1α selectively attenuates H2O2-induced JNK activation with preserved p38 MAP kinase activation (Fig. 5F). In total, these data link stimulation of mitochondrial biogenesis to endothelial cell protection from cell death and indicate that PGC-1α overexpression is both necessary and sufficient for this response.
Figure 5. Mitochondrial biogenesis protects endothelial cells from H2O2-mediated toxicity.
(A) PAECs were incubated with AICAR or metformin (MET) for 24h before exposure to TNF-α as indicated. Cells were lysed and immunoblotted for c-Jun or its phosphorylated form. (B and C) HUVECs were treated with 24h of AICAR (1mM) or metformin (MET; 5mM) or transfected with control (LacZ) or PGC-1α adenovirus. Cells were then treated with H2O2 as indicated for 2h and cell death or viability determined by LDH release or MTT assay, respectively (*p<0.05 vs. CTL, ‡P<0.05 vs. H2O2 alone by one-way ANOVA with Tukey test, n=5). (D and E) HUVECs were incubated (72h) in media alone or media with siRNA against α1-AMPK, PGC-1α, or scrambled control (Scr) before incubation with or without AICAR for 24h. After incubation, cells were treated with H2O2 and LDH release or cell survival assessed as in B and C, respectively (*p<0.05 vs. no additions by one-way ANOVA with Dunnett's test). (F) HUVECs were incubated with control (Ad-LacZ) or PGC-1α adenovirus (Ad-PGC-1α) for 48h before exposure to H2O2 or TNF-α (10ng/mL) as indicated. Cells were lysed and immunoblotted for phosphorylated c-Jun as well as total PGC-1α, and actin.
In Vivo AMPK Activation Prevents Endothelial Dysfunction in a PGC-1α-Dependent Manner
To determine if our cell culture data are operative in vivo, we utilized a model of angiotensin infusion that produces vascular dysfunction, in part, through increased vascular oxidative stress via reactive oxygen species production.32 Accordingly, we induced endothelial dysfunction in mice with a 7 day infusion of angiotensin II. Aortae were harvested and examined for endothelial function as acetylcholine-induced endothelium-dependent arterial relaxation. We found that angiotensin II-induced endothelial dysfunction (Fig. 6A) was characterized by an increased superoxide signal in media of the thoracic aorta (Fig. 6B), increased myocardial NADPH oxidase activity (Fig. 6C),32 and JNK activation (Fig. 6D). In this model, chronic AMPK activation with AICAR attenuated endothelial dysfunction (Fig. 6A) and NADPH oxidase activation (Figs. 6B and C) without material alteration in the blood pressure response (Fig. S3). Moreover, AICAR was ineffective in preserving endothelial function in mice lacking either the α1-AMPK isoform (Fig. 6E) or PGC-1α (Fig. 6F), validating our paradigm in vivo.
Figure 6. Metabolic stress protects the endothelium from angiotensin II-mediated dysfunction.
Mice (C57Bl6) were infused with angiotensin II (ATII; 1.0 mg/kg/d) or vehicle (CTL) via osmotic minipumps for 7d and each group was also treated with AICAR (200 mg/kg/d) or vehicle by sc injection once daily. Aortae were harvested and assessed for (A) endothelium-dependent relaxation to acetylcholine, (B) superoxide by dihydroethidium staining (E=endothelium; A=adventitia), and (D) JNK activation as c-Jun phosphorylation; (*p<0.05 vs. vehicle alone; ‡P<0.05 vs. angiotensin II alone both by two-way ANOVA interaction term). Hearts were also harvested for (C) NADPH oxidase activity using NADPH-driven lucigenin chemiluminescence as described.22 Mice lacking α1-AMPK (E) or PGC-1α (F) and littermate controls were infused with angiotensin II or vehicle and each group was also treated with either AICAR or vehicle as in (A; *p<0.05 vs vehicle infusion).
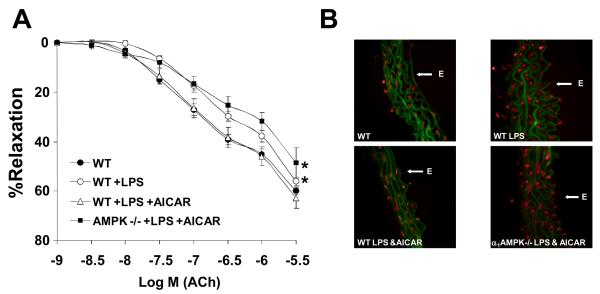
To determine if chronic AMPK activation was generally protective, we utilized an LPS-induced model of endothelial dysfunction.33 Treatment of mice with LPS induced both endothelial dysfunction (Fig. 7A) and an increased vascular superoxide signal (Fig. 7B) that were prevented by chronic AMPK activation in wild-type, but not α1-AMPK-null mice. Thus, taken together, these data indicate that chronic AMPK activation in vivo also protects the endothelium against the injurious actions of angiotensin II and LPS.
Figure 7. Metabolic stress protects the endothelium from LPS-induced dysfunction.
Wild-type (C57Bl6) or α1-AMPK-null mice were injected with intravenous LPS (10 mg/kg/d) or vehicle (CTL) via tail vein as indicated. The indicated groups were also pre-treated (7d) with AICAR (200 mg/kg/d) by sc injection once daily. Aortae were harvested and assessed for (A) endothelium-dependent relaxation to acetylcholine or (B) superoxide by dihydroethidium staining as in Fig. 6. (*p<0.05 vs. vehicle alone by two-way ANOVA interaction term).
Discussion
In this study, we found that induction of metabolic stress in the form of chronic AMPK activation was highly effective in protecting endothelial cells against both H2O2 and TNF-α. This stress resistance was related to AMPK-mediated modulation of mitochondrial redox state that was dependent upon the transcriptional co-activator, PGC-1α. Indeed, PGC-1α was sufficient for this response as determined by overexpression of PGC-1α and the redox modulation was mimicked by a mitochondrial-targeted antioxidant. We also found that the AMPK-PGC-1α pathway selectively attenuated stress-related JNK activation, an effect one might expect to suppress stress-related cellular injury and death. Our findings were physiologically relevant as metabolic stress induction via AICAR in vivo prevented angiotensin II-mediated JNK activation and endothelial injury, a process dependent upon oxidative stress and vascular ROS production.34 Moreover, LPS-induced endothelial dysfunction was also prevented by chronic AMPK activation. This protective effect of AICAR was lost in mice lacking α1-AMPK (the isoform that predominates in the endothelium) and PGC-1α. Thus, these data indicate that AMPK can direct adaptive changes in the mitochondrion via PGC-1α that enhances mitochondrial biogenesis and cellular resistance to stress.
Previous studies addressing the role of AMPK in cell death have yielded equivocal results.35-37 Some of these previous negative studies relied solely on AICAR as the means to increase AMPK activity.35, 37 However, AICAR may affect other AMP-dependent enzymes and long-term AICAR treatment might increase cellular nucleotide levels.38 In this study, we used metformin as a complementary means of AMPK activation and found comparable effects on endothelial cell survival. Since the mechanism of metformin-mediated AMPK activation differs from that of AICAR,39 there is confidence that results with AICAR are indeed due to AMPK activation. This contention is supported by our observations that both genetic and pharmacological approaches to block AICAR-mediated AMPK activation reversed the protective effects of AICAR in vitro and in vivo.
It is now clear that different AMPK isoform compositions may dictate distinct sequelae of AMPK activation. For example, metabolic consequences of AMPK activation such as insulin sensitivity25 have largely been attributed to the α2-isoform. The AMP-dependency of AMPK is also greater in α2-bearing enzyme complexes.40 Moreover, differences in substrate specificity have been observed for the two α-subunits in vitro.24 Thus, the fact that endothelial cells exclusively harbor the α1-AMPK isoform may indicate a functional role distinct from the well-characterized metabolic features of AMPK. For example, one could speculate that the α1 AMPK enzyme is protective against cell death, whereas α2 containing enzymes might promote it. In this regard, studies in neuronal or pancreatic cells (with a preponderance of the α2 isoform) exhibit increased cell death upon AMPK activation,41 whereas data presented here and another study in endothelial cells (with the α1-isoform) show that AMPK activation is cytoprotective.36
In the current study, we found that AMPK activation induced mitochondrial biogenesis in the endothelium, consistent with reports from skeletal muscle.12 A previous report suggests that modulation of NO levels in endothelium parallel PGC-1α-dependent mitochondrial biogenesis.42 Since previous in vivo data implicate endothelial nitric oxide synthase (eNOS)-derived NO in mitochondrial biogenesis,43 it is tempting to speculate that our effects with AICAR are due to eNOS activation. Such speculation would be consistent with reports that AMPK is responsible for VEGF-mediated eNOS activation.44 However, recent data that AMPK directly phosphorylates PGC-1α 45 would seem to refute the requirement for NO production. Understanding the precise details of AMPK-mediated PGC-1α activation in the endothelium will require further investigation.
Signaling cascades that lead to cell death often require mitochondrial ROS production and a loss of mitochondrial membrane potential.46 We have implicated stabilization of the mitochondrial membrane potential and reduced mitochondrial ROS as key targets for metabolic stress-mediated prevention of endothelial cell death. Our findings implicate PGC-1α in this process and provide a mechanism whereby the observed contribution of PGC-1α to cellular oxidant defense leads to attenuation of cell death.47 Indeed, the fact that a mitochondrial-targeted antioxidant (MitoQ) also limits stress-induced mitochondrial ROS and cell death supports our contention that the mitochondrion is a key component of AMPK-mediated protection.
Our data indicate that metabolic stress provides cellular protection, at least in part, through the attenuation of JNK activation. It is well-known that JNK mediates apoptosis and cell death in response to environmental stress,48 thus it is plausible the salutary effects of metabolic stress stem from JNK inhibition. However, the specific means whereby AMPK and PGC-1α signaling impact JNK is not yet clear. One possibility relates to observations that prolonged JNK activation requires intracellular ROS, that inactivate JNK phosphatases.49 In our study, we found that AMPK activation attenuated intracellular and mitochondrial ROS signals, perhaps preventing the phosphatase inhibition needed for JNK activation. Published data indicate that MnSOD prevents TNF-α-induced JNK activation,49 consistent with our findings that AMPK increases endothelial cell MnSOD content. Thus, suppression of intracellular ROS appears a plausible explanation for our findings that AMPK and PGC-1α attenuate endothelial JNK activation and injury.
The current study has important implications for endothelial cell biology. Oxidative stress is a common feature of many vascular diseases and is known to impair endothelial function. Indeed, endothelial damage and reendothelialization are important factors that determine endothelial function. In this context, the endothelium has emerged as a target for the development of new therapies for vascular disease. However, the implications of increased oxidative stress on endothelial cell viability has garnered surprisingly little attention. We show here that moderate levels of oxidative stress (∼10 μM H2O2) lead to significant endothelial cell necrosis and thus, it seems reasonable that finding new molecular targets to limit endothelial cell death should favorably impact vascular disease. Our study identifies PGC-1α, a key regulator of mitochondrial biogenesis, as a potential molecular target to improve endothelial cell viability and function in vascular disease.
Supplementary Material
Acknowledgments
We thank Ana Sharma and Nikhiel Rau for excellent technical assistance. This work was supported by NIH grants HL68758, and HL81587 (to J.F.K) and a Deutsche Forschungsgemeinschaft grants SCHU 1486/1-1 and SCHU 1486/2-1 (to E.S.).
The abbreviations used are
- AMP
adenosine monophosphate
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- AMPK
AMP-activated protein kinase
- ACC
actetyl CoA carboxylase
- AICAR
5-aminoimidazole-4-carboxamide riboside
- SOD
superoxide dismutase
- LDH
lactate dehydrogenase
- ERK 1/2
extracellular signal-regulated kinase forms 1/2
- JNK
c-jun NH2-terminal kinase
- PI-3K
phosphoinositide 3-kinase
- TNFα
tumor necrosis factor alpha
- ANOVA
analysis of variance
- S.E.M.
standard error of the mean
- RT-PCR
reverse transcriptase polymerase chain reaction
- DHR
dihydrorhodamine
- FBS
fetal bovine serum
- H2O2
hydrogen peroxide
- PAEC
porcine aortic endothelial cells
- HAEC
human aortic endothelial cells
- BAEC
bovine aortic endothelial cells
- HUVEC
human umbilical vein endothelial cells
- PSS
HEPES-buffered physiologic salt solution
- ROS
reactive oxygen species
- DHR
dihydrorhodamine 123
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
Footnotes
Disclosures: None
Reference List
- 1.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007 October;7(10):803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 2.Widlansky ME, Gokce N, Keaney JF, Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003 October 1;42(7):1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 3.Gokce N, Keaney JF, Jr., Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. J Am Coll Cardiol. 2003 May 21;41(10):1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 4.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–51. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000 January 20;342(3):154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005 September;126(9):987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Ingram DK, Anson RM, de CR, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004 June;1019:412–23. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 8.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003 December;144(12):5179–83. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 9.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007 October;8(10):774–85. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 10.Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989 June 15;1012(1):81–6. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- 11.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000 June;88(6):2219–26. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 12.Zong H, Ren JM, Young LH, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002 December 10;99(25):15983–7. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu J, Brozinick JT, Jr., Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001 May;7(5):1085–94. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Thomas SR, Albano A, Murphy MP, Keaney JF., Jr Mitochondrial function Is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J Biol Chem. 2004 June 4;279(33):35079–86. doi: 10.1074/jbc.M404859200. [DOI] [PubMed] [Google Scholar]
- 15.Schulz E, Anter E, Zou MH, Keaney JF., Jr Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation. 2005 June 28;111(25):3473–80. doi: 10.1161/CIRCULATIONAHA.105.546812. [DOI] [PubMed] [Google Scholar]
- 16.Smiley ST, Reers M, Mottola-Hartshorn C, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3671–5. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005 February;25(4):1354–66. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maftah A, Petit JM, Ratinaud MH, Julien R. 10-N nonyl-acridine orange: a fluorescent probe which stains mitochondria independently of their energetic state. Biochem Biophys Res Commun. 1989 October 16;164(1):185–90. doi: 10.1016/0006-291x(89)91700-2. [DOI] [PubMed] [Google Scholar]
- 19.Metivier D, Dallaporta B, Zamzami N, et al. Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunol Lett. 1998 April;61(23):157–63. doi: 10.1016/s0165-2478(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 20.Viollet B, Andreelli F, Jorgensen SB, et al. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003 February;31(Pt 1):216–9. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 21.Leone TC, Lehman JJ, Finck BN, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005 April;3(4):e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sydow K, Daiber A, Oelze M, et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest. 2004 February;113(3):482–9. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Keaney JF, Jr., Schulz E, et al. Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proc Natl Acad Sci U S A. 2004 August 17;101(53):13014–9. doi: 10.1073/pnas.0405389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods A, Salt I, Scott J, Hardie DG, Carling D. The alpha1 and alpha2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996 November 18;397(23):347–51. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- 25.Viollet B, Andreelli F, Jorgensen SB, et al. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003 January;111(1):91–8. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989 December 8;186(12):129–36. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001 October;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000 May 12;275(19):14624–31. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 29.Tournier C, Hess P, Yang DD, et al. Requirement of JNK for stress-induced activation of the cytochrome c- mediated death pathway. Science. 2000 May 5;288(5467):870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 30.Smith RA, Kelso GF, Blaikie FH, et al. Using mitochondria-targeted molecules to study mitochondrial radical production and its consequences. Biochem Soc Trans. 2003 December;31(Pt 6):1295–9. doi: 10.1042/bst0311295. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999 July 9;98(1):115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopalan S, Kurz S, Münzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/HADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachschmid M, Thurau S, Zou MH, Ullrich V. Endothelial cell activation by endotoxin involves superoxide/NO-mediated nitration of prostacyclin synthase and thromboxane receptor stimulation. FASEB J. 2003 May;17(8):914–6. doi: 10.1096/fj.02-0530fje. [DOI] [PubMed] [Google Scholar]
- 34.Keaney JF., Jr Oxidative stress and the vascular wall: NADPH oxidases take center stage. Circulation. 2005 October 25;112(17):2585–8. doi: 10.1161/CIRCULATIONAHA.105.578146. [DOI] [PubMed] [Google Scholar]
- 35.Campas C, Lopez JM, Santidrian AF, et al. Acadesine activates AMPK and induces apoptosis in B-cell chronic lymphocytic leukemia cells but not in T lymphocytes. Blood. 2003 May 1;101(9):3674–80. doi: 10.1182/blood-2002-07-2339. [DOI] [PubMed] [Google Scholar]
- 36.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002 January;51(1):159–67. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki A, Kusakai G, Kishimoto A, Lu J, Ogura T, Esumi H. ARK5 suppresses the cell death induced by nutrient starvation and death receptors via inhibition of caspase 8 activation, but not by chemotherapeutic agents or UV irradiation. Oncogene. 2003 September 18;22(40):6177–82. doi: 10.1038/sj.onc.1206899. [DOI] [PubMed] [Google Scholar]
- 38.Longnus SL, Wambolt RB, Parsons HL, Brownsey RW, Allard MF. 5-Aminoimidazole-4-carboxamide 1-beta -D-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol Regul Integr Comp Physiol. 2003 April;284(4):R936–R944. doi: 10.1152/ajpregu.00319.2002. [DOI] [PubMed] [Google Scholar]
- 39.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002 August;51(8):2420–5. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 40.Salt I, Celler JW, Hawley SA, et al. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998 August 15;334(Pt 1):177–87. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung JE, Lee J, Ha J, et al. 5-Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative stress-induced apoptosis through activation of nuclear factor-kappaB in mouse Neuro 2a neuroblastoma cells. Neurosci Lett. 2004 January 16;354(3):197–200. doi: 10.1016/j.neulet.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Borniquel S, Valle I, Cadenas S, Lamas S, Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J. 2006 September;20(11):1889–91. doi: 10.1096/fj.05-5189fje. [DOI] [PubMed] [Google Scholar]
- 43.Nisoli E, Clementi E, Paolucci C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003 February 7;299(5608):896–9. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 44.Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun. 2007 March 23;354(4):1084–8. doi: 10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007 July 17;104(29):12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-x(L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2000 August;20(15):5680–9. doi: 10.1128/mcb.20.15.5680-5689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006 October 20;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 48.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000 October 13;103(2):239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 49.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005 March 11;120(5):649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 50.Chen K, Vita JA, Berk BC, Keaney JF., Jr c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves src-dependent EGF receptor transactivation. J Biol Chem. 2001;276:16045–50. doi: 10.1074/jbc.M011766200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.