Abstract
Warnericin RK is the first antimicrobial peptide known to be active against Legionella pneumophila, a pathogen bacterium that is responsible for severe pneumonia. Strikingly, this peptide displays a very narrow range of antimicrobial activity, almost limited to the Legionella genus, and a hemolytic activity. A similar activity has been described for δ-lysin, a well-known hemolytic peptide of Staphylococci that has not been described as antimicrobial. In this study we aimed to understand the mode of action of warnericin RK and to explain its particular target specificity. We found that warnericin RK permeabilizes artificial membranes in a voltage-independent manner. Osmotic protection experiments on erythrocytes showed that warnericin RK does not form well-defined pores, suggesting a detergent-like mode of action, as previously described for δ-lysin at high concentrations. Warnericin RK also permeabilized Legionella cells, and these cells displayed a high sensitivity to detergents. Depending on the detergent used, Legionella was from 10- to 1000-fold more sensitive than the other bacteria tested. Finally, the structure of warnericin RK was investigated by means of circular dichroism and NMR spectroscopy. The peptide adopted an amphiphilic α-helical structure, consistent with the proposed mode of action. We conclude that the specificity of warnericin RK toward Legionella results from both the detergent-like mode of action of the peptide and the high sensitivity of these bacteria to detergents.
Introduction
Antimicrobial peptides (AMPs) are ubiquitous molecules that are part of the intrinsic defenses of most organisms (1–5). In multicellular organisms, AMPs such as defensins and cathelicidins provide a coordinated protective response against infection, and they are a principal component of innate immunity in vertebrates. AMPs likely help unicellular organisms struggle against competitor species. AMPs are classified on the basis of their primary structure, biochemical features, or spectrum of activity. They act against bacteria by permeabilizing the membrane or inhibiting metabolism (6). Many studies have focused on peptide-membrane interactions, leading to two distinct mechanisms (7,8). First, in the toroidal or barrel-stave pore models, the peptides form well-defined transmembrane pores. Second, in the carpet or detergent-like models, peptide monomers or micelles interact with and translocate across the membrane, leading to transient permeabilization. At high concentration, the membrane is disrupted, leading to cell lysis. In this model, even peptides that are too short to form transmembrane pores might permeabilize the membrane. We recently identified three peptides, produced by the Staphylococcus warneri RK strain, that are active against Legionella (9). To our knowledge, they are the first anti-Legionella peptides to be described. Legionella pneumophila is the pathogenic bacterium responsible for legionellosis. Legionella is found in freshwater environments and eventually infects humans who inhale contaminated droplets (10,11). Two of these three anti-Legionella peptides belong to the δ-lysin family, and one—warnericin RK—had not been described before, in the literature. δ-Lysins are peptides that are produced by various Staphylococcal strains and possess a hemolytic activity (12). Warnericin RK does not share high sequence similarity with other peptides. Of interest, the three peptides display a very narrow spectrum of activity, almost limited to the Legionella genus. However, δ-lysins had not been described to be active against bacteria (13,14). Furthermore, warnericin RK has been shown to have a hemolytic activity (9). Although warnericin RK and δ-lysins do not share a high similarity in their primary sequence, they do share biological activities. As δ-lysin activity has been extensively studied (for review, see Verdon et al. (15)), it could be useful to understand warnericin RK's mode of action. δ-Lysin is a linear amphiphilic α-helical peptide (22 aa) that interacts with membranes and, depending on the concentration, leads to permeabilization or cell lysis (16). It has been proposed that δ-lysin has a detergent-like mode of action (7). Some membrane features, such as the presence of liquid-disordered domains, phospholipid acyl chain structure, and elastic properties, particularly influence the interaction with δ-lysin (17–19).
In an attempt to unravel the mode of action of warnericin RK and understand its narrow spectrum of activity, we studied its activity on artificial membranes and on L. pneumophila cells. The antimicrobial activity of warnericin RK and several detergents were compared for various bacteria. Finally, the structure of warnericin RK was examined by means of circular dichroism (CD) and NMR.
Materials and Methods
Bacterial strains, growth conditions, and antimicrobials
The bacterial strains used in this study are listed in Table 1. Legionella strains were routinely cultured in buffered yeast extract (BYE) broth supplemented with l-cysteine (0.4 g/L) and ferric pyrophosphate (0.25 g/L) or on buffered charcoal yeast extract (BCYE) agar plates for 4 days. Solid medium was obtained by adding charcoal (2 g/L) and agar (15 g/L) to nonsterilized BYE. Nonlegionellae bacteria were grown on brain heart infusion (BHI) agar plates. Cultures were incubated in BHI broth at 37°C for 24–96 h under agitation (150 rpm). Synthetic warnericin RK, with the amino acid sequence MQFITDLIKKAVDFFKGLFGNK, was purchased from GenScript Corporation (Piscataway, NJ).
Table 1.
Minimum inhibitory concentration of various detergents against L. pneumophila and selected bacterial strains
| SDS | Tween 20 | Tween 80 | CHAPS | Triton X-100 | |
|---|---|---|---|---|---|
| B. megaterium F04 | 0.007 | >1 | >1 | 0.7 | 0.004 |
| Escherichia coli MG1655 | 0.1 | >1 | >1 | 1 | >1 |
| Legionella longbeachae ATCC 33484 | 0.001 | 0.001 | 0.001 | 0.1 | 0.001 |
| L. pneumophila Lens | 0.001 | 0.001 | 0.001 | 0.1 | 0.0007 |
| Listeria monocytogenes EGDe | 0.007 | >1 | >1 | >1 | >1 |
| Staphylococcus aureus 971 | 0.01 | >1 | >1 | >1 | >1 |
| Salmonella typhimurium | >1 | >1 | >1 | 1 | >1 |
| Staphylococcus warneri RK | 0.01 | >1 | >1 | >1 | >1 |
All detergents were used up to 1% concentration. The results are the mean of three independent experiments.
Planar bilayer assays
The methods used for the lipid bilayer experiments have previously been described in detail (20). In the experimental setup, two compartments of a Teflon cell filled with 5 mL electrolyte solution were connected by a small circular hole with an area of ∼0.3 mm2. The black lipid bilayer membrane was made by painting a 1% (w/v) diphytanoyl phosphatidylcholine (DPPC)/n-decane solution (Avanti Polar Lipids, Alabaster, AL) across the hole. Warnericin RK was added from a concentrated stock solution (1 mg/mL) to the aqueous phase on both sides, bathing the black membrane. Ag/AgCl electrodes (with salt bridges) were connected in series to a voltage source and an in-house-made current-to-voltage converter for the electrical measurements. The amplified signal was recorded with a strip chart recorder. All salts were obtained from Merck (Darmstadt, Germany) or Sigma-Aldrich (St. Louis, MO) at analytical grade. The aqueous salt solutions were unbuffered and, if not noted otherwise, had a pH of ∼6. The temperature during all experiments was maintained at 20°C. The zero-current membrane potential measurements were performed as been described previously (21) by establishing a salt gradient starting from 0.1 M KCl across black membranes containing 10–1000 channels. Zero-current potentials were measured using a high-impedance electrometer (Keithley 617). Analysis of the zero current membrane potential was performed using the Goldman-Hodgkin-Katz equation.
Hemolysis assay and osmotic protection experiments
The hemolytic activity of warnericin RK and osmotic protection were measured by detection of released hemoglobin from human erythrocytes as described previously (22). Fresh human blood (4 × 1 mL) from a healthy volunteer was centrifuged (12000 × rpm, 1 min, 4°C) to collect erythrocytes. The cells were washed with saline buffer (0.9% NaCl, 5 mM Tris, pH 7) until the supernatant became clear, pooled, and adjusted to a final volume of 30 mL with saline buffer. Reactions were performed in 1 mL mixtures containing 250 μL of the erythrocytes solution, 500 μL of saline buffer, and 250 μL of a variable amount of peptides or an equivalent volume of saline buffer. Serial twofold dilutions of peptides were performed in saline buffer and added to the mixtures. The reaction mixtures were incubated at 37°C for 30 min. Erythrocytes were removed by centrifugation (12000 × rpm, 1 min, 4°C) and the OD543 of the supernatant was measured by spectrophotometry. To determine 100% cell lysis, the erythrocytes were resuspended in 0.1% Triton X-100.
For osmotic protection, we used the following polyethyleneglycols (PEGs): PEG 200 (diameter 0.75 nm), PEG 400 (diameter 1.07 nm), PEG 600 (diameter 1.32 nm), PEG 1000 (diameter 1.72 nm), PEG 2000 (diameter 2.47 nm), PEG 4000 (diameter 3.54 nm), PEG 6000 (diameter 4.37 nm), PEG 8000 (diameter 5.04 nm), and PEG 10000 (diameter 5.70 nm) at a final concentration of 30 mM. Human erythrocytes were incubated with warnericin RK at 37°C for 30 min. Lysis of the human erythrocytes was quantified by hemoglobin release as determined at OD543. The results were expressed as a percentage of the total hemoglobin release as compared to 0.1% Triton X-100.
Detergent antibacterial activity
The minimal inhibitory concentration (MIC) of the detergents was determined by a microdilution susceptibility test as described below. Bacteria cells were inoculated in BYE or BHI broth to ∼106 CFU/mL. Bacterial suspensions were put into a sterile microtiter plate (Nunclon Delta Surface, Nunc, Illkirch, France). Serial 10-fold dilutions of detergents were performed in sterile water and added to bacterial suspensions at a starting concentration of 1%. Microtiter plates were incubated at 37°C for 24 or 96 h depending on the tested strain. The MIC was determined by monitoring the OD595 with a microtiter plate reader Sunrise (TECAN, Lyon, France).
Membrane permeabilization assays
L. pneumophila cells in the exponential growth phase (OD600 ∼0.5–0.8) were adjusted at 106 CFU/mL and treated with several concentrations of warnericin RK. Samples were incubated 45 min at 37°C. Then the cells were stained with a Live/Dead BacLight kit (Invitrogen, Cergy Pontoise, France) as recommended by the supplier. The two fluorescent dyes (SYTO 9 and propidium iodide (PI)) were added either alone or in combination to stain the cells. Staining was allowed for 15 min at room temperature in the dark. Flow cytometric measurements were performed on a FACSCanto II flow cytometer (BD Biosciences, Le Pont de Claix, France) with a 488 nm argon excitation laser. A total of 50,000 events were analyzed in each sample, using BD FACSDiVa 6 software (BD Biosciences) for data acquisition and analysis. Optical filters were set up such that PI fluorescence was measured at 670 LP nm and SYTO 9 fluorescence was measured at 530/30 BP nm. A SYTO 9+/PI-gate was drawn to determine the percentage of viable cells in the control, and the antimicrobial activity of warnericin RK was measured as the percentage of remaining nonpermeabilized bacteria with respect to the untreated control. All experiments were repeated at least three times, and the patterns were reproducible.
Preparation of small unilamellar vesicles
Deuterated 1.2-dimyristoyl-sn-glycero-3-phosphocholine d54 (DMPC) was purchased from Avanti Polar Lipids. DMPC dissolved in chloroform solution was first evaporated under nitrogen. The residual lipid film was resuspended in 10 mM sodium phosphate buffer to a final concentration of 10 mg/mL. Small unilamellar vesicles (SUVs) were prepared from this suspension by bath sonication for 15–30 min.
CD spectra
CD measurements were performed using a Jasco J-715 spectrometer. For the experiments, lyophilized warnericin RK was dissolved in the measurement buffer. CD spectra were recorded from solutions containing 39 μM warnericin RK in pure 10 mM sodium phosphate buffer at pH 5.2 and in the same buffer with 8% deuterated trifluoroethanol (TFE-d3) added as cosolvent, as well as from solutions containing 50 μM warnericin RK and deuterated DMPC in molar ratios of warnericin RK/DMPC of 1:1, 1:2.5, 1:5, and 1:10 in 10 mM sodium phosphate buffer at pH 5.5.
To determine the secondary structure content of warnericin RK by decomposition of the measured CD spectra, CDSSTR analysis software was used via the DichroWeb server (DichroWeb—On Line Circular Dichroism Analysis, http://www.cryst.bbk.ac.uk/cdweb/html/home.html).
NMR spectra
Three different samples were used for NMR spectroscopy of warnericin RK in pure buffer solution, in buffer with 8% TFE, and in a DMPC SUVs solution. For all samples, 10 mM sodium phosphate solution at pH 5.5 with 10% D2O was used as buffer.
Correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY), and nuclear Overhauser effect spectroscopy (NOESY) NMR spectra from a 1.3 mM solution of warnericin RK in the pure aqueous buffer were acquired on a Bruker Avance 800 MHz spectrometer with a cryo-probehead (Bruker Biospin, Rheinstetten, Germany).
COSY, TOCSY, and NOESY NMR spectra of the sample containing 1.4 mM warnericin RK and 13.6 mM DMPC SUVs were measured on a Bruker Avance 750 MHz wide-bore spectrometer equipped with a TXI probehead.
To obtain the spectra of warnericin RK (1.7 mM) in a buffer containing 8% TFE-d3 as cosolvent, COSY, TOCSY, and two NOESY spectra with different mixing times (150 ms and 300 ms) were acquired on a Bruker Avance 600 MHz with a TXI probehead. The concentration of 8% TFE was chosen from an NMR titration, representing the concentration at which line-broadening was substantially reduced but changes in the chemical shift of individual resonances were still minimal.
1H resonances of the NMR spectra of warnericin RK in 8% TFE solution were assigned using NMRView and according to a standard sequential assignment procedure (23).
Structure calculations were performed with ARIA (version 1.2) (24). Structures were calculated from random starting conformations using standard restrained molecular-dynamics and simulated-annealing protocols. A single ARIA run consisted of eight iterations. In each iteration, 100 structures were calculated, and in the final iteration the 20 structures with the lowest energy were selected for further analysis.
Results
Interaction of warnericin RK with lipid bilayer membranes
To provide the basis for understanding the interaction of warnericin RK with Legionella cells, we analyzed its ability to influence the conductance of planar bilayer membranes. Black lipid membranes were formed from 100% zwitterionic lipid (DPPC) dissolved in n-decane, and warnericin RK was added in low concentration to one or both sides of black lipid bilayer membranes. The results suggest that warnericin RK was able to increase the conductance of the model membranes by several orders of magnitude. Of interest, in contrast to measurements with other cationic peptides (1,24), an increase in conductance was observed at very low transmembrane voltages, which means that voltages of 10 or 20 mV were sufficient to detect conductance increase of the membranes (data not shown).
Studies on the single-channel level
To determine whether warnericin RK is a pore-forming compound, we repeated the membrane experiments at low peptide concentration and high current resolution to observe single-channel events. A low concentration of warnericin RK (0.4 μM) was added to a black lipid membrane in 1 M KCl while stirring to allow equilibration; ∼1 min later, the membrane conductance started to increase (Fig. 1). The conductance increase occurred partially in small steps, but also sometimes continuously, indicating that, rather than forming pores, warnericin RK destabilizes the membrane. Fig. 2 shows a distribution of single events measured in experiments similar to that shown in Fig. 1. The histogram indicates that warnericin RK induced conductance over a considerable conductance range from 100 to ∼900 pS, with a maximum at 300 pS. Measurements were also performed at a KCl concentration of 0.1 M. Under these conditions, it was more or less impossible to detect single-channel events. However, the conductance increase was smoother than at 1 M KCl, suggesting that the conductance was much smaller at 0.1 M KCl.
Figure 1.
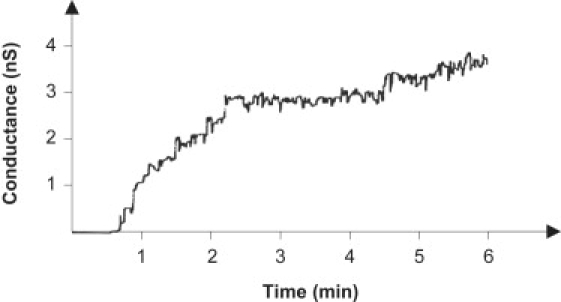
Single-channel analysis of warnericin RK in black lipid membranes, showing the increase of the membrane current (expressed as nS) after addition of warnericin RK at 0.4 μM. The aqueous phase contained 1 M KCl. The membrane was formed from DPPC/n-decane. The voltage applied was 20 mV; T = 20°C.
Figure 2.
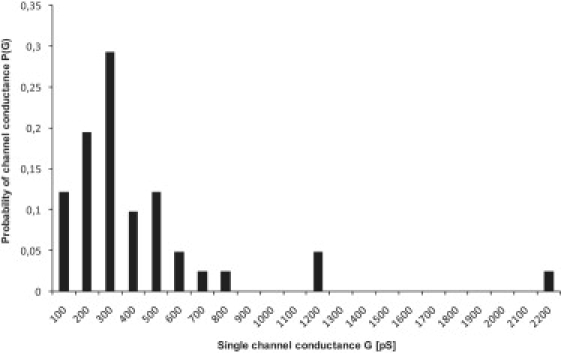
Histogram of the probability for the occurrence of a given conductivity unit observed with membranes formed of DPPC/n-decane in the presence of warnericin RK. P(G) indicates the probability with which a given conductance increment G was observed in the single-channel experiments. It was calculated by dividing the number of transitions with a given conductance increment by the total number of conductance fluctuations. The average single-channel conductance of the right-hand maximum was ∼500 pS for 70 conductance fluctuations. The aqueous phase contained 1 M KCl and 0.4 μM warnericin RK, and the applied voltage was 20 mV; T = 20°C.
Voltage dependence of warnericin RK's conductance
High voltages tend to induce the peptide-mediated conductance of defensin-like molecules, particularly when the cis side (the side of the addition of the peptides) has a positive voltage, because the electric field tends to force the cationic peptides into the membrane (4,25,26). Surprisingly, the electric field strength had almost no influence on warnericin RK-induced membrane conductance, as Fig. 3 clearly indicates. Up to ∼60 mV, a linear current-voltage relationship was observed when warnericin RK was added to either one or both sides of neutral DPPC/n-decane membranes. At higher transmembrane voltage, the membranes became unstable and broke; therefore, it is not clear whether higher voltages influenced warnericin RK-induced membrane conductance.
Figure 3.
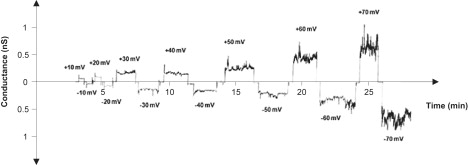
Current-voltage dependence of warnericin RK in multichannel experiments. Warnericin RK was added in a concentration of 0.4 μM to the 1 M KCl solution bathing a black DPPC/n-decane membrane. Then, 20 min after the addition of the peptide, most of the conductance increase was over and voltages of positive and negative sign were applied to the membrane (T = 20°C). Note that a linear current voltage curve was obtained in the experiments, indicating that the warnericin RK-induced conductance was not voltage-dependent.
Ionic selectivity of warnericin RK-induced conductance
Zero-current membrane potential measurements were performed to obtain further information on the structure of the channel formed by warnericin RK. To that end, membranes were formed in 0.1 M KCl, and warnericin RK was added to the aqueous phase to a concentration of 0.2 μM. Subsequently, the membrane conductance started to increase. After ∼20 min, the conductance nearly reached a steady state. At that time, the KCl concentration on one side of the membranes was increased fivefold, starting at 0.1 M, by adding concentrated KCl solution. The zero-current potentials were measured 5 min after every increase of the salt gradient across the membrane. For KCl the more diluted side of the membrane (0.1 M) always became positive, which indicated preferential movement of potassium ions through the membrane. This result indicated that warnericin RK made the membranes permeable for cations, in contrast to its positive net charge (data not shown). Analysis of the membrane potential using the Goldman-Hodgkin-Katz equation (21,27) allowed us to calculate the ratio of cation permeability divided by anion permeability, which was on average (four experiments) ∼2.5 for KCl.
Warnercin RK permeabilizes Legionella cells
Since warnericin RK permeabilized the model membranes, we tested whether warnericin RK could also permeabilize Legionella cells. The Baclight kit was used to assess the permeability of L. pneumophila. This kit employs two nucleic acid fluorochromes: the green-fluorescent SYTO 9 and the red-fluorescent PI. SYTO 9 labels every bacteria, whereas PI penetrates only bacteria with damaged membranes. L. pneumophila were treated for 45 min with various warnericin RK concentrations, stained with the Baclight kit, and analyzed by flow cytometry. The results show that PI penetrated treated cells, suggesting permeabilization (Fig. 4). Furthermore, the number of permeabilized (i.e., PI-positive) cells increased with warnericin RK concentrations. The concentrations necessary to induce permeabilization of both Legionella and artificial membranes are in the micromolar range. Thus, warnericin RK is rather efficient as compared to other AMPs whose MICs range from 0.3 to 50 μM (28).
Figure 4.
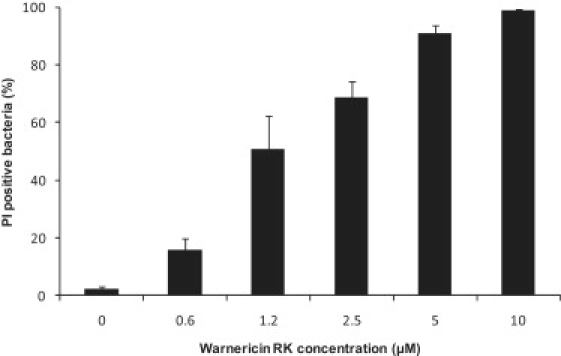
Membrane permeabilization of L. pneumophila by warnericin RK. A flow cytometry assay of L. pneumophila membrane permeabilization by warnericin RK is shown. Exponential cells (106 CFU/mL) were incubated at various warnericin RK concentrations for 45 min at 37°C. Cells were stained with the Baclight kit (SYTO 9 and PI) and analyzed by flow cytometry. PI penetrates in membrane-damaged cells. A total of 50,000 events were recorded for each sample. Results represent the mean of three independent experiments.
Osmotic protection experiments
We previously showed that warnericin RK lyses erythrocytes (9). To estimate the pore size induced by warnericin RK, we performed osmotic protection experiments. Fig. 5 A shows the dose response curve of the hemolytic activity of warnericin RK on human erythrocytes. Hemolytic activity on human erythrocytes started at a low concentration of ∼0.8 μM, and total hemolysis was obtained at a concentration of 200 μM. This concentration was used in the osmotic protection experiments. Such experiments are based on the fact that incubation of the human erythrocytes with the peptide may cause channels of a given size. This allows the rapid exchange of solutes across the membrane, and since the colloid osmotic pressure is no longer balanced, the cells will swell and lyse. Nonpermeating molecules are able to balance the osmotic pressure and thereby protect the cells from lysis. In contrast, molecules that permeate the channels do not balance the osmotic stress, which leads to cell lysis. Human erythrocytes were suspended either in saline solution or in saline solution supplemented with 30 mM PEGs of different molecular masses. The cells were incubated with 200 μM warnericin RK for 30 min and then centrifuged for 1 min. Finally, OD543 was measured in the supernatant. PEGs 200–4000 (3.54 nm) were not able to protect human erythrocytes from lysis (Fig. 5 B). Only partial protection was obtained at higher molecular masses of PEG, ranging from 4000 to 10,000 Da (corresponding to diameters of 3.54–5.70 nm). This result suggests that warnericin RK did not form pores with defined sizes.
Figure 5.
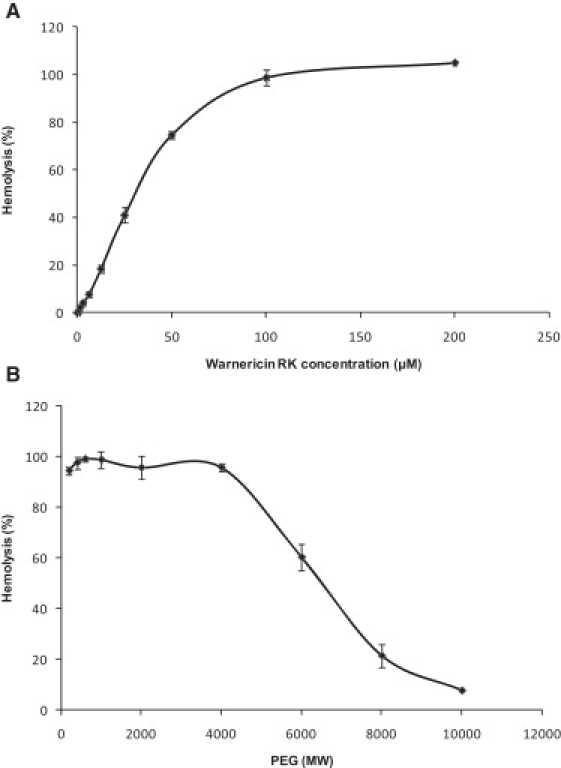
Osmotic-protection analyses with warnericin RK shown as a function of the molecular mass of the PEGs. (A) Hemolysis as a function of warnericin RK concentration was first established to determine which concentration corresponded to 100% hemolysis. (B) This concentration (i.e., 200 μM) was used in the osmotic-protection assays. Hemolytic activity was measured as OD543 relative to total lysis of the erythrocytes in 0.1% Triton X-100, which was set to 100%. Human erythrocytes were incubated with the peptide at 37°C in saline solution (control) or in saline solution supplemented with 30 mM of PEGs of different molecular masses (PEG 200, 400, 600, 1000, 2000, 4000, 6000, 8000, and 10,000) for 30 min. Data are the means ± standard deviations of two independent experiments.
Detergent sensitivity
Given the previous results and the similarity between warnericin RK and δ-lysin, we hypothesized that warnericin RK might have a detergent-like mode of action. If this were true, Legionella should be particularly sensitive to detergents compared to other bacteria, which would explain why warnericin RK is specifically active against the Legionella genus. To test this hypothesis, we assessed L. pneumophila's sensitivity to various detergents (SDS (ionic), Tween 20 (nonionic), Tween 80 (nonionic), CHAPS (zwitterionic), and Triton X-100 (nonionic)). Their MIC was measured for several Gram-negative and Gram-positive bacteria. The results show that the two strains of Legionella were highly sensitive to detergents as compared to the other bacteria (Table 1). Depending on the detergent used, Legionella were 10-fold (CHAPS) to >1000-fold (Tween and Triton X-100) more sensitive than other bacteria. Bacillus megaterium displays an intermediate phenotype because this strain is more sensitive to SDS and Triton X-100 than most bacteria. Of interest, it has been shown that warnericin RK has a slight activity against this B. megaterium strain (9). Taken together, these results indicate that there is a strong correlation between warnericin RK and detergent sensitivity, suggesting that warnericin RK could have a detergent-like mode of action.
Structure of warnericin RK
Since warnericin RK and δ-lysin share similar activity, we investigated the structure of warnericin RK by CD and NMR spectroscopy to compare it with the known structure of δ-lysin (29,30). CD spectroscopy suggested that the peptide did not have a defined secondary structure in aqueous solution (Fig. 6 A). In a membrane-like environment, mimicked by addition of DMPC vesicles, a defined α-helical secondary structure was formed. At an equimolar concentration of DMPC, a >50% helical content was found, which increased only slightly for higher DMPC concentrations up to a 10-fold excess. A similar CD spectrum indicating a nearly identical secondary structure was observed for the peptide in the presence of 8% TFE (Fig. 6 B). Thus we concluded that, similarly to DMPC, 8% TFE mimics the membrane environment and is suitable for investigating the peptide structure.
Figure 6.
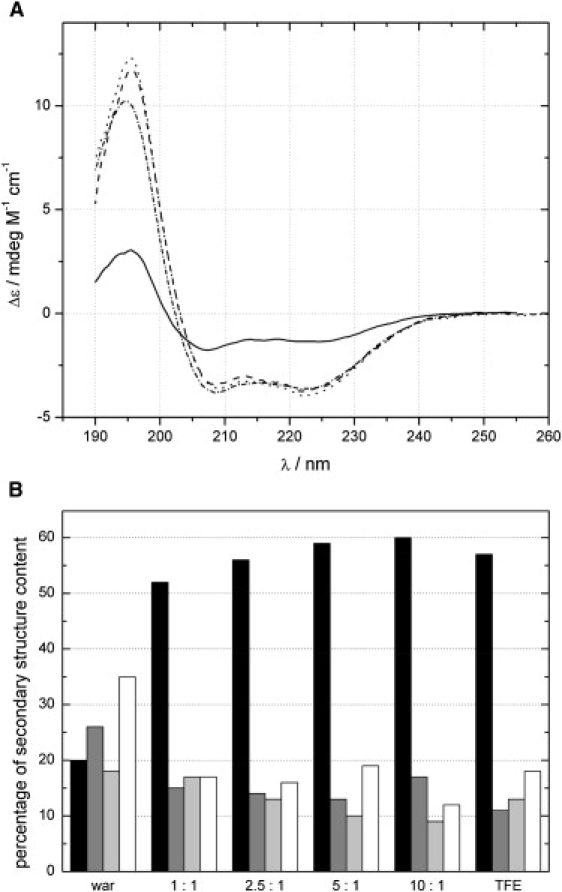
CD spectroscopy of warnericin RK. (A) CD spectra of pure warnericin RK (solid line), with 8% TFE (dashed-dotted line) and in the presence of DMPC (1:2.5, dashed line; 1:10, dotted line). (B) Content of secondary structure as derived from the CD spectra. Black bars: helical; dark gray bars: extended conformation; light gray bars: turn; empty bars: random coil.
The results obtained from CD spectra were well confirmed by NMR spectroscopy. Warnericin RK in aqueous solution displayed broadened resonance lines in 1D NMR spectra (see Fig. S1 in the Supporting Material). Only a few cross peaks were observed in the 2D spectra, suggesting that the peptide was subjected to fast structural dynamics, which strongly affected the NMR relaxation times. In the presence of a 10-fold excess of DMPC, the resonance lines of the peptide were extremely broadened (Fig. S1). In all of the 2D spectra, only weak diagonal peaks and no cross peaks were observed. These observations suggest that molecular tumbling of the peptide was severely restricted by binding to the large DMPC vesicles. In contrast, warnericin RK in 8% TFE showed sharp resonance lines, allowing for the acquisition of well-resolved 2D NMR spectra. From the NOESY spectra, 238 cross peaks were identified and used for the structure calculation, which revealed a well-defined α-helix extending from residue 4 to residue 16 (Fig. 7 A and Table 2). The helix showed a pronounced amphiphilic character (Fig. 7 B), suggesting that the peptide is embedded in the membrane with its hydrophobic side. Other structural details, such as the exact length of the helix or the curvature, were not further investigated, since these depend on the actual environment and may vary slightly in different model systems. The common and important structural feature is amphiphilicity, which renders the peptide capable of destabilizing membranes.
Figure 7.
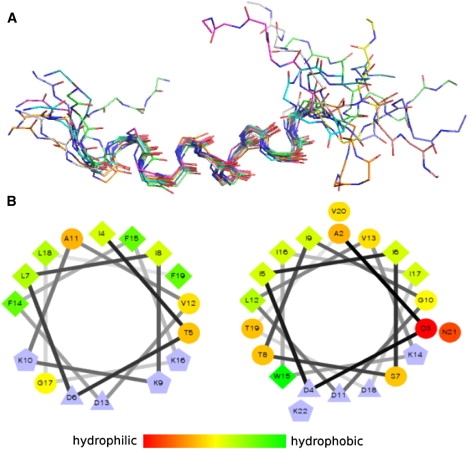
Structural model of warnericin RK in a membrane-like environment. (A) Superposition of the 10 lowest-energy structures calculated from the NMR data acquired in the presence of 8% TFE. (B) Helix wheel representation shows the amphiphilic character of warnericin RK residues 4–16 (left) and δ-lysin residues 2–21 (right). Hydrophobicity according to the color bar (charged residues are colored blue). Symbols: circles, hydrophilic; diamonds, hydrophobic; triangles, negatively charged; pentagons, positively charged.
Table 2.
NOE statistics and average root mean-square deviation (RMSD) in angstroms from the average structure for the 20 lowest-energy structures
| Distance restraints from 238 NOEs | |
| Intraresidual | 127 |
| Sequential | 70 |
| Medium range | 41 |
| NOE violations | |
| > 0.5 Å | 0 |
| > 0.3 Å | 0.2 ± 0.5 |
| > 0.1 Å | 1.3 ± 1.3 |
| RMSD values in angstroms | |
| All backbone atoms | 3.9 ± 0.9 |
| All heavy atoms | 4.0 ± 0.9 |
| Backbone atoms residues 4–16 | 0.6 ± 0.3 |
| Heavy atoms residues 4–16 | 1.3 ± 0.3 |
Discussion
Warnericin RK is an AMP that is produced by S. warneri RK, acts specifically on the Legionella genus, and possesses hemolytic activity (9). The S. warneri RK strain also produces two δ-lysins that display anti-Legionella and hemolytic activities. To understand the specificity against Legionella, we decided to study the structure and function of warnericin RK. Planar lipid bilayer studies with warnericin RK indicated that the peptide interacts with model lipid membranes, suggesting that this peptide is membrane active. At low concentration (0.4 μM), warnericin RK increased conductance of a black lipid membrane, whereas at high concentration (>2 μM) the peptide induced membrane disruption. Similar observations were reported previously for δ-lysin, which induces permeabilization in the concentration range of 0.1–2 μM (31). The conductance ranges from 100 to 900 pS with warnericin RK and from 70 to 450 pS with δ-lysin (31). In contrast to measurements with other AMPs, such as the lantibiotics or the defensins with high cationic charge densities, which need high transmembrane voltages for membrane activity (4,25,26), warnericin RK is active with lipid bilayer membranes at a relatively low voltage. δ-Lysin also shows a voltage-independent activity when the voltage is maintained below 60 mV, suggesting that both warnericin RK and δ-lysin permeabilize the membrane even in the absence of an applied potential. Membrane activity at low transmembrane potentials is most likely also the reason for lysis of erythrocytes by warnericin RK. Nevertheless, the conductance events observed for warnericin RK varied widely in magnitude and duration of existence, similarly to other AMPs. Warnericin RK displayed a cation selectivity (permeability ratio: 2.5) that has also been observed for δ-lysin (31,32). Altogether, the results from the conductance experiments with warnericin RK were similar to the previous observations made with δ-lysin.
In addition to the interaction with model membranes, we studied the effect of warnericin RK on L. pneumophila using a fluorochrome (PI) that penetrates membrane-damaged bacteria. The results clearly demonstrate that warnericin RK rapidly permeabilized these bacteria in a concentration-dependent manner. Moreover, warnericin RK permeabilized black lipid membrane and bacteria in a similar concentration range, whereas hemolysis required a higher concentration. It has been suggested that, with δ-lysin, a low concentration induces channel formation and a high concentration induces hemolysis (32). At high concentration, δ-lysin seems to induce a detergent-like solubilization of the membrane (7). This hypothesis is consistent with our results regarding osmotic protection with warnericin RK. Indeed, a high peptide concentration was used and the erythrocytes were not fully protected by PEG, even with the highest molecular mass (PEG 10,000). These results indicate that, at this concentration, warnericin RK forms large channels of various sizes, in a manner similar to the action of detergents. This means that warnericin RK likely has a detergent-like mode of action. The peptides self-associate and transiently destabilize the membrane. At higher concentrations, this destabilization can lead to cell lysis.
Furthermore, we found that Legionella is more sensitive to detergent (by 10- to 1000-fold) compared to other genera, which is fully consistent with a putative detergent-like mode of action for warnericin RK. Consequently, we conclude that Legionella is highly sensitive to warnericin RK, because these bacteria are highly sensitive to detergents in general. A feature of membrane composition may explain this high sensitivity of Legionella as compared to other bacteria. It has been reported that L. pneumophila membrane is rich in branched-chain fatty acids (33). Furthermore, δ-lysin activity is dependent of the phospholipid acyl chain structure or elastic properties of the membrane (19). Thus, a possible explanation is that a high rate of branched-chain fatty acids modifies the elastic properties of the membrane and thereby increases the sensitivity to warnercin RK.
The destabilizing mode of action of warnericin RK is further corroborated by our structural data. The central part of the peptide forms a nearly perfect amphiphilic helix, as also observed for δ-lysin (29) (Fig. 7 B). In contrast to δ-lysin, however, warnericin RK is four residues shorter and does not form an extended helix over its full length. Therefore, the structural data suggest that the peptide interacts with the membrane in a detergent-like manner, rather than forming well-defined, pore-forming multimers. However, spectroscopic methods require micro- and millimolar concentrations, and therefore do not exclude channel formation in the submicromolar range as observed in the conductivity measurements.
In conclusion, we have provided ample evidence that warnericin RK acts as a specific anti-Legionella AMP by permeabilizing the bacterial membrane. The high specificity and activity against Legionella results on the one hand from the detergent-like mode of action of the peptide, and on the other hand from the particular detergent-sensitivity of this pathogen. Warnericin RK is therefore a promising candidate for a novel specific drug against Legionella.
Supporting Material
A figure is available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(09)01281-8.
Supporting Material
Acknowledgments
We thank Prof. Franck Morel (University of Poitiers) for technical assistance with the flow cytometry, Prof. Paul Rösch (University of Bayreuth) for access to his NMR spectrometers, and Jean-Marc Berjeaud and Christian Lacombe (University of Poitiers) for fruitful scientific discussions.
This work was supported by grants from the Agence Française de Sécurité Sanitaire de l'Environnement et du Travail (ARCL-2005-005 to Y.H.), the Deutsche Forschungsgemeinschaft (SFB630 projects B1 to M.S., and C2 to C.F.), and the Fonds der Chemischen Industrie (to R.B.). J.V. was supported by a grant from the French Minister of Research.
References
- 1.Aerts A.M., Francois I.E., Cammue B.P., Thevissen K. The mode of antifungal action of plant, insect and human defensins. Cell. Mol. Life Sci. 2008;65:2069–2079. doi: 10.1007/s00018-008-8035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowdish D.M., Davidson D.J., Hancock R.E. Immunomodulatory properties of defensins and cathelicidins. Curr. Top. Microbiol. Immunol. 2006;306:27–66. doi: 10.1007/3-540-29916-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock R.E., Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 4.Kagan B.L., Ganz T., Lehrer R.I. Defensins: a family of antimicrobial and cytotoxic peptides. Toxicology. 1994;87:131–149. doi: 10.1016/0300-483x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 5.Lehrer R.I. Primate defensins. Nat. Rev. Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 6.Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 7.Bechinger B., Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 9.Verdon J., Berjeaud J.M., Lacombe C., Hechard Y. Characterization of anti-Legionella activity of warnericin RK and δ-lysin I from Staphylococcus warneri. Peptides. 2008;29:978–984. doi: 10.1016/j.peptides.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Fields B.S., Benson R.F., Besser R.E. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinert M., Hentschel U., Hacker J. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol. Rev. 2002;26:149–162. doi: 10.1111/j.1574-6976.2002.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman G.M. The hemolysins of Staphylococcus aureus. Bacteriol. Rev. 1975;39:317–344. doi: 10.1128/br.39.4.317-344.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhople V.M., Nagaraj R. δ-Toxin, unlike melittin, has only hemolytic activity and no antimicrobial activity: rationalization of this specific biological activity. Biosci. Rep. 1993;13:245–250. doi: 10.1007/BF01123506. [DOI] [PubMed] [Google Scholar]
- 14.Kreger A.S., Kim K.S., Zaboretzky F., Bernheimer A.W. Purification and properties of staphylococcal δ hemolysin. Infect. Immun. 1971;3:449–465. doi: 10.1128/iai.3.3.449-465.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdon J., Girardin N., Lacombe C., Berjeaud J.M., Hechard Y. δ-Hemolysin, an update on a membrane-interacting peptide. Peptides. 2009;30:817–823. doi: 10.1016/j.peptides.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Thelestam M. Membrane damage by staphylococcal α-toxin to different types of cultured mammalian cell. Biochim. Biophys. Acta. 1983;762:481–488. doi: 10.1016/0167-4889(83)90050-2. [DOI] [PubMed] [Google Scholar]
- 17.Pokorny A., Almeida P.F. Permeabilization of raft-containing lipid vesicles by δ-lysin: a mechanism for cell sensitivity to cytotoxic peptides. Biochemistry. 2005;44:9538–9544. doi: 10.1021/bi0506371. [DOI] [PubMed] [Google Scholar]
- 18.Pokorny A., Birkbeck T.H., Almeida P.F. Mechanism and kinetics of δ-lysin interaction with phospholipid vesicles. Biochemistry. 2002;41:11044–11056. doi: 10.1021/bi020244r. [DOI] [PubMed] [Google Scholar]
- 19.Pokorny A., Kilelee E.M., Wu D., Almeida P.F. The activity of the amphipathic peptide δ-lysin correlates with phospholipid acyl chain structure and bilayer elastic properties. Biophys. J. 2008;95:4748–4755. doi: 10.1529/biophysj.108.138701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benz R., Janko K., Boos W., Lauger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta. 1978;511:305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- 21.Benz R., Janko K., Lauger P. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta. 1979;551:238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig A., Schmid A., Benz R., Goebel W. Mutations affecting pore formation by haemolysin from Escherichia coli. Mol. Gen. Genet. 1991;226:198–208. doi: 10.1007/BF00273604. [DOI] [PubMed] [Google Scholar]
- 23.Wütrhrich K. Wiley; New York: 1986. NMR of Proteins and Nucleic Acids. [Google Scholar]
- 24.Linge J.P., Habeck M., Rieping W., Nilges M. ARIA: automated NOE assignment and NMR structure calculation. Bioinformatics. 2003;19:315–316. doi: 10.1093/bioinformatics/19.2.315. [DOI] [PubMed] [Google Scholar]
- 25.Duclohier H., Wroblewski H. Voltage-dependent pore formation and antimicrobial activity by alamethicin and analogues. J. Membr. Biol. 2001;184:1–12. doi: 10.1007/s00232-001-0077-2. [DOI] [PubMed] [Google Scholar]
- 26.Sahl H.G., Kordel M., Benz R. Voltage-dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch. Microbiol. 1987;149:120–124. doi: 10.1007/BF00425076. [DOI] [PubMed] [Google Scholar]
- 27.Benz R., Schmid A., Hancock R.E. Ion selectivity of Gram-negative bacterial porins. J. Bacteriol. 1985;162:722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melo M.N., Ferre R., Castanho M.A. Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009;7:245–250. doi: 10.1038/nrmicro2095. [DOI] [PubMed] [Google Scholar]
- 29.Lee K.H., Fitton J.E., Wuthrich K. Nuclear magnetic resonance investigation of the conformation of δ-haemolysin bound to dodecylphosphocholine micelles. Biochim. Biophys. Acta. 1987;911:144–153. doi: 10.1016/0167-4838(87)90003-3. [DOI] [PubMed] [Google Scholar]
- 30.Tappin M.J., Pastore A., Norton R.S., Freer J.H., Campbell I.D. High-resolution 1H NMR study of the solution structure of δ-hemolysin. Biochemistry. 1988;27:1643–1647. doi: 10.1021/bi00405a038. [DOI] [PubMed] [Google Scholar]
- 31.Mellor I.R., Thomas D.H., Sansom M.S. Properties of ion channels formed by Staphylococcus aureus δ-toxin. Biochim. Biophys. Acta. 1988;942:280–294. doi: 10.1016/0005-2736(88)90030-2. [DOI] [PubMed] [Google Scholar]
- 32.Kerr I.D., Dufourcq J., Rice J.A., Fredkin D.R., Sansom M.S. Ion channel formation by synthetic analogues of staphylococcal δ-toxin. Biochim. Biophys. Acta. 1995;1236:219–227. doi: 10.1016/0005-2736(95)00051-4. [DOI] [PubMed] [Google Scholar]
- 33.Finnerty W.R., Makula R.A., Feeley J.C. Cellular lipids of the Legionnaires' disease bacterium. Ann. Intern. Med. 1979;90:631–634. doi: 10.7326/0003-4819-90-4-631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


