Abstract
Clinical management of patients with type 2 diabetes (T2D) successfully prevents extreme hyperglycemia but does not precisely control glucose levels throughout the day. The pathogenesis of T2D is akin to a double barrel shotgun. The first trigger causes an explosion that sets genetic expression of the disease in motion; the second trigger discharges a host of environmental factors that worsen its clinical course. Candidate shells include glucolipotoxicity, cytokines, oxidative and endoplasmic reticulum stress, and insulin resistance. This review considers how each candidate adversely impacts β-cell function to create the downward spiral of glycemic control. Their roles in pathogenesis raise possibilities for new drug therapies designed to protect against adverse effects of residual hyperglycemia in patients treated with conventional drugs.
The β-cell in type 2 diabetes (T2D): culprit and victim
The causes of T2D and the reasons for its clinical variability are poorly understood. For example, some patients treat mild T2D with dietary measures, weight control and exercise, and glycemia nicely stabilizes for years. However, this is usually not the case, and most people must turn to an increasing variety of drugs, including insulin, to manage hyperglycemia. Recently, T2D has added a new twist. It now occurs with increasing frequency in children, who in the past, typically developed type 1 diabetes (T1D) only. We know T1D is an autoimmune disease in which specific antibodies commonly are found in blood and very likely, T cells and cytokines are major pathogenic factors that cause β-cell death. While T1D results in complete dependency on insulin therapy for survival, this is not true for T2D.
The clinical course for T2D is more highly variable than T1D, acting more as a syndrome than a disease. Diseases (e.g. pneumonia, anemia, and hypothyroidism) typically have discrete, identifiable causes that respond to definitive treatment. T2D may in fact be a large, poorly defined category of tens or hundreds of different etiologies that simply have hyperglycemia in common. Often T2D is associated with obesity, but sometimes not. It has no known markers of autoimmunity. Although apoptosis becomes an important feature, β-cells are not killed over a short period of time; instead, they usually remain functional and secrete insulin. When clinically profiling T2D, glucose appears to be the only intravenous stimulus that β-cells do not respond to. They retain their response to intravenous amino acids, sulfonylurea drugs, and β-adrenergic agonists. Although T2D has been distinguished from T1D for almost a century and we know the primary cause of T2D is almost certainly genetic, our basic understanding of T2D pathogenesis is rudimentary.
The etiology of T2D can be compared to a gunfight in an Old West movie. In these scenes, shots are fired, perhaps resulting in death. The targets that take a hit but avoid death still bear scars of the injury and remain vulnerable in some manner. This same chain of events occurs with β-cells and T2D. From the point of view of the β-cell, the onslaught of diabetes can either be deadly or a simple brush with disaster. An example might be a brief period of cytokine-induced accelerated apoptosis that later diminishes and resolves as opposed to chronic β-cell dysfunction due to uncompensated glucose toxicity.
Against the background of our burgeoning knowledge about the initial polygenic causes of T2D [1-6], recent developments have provided a clearer understanding of why this disease is so variable in progression after its onset. The weapon in this case is most probably akin to a double barrel shotgun. After the first trigger of the genomic barrel has been fired, the trigger that discharges the second barrel likely activates environmental factors that create gene-environment interactions in the β-cell that amplify the initial genetic damage, ultimately worsening the hyperglycemia. Potential shells in the second barrel include hyperglycemia, hyperlipidemia, oxidative and endoplasmic reticulum (ER) stress, cytokines, and worsening insulin resistance (Figure 1). This review focuses on the contents of the second barrel that prey on the β-cell after it has been weakened by the initial genetic shell, thereby worsening the clinical outcomes of the disease we call T2D.
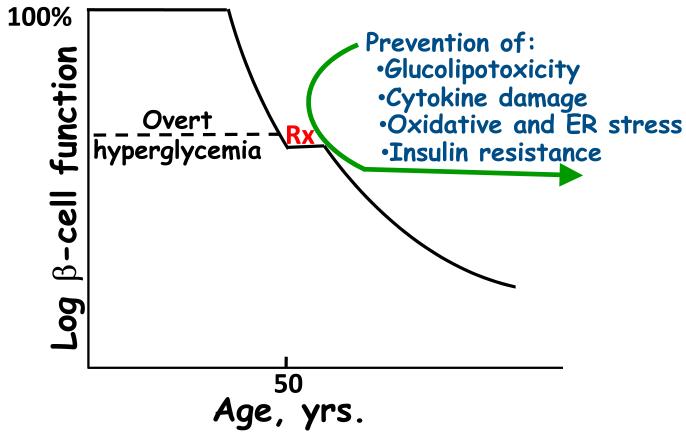
Figure 1. Natural history of β-cell deterioration in type 2 diabetes (T2D).
This schema proposes that T2D is primarily a polygenic disorder that eventually results in clinical hyperglycemia, which is then made worse by environmental factors, resulting in a relentless decline in β-cell function. Environmental factors likely to contribute to this process include glucolipotoxicity, cytokines, oxidative and endoplasmic reticulum (ER) stress, and insulin resistance. Intervention with conventional therapy (Rx) usually arrests the initial decline in β-cell function, but characteristically after a brief return to improved function, the β-cell once again continues down its path of declining function. This schema proposes that prevention of the adverse consequences of environmental factors might arrest this decline.
Glucose toxicity and oxidative stress
Hyperglycemia, the hallmark of diabetes, is the clinical outcome of β-cell dysfunction that causes acute symptoms leading to its diagnosis and chronic complications (blindness, kidney failure, neuropathies, and vascular disease). It is not generally appreciated that the β-cell itself becomes a secondary complication. Inadequate insulin secretion from decompenated β-cells causes increasingly higher blood glucose levels that continually bathe the islet. This leads to a spectrum of consequences for the β-cell, including glucose desensitization, β-cell exhaustion, and eventually glucose toxicity - a spectrum of physiology through pathophysiology. Desensitization is a pharmacologic concept involving a cellular response to protect itself from excessive stimulation. In the case of the β-cell, this desensitization is homologous in nature. Repeated challenges by high glucose concentrations rapidly lead to diminished insulin secretory responses to glucose but not to other agents. However, upon cessation of glucose stimulation, this refractoriness to glucose stimulation eventually recovers, and the β-cell once again responds normally to glucose [7,8]. However, more prolonged exposure to high glucose concentrations leads to β-cell exhaustion. This state is usually described as depletion of available insulin stores due to prolonged stimulation of insulin secretion without a sufficient compensatory increase in insulin synthesis. In this case, it is likely that insulin granules in the exocytotic apparatus become depleted. However, prolonged periods of rest under conditions of normal glucose levels permit the β-cell to recover and once again respond normally to glucose stimulation [7,8]. At the extreme of this spectrum is glucose toxicity [9]. This term implies a toxocologic rather than a pharmacologic condition wherein the β-cell has been bombarded with excessive levels of glucose molecules for an extended period of time. In this state, important mechanisms such as glucose regulation of the insulin promoter and insulin synthesis are deranged. The β-cell nearly shuts down, and insulin secretion is greatly compromised, especially post-prandially. Molecular studies demonstrated that extensive exposure to high glucose concentrations renders the β-cell incapable of producing insulin mRNA, a consequence of the loss of two critical insulin gene transcription factors, musculoaponeurotic fibrosaracoma protein A (MafA) and pancreatic duodenal homeobox-1 (PDX-1) (Figure 2, [10-12]). The loss of MafA involves a post-translational defect [13], whereas loss of PDX-1 involves a post-transcriptional defect [10] in synthesis. That these losses in MafA and PDX-1 can be devastating has been confirmed by in vitro experiments using cell lines showing that mutation of the DNA binding sites of either factor leads to marked decreases in insulin reporter activity [11,12]; furthermore, reconstitution of glucotoxic cells with MafA and PDX-1 normalizes insulin reporter activity and improves defective insulin gene expression [13]. However, even after these severe injuries, the β-cell is not yet in extremis. Use of extended cultures of a β-cell line has shown that, for a finite period of time, returning glucotoxic cells to physiologic glucose conditions allows the cells to heal and restore MafA and PDX-1 levels [14]. This corrects the defects in insulin gene expression, insulin content, and glucose-induced insulin secretion. Transplantation of isolated human islets in hyperglycemic nude mice causes failure of β-cells to respond to glucose stimulation during perfusion of the graft [15]. Recipient mice that were made hyperglycemic for 4 weeks and then returned to normoglycemia for two weeks reestablished insulin stores but retained abnormally low glucose-induced insulin secretion.
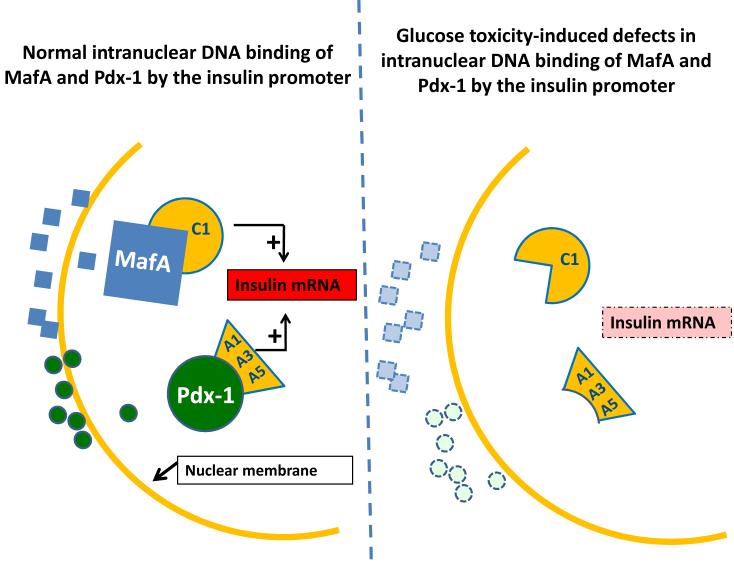
Figure 2. Molecular mechanism of glucotoxicity at the level of insulin gene expression.
(a) Under physiologic conditions, musculoaponeurotic fibrosaracoma protein A (MafA) and pancreatic duodenal homeobox-1 (PDX-1) are two critically important proteins that are synthesized in the cytoplasm and transported across the nuclear membrane to interact with their DNA binding sites on the insulin promoter. MafA is bound by the C1 box and PDX-1 is bound by the A1, A3, and A5 sites on the insulin promoter. (b) Glucotoxicity greatly diminishes protein levels of MafA and PDX-1, the former through post-translational and the latter through post-transcriptional mechanisms. This pathophysiologic process likely involves multiple steps (not shown) that initially involve exclusion of the proteins from the nucleus and later involve decreased synthesis and ultimate disappearance of the proteins from the cytoplasm. These abnormalities lead to decreased insulin mRNA, insulin content, and glucose-induced insulin secretion, and are reversible only in the early stages of glucose toxicity.
Similarly, exposure to supraphysiologic glucose concentrations can cause irreversible β-cell damage cell lines in a time- and glucose concentration-dependent manner [14]. One established mechanism for this terminal event is that of oxidative stress. This conclusion is based on experimental evidence that chronic exposure to high glucose concentrations floods metabolic pathways to cause excessive production of reactive oxygen species (ROS) via several biochemical pathways [16]. These include glucose autoxidation, protein kinase C activation, methylglyoxal formation and glycation, hexosamine metabolism, sorbitol formation and oxidative phosphorylation.
The overabundance of ROS in the microenvironment of the β-cell is compounded by a profound irony. This islet cell that is the primary cause of and eventually becomes damaged by hyperglycemia contains poor host defenses mechanisms against oxidative stress. In comparison with other mammalian cell types, β-cells contain only modest levels of superoxide dismutases and virtually no catalase or glutathione peroxidase [17, 18]. The meaning behind this peculiar set of circumstances is not clear. One possibility is that the β-cell was created to thrive in an environment with higher hydrogen peroxide (H2O2) levels than most cells [19,20], because superoxide dismutases form H2O2 from superoxide and there are no means within the β-cell to catabolize H2O2. Adenoviral overexpression of glutathione peroxidase (GPx) in isolated islets prevents the compromised β-cell function caused by high ROS levels formed in response to elevated glucose concentrations [21]. Adverse effects of oxidative stress can also be prevented by the potent antioxidant, n-acetylcysteine (NAC). The mechanism of protection is likely enhanced generation of endogenous glutathione because NAC contains cysteine, the rate-limiting amino acid for glutathione synthesis. Moreover, the favorable effects of NAC can be blocked by buthionine sulfoximine, a drug that interferes with glutathione synthesis [21]. It has also been demonstrated that excess levels of D-glyceraldehyde generate ROS and inhibit insulin secretion, effects that are prevented by NAC and enhanced by koningic acid, a drug that inhibits glyceraldehyde catabolism [22].These studies reinforce the concept that enhancing antioxidant protection of the β-cell protects it from ROS generated via different sources.
Studies treating cell lines and animal models of T2D with exogenous antioxidants and experiments overexpressing endogenous antioxidant enzymes have shown that increasing antioxidant intrinsic defense mechanisms ameliorates β-cell damage caused by prolonged exposure to supraphysiologic glucose concentrations. Examples are NAC and aminoguanidine, which ameliorate the development of hyperglycemia in Zucker Diabetic Fatty rats and db/db mice [23,24], both leptin receptor-deficient rodent models of T2D that are prone to obesity . Tempol, a superoxide dismutase inhibitor, has also been used to prevent the increase in total and mitochondrial superoxide and β-cell dysfunction in Wistar rats induced by prolonged exposure to glucose [25]. Consistent with these observations, recently published pilot studies in subjects with type 2 diabetes report improved β-cell function and glycemic control during antioxidant treatment [26, 27]. The overall message from these findings is that chronic exposure to supraphysiologic levels of glucose induces oxidative stress that in turn causes a relentless decline in β-cell function that can be prevented by enhanced antioxidant protection (Figure 3). It is important to point out, however, that glucose toxicity and oxidative stress in diabetes has also been associated with increased insulin resistance [28], a factor that could play an important indirect role in β-cell dysfunction.
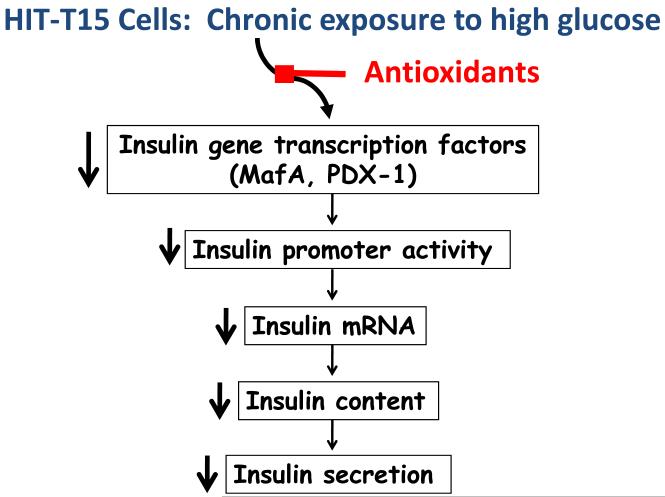
Figure 3. Prevention of glucose toxicity by antioxidants.
The adverse effects of glucose toxicity on processes supporting insulin synthesis and secretion, starting with insulin gene transcription factor availability have been shown to be preventable by intervention in vitro using overexpression of antioxidant enzymes in isolated islets and in vivo by treating animals with antioxidant drugs.
Lipotoxicity or glucolipotoxicity?
The close clinical association of T2D with obesity led to research interest in fatty acids, a major product of lipolysis, as players in the deterioration of β-cell function in T2D [9, 29]. Insulin is the major antilipolytic hormone, so when it is less available, one might expect excess levels of fatty acids released from adipocytes into the circulation to reach the rest of the body, including the pancreatic islet. Additionally, T2D is not uncommonly associated with elevated triglyceride and free fatty acid levels. After lipoprotein synthesis and release from the liver, lipid uptake into adipocytes is regulated by the insulin-activated enzyme lipoprotein lipase. A major report suggesting a role for lipotoxicity documented that ZDF rats had increased blood glucose and lipid levels, both of which were lowered with troglitazone treatment [30]. However, the question remained whether these two beneficial outcomes were related. Studies addressing this question, again using ZDF rats, observed that treatment with bezafibrate lowered blood triglyceride but not glucose levels, while treatment with phlorizin resulted in lowered blood glucose but not triglyceride levels. In this study, the prevention of hyperglycemia was associated with prevention of loss of insulin gene expression, but prevention of hypertriglyceridemia did not [31]. The conclusion was that hyperglycemia for prolonged periods of time, or glucose toxicity, is an independent cause of continual β-cell deterioration in T2D. On the other hand, hyperlipidemia, or lipotoxicity, does not adversely affect β-cell function unless hyperglycemia has already been established. The same conclusions were reached in other experiments utilizing isolated islets exposed to low and high concentrations of glucose and palmitic acid [9].
This interplay between adverse effects of high concentrations of glucose and lipid can be explained by the competition between glucose and fatty acids for metabolism by the glycolytic pathway. After prolonged hyperglycemia, oxidative phosphorylation is supersaturated with glucose products, inducing formation of high levels of cytosolic malonyl-CoA, a compound that inhibits β-oxidation of fatty acids. This causes the β-cell to shunt fatty acids to alternate pathways for its metabolism [32]. One outcome of this shunting is formation of excessive fatty acid esterification products capable of damaging β-cells, such as ceramide [33,34]. Such information has intensified interest in the possible relationships among diabetes, lipotoxicity, and endoplasmic reticulum stress [35-40].
Cytokines and β-cell failure
For decades, cytokines have played a central role in explaining T1D pathogenesis. Usually they are described as factors that exert negative effects on β-cells. Important cytokines in this regard include interleukin-1-beta IL-1-β), tissue necrosis factor-1-alpha (TNF-1-α), and interferon-gamma (INF-γ) [41]. While early observations described both positive and negative effects of IL-1-β on β-cells, the combination of all three cytokines consistently exerted negative effects. The clinical context in which these cytokines have been classically thought to adversely impact β-cells has been T1D and immune rejection of transplanted islets [41]. More recently however, reports describe evidence of macrophages and cytokine production in islets from animal models of T2D and in pancreatic tissue from patients with T2D [42, 43], although these findings are debated because of failure by others to observe an increase in islet IL-1 production by human islets cultured in media with high glucose concentrations, as well as failure to observe increased IL-1 levels in human islets isolated from T2D subjects compared to islets isolated from non-diabetic subjects [44]. An endogenous antagonist to the IL-1β receptor (IL-1Ra) is reported to protect against leptin-induced apoptosis in human islets [45]. Most recently, a human trial with a drug that blocks binding of IL-1β to its receptor led to improvement in β-cell function and amelioration of hyperglycemia in human T2D [46]. It will be important to conduct follow-up studies for greater periods of time in larger groups of people with T2D to confirm these observations
The association between elevated cytokine levels and cell death raises the possibility that cytokines contribute to accelerated β-cell apoptosis and decreased β-cell mass in T2D. The relationship of the evolution of T2D to changes in β-cell mass has received a great deal of attention in recent years. In studies of human pancreases obtained at autopsy, obese individuals had greater β-cell volume and neogenesis than lean subjects [47]. Furthermore, both obese and lean individuals with T2D had decreased β-cell volume compared to non-diabetic obese and lean controls. Frequency of β-cell replication was very low in all cases, and neogenesis was similar in diabetic vs. non-diabetic groups. However, there was a striking increase in β-cell apoptosis in the lean and obese diabetic subjects compared to their respective controls. This suggests that T2D is characterized by ongoing acceleration of β-cell apoptosis that is not compensated by a matched increase in β-cell replication.
Returning to the double barreled shotgun analogy, islet amyloid may be another component/shell of the environmental barrel [48]. A transgenic mouse model that overexpresses human islet amyloid polypeptide (IAPP) develops islet pathology similar to that of humans with T2D. A 10-fold elevation in β-cell apoptosis was found in this IAPP mouse model, and the frequency of β-cell apoptosis was related to the rate of increased islet amyloid [49]. This is of major interest because islet amyloid is observed in pancreases of humans with T2D [48]. These observations have set the stage to consider possible mechanisms of accelerated apoptosis that are associated with cytokines and IAPP as well as glucolipotoxicity and oxidative and endoplasmic reticulum stress [50,51]. Thioredoxin-interacting protein has been reported to be a potentially important link between glucose toxicity and apoptosis based on the observation that high glucose concentrations induce apoptosis and increased levels of this protein in INS-1 cells; similarly, HcB-19 islets deficient in thioredoxin-interacting protein were protected against glucose-induced apoptosis [52].
Obesity and insulin resistance
A major debate in diabetes research is whether insulin resistance or intrinsic β-cell defects is the primary cause of T2D. The consensus is that one cannot develop T2D without having inherently dysfunctional β-cells. Insulin resistance secondary to obesity places additional demands on β-cells to secrete more insulin. However, in most cases of severe human obesity, the islet is capable of increasing sufficient insulin production to maintain normoglycemia. It is assumed that the minority of obese people who succumb to T2D do not have a sufficient β-cell mass to meet the metabolic demands of insulin resistance. It should be noted, however, that not all T2D is triggered by obesity and insulin resistance. Many lean people with T2D have never been obese and are not resistant to insulin. Consequently, T2D is most accurately viewed as a genetic disease characterized by inadequate functional β-cell mass that cannot compensate for insulin resistance.
Conclusion and therapeutic implications
This review focuses on T2D in its second stages, i.e. after it initially manifests due to genetic causes. If not adequately treated by behavior modification and/or drugs and glycemic control is not achieved, the secondary triggers of glucolipotoxicty, cytokines, oxidative/endoplasmic reticulum stress, and insulin resistance conspire to make the β-cell even more dysfunctional. Eventually, accelerated apoptosis ensues which worsens the clinical syndrome. This understanding calls for intensive medical management of T2D, with a full realization that the best therapy and compliance usually will not keep post-prandial glucose levels normal. Recent findings from basic research point to potentially fruitful new areas of therapeutic development, involving drugs directed against glucose toxicity, cytokine effects, oxidative stress, and accelerated apoptosis.
A major handicap in the case of advancing antioxidant therapy is the bias that these common compounds, often found in the foods we eat, are insufficiently strong to be therapeutically efficacious. Trials using vitamins as antioxidants to protect against cardiovascular disease are often cited to make this point [53]. However, such trials are not adequate to provide insight into whether antioxidants protect patients with diabetes against the adverse effects of glucolipotoxicity. These studies were not designed with diabetes in mind, nor were the endpoints selected to reflect β-cell function or insulin resistance. Moreover, the vitamin doses used do not provide maximal antioxidant protection. Therefore, the concept of whether antioxidants serve as an ancillary form of treatment to protect tissues against the hyperglycemic background that persists in patients with diabetes despite their treatment with conventional antihyperglycemic drugs remains untested. More work is needed using potent antioxidants in human trials to ascertain whether these drugs provide added protection against the oxidant-promoting contents of the second barrel that contribute to the inexorable deterioration in β-cell function in patients with T2D.
Acknowledgments
Supported by NIH NIDDK grant R01 85325
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 2.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 4.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, Doumatey A, So WY, Ma RC, Andersen G, Borch-Johnsen K, Jorgensen T, van Vliet-Ostaptchouk JV, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Rotimi C, Gurney M, Chan JC, Pedersen O, Sigurdsson G, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- 6.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilpatrick ED, Robertson RP. Differentiation between glucose-induced desensitization of insulin secretion and beta-cell exhaustion in the HIT-T15 cell line. Diabetes. 1998;47:606–611. doi: 10.2337/diabetes.47.4.606. [DOI] [PubMed] [Google Scholar]
- 8.Vague P, Moulin JP. The defective glucose sensitivity of the B cell in non insulin dependent diabetes. Improvement after twenty hours of normoglycaemia. Metabolism. 1982;31:139–142. doi: 10.1016/0026-0495(82)90125-1. [DOI] [PubMed] [Google Scholar]
- 9.Poitout V, Robertson P. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J Clin Invest. 1992;89:1761–1766. doi: 10.1172/JCI115865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson LK, Redmon JB, Towle HC, Robertson RP. Chronic exposure of HIT cells to high glucose concentrations paradoxically decreases insulin gene transcription and alters binding of insulin gene regulatory protein. J Clin Invest. 1993;92:514–9. doi: 10.1172/JCI116596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A, Olson LK, Robertson RP, Stein R. The reduction of insulin gene transcription in HIT-T15 β-cells chronically exposed to high glucose concentration is associated with the loss of RIPE3b1 and STF-1 transcriptioin factor expression. Mol Endocrinol. 1995;9:1127–1134. doi: 10.1210/mend.9.9.7491105. [DOI] [PubMed] [Google Scholar]
- 13.Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005;280:11107–11113. doi: 10.1074/jbc.M410345200. [DOI] [PubMed] [Google Scholar]
- 14.Gleason CE, Gonzales M, Harmon JS, Robertson RP. Determinants of glucose toxicity and its reversibility in the pancreatic islet beta-cell line, HIT-T15. Am J Physiol Endocrinol Metab. 2000;279:E997–1002. doi: 10.1152/ajpendo.2000.279.5.E997. [DOI] [PubMed] [Google Scholar]
- 15.Jansson L, Eizirik DL, Pipeleers DG, Borg LA, Hellerstrom C, Anderssson A. Impairment of glucose-induced insulin secretion in human pancreatic islets transplanted to diabetic nude mice. J Clin Invest. 1995;96:721–726. doi: 10.1172/JCI118115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson RP. Chronic Oxidative Stress as a Central Mechanism for Glucose Toxicity in Pancreatic Islet Beta Cells in Diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 17.Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981;199:393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 19.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 20.Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani A-L, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002;99:12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi H, Tran PO, LeRoy E, Harmon JS, Tanaka Y, Robertson RP. D-Glyceraldehyde causes production of intracellular peroxide in pancreatic islets, oxidative stress, and defective beta cell function via non-mitochondrial pathways. J Biol Chem. 2004;279:37316–37323. doi: 10.1074/jbc.M403070200. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka Y, Gleason CE, Tran POT, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes. 1999;48:927–932. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 25.Tang C, Han P, Oprescu AI, Lee SC, Gyulkhandanyan AV, Chan GN, Wheeler MB, Giacca A. Evidence for a role of superoxide generation in glucose-induced beta-cell dysfunction in vivo. Diabetes. 2007;56:2722–2731. doi: 10.2337/db07-0279. [DOI] [PubMed] [Google Scholar]
- 26.Robertson RP, Zhou H, Zhang T, Harmon JS. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys. 2007;48:139–146. doi: 10.1007/s12013-007-0026-5. [DOI] [PubMed] [Google Scholar]
- 27.Hussain SA. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J Med Food. 2007;10:543–547. doi: 10.1089/jmf.2006.089. [DOI] [PubMed] [Google Scholar]
- 28.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 29.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 30.Shimabukuro M, Zhou YT, Lee Y, Unger RH. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J Biol Chem. 1998;273:3547–3550. doi: 10.1074/jbc.273.6.3547. [DOI] [PubMed] [Google Scholar]
- 31.Harmon JS, Gleason CE, Tanaka Y, Poitout V, Robertson RP. Antecedent hyperglycemia, not hyperllipidemia, is associated with increased islet triacylglycerol content and decreased insulin gene mRNA level in Zucker Diabetic Fatty rats. Diabetes. 2001;50:2481–2486. doi: 10.2337/diabetes.50.11.2481. [DOI] [PubMed] [Google Scholar]
- 32.Prentki M, Corkey BE. Are the ß-cell signaling molecules malonyl-CoA and cytosolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes. 1996;45:273–283. doi: 10.2337/diab.45.3.273. [DOI] [PubMed] [Google Scholar]
- 33.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278:30015–30021. doi: 10.1074/jbc.M302548200. [DOI] [PubMed] [Google Scholar]
- 34.Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335(Pt 3):465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 36.Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, Overbergh L, Mathieu C, Lupi R, Hai T, Herchuelz A, Marchetti P, Rutter GA, Eizirik DL, Cnop M. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 38.Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 39.Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–2474. doi: 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- 40.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donath MY, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and β-cell biology: from concept to clinical translation. Endocr Rev. 2008;29:334–350. doi: 10.1210/er.2007-0033. [DOI] [PubMed] [Google Scholar]
- 42.Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 43.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh N, Cnop M, Kharroubi I, Bugliani M, Lupi R, Marchetti P, Eizirik DL. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes millieu to human pancreatic islets? Diabetes. 2005;54:3238–3244. doi: 10.2337/diabetes.54.11.3238. [DOI] [PubMed] [Google Scholar]
- 45.Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci USA. 2004;101:8183–8143. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 47.Butler AE, Janson J, Wonner-Weir S, Ritzel R, Rizza RA, Butler PC. β -cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 48.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;293:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler AE, Janson J, Soeller WC, Butler PC. Increased β-cell apoptosis prevents adaptive increase in β-cell mass in mouse model of type 2 diabetes. Diabetes. 2003;52:2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 50.Zraika S, Hull RL, Udayasankar J, Aston-Mourney K, Subramanian SL, Kisilevsky R, Szarek WA, Kahn SE. Oxidative stress is induced by islet amyloid formation and time-dependently mediate amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hull RL, Zraika S, Udayasankar J, Aston-Mourney K, Subramanian SL, Kahn SE. Amyloid formation in human IAPP transgenic mouse islets and pancreas, and human pancreas, is not associated with endoplasmic reticulum stress. Diabetologia. 2009 doi: 10.1007/s00125-009-1329-4. .Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes. 2008;57:938–44. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC, HOPE Study. MICRO-HOPE Study Diabetes Care. 2002;25:1919–1927. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]