Abstract
Acute myelogenous leukemias (AMLs) are characterized by medullary and extramedullary invasion. We hypothesized that a supramolecular complex, the leukemia-cell invadosome, which contains certain integrins, matrix metalloproteinases (MMPs), and other as-yet unidentified proteins, is essential for tissue invasion and may be central to the phenotypic diversity observed in the clinic. Here we show that the specific binding of MMP-9 to leukocyte surface β2 integrin is required for pericellular proteolysis and migration of AML-derived cells. An efficient antileukemia effect was obtained by the hexapeptide HFDDDE, a motif of the MMP-9 catalytic domain that mediates integrin binding: HFDDDE prevented proMMP-9 binding, transmigration through a human endothelial cell layer, and extracellular matrix degradation. Notably, the functional protein anchorage between β2 integrin and proMMP-9 described in this study does not involve the enzymatic active sites targeted by known MMP inhibitors. Taken together, our results provide a biochemical working definition for the human leukemia invadosome. Disruption of specific protein complexes within this supramolecular target complex may yield a new class of anti-AML drugs with anti-invasion (rather than or in addition to cytotoxic) attributes.
Introduction
Acute myelogenous leukemia (AML) results in lethal overgrowth of the myeloid progeny in the bone marrow. Typically, AML cells replace most of the normal hematopoietic lineages and lead to bone-marrow failure and death from infection and/or hemorrhage. However, AML cells also circulate through the bloodstream and can invade virtually any tissues; certain patterns are characteristic of specific subtypes of AML.1 Currently, a significant cause of morbidity and mortality in some patients with AML is pulmonary hemorrhage and acute respiratory distress syndrome secondary to lung involvement. Moreover, extramedullary infiltration accounts for splenomegaly, lymph node enlargement, gum involvement, and diffuse infiltration of the skin.
Clinically, the subtypes of AML (termed M1-M7 variants) share many similar features, but there is epidemiologic and molecular diversity among the variants.2 For example, in the M4 variant, one of the most frequent AML subtypes, populations of abnormal myelocytes and monocytes coexist in variable proportions; however, soft-tissue infiltration is a common feature of the M5 variant, a relatively uncommon AML subtype.
The molecular basis for AML phenotypic diversity remains largely unknown. Because trafficking through the vascular and lymphatic systems and homing to selective lymphoid organs are functions of normal leukocytes, the clinical manifestations observed in AML could be the result of aberrant leukemia-cell adhesion phenomena involving αMβ2 and other integrins. Given our previous work on the molecular interplay between β2 integrins and MMPs,3–5 we attempted to recapitulate these functional protein-protein interactions in vitro, in leukemia-derived cells, and in animal AML models. Because of the clinical and epidemiologic manifestations of AML, we chose the leukemia cells OCI-AML-3 (an M4 variant) and THP-1 (an M5 variant) as representative human AML-derived cell lines.6,7
Among the many known MMPs, the expression of MMP-9 may correlate with the progression and invasiveness of acute and chronic leukemias.8–10 In effect, we have shown that proMMP-9 binds via its procatalytic domain to I domains of αMβ2 and αLβ2 integrins; we have also shown that the interaction with the αM I domain is stronger than that with the αL I domain.11 Moreover, the β2 integrin-proMMP9 interaction can be recapitulated with a peptide as short as the hexapeptide HFDDDE, derived from the MMP-9 catalytic domain sequence, to show that perturbation of this interaction inhibits leukemia cell migration in vitro.12 Another peptide capable of inhibiting proMMP-9 binding is the β2 integrin ligand ADGACILWMDDGWCGAAG (referred to as “DDGW”).11–13
We set out to study the potential functional role of a supramolecular complex composed of β2 integrins and MMP-9 on the extramedullary leukemia. We show that disruption of this protein interaction inhibits extravasation and extramedullary invasion in murine models of human disease; these findings add support to the term “leukemia invadosome” describing complexes among integrins, MMPs, and ligands/substrates that are temporarily formed and disrupted on the surface of leukemia cells as they invade tissues14; this term was coined to describe the biology of round-shaped leukemia cells, in which filamentous actin-based structures (such as filopodia) either do not exist or are not stable to the same extent they are in stationary adherent cells.15 Currently, there is no treatment strategy aimed at tissue invasion in the setting of leukemia. In effect, extramedullary leukemia can even precede systemic AML; thus, isolated extramedullary disease is treated systemically with chemotherapy. Given that the selected peptide motifs evaluated here are active against cell invasion in the preclinical setting, inhibiting the leukemia invadosome may have translational value for the development of new mechanism-based agents in addition to chemotherapy or targeted drugs.
Methods
Cell culture
OCI-AML3 cell line, derived from the primary blast of an AML patent,6 was maintained in RPMI containing 10% fetal bovine serum (FBS) and supplemented with l-glutamine, penicillin, and streptomycin. The human THP-1 and Jurkat leukemia cell lines were obtained from ATCC and maintained as described.16–18 To mimic in vivo conditions, all the cell cultures were maintained at 5% oxygen in a hypoxia chamber (Heraeus Instruments).
Antibodies, peptides, and small molecules
Rat antibody against the mouse αMβ2 integrin (MCA74) and fluorescein isothiocyanate (FITC)–conjugated anti-rat (Fab′)2 were from Serotec and rat antibody against CD31 (MEC 13.3) was from BD Biosciences. Polyclonal antibodies against MMP-9 were from Santa Cruz Biotechnology or as described.19 Rabbit antibodies against the MMP-9 sequence YQGDAHFDDDE were generated at Neosystems (NeoMPS) and purified by peptide affinity chromatography. Preimmune IgG was affinity-purified by the use of protein G. Other antibodies and peptides were as described.4,11–13,17 The αMβ2 integrin-binding small-molecule IMB-10 was obtained from Calbiochem, and the control IMB-8 compound was obtained as described.4
Nude and Balb/c mouse experiments
The institutional ethical committee of The University of Texas M. D. Anderson Cancer Center (UTMDACC) and approved all animal experiments. Immunodeficient nude mice received 106 cells subcutaneously in both flanks. At day 4, OCI-AML-3 cohorts (n = 6-8 mice per group) received 10 mg/kg/day peptide or saline intravenously 5 days a week for 3 weeks. Treatments of THP-1 cohorts (n = 10 mice per group) began at day 14 with HFDDDE (at 20 mg/kg/day) or DDGW (at 10 mg/kg/day). Actuarial survival was analyzed by the Kaplan-Meier methodology. For statistical analysis, either the Student t test or the log rank test (as indicated) was used to compare differences between the test peptide- or control peptide-treated groups. The F test (analysis of variance) also was used when indicated. The statistical software used was SPSS for Windows, Version 8.0 (SPSS Institute). For tracking leukemia cell dissemination, OCI-AML-3 cells were labeled with 125I by the lactoperoxidase method and administered intravenously (106 cells in 100 μL) into immunocompetent Balb/c mice (n = 3 per group) with or without peptides or antibodies as indicated. Mice were killed 1 hour after inoculation. Organs were collected, weighed, and 125I was determined in a γ-radiation counter. To determine the effect of peptides on circulating MMP-9 levels, peptide (200 μg) was injected intravenously into 3 Balb/c mice, and MMP-9 was determined by gelatin zymography.
Cell migration, adhesion, and proliferation assays
For transendothelial migration, OCI-AML3 cells (5 × 104) were plated on an established layer of endothelial cells (EaHy926 grown for 1 week in Transwells of 5-μm pore size) and cultured for 24 hours at 5% oxygen. The transmigration of OCI-AML3 cells through the endothelial layer was determined by cellular phosphatase assay11 or glyceraldehyde 3-phosphate dehydrogenase assay (Ambion Inc). Primary AML-M4 cells were obtained from cryopreserved peripheral blood cells from leukemia patients treated at UTMDACC and studied in collagen-coated 3-μm pore size filters (Costar). A total of 105 cells were allowed to migrate for 24 hours in 10% FBS/RPMI, after which the cells migrated to the bottom well were counted. For adhesion studies, 96-well plates were coated with 2% gelatin (Sigma-Aldrich) and blocked with 2% bovine serum albumin (BSA) for 30 minutes. Primary AML-M4 cells (5 × 104) were stimulated with 40 nmol/L phorbol 12,13-dibutyrate (PDBu) and plated onto the wells in 100 μL RPMI containing 0.1% BSA. After incubation for 1 hour at 37°C in hypoxia, unbound cells were removed by gently washing with PBS. The adherent cells were determined by the measurement of mitochondrial dehydrogenase activity by the use of DHL cell viability and proliferation assay according to the instructions of the manufacturer (AnaSpec). For cell-proliferation studies, 5 × 104 cells per well were cultured in 10% FBS/RPMI in 96-plates under hypoxic conditions for 1, 2 or 7 days, and the cells were determined by the DHL assay.
RNA interference
Targeted small interfering (si)RNAs against the αM integrin (siRNA ID: s7567, or 5′-GGGUGUCCUCAAGAGGAUATT-3′) and proMMP-9 (siRNA ID: s8862, or 5′- UAUCCUCUUGAGGACACCCTC-3′) were all obtained from Ambion. Nontargeted siRNA, negative control 1 also was obtained from Ambion (catalog no. 4390844). siRNAs were transfected at 160 nmol/L concentrations to OCI-AML3 cells by siPORT Amine (Ambion) in serum-free medium or by Lipofectamine 2000 (Invitrogen Life Technologies) in complete medium. After 72 hours, the cells were analyzed by different methods as described. For statistical analysis, the Student t test was used, and a P value of less than .001 was considered statistically significant.
Immunoblotting
Equal amounts of total protein (30 μg) from siRNA-transfected OCI-AML3 cells were separated on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transfected to nitrocellulose membrane, and probed with anti-αM 2LPM19c antibody. The membrane was preprobed with an antibody against α-actinin to demonstrate equal protein sample loading.
Flow cytometry
OCI-AML3 cells were grown on top of EaHy926 cells in the presence of 50 nmol/L PDBu for 2 hours at 5% oxygen. The medium was removed, and the cells were fixed with 4% paraformaldehyde for 15 minutes. The cells were incubated in labeling buffer (PBS containing 0.1% BSA, 0.1% NaN3, and 5% heat-inactivated donkey serum) for 30 minutes and washed (0.1% BSA, 0.1% NaN3 in PBS). Subsequently, the cells were incubated with 20 μg/mL of primary antibody for 1 hour. The primary antibodies used were anti-αL MEM83, anti-αM MEM170, and anti-MMP-9 H-129 (polyclonal; all from Santa Cruz). The cells were washed again and incubated with secondary antibody for 30 minutes. The secondary antibodies used were 1:60 of FITC-conjugated donkey anti–mouse (715-095-150; Jackson ImmunoResearch) or 1:120 of Cy3-conjugated donkey anti–rabbit (711-165-152; Jackson ImmunoResearch). After washing, the cells were resuspended to in PBS containing 0.1% BSA and analyzed by flow cytometry (BD FACSCalibur).
Immunofluorescence
OCI-AML3 cells were transferred on top of an 8-chamber slide coated with poly-L-lysine. Cells were stimulated with 50 nmol/L PDBu for 30 minutes, fixed with 4% paraformaldehyde, and labeled with primary antibodies, anti-αL TS1/22, anti-αM MEM170, anti-β2 R7E4, or affinity purified anti-MMP-9 antibodies19 for 1 hour at room temperature (RT; see Figure 5C). Alternatively, OCI-AML3 cells were transferred on top of an 8-chamber slide coated with EaHy926 endothelial cells. Cells were stimulated with 50 nmol/L PDBu for 2 hours at 5% oxygen, fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and labeled with primary antibodies MEM170 and H-129 (both from Santa Cruz Biotechnology) for 1 hour at RT (see Figure 5E). The cells were incubated with secondary antibodies, 1:150 FITC goat anti–mouse (sc-3699; Santa Cruz Biotechnology), and 1:300 TRICT goat anti–rabbit (sc-3841; Santa Cruz Biotechnology) for 30 minutes at RT. The confocal microscope used was Leica DM 6000 B; data acquisition software used was Leica, LAS AF-TCS SP5.
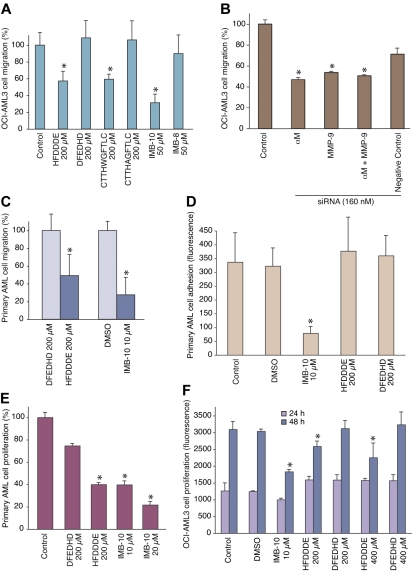
Figure 5.
Inhibition of AML cell migration, adhesion, and proliferation in vitro. (A) OCI-AML3 cells treated with 200 μmol/L peptide or 20 μmol/L small-molecule were allowed to migrate through an endothelial-cell monolayer. The results show means ± SD from triplicate wells. *P < .001 by Student t test. (B) Cells pretreated with RNAi oligomers were assessed as in panel A; *P < .001. (C) AML-M4 primary human leukemia-derived cells were subjected to migration in collagen-coated chambers for 24 hours, and the migrated cells were counted. DMSO indicates dimethylsulfoxide. *P < .05. (D) AML-M4 primary human leukemia-derived cells were allowed to bind to gelatin-coated microtiter wells for 60 minutes after which the bound cells were determined via the DHL assay; *P < .001. (E) AML-M4 primary cells were cultivated in suspension for 7 days with the compounds as described and the growth was determined via the DHL assay; *P < .02. (F) OCI-AML3 cells were cultivated in suspension for 24 hours or 48 hours, and the growth was determined via the DHL assay; *P < .01.
Phage-binding assay
Bone-marrow samples were obtained after informed consent was provided in accordance with the Declaration of Helsinki and UTMDACC Institutional Research Board approval from 8 AML patients at UTMDACC and from a healthy donor volunteer. Phage binding to the bone-marrow samples and cell lines was performed as described20 with 107 transducing units phage input per 106 cells. After an oil/water phase separation assay20 the bound phage (in transducing units) was determined by infecting host K91kan bacteria.
Cell binding of MMP-9 domains
125I-labeled MMP-9 domains ΔproMMP-9 and PEX12,13 were incubated with cells on ice for 3 hours in the absence or presence of competitors. Cells were fixed with 3% paraformaldehyde followed by washing. Samples were analyzed in a γ-counter. The dissociation constant (Kd) was determined with Prism software, Version 2.01 (GraphPad).
Pericellular proteolysis
For gelatinolysis assays, phorbol ester–activated OCI-AML-3 cells were incubated with FITC-labeled gelatin coated on 96-wells,13 or alternatively with FITC-labeled gelatin–Sepharose in suspension. The released FITC label was analyzed in the supernatant by fluorometry (Wallac Victor Fluorometer). To prepare [3H]proline-labeled ECM, EaHy926 cells were grown in the presence of 2.5 mCi/mL [3H]proline for 1 week in 24-well plates and then detached by mild alkaline hydrolysis. OCI-AML3 cells (at 2 × 105 per well) were incubated for 6 hours in 1 mL serum-free RPMI, and the supernatants were analyzed by liquid scintillation counting. To study the stability of endogenous membrane proteins, 4 × 107 cells were surface-biotinylated with Sulfo-NH-SS-Biotin. After washing, the cells were incubated in the absence or presence of peptides (200 μmol/L) in complete medium for 16 hours. Cell surface-biotinylated proteins were isolated with a protein biotinylation and purification kit (Pierce) and analyzed on sodium dodecyl sulfate gels with silver staining and immunoblotting with an anti-β2 integrin (R2E7) antibody. In flow cytometry, cell-surface biotinylated proteins were determined with streptavidin-phycoerythrin (BD Biosciences).
Results
Expression of MMP-9 and αMβ2 in AML cells
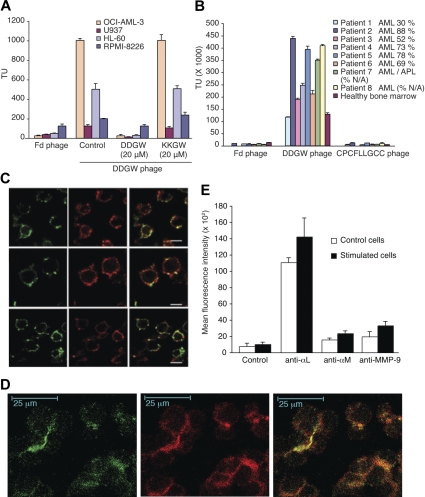
The β2 integrin-binding and invadosome-targeting DDGW peptide was chosen for our studies because of its remarkable ability to recognize primary AML cells and cell lines. In a Biopanning and Rapid Analysis of Selective Interactive Ligands (BRASIL) assay,20 phage displaying DDGW sequence bound most strongly to OCI-AML3 cells of the 4 leukemia cell lines studied (Figure 1A). The binding was specifically inhibited by the DDGW peptide but not by a control peptide ADGACILWMKKGWCGAAG (referred to as “KKGW”). Insertless Fd phage was used as a control. Similarly, DDGW phage bound strongly to bone-marrow samples of AML patients (Figure 1B). A total of 7 of 8 studied AML specimens bound significantly more DDGW phage than a bone-marrow control from a healthy donor. These 7 bone-marrow samples contained 52% to 88% AML cells, indicating that DDGW does recognize the primary tumor cells. We also observed that DDGW demonstrated much stronger binding to the bone-marrow samples than another β2 integrin-recognizing peptide, CPCFLLGCC, which does not inhibit integrin-proMMP-9 interaction.11
Figure 1.
Expression of MMP-9 and integrin on AML cells and binding activity of DDGW peptide. (A) Phage displaying DDGW was allowed to bind to 4 leukemia cell lines in the absence or presence of DDGW or KKGW peptide. Insertless Fd phage was a control. The bound phages were determined by titering in bacteria. (B) Fd, DDGW, or CPCFLLGCC phages were examined for binding to bone marrow smears from AML patients and one healthy donor. The percentage of tumor cells is indicated. The bound phages were determined by bacterial infection. The results show mean ± SD. (C) OCI-AML3 cells were treated with 50 nmol/L PDBu for 30 minutes and stained with anti-αL TS1/22, anti-αM MEM170, and anti-β2 R7E4 antibodies (in green in top, middle, and bottom panels, respectively) and affinity purified antibodies against MMP-9 (in red). Yellow indicates the colocalization of αL/αMβ2 integrins and MMP-9. (D) OCI-AML3 cells were transferred onto the slides coated with endothelial cells (EaHy926), and the cells were treated with 50 nmol/L PDBu for 2 hours. Coculture was stained with anti-αM MEM170 (green) and polyclonal anti-MMP-9 H-129 (red) antibodies. Yellow indicates colocalization. (E) Cells were grown in the absence or presence of an endothelial cell monolayer and 50 nmol/L PDBu for 2 hours. The cells were stained with anti-αL MEM83, anti-αM MEM170, or anti-MMP-9 H-129 antibodies and analyzed by flow cytometry. Shown are mean ± SD from 3 separate experiments.
The OCI-AML3 cell line was chosen for further studies because of its strong binding to the DDGW peptide. Colocalization of proMMP-9 and β2 integrin was observed in OCI-AML3 cells when they were stimulated with phorbol ester (Figure 1C) or with endothelial-cell coculture (Figure 1D). Colocalization was detectable with antibodies to each integrin subunit αM, αL, or β2 as studied by confocal microscopy. Interestingly, in the endothelial-leukemia cell coculture, the colocalization of proMMP-9 and αMβ2 occurred in apparent contact areas between the leukemia cells (Figure 1D). Furthermore, the coculture with an endothelial cell monolayer increased the membrane expression of αLβ2, αMβ2, and proMMP-9 on OCI-AML3 cells as studied by flow cytometry (Figure 1E). OCI-AML3 cells appeared to express more αL chain on the cell surface than αM chain and, in fact, the cell-surface expression of αM chain became readily detectable only after the stimulation.
Inhibition of subcutaneous leukemia xenografts by HFDDDE and DDGW peptides
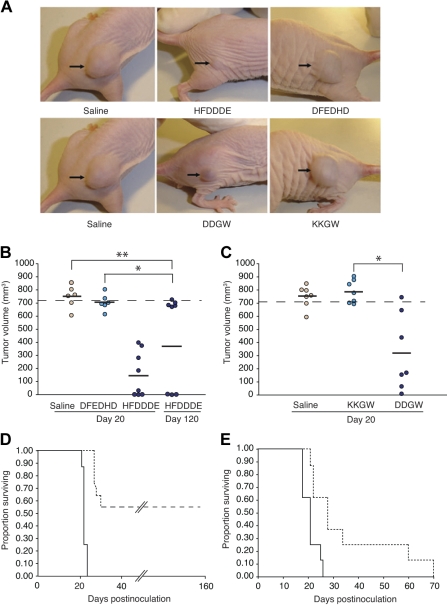
The ability of DDGW to recognize AML-derived cells led us to study the effects of invadosome-targeting peptides on AML cell invasion in murine models. When the cells were grown as subcutaneous xenografts in nude mice, a process that requires cell invasion and tissue remodelling, both peptides—but not corresponding negative control peptides—markedly decreased the growth of extramedullary leukemia (Figure 2A). HFDDDE administered intravenously induced tumor regression in 3 of 8 mice bearing OCI-AML3 xenografts (Figure 2B). A tumor response induced by HFDDDE was significant at early (20 days) and later (120 days) time points (t test, P < .001); a scrambled peptide (DFEDHD) had no detectable effects. Moreover, after the HFDDDE peptide treatment, 3 of the mice remained healthy for 6 months, after which time they were killed. Tumor response was shorter (∼ 3 weeks) for DDGW (Figure 2C); KKGW had no activity. Treatment with either HFDDDE or DDGW led to a significant increase in the actuarial survival of leukemia-bearing mice in comparison with control mice (Figure 2D-E). Unlike the cohort of DDGW-treated mice, discontinuation of treatment with HFDDDE did not result in leukemia regrowth. The peptides induced no weight loss or other evident untoward effects at the doses used.
Figure 2.
Antileukemia activity of invadosome-targeting peptides in OCI-AML3 xenografts. (A) Shown are representatives of peptide-treated leukemia-bearing mice 20 days after OCI-AML-3 cell inoculation. (B and C) Tumor sizes of OCI-AML-3–derived xenografts. Bars represent means from each peptide group. *Student t test, P < .001 of either HFDDDE- or DDGW-treated mice compared with control mice. No significant differences were detected between DFEDHD vs the saline group (t test, P = .107) or KKGW vs the saline group (t test, P = .65). Mice were killed when leukemia-derived xenograft volume reached 700 mm3 as indicated. (D-E) Kaplan-Meier actuarial survival analysis of the cohorts is shown. Differences were statistically significant at P < .001 and P = .004 for HFDDDE-treated group (D, dashed line) and DDGW-treated group (E, dashed line), respectively, compared with control DFEDHD-treated mice (D, solid line) or KKGW peptide-treated group (E, solid line).
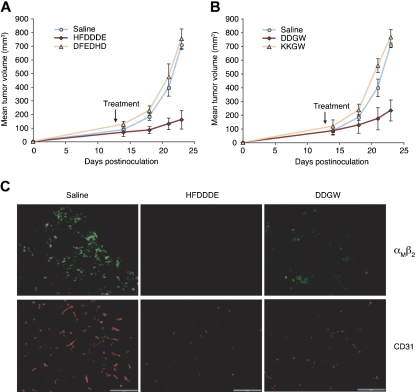
Similarly, peptide treatments of nude mice bearing THP-1 xenografts led to a subsequent tumor response: HFDDDE-treated (Figure 3A) or DDGW-treated (Figure 3B) mice had smaller tumor xenografts compared with control mice. Immunostaining of the xenografts showed signs of extensive tissue remodeling, including recruitment of host leukocytes and neovascularization (Figure 3C). Notably, the number of αMβ2-positive tumor-infiltrating leukocytes and CD31-positive endothelial cells were markedly decreased by either HFDDDE or DDGW treatment.
Figure 3.
Invadosome-targeting peptides reduce THP-1 xenograft growth and host cell infiltration. In THP-1–derived xenograft differences were statistically significant at P < .001 and P = .004 for HFDDDE- (A) or DDGW-treated groups (B), respectively, compared with vehicle-treated group. (C) Staining of tumor-infiltrating leukocytes with an αMβ2 integrin antibody (top panel) and tumor vasculature with an anti-CD31 monoclonal antibody (bottom panel). Representative tissue sections are shown from mice treated with saline, HFDDDE, or DDGW. Scale bar, 200 μm.
Inhibition of leukemia cell extravasation by HFDDDE
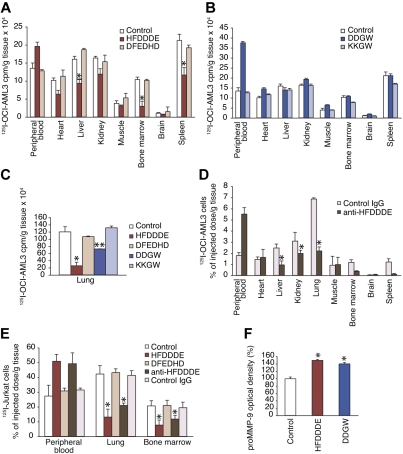
In another experimental approach relevant to leukemia we studied leukemia-cell extravasation by coadministering 125I-surface–labeled OCI-AML-3 cells with or without the competitor peptide intravenously into Balb/c mice. We measured the cell-associated radiolabel accumulated in blood and organs 1 hour after the injection. HFDDDE and DDGW both caused a simultaneous shift in the leukemia-cell distribution, coupled with a decrease in leukemia-cell migration and increase in the cell pool remaining in the peripheral circulation. Again, HFDDDE was the more active peptide and inhibited accumulation of leukemia cells not only into the bone-marrow cavity but also in commonly invaded extramedullary organ sites such as liver and spleen, whereas the scrambled control peptide had no detectable effects (Figure 4A). DDGW caused no inhibition of tissue infiltration by leukemia cells except in the lungs (Figure 4B-C). Because the MMP-9–derived HFDDDE proved so bioactive and relatively potent against experimental models of AML, we tested antibodies raised against the peptide. Anti-HFDDDE antibodies recognized proMMP-9 in enzyme-linked immunosorbent assay and in immunoblotting (data not shown). Furthermore, anti-HFDDDE antibodies had an effect with similar magnitude to the cognate peptide itself when coadministered with the cells (Figure 4D).
Figure 4.
Effect of invadosome-inhibiting peptides on leukemia-cell extravasation and circulating MMP-9 levels. (A-D) 125I-labeled OCI-AML-3 cells were administered intravenously into Balb/c mice with peptide (200 μg), anti-HFDDDE (20 μg), or preimmune IgG. At 1 hour after inoculation, mice were killed, and tissues were harvested, weighed in, and subjected to a γ-counter. (E) 125I-labeled Jurkat leukemia T cells were administered as described previously. (F) Peptide alone was administered intravenously, and serum MMP-9 activity was measured by gelatin zymography. (A-F) Shown are means ± SD from triplicates. *P < .005 (t test) of HFDDDE or antibody-treated mice compared with control mice.
The leukemia cells remained largely within the peripheral-blood circulation and were prevented from infiltrating bone marrow and most extramedullary sites. HFDDDE and anti-HFDDDE antibodies similarly inhibited the infiltration of Jurkat cells to bone marrow and to extramedullary sites of invasion, suggesting that the major integrin, αLβ2, expressed by these cells in association with MMP-9, may play a role in T-cell leukemia extravasation (Figure 4E). Because HFDDDE and DDGW release proMMP-9 from leukocytic cells in vitro,11–13 we examined the serum MMP-9 levels in Balb/c mice injected intravenously with either peptide. Consistently, we found that the serum MMP-9 levels were greater in peptide-injected than in control mice, suggesting that both peptides prevent cell-surface binding of proMMP-9 in vivo as well (Figure 4F).
HFDDDE inhibits migration and proliferation of AML cells in vitro
To obtain further evidence that proMMP-9 and β2 integrin together are central for AML cell extravasation, we examined the cells in a transendothelial migration assay. Blockage of either the integrin (with the HFDDDE peptide) or of proMMP-9 (with the CTTHWGFTLC peptide; Koivunen et al17) inhibited OCI-AML3 cell migration through an EaHy926 endothelial cell layer (Figure 5A). DFEDHD and CTTHAGFTLC served as controls. A strong effect on cell migration also was obtained by IMB-10, a pharmacologic αMβ2 integrin inhibitor/modulator, but not by the control compound IMB-8, indicating specificity. Similarly, siRNA against αMβ2 or proMMP-9 inhibited significantly (P < .001), whereas a control siRNA did not. Furthermore, the combined effect of the siRNAs was not additive (Figure 5B).
HFDDDE and IMB-10 also inhibited migration of primary AML cells as studied in collagen-coated migration chambers (Figure 5C). However, initial adhesion of primary cells was not affected by HFDDDE in a 60-minute assay where IMB-10 was antiadhesive (Figure 5D). Further analysis of AML cells indicated that their long-term proliferation in suspension was sensitive to inhibition by IMB-10 or HFDDDE. Figure 5E shows the results for primary AML cells after a 7-day culture, where IMB-10 and HFDDDE, but not DFEDHD, significantly diminished cell proliferation. In OCI-AML3 cell culture, HFDDDE had no detectable effects at 24 hours but began to affect cell growth when incubated for 48 hours (Figure 5F). IMB-10 inhibited OCI-AML3 cell proliferation even more effectively than HFDDDE.
Major role of αMβ2 in binding proMMP-9 and mediating pericellular proteolysis
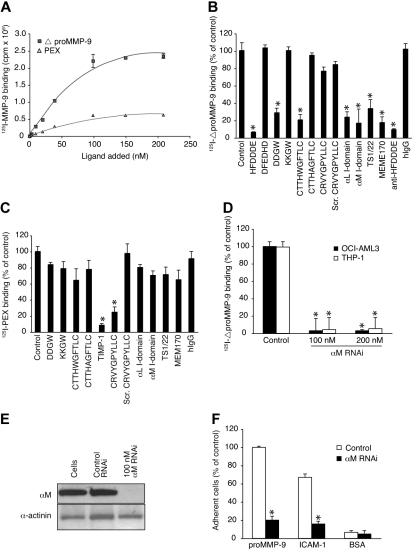
We determined the affinity of proMMP-9 to the cell surface by using separate MMP-9 procatalytic and C-terminal PEX domains. 125I-labeled ΔproMMP-9 and 125I-labeled PEX bound to OCI-AML-3 cells with dissociation constants of 93 plus or minus 18 nmol/L (n = 3) and 100 plus or minus 19 nmol/L (n = 3), respectively (Figure 6A). Unlabeled ΔproMMP-9 (50 mu]g/mL) inhibited the binding of 125I-ΔproMMP-9 by more than 90% and, correspondingly, unlabeled PEX competed with 125I-PEX (not shown). Inhibition experiments with integrin antibodies (MEM170, TS1/22) and soluble integrin I domains indicated that αMβ2 and αLβ2 serve as receptors for ΔproMMP-9 (Figure 6B). Moreover, HFDDDE and DDGW as well as anti-HFDDDE antibodies inhibited efficiently and confirmed our hypothesis. In addition, ΔproMMP-9 binding was inhibited with the ΔproMMP-9-binding peptide CTTHWGFTLC but not with the PEX-domain binding peptide CRVYGPYLLC13 or the natural MMP-9 inhibitor TIMP-1.
Figure 6.
The integrin I-domain anchors proMMP-9 at the cell surface. (A) 125I-labeled MMP-9 domains bind to OCI-AML-3 cells in a dose-dependent manner. (B) 125I-ΔproMMP-9 binding was competed with peptides (200 μmol/L), αL and αM I domains (20 μg/mL), or the antibodies (20 μg). (C) Binding of 125I-PEX domain was studied as in panel B. (D) 125I-ΔproMMP-9 binding to siRNA-treated or untreated cells. (E) Immunoblots of siRNA-treated cells with αM antibodies and control α-actinin antibodies. (F) Binding of siRNA-treated cells to immobilized proMMP-9, intercellular adhesion molecule-1, or BSA. Adherent cells were quantitated by phosphatase assay. Data are means ± SD from triplicates. *P < .001 by t test.
In contrast, CRVYGPYLLC and TIMP-1 prevented the cell-surface binding of the PEX domain, which thus has a binding site different from that of the procatalytic domain (Figure 6C). Genetic knockdown with αM siRNA confirmed that that αMβ2 serves as a receptor for ΔproMMP-9 (Figure 6D). At the concentrations indicated, αM siRNA but not control siRNA markedly suppressed integrin expression as determined by protein levels (Figure 6E) and by flow cytometry (data not shown). αM siRNA did not affect proMMP-9 protein levels (data not shown). RNAi-treated cells showed decreased binding to intercellular adhesion molecule-1– or proMMP-9–coated surfaces, indicating an integrin loss-of-function effect (Figure 6F).
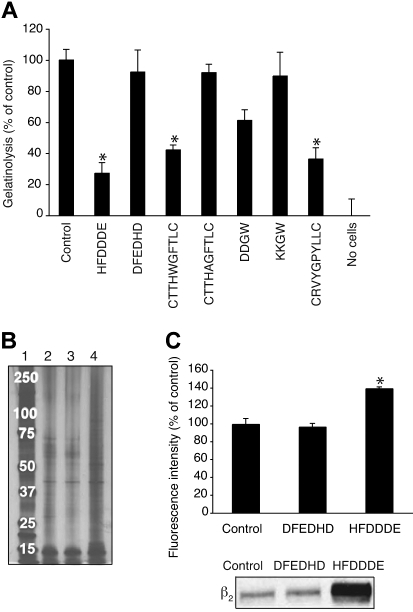
Finally, we studied whether the leukemia cell invadosome is important for pericellular proteolysis. Gelatinolysis by OCI-AML-3 cells was inhibited by HFDDDE (100 μmol/L), although the peptide itself lacks gelatinase-inhibiting activity (Figure 7A). As expected, gelatinolysis was inhibited by CTTHWGFTLC or CRVYGPYLLC, confirming that the process is mediated by gelatinase. We obtained similar results by studying the stability of biotin-labeled endogenous cell-surface proteins. More biotinylated proteins were recovered from HFDDDE-treated cells compared with DFEDHD-treated or control cells (Figure 7B). DDGW, but not the control KKGW, had a similar stabilizing effect (not shown). In a third proteolysis assay, we examined the degradation of [3H]proline-labeled endothelial ECM by OCI-AML-3 cells. HFDDDE and CTTHWGFTLC inhibited the [3H]proline label release by 32% and 83%, respectively, and DFEDHD or CTTHAGFTLC had no effects (data not shown). Moreover, we confirmed this HFDDDE peptide-mediated stabilization of cell-surface proteins by flow cytometry and identified the β2 integrin chain itself as one of the stabilized proteins by immunoblotting (Figure 7C).
Figure 7.
Inhibition of pericellular proteolysis of OCI-AML3 cells. (A) Effects of peptides (200 μmol/L) on the release of gelatin fragments from coated FITC-labeled gelatin. The results show means ± SD from triplicates. *P < .005. (B) Biotinylated cell surface proteins were isolated from cells incubated without peptide (lane 2), with DFEDHD (lane 3), or with HFDDDE (lane 4). Samples were run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by silver staining. Molecular weight markers are shown in lane 1. (C) Quantitation of cell-surface biotinylated proteins with streptavidin-phycoerythrinin flow cytometry (top panel). Immunoblotting with β2 integrin antibody (bottom panel).
Discussion
As per the definition used in this work, the invadosome is a supramolecular MMP-integrin complex required for leukemia-cell invasion. We show that inhibition of the leukemia invadosome is a new and attractive strategy for developing antileukemic agents; such drugs may be useful against certain human AML extramedullary disease phenotypes. These studies raise the possibilities of designing a new class of more specific proMMP-9 targeting drugs that are not based on inhibition of the conserved catalytic mechanism but on the distinct HFDDDE motif interacting with integrins. The MMP-9 mimetic hexapeptide was the most active of the peptides studied here and inhibited proMMP-9 binding to the cell surface, prevented pericellular proteolysis, affected long-term cell proliferation, and decreased medullary and extramedullary AML cell dissemination in murine models in vivo. Because the αMβ2 integrin, in particular, is known of its promiscuous binding activity toward many types of ligands,21 we cannot exclude the possibility that the peptides studied here also affect binding of other ligands than proMMP-9. However, the similar activity of anti-HFDDDE antibodies to that of the synthetic peptide does suggest that proMMP-9 is a critical factor in AML progression and dissemination.
In essence, our findings that coculturing AML cells with endothelial cells up-regulates the surface expression and colocalization of αLβ2 and αMβ2 with proMMP-9 on AML cells indicate that mere contact with endothelial cells may harness AML cells with full invasion capacity. Transendothelial cell migration was inhibited in vitro and in vivo by blocking αMβ2 or proMMP-9. Moreover, by studying the binding of recombinant MMP-9 forms to leukemia-cell surfaces, we confirm our findings11–13 that the I-domain of αMβ2 serves as the major receptor for the procatalytic MMP-9 domain whereas the C-terminal PEX domain has another binding site. Thus, MMP-9 can in principle bind in at least 2 different ways to AML cell surfaces; whether the binding arrangements complement each other or have altogether different functions remains an open question at this point. The MMP-9 PEX domain has been found to interact with α4β1 integrin and CD44 in B lymphoma cells22 and with the DNA repair protein Ku in AML cells.23 Therefore, although our preclinical studies have unequivocally pointed to a central role for functional protein interaction between β2 integrin and proMMP-9 in the extramedullary AML phenotype, further refinement of the biochemical working definition of the leukemia invadosome may lead to the recognition that other proteins also are involved.
Notably, despite not inhibiting MMP-9 enzyme activity directly, HFDDDE was approximately as effective as the specific gelatinase-inhibiting peptide CTTHWGFTLC in inhibiting pericellular proteolysis and cell migration. These results suggest that integrins and proMMP-9 act in concert and that binding of proMMP-9 to the cell-surface integrins is central for localized proteolytic activity. We found that the β2 integrin chain itself was one of the proteins stabilized by the treatment with HFDDDE. This raises the question whether MMP-9 degrades the β2 integrin chain and whether continuous turnover of αMβ2 could be a reason for its apparent low surface expression in OCI-AML3 cells. Consistently with this interpretation, the authors of a recent study24 show that the β2 integrin chain is cleaved and shed in MMP-9 transfected macrophages and that αMβ2 can be a substrate for MMP-9. Thus, one can envisage that such a proteolytic cleavage is indeed relevant for the ability of leukemia cells to be mobile and to generate new adhesion sites. Such a steady proteolytic activity may not be relevant only for extravasation but also for the primary invasion of leukemia cells within human bone marrow. Moreover, the data presented here suggest that pericellular proteolysis is also important for long-term survival and proliferation of AML cells because their proliferation was decreased when incubated with HFDDDE for 2 days or longer. Finally, pharmacologic inhibition of αMβ2 integrin with the small molecule IMB-10 diminished leukemia-cell proliferation even more efficiently. In effect, progression of AML and its chemosensitivity has been reported to depend on integrin-mediated signaling and adhesion.25–27
We also observed here that not only the leukemia cells but also tumor-infiltrating leukocytes were markedly diminished after treatment with HFDDDE or DDGW. Although we cannot entirely rule out the possibility that this decrease is merely a direct consequence of decreased AML cells, host leukocytes, including neutrophils12 are also likely to migrate in a β2 integrin-proMMP-9 complex-dependent manner and thus be inhibited by HFDDDE. Thus, reagents affecting the invadosome or the αM/αLβ2 integrin5 may also have anti-inflammatory effects, which could conceivably be either beneficial or harmful depending on the microenvironmental context. However, a recent report shows that those bone marrow–derived myelomonocytic cells that enhance tumor growth and progression are double-positive for MMP-9 and αMβ2.28 Therefore, an agent capable of blocking both the tumor cells and tumor-infiltrating host cells could theoretically be a superior therapeutic, at least under certain conditions. Further studies will likely shed light into these open questions.
In conclusion, we report a potent antileukemia effect of peptides that block the functional protein complex between αM/αLβ2 integrin and proMMP-9 in preclinical settings. Our findings suggest a potential role for the β2 integrin-proMMP-9 complex in the extramedullary phenotype of AML cells. Inhibition of the leukemia invadosome as biochemically defined here is a promising new approach to be exploited in further translational studies.
Acknowledgments
This work was supported by grants from the Academy of Finland and the Finnish Cancer Society (to E.K. and C.G.G.), the Magnus Ehrnrooth Foundation, the Helsingin Sanomat Foundation and Developmental SPORE award (to E.K.), and by the Specialized Program in Research Excellence (SPORE) in Leukemia and National Institutes of Health/National Cancer Institute P50CA100632 at The University of Texas M. D. Anderson Cancer Center (R.P. and W.A.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contributions: M.S., K.K., and D.E.J. performed research and analyzed data; and C.G.G., S.O., R.P., W.A., and E.K. designed the research and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Erkki Koivunen, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ekoivunen@mdanderson.org or Wadih Arap, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail:warap@mdanderson.org.
References
- 1.Scheinberg D, Maslak P, Weiss M. In: Cancer: Principles and Practice of Oncology. Vita V, Hellman S, Rosenberg S, editors. Philadelphia, PA: Lippincott-Raven; 2005. pp. 2404–2432. [Google Scholar]
- 2.Jandl JH. Blood: Textbook of Haematology. 2nd ed. Toronto: Little, Brown and Co; 1996. pp. 1–1200. [Google Scholar]
- 3.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108(5):1441–1450. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 4.Björklund M, Aitio O, Stefanidakis M, et al. Stabilization of the activated aMb2 integrin by a small-molecule inhibits leukocyte migration and recruitment. Biochemistry. 2006;45(9):2862–2871. doi: 10.1021/bi052238b. [DOI] [PubMed] [Google Scholar]
- 5.Suojanen J, Salo T, Sorsa T, Koivunen E. aMb2 integrin modulator exerts antitumor activity in vivo. Anticancer Res. 2007;27(6B):3775–3782. [PubMed] [Google Scholar]
- 6.Wang C, Curtis JE, Minden MD, McCulloch EA. Expression of a retinoic acid receptor gene in myeloid leukemia cells. Leukemia. 1989;3(4):264–269. [PubMed] [Google Scholar]
- 7.Tsuchiya S, Kobayashi Y, Goto Y, et al. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42(4):1530–1536. [PubMed] [Google Scholar]
- 8.Lin LI, Lin DT, Chang CJ, Lee CY, Tang JL, Tien HF. Marrow matrix metalloproteinases (MMPs) and tissue inhibitors of MMP in acute leukemia: potential role of MMP-9 as a surrogate marker to monitor leukaemic status in patients with acute myelogenous leukemia. Br J Haematol. 2002;117(4):835–841. doi: 10.1046/j.1365-2141.2002.03510.x. [DOI] [PubMed] [Google Scholar]
- 9.Travaglino E, Benatti C, Malcovati L, et al. Biological and clinical relevance of matrix metallorproteinases 2 and 9 in acute myeloid leukemias and myelodysplastic syndromes. Eur J Hematol. 2008;80(3):216–226. doi: 10.1111/j.1600-0609.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 10.Nieborowska-Skorska M, Hoser G, Rink L, et al. Id1 Transcription inhibitor–matrix metalloproteinase 9 axis enhances invasiveness of the breakpoint cluster region/abelson tyrosine kinase–transformed leukemia cells. Cancer Res. 2006;66(8):4108–4116. doi: 10.1158/0008-5472.CAN-05-1584. [DOI] [PubMed] [Google Scholar]
- 11.Stefanidakis M, Björklund M, Ihanus E, Gahmberg CG, Koivunen E. Identification of a negatively charged peptide motif within the catalytic domain of progelatinases that mediates binding to leukocyte β2 integrins. J Biol Chem. 2003;278(36):34674–34684. doi: 10.1074/jbc.M302288200. [DOI] [PubMed] [Google Scholar]
- 12.Stefanidakis M, Ruohtula T, Borregaard N, Gahmberg CG, Koivunen E. Intracellular and cell surface localization of a complex between alpha(M)beta(2) integrin and promatrix metalloproteinase-9 progelatinase in neutrophils. J Immunol. 2004;172(11):7060–7068. doi: 10.4049/jimmunol.172.11.7060. [DOI] [PubMed] [Google Scholar]
- 13.Bjorklund M, Heikkila P, Koivunen E. Peptide inhibition of catalytic and noncatalytic activities of matrix metalloproteinase-9 blocks tumor cell migration and invasion. J Biol Chem. 2004;279(28):29589–29597. doi: 10.1074/jbc.M401601200. [DOI] [PubMed] [Google Scholar]
- 14.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755(1):37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Gupton SL, Gertler FB. Filopodia: the fingers that do the walking [review]. Sci STKE. 2007;2007(400):re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Xie J, Kantor C, et al. A peptide derived from the intercellular-adhesion molecule-2 regulates the avidity of the leukocyte integrins CD11B/CD18. J Cell Biol. 1995;129(4):1143–1153. doi: 10.1083/jcb.129.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koivunen E, Arap W, Valtanen H, et al. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17(8):768–774. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 18.Tian L, Yoshihara Y, Mizuno T, Mori K, Gahmberg CG. The neuronal glycoprotein telencephalin is a cellular ligand for the CD11a/CD18 leukocyte integrin. J Immunol. 1997;158(2):928–936. [PubMed] [Google Scholar]
- 19.Kjeldsen L, Bjerrum OW, Askaa J, Borregaard N. Subcellular localization and release of human neutrophil gelatinase, confirming the existence of separate gelatinase-containing granules. Biochem J. 1992;287(Pt 2):603–610. doi: 10.1042/bj2870603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giordano RJ, Cardo-Vila M, Lahdenranta J, Pasqualini R, Arap W. Biopanning and rapid analysis of selective interactive ligands. Nat Med. 2001;7(11):1249–1253. doi: 10.1038/nm1101-1249. [DOI] [PubMed] [Google Scholar]
- 21.Yakubenko VP, Lishko VK, Lam SCT, Ugarova TP. A molecular basis for integrin alphaMbeta2 ligand binding promiscuity. J Biol Chem. 2002;277(50):48635–48642. doi: 10.1074/jbc.M208877200. [DOI] [PubMed] [Google Scholar]
- 22.Redondo-Munoz J, Ugarte-Berzal E, Garcia-Marco JA, et al. {alpha}4{beta}1 integrin and 190 kDa CD44v constitute a cell surface docking complex for gelatinase B/MMP-9 in chronic leukemic but not in normal B cells. Blood. 2008;112(1):169–178. doi: 10.1182/blood-2007-08-109249. [DOI] [PubMed] [Google Scholar]
- 23.Monferran S, Paupert J, Dauvillier S, Salles B, Muller C. The membrane form of the DNA repair protein Ku interacts at the cell surface with metalloproteinase 9. EMBO J. 2004;23(19):3758–3768. doi: 10.1038/sj.emboj.7600403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaisar T, Kassim SY, Gomez IG, et al. MMP-9 sheds the β 2 integrin subunit (DC18) from macrophages. Mol Cell Prot. 2009;8(5):1044–1060. doi: 10.1074/mcp.M800449-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsunaga T, Takemoto N, Sato T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9(9):1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 26.Becker PS, Kopecky KJ, Wilks AN, et al. Very late antigen-4 of myeloblast correlates with improved overall survival for patients with acute myeloid leukemia. Blood. 2009;113(4):866–874. doi: 10.1182/blood-2007-12-124818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Toni F, Racaud-Sultan C, Chicanne G, et al. A crosstalk between the Wnt and the adhesion-dependent signalling pathways govern the chemosensitivity of acute myeloid leukemia. Oncogene. 2006;25(22):3113–3122. doi: 10.1038/sj.onc.1209346. [DOI] [PubMed] [Google Scholar]
- 28.Ahn G-O, Brown M. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: Role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13(3):193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]