Abstract
Purpose
To investigate the changes of pattern electroretinogram (PERG) after intraocular pressure lowering in glaucoma patients and normal controls.
Design
Interventional retrospective cross-sectional study.
Participants
Twenty-five patients (49 eyes) with ocular hypertension or glaucoma undergoing topical treatment to lower IOP served as a study group; 22 patients (44 eyes) with ocular hypertension or glaucoma observed without treatment served as a control group for treated glaucoma patients; 9 normal subjects (18 eyes) receiving a 250-mg acetazolamide tablet served as a second study group; and 17 normal subjects (34 eyes) from a previous study served as a second control group for treated normal subjects.
Method
Pattern electroretinograms were recorded simultaneously from both eyes using skin electrodes and automated analysis. Visual field (VF) analyses were performed with white-on-white standard automated perimetry (SAP). Intraocular pressure was measured with Goldmann applanation tonometry; central corneal thickness was measured with pachymetry.
Main Outcome Measures
Pattern electroretinogram amplitude (microvolts), phase (π rads), and test–retest variability (test 2–to–test 1 ratio, in decibels), SAP mean deviation (decibels), and IOP (millimeters of mercury).
Results
In 56% of right eyes and 21% of left eyes of the treated glaucoma subgroup, the PERG amplitude and/or phase improved beyond the 95% confidence intervals of the test–retest variability of the untreated glaucoma control group. Pattern electroretinogram improvement with IOP lowering occurred in both high- and low-tension glaucoma eyes. Eyes with severely impaired VFs showed little improvement in PERG; however, eyes of normal subjects treated with acetazolamide did not show significant PERG changes relative to the test–retest variability of normal controls.
Conclusions
Retinal ganglion cell function can be at least partially restored after IOP reduction in glaucomatous eyes with early VF impairment.
Glaucoma is an optic neuropathy characterized by degeneration of retinal ganglion cells (RGCs) resulting in progressive loss of vision. The current opinion is that structural changes of the optic nerve fiber layer precede the appearance of visual dysfunction, as measured by standard automated perimetry (SAP).1,2 Alternative hypotheses cannot be excluded, however.3 An appealing alternative hypothesis is that, before irreversible cell death occurs, RGCs undergo a stage of reversible dysfunction due to intraocular pressure (IOP) elevation and, possibly, other factors that remain unknown. If IOP is a major cause of dysfunction, then lowering it should result in an improvement of RGC function.
Retinal ganglion cell function can be evaluated objectively by means of the pattern electroretinogram (PERG). The PERG is a special kind of electroretinogram that uses a stimulus of contrast-reversal gratings rather than uniform flashes of light. Retinal ganglion cell death and/or RGC dysfunction can alter the waveform of the PERG. Several studies in different experimental mammals with optic nerve lesions causing retrograde RGC degeneration4–7 and several case reports of human patients with corresponding clinical conditions8–10 have demonstrated a strong correlation between the PERG losses and RGC losses. A linear correlation between the PERG loss and RGC loss has also been reported in monkeys with experimental glaucoma.11 An important characteristic of the PERG is that it requires physiologic integrity of viable RGCs to be generated. The PERG amplitude is much reduced during a transient blockade of RGC spiking activity induced by intravitreal injections of tetrodotoxin in cats and monkeys.12,13 The PERG amplitude also may be reduced reversibly in human subjects by a transient increase of the IOP to ≥30 mmHg14–16 with a suction cup or with body inversion. Thus, a reduction in PERG amplitude may represent the cumulative effects of RGC death and dysfunction of viable RGCs. Assuming that RGC dysfunction is due in part to IOP elevation, a reduction of IOP should improve RGC function, resulting in augmented PERG amplitude and/or shortening of PERG latency (advancement of PERG phase).
This is a retrospective study of patients with ocular hypertension or glaucoma followed up in the previous 2 years. We selected patients who had been candidates for treatment and had previous baseline PERG measurements. We were able to identify a subgroup of patients who received topical treatment to reduce IOP and another subgroup of patients with approximately the same mean age and clinical breakdown of the treated subgroup and who were still under observation before treatment. To facilitate the PERG technique, we utilized a recently developed user-friendly paradigm for PERG recording.17 In approximately 40% of eyes of the treated subgroup, the PERG amplitude increased and/or phase advanced beyond the test–retest variability of the nontreated glaucoma subgroup. This result suggests that, in some eyes with ocular hypertension or glaucoma, a population of viable RGCs has abnormal function and that RGC function may be restored, at least in part, by lowering the IOP.
Materials and Methods
Subjects
Patients were selected from a larger group of glaucomatous patients (n>300) observed with PERG.18 Candidates for treatment with pressure lowering who had 2 baseline PERGs and who were still under observation were used as a control group for test–retest variability without treatment. Patients who had one baseline PERG and another PERG after treatment were used as a study group (test–retest with treatment).
Study Group
Forty-nine eyes of 25 patients (mean age, 65±12 years) received topical treatment for glaucoma based on standard clinical considerations. Five had ocular hypertension, defined by IOP of >21 mmHg without glaucomatous changes of SAP and the optic disc; 8 had chronic open-angle glaucoma (COAG), defined by progressive glaucomatous abnormalities of the optic disc (vertical cup-to-disc [C/D] ratio > 0.5, C/D asymmetry ≥ 0.2, localized thinning of the disc, splinter disc hemorrhages) and/or glaucomatous changes of the SAP with IOP of >21 mmHg; 11 had normal-tension glaucoma (NTG), defined by progressive glaucomatous abnormalities of the optic disc and/or glaucomatous changes of SAP with IOP of <21 mmHg; and 1 had primary closed-angle glaucoma (IOP of >21 mmHg with no SAP or optic disc abnormalities) and also received bilateral iridotomy. Clinical follow-up included stereo disc photography, white-on-white SAP (Humphrey), IOP measured with Goldmann applanation tonometry, central corneal thickness measured with pachymetry, and PERG. Patients were tested before treatment and 1 to 3 months after.
Control Group
Forty-four eyes of 22 patients (mean age, 57±12 years) were candidates for treatment based on standard clinical considerations, but were still under observation before treatment. Two patients had ocular hypertension, 10 had NTG, 2 had COAG, and 7 were glaucoma suspects based on a glaucomatous appearance of the optic disc associated with ≥1 risk factors for glaucoma (IOP of >21 mmHg, black race, positive family history) but normal SAP.
Normal Subjects
To understand whether substantial IOP lowering might cause PERG changes in nonglaucomatous subjects, 18 eyes of 9 normal subjects (mean age, 38±15 years) were also included. Normal subjects were tested on the same day before and 2 to 3 hours after receiving 1 tablet of 250-mg acetazolamide (Diamox, Lederle Laboratories, Pearl River, NY), which is known to induce IOP reductions by approximately 30% in 2 to 3 hours.19 Test–retest changes in acetazolamide-treated normal subjects were compared with previously published test–retest changes of 17 untreated normal subjects of comparable mean age (43±12 years).17
Overall, 4 groups of subjects were studied: untreated controls (n = 17, 34 eyes), treated controls (n = 9, 18 eyes), untreated glaucoma (n = 22, 44 eyes), and treated glaucoma (n = 25, 49 eyes).
The research followed the tenets of the Declaration of Helsinki. The study was approved by the institutional review board of the University of Miami. Informed written consent was obtained from all subjects after the nature of the study and possible risks were explained in detail.
Pattern Electroretinogram Technique
Pattern electroretinograms were recorded simultaneously from both eyes according to a user-friendly paradigm recently described using skin electrodes.17 This paradigm overcomes some limitations of current standard PERG techniques with corneal electrodes,20 whose reproducibility depends on both operator skill and patient compliance (e.g., Jacobi et al21). In addition, with this new paradigm, the PERG waveforms recorded in individual eyes are not contaminated by the responses originating from the contralateral eyes, as has been shown to occur with some corneal electrodes.22,23 In summary, flat-cup electrodes were taped on the lower eyelids, whereas reference and ground electrodes were taped on ipsilateral temples and the central forehead, respectively. The pattern stimulus consisted of horizontal gratings (1.7 cup-to-disc ratio, 25° circular field, 95% contrast, 40 candelas per square meter of mean luminance), reversed in counterphase at 8.14 Hz (16.28 reversals per second). Signals were band pass filtered (1–30 Hz), amplified (100 000 fold), and averaged (600 sweeps). Subjects with undilated pupils were refracted for the viewing distance (30 cm) and were asked to fixate on a target at the center of the stimulus. Subjects were allowed to blink freely. The recording time was approximately 3 minutes. Sweeps contaminated by eye blinks or gross eye movements were automatically rejected over a threshold voltage of 25 microvolts.
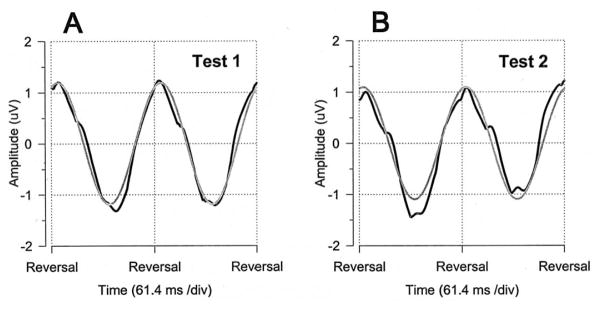
An example of PERG waveforms recorded from the right eye of a normal subject in 2 different sessions (tests 1 and 2) is shown in Figure 1. For fast (16.28 Hz) contrast-reversing patterns, PERG waveforms (black tracings) have an approximately sinusoidal shape, and their frequency corresponds to the reversal rate. Pattern electroretinogram waveforms are automatically analyzed by discrete Fourier transform to isolate the sinusoidal component (gray tracings) at the reversal frequency and measure its amplitude in microvolts and phase lag in π rads. The PERG amplitude is evaluated as half of the peak-to-trough amplitude, and the PERG phase is evaluated relative to the reversal period (vertical grid, 61.4 milliseconds = 2 π rads). Isolation of the contrast-reversal component is a fundamental process to distinguish the relevant signal from the additive noise, and to eliminate the ambiguity of choosing a particular peak or trough of the waveform, thereby increasing the reliability of the measurements. This is particularly important for evaluating responses of small amplitude. In the example of Figure 1, a small difference in amplitude and phase between the PERGs recorded from the right eye of a normal subject in 2 different sessions is noted. The amplitudes and phases of the PERG of test 1 and test 2 were, respectively, 0.93 microvolts, 1.74 π rads, and 1.08 microvolts, 1.72 π rads.
Figure 1.
Example of pattern electroretinogram (PERG) waveforms and response analysis. A, B, Pattern electroretinogram waveforms (black tracings) recorded for the right eye of a normal subject in 2 different sessions. Gray sinusoidal tracings superimposed on the PERG waveforms represent the response component at the contrast-reversal frequency isolated by means of digital Fourier transform analysis. ms = milliseconds; div = division.
Statistics
To provide a measure of repeatability of PERG amplitude and phase in 2 different sessions, test–retest changes in individual eyes have been calculated as test 2–to–test 1 ratios. Values were log transformed to obtain equivalent numbers independently of whether test 1 or test 2 was larger, and multiplied by 10 to convert them to decibel (dB) units [R = 10*log (T2/T1)].24 Signed ratios have the advantage of (1) minimizing the problem of large inter-subject PERG amplitude differences due to different ages and pathologic conditions and (2) providing a normalized index of test–retest variability (standard deviation [SD]) to be compared with corresponding PERG measures obtained under different experimental conditions as well as with different visual tests like multifocal visual evoked potentials or SAP. In normal subjects and untreated glaucoma patients, the test–retest variability is expected to originate from random changes. Therefore, mean log PERG amplitude and phase ratios should not differ significantly from zero. By contrast, a treatment-induced PERG change should result in a biased mean significantly different from zero. The SDs of log PERG amplitude and phase ratios in normal controls and untreated glaucoma patients represent corresponding test–retest variabilities. In both treated glaucoma patients and treated normal controls, eyes with PERG amplitude and phase ratios exceeding +2 SDs of the test–retest variability of the corresponding control group (untreated glaucoma patients and untreated normal controls, respectively) have been considered significantly improved. We performed an analysis of the associations between PERG changes and visual field (VF) and IOP for right and left eyes separately or using all eyes after adjusting the variance for the correlation between the two eyes.25
Results
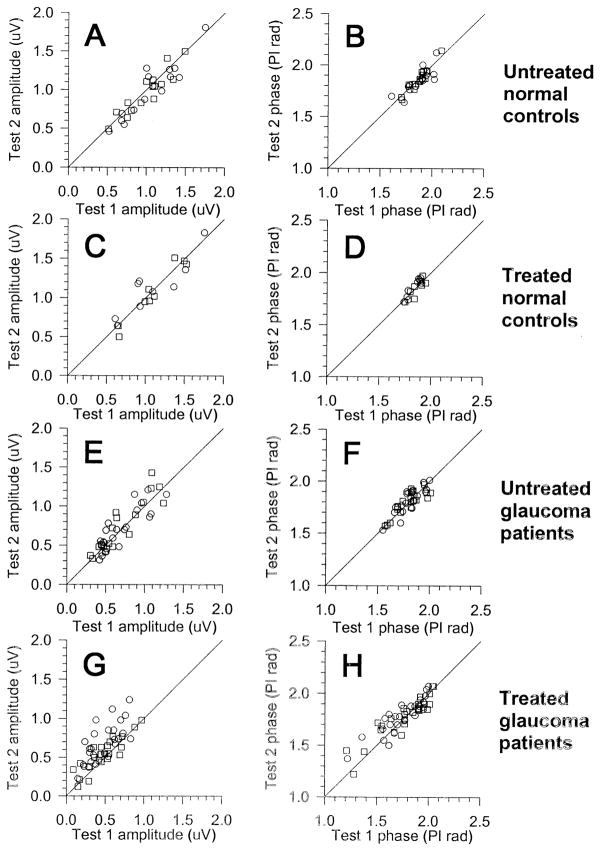
The amplitude and phase of PERGs recorded in individual eyes at test 1 have been compared with corresponding measures recorded at test 2 in a test–retest scatterplot (Fig 2). In all diagrams, the diagonal has a slope of 1, which corresponds to identical test–retest measures. It can be noted in Figure 2 that amplitude and phase measures of untreated normal controls (Fig 2A, B), treated normal controls (Fig 2C, D), and untreated glaucoma patients (Fig 2E, F) are distributed rather symmetrically on both sides of the diagonal. This would suggest that test–retest changes are due to random variability. By contrast, as shown in Figure 2G, amplitudes of treated glaucoma patients tend to be more frequent on the left side of the diagonal, indicating that in some eyes PERG amplitudes recorded at test 2 are greater (improvement) relative to baseline test 1 values. The comparison (paired t test) between tests 2 and 1 is highly significant for PERG amplitude (right eye, P<0.001; left eye, P = 0.006) but not for PERG phase (right eye, P = 0.07; left eye, P = 0.5).
Figure 2.
Scatterplots of test versus retest PERG amplitude (microvolts; left panels) and phase (π rads; right panels). Circles represent right eyes, and squares, left eyes.
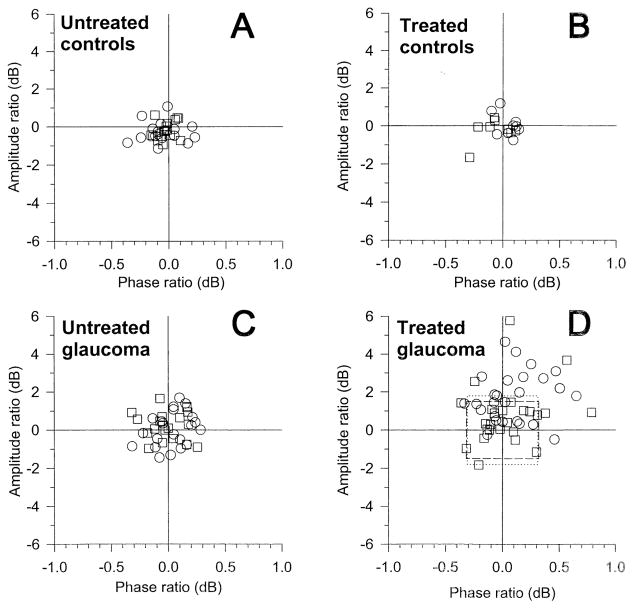
Test–retest ratios [10*log (T2/T1)] for amplitude and phase of individual eyes have been calculated and displayed as amplitude/phase scatterplots (Fig 3; circles, right eyes; squares, left eyes). Average ratios and corresponding SDs are summarized in Table 1. It can be noted in Figure 3A and C that ratios cluster around zero in both untreated controls and untreated glaucoma. The spread of data points, however, is larger in untreated glaucoma eyes (SD of amplitude, 0.7–0.9 dB) than in untreated control eyes (SD of amplitude, ~0.5 dB). For both right and left eyes of untreated controls and untreated glaucoma patients, mean amplitude and phase ratios did not differ significantly from zero (Table 1). Data points of treated control eyes (Fig 3B) also cluster around zero, and their variability is similar to that of untreated control eyes. Mean amplitude and phase ratios do not significantly differ from zero. This indicates that acetazolamide-induced IOP reduction in normal subjects has no significant effect on the PERG.
Figure 3.
Scatterplots of amplitude ratio versus phase ratio. Amplitude and phase ratios were evaluated as 10*log (test 2/test 1). Circles represent right eyes, and squares, left eyes. Dotted and dashed boxes represent the 95% tolerance intervals for right and left eyes, respectively, derived from data displayed in C. dB = decibels.
Table 1.
Summary of Amplitude and Phase Ratios in Different Groups of Subjects
| Mean | SD | n | H0 | |
|---|---|---|---|---|
| UC | ||||
| RE amplitude | −0.27 | 0.54 | 17 | NS |
| LE amplitude | −0.15 | 0.47 | 17 | NS |
| RE phase | −0.048 | 0.15 | 17 | NS |
| LE phase | −0.028 | 0.07 | 17 | NS |
| TC | ||||
| RE amplitude | 0.087 | 0.63 | 9 | NS |
| LE amplitude | −0.177 | 0.48 | 9 | NS |
| RE phase | 0.048 | 0.091 | 9 | NS |
| LE phase | −0.044 | 0.11 | 9 | NS |
| UG | ||||
| RE amplitude | 0.014 | 0.89 | 22 | NS |
| LE amplitude | 0.24 | 0.74 | 22 | NS |
| RE phase | 0.032 | 0.15 | 22 | NS |
| LE phase | −0.007 | 0.15 | 22 | NS |
| TG | ||||
| RE amplitude | 1.7 | 1.3 | 25 | P<0.001 |
| LE amplitude | 0.74 | 1.54 | 24 | P<0.05 |
| RE phase | 0.092 | 0.25 | 25 | NS |
| LE phase | 0.046 | .27 | 24 | NS |
H0 = hypothesis that the mean ratio does not differ from zero; LE = left eye; NS = nonsignificant; RE = right eye; TC = treated normal controls; TG = treated glaucoma patients; UC = untreated normal controls; UG = untreated glaucoma patients.
By contrast, in treated glaucoma (Fig 3D) the distribution of ratios appears skewed towards positive changes of both amplitude and phase. For both right and left eyes, the mean amplitude ratio significantly differed from zero (right eyes, P<0.001; left eyes, P<0.05). Mean phase ratios did not differ significantly from zero. Several eyes had positive changes exceeding the range of variability of corresponding untreated glaucoma eyes (+2 SDs of mean amplitude and phase ratios: dotted and dashed boxes in Fig 3D). Twelve right eyes of 25 (48%) improved in amplitude, 5/25 (20%) in phase, and 3/25 (12%) in both amplitude and phase. Overall, 14 of 25 (56%) right eyes showed PERG improvement in either amplitude or phase. Three left eyes of 24 (13%) improved in amplitude, 3/24 (12%) in phase, and 1/24 (4%) in both amplitude and phase. Overall, 5/24 (21%) left eyes showed PERG improvement in either amplitude or phase.
Association between Pattern Electroretinogram Changes and Baseline Mean Deviation and Intraocular Pressure
To understand the relationship between PERG changes in treated glaucoma patients and some of the relevant baseline factors, PERG amplitude before treatment was compared with PERG amplitude after treatment across different levels of baseline IOP and baseline VFs. Standard automated perimetry mean deviation (MD) was chosen among different SAP indices because, like the PERG, it is an integrated measure of visual function.
Figure 4 displays PERG amplitude changes as a function of 2 different categories of baseline VFs. The first category represents no or minor VF loss (MD, −1±1.3 dB, n = 31). The second category represents severe VF loss (MD, −10±9 dB, n = 18). Figure 4 illustrates 3 points. Compared with treated normal controls, the PERG amplitude of glaucoma patients is smaller and progressively decreases with increasing VF defect. In addition, treated glaucoma patients, but not treated normal controls, show some improvement in PERG amplitude after IOP reduction. Finally, PERG improvement after treatment in glaucoma patients is substantial for little or no VF defects (paired t test, P<0.01), whereas for severe defects the improvement is of borderline significance (paired t test, P = 0.07).
Figure 4.
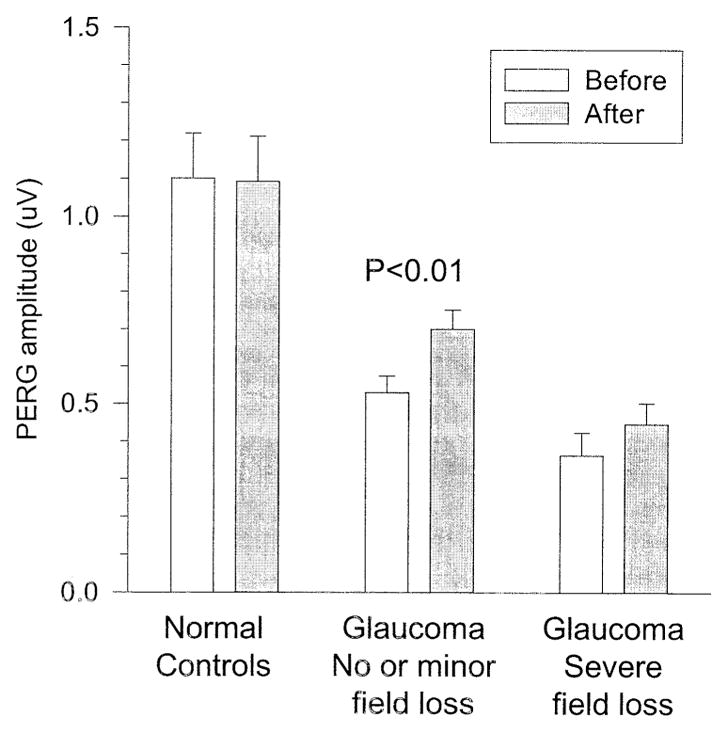
Pattern electroretinogram (PERG) amplitude (microvolts) before and after intraocular pressure (IOP)–lowering treatment in normal controls and glaucoma patients with 2 different levels of severity of visual field (VF) defects. Note that PERG amplitude improvements are larger in eyes with little or no VF defects than in eyes with severe defects.
Figure 5 displays PERG amplitude changes as a function of 2 different categories of baseline IOP (<21 mmHg, average of 15±3, n = 27; ≥21 mmHg, average of 29±12, n = 22). It can be noted that substantial PERG improvements can be found in eyes with baseline IOPs either lower than or higher than 21 mmHg. This would suggest that RGCs with reversible dysfunction can be found in both NTG and COAG. We did not find, however, a significant linear correlation between PERG amplitude and IOP reduction expressed either as Δ IOP (Pearson product moment correlation, P = 0.57) or as percent IOP change (Pearson product moment correlation, P = 0.7). This would suggest that the relationship between PERG improvement and IOP reduction is complex, and probably depends on other variables and terms not envisioned here.
Figure 5.
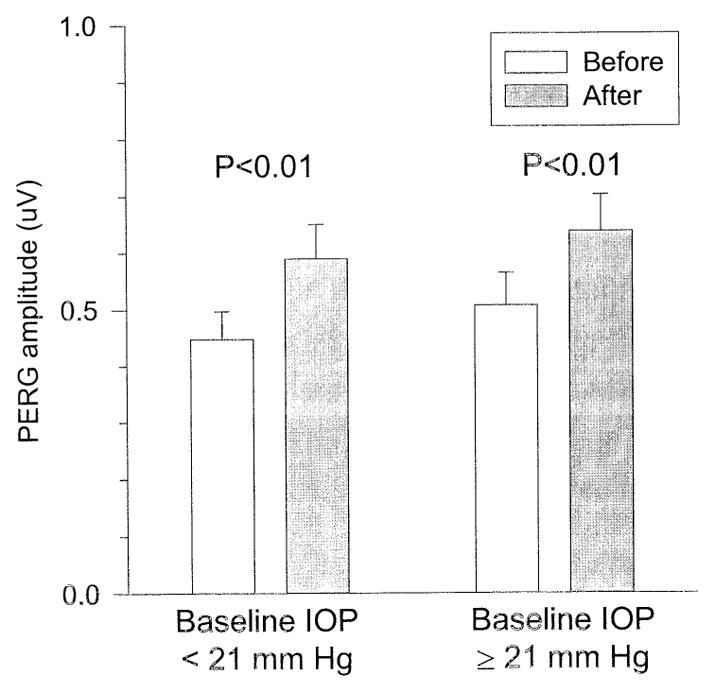
Pattern electroretinogram (PERG) amplitude (microvolts) before and after intraocular pressure (IOP)–lowering treatment in glaucoma patients with 2 different levels of baseline IOP. Note that significant PERG improvement may occur for baseline IOP either lower than or higher than 21 mmHg.
Discussion
This study shows that reducing the IOP in patients with ocular hypertension or glaucoma may result in a significant improvement of PERG amplitude and/or phase. Because the PERG reflects both RGC death and dysfunction of viable RGCs, our results imply that in our particular group of patients a population of RGCs was dysfunctional, at least in part as a consequence of increased IOP. Retinal ganglion cell function could be restored to some degree by reducing the IOP. We cannot exclude, however, that the PERG effects also may be due to changes occurring in the inner retina that do not directly involve RGCs.
Improvement due to IOP lowering may occur not only in ocular hypertension and COAG eyes, but also in NTG eyes. To the best of our knowledge, this is the first report of IOP-dependent PERG improvement in NTG. Pattern electroretinogram improvements in NTG, compared with COAG eyes, are associated with smaller IOP reductions. These results might be explained by assuming that RGCs of NTG eyes are more susceptible to IOP insult than those of COAG eyes. Consequently, RGC dysfunction occurs at lower IOP levels in NTG as compared with COAG, and PERG improvement occurs with smaller IOP reductions.
In addition to IOP lowering, PERG improvement seems to depend on other factors. To have a substantial improvement, the PERG must be abnormal. No significant changes occured in acetazolamide-treated normal subjects, despite an IOP reduction of approximately 30%. This finding may explain why previous studies of ocular hypertension eyes with normal or slightly abnormal baseline PERG and treated with timolol showed slight PERG amplitude increases of 10% to 20% (i.e., in the range of test–retest variability).26–28 In these studies, PERG changes displayed a weak negative correlation with IOP changes, indicating that the PERG was somehow sensitive to IOP reduction in ocular hypertension. In another study by Papst et al,29 7 patients with protracted elevation of IOP (range, 32–56 mmHg) and severe PERG reduction but normal VF and optic discs had substantial PERG improvements (range, 30%–200%) after treatment with acetazolamide.
Our results indicate that greater PERG improvement is associated with normal or early altered VFs. Less PERG improvement occurs in eyes with severe VF defects. This may explain why previous PERG studies in patients with advanced glaucoma did not show significant PERG improvement after glaucoma surgery.30,31 A possible explanation for the interaction between PERG improvement and the extent of VF loss is that, in eyes with preserved VFs, a larger population of RGCs is still viable, as compared with advanced disease states. A fraction of these RGCs have an IOP-dependent dysfunction causing PERG reduction that is reversible with IOP-lowering treatment. This implies that, in the early stages of glaucoma, reversible RGC dysfunction may precede RGC death. This conclusion is in apparent contradiction to the current opinion that structural changes of the optic nerve fiber layer precede the appearance of visual dysfunction, as measured by SAP.1,2 It is important to consider that PERG and VF techniques probe different retinal regions and different aspects of visual function. Quigley et al32 reported that, within 30° eccentricity, a 5-dB sensitivity loss corresponds to a 20% RGC loss. Within the central 12° (the region subserving the PERG response), a 5-dB sensitivity loss corresponds to a 50% RGC loss. Thus, the SAP is relatively insensitive to RGC death in the central area, where there are high RGC density and redundancy. In contrast, the PERG, as a suprathreshold mass response of central RGCs, may be able to signal early dysfunction of viable RGCs.
We realize that this retrospective study has several limitations. The first is that the control group does not accurately match the study group in terms of mean age and clinical breakdown. As mentioned above, the control group represented an approximation to the ideal based on ethics, because treatment cannot be denied to patients who need it. The sensitivity of detection of PERG improvement in the study group was limited by the confidence intervals of test–retest variability of the external control group. The test–retest variability of amplitude is larger in untreated glaucoma patients (SD, 0.7–0.9 dB) than in untreated controls (SD, ~0.5 dB). These variabilities are somewhat smaller than those reported for either multifocal visual evoked potentials24 or SAP.33 The limitations of the external control group can be overcome in a prospective study with an internal control, in which the test–retest variability in individual eyes may be determined in a series of pretreatment measures. The second limitation is that the study group included 4 different manifestations of glaucoma with a varied spectrum of disease severity. Due to the relatively small sample of patients, we were unable to perform a multivariate statistical analysis to separate the effects of possible variables such as type and severity of disease and the effects of different pharmacological agents. In future prospective studies on a larger sample of patients, we will also seek to model the relationship between PERG improvement and IOP reduction, considering the interaction of factors including baseline IOP, baseline VF, and baseline optic disc cupping.
In conclusion, this pilot study suggests that lowering the IOP in patients with early glaucoma may restore, at least partially, the activity of dysfunctional RGCs. Without treatment, these dysfunctional RGCs may be destined to a premature death. Our results provide a neurophysiological explanation for several multicenter studies indicating that IOP reduction delays the onset or the progression of VF deterioration in ocular hypertension,34 early manifest glaucoma,35 advanced glaucoma,36 and NTG.37 In addition, the present results suggest that the PERG could be used as a tool to target the neuroprotective effects of IOP-lowering drugs based on RGC function.
Acknowledgments
The authors thank William J. Feuer, MS, for providing statistical assistance and for reviewing and discussing the manuscript.
Supported by National Institutes of Health, Bethesda, Maryland (center grant no.: P30-EY14801); an unrestricted grant to the University of Miami from Research to Prevent Blindness, Inc., New York, New York; The Glaucoma Foundation, New York, New York; Fight for Sight, New York, New York; and Rotary International, Miami, Florida.
Footnotes
Presented at: American Academy of Ophthalmology Annual Meeting, November, 2003; Anaheim, California.
Dr Porciatti has a patent pending application (no. PCT/US03/03155) on the algorithm to record the pattern electroretinogram.
References
- 1.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 2.Tuulonen A, Lehtola J, Airaksinen PJ. Nerve fiber layer defects with normal visual fields. Do normal optic disc and normal visual field indicate absence of glaucomatous abnormality? Ophthalmology. 1993;100:587–97. doi: 10.1016/s0161-6420(93)31598-8. discussion 597–8. [DOI] [PubMed] [Google Scholar]
- 3.Harwerth RS, Crawford ML, Frishman LJ, et al. Visual field defects and neural losses from experimental glaucoma. Prog Retin Eye Res. 2002;21:91–125. doi: 10.1016/s1350-9462(01)00022-2. [DOI] [PubMed] [Google Scholar]
- 4.Maffei L, Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science. 1981;211:953–5. doi: 10.1126/science.7466369. [DOI] [PubMed] [Google Scholar]
- 5.Maffei L, Fiorentini A, Bisti S, Hollander H. Pattern ERG in the monkey after section of the optic nerve. Exp Brain Res. 1985;59:423–5. doi: 10.1007/BF00230925. [DOI] [PubMed] [Google Scholar]
- 6.Berardi N, Domenici L, Gravina A, Maffei L. Pattern ERG in rats following section of the optic nerve. Exp Brain Res. 1990;79:539–46. doi: 10.1007/BF00229323. [DOI] [PubMed] [Google Scholar]
- 7.Porciatti V, Pizzorusso T, Cenni MC, Maffei L. The visual response of retinal ganglion cells is not altered by optic nerve transection in transgenic mice overexpressing Bcl-2. Proc Natl Acad Sci U S A. 1996;93:14955–9. doi: 10.1073/pnas.93.25.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorentini A, Maffei L, Pirchio M, et al. The ERG in response to alternating gratings in patients with diseases of the peripheral visual pathway. Invest Ophthalmol Vis Sci. 1981;21:490–3. [PubMed] [Google Scholar]
- 9.Dawson WW, Maida TM, Rubin ML. Human pattern-evoked retinal responses are altered by optic atrophy. Invest Ophthalmol Vis Sci. 1982;22:796–803. [PubMed] [Google Scholar]
- 10.Harrison JM, O’Connor PS, Young RS, et al. The pattern ERG in man following surgical resection of the optic nerve. Invest Ophthalmol Vis Sci. 1987;28:492–9. [PubMed] [Google Scholar]
- 11.Johnson MA, Drum BA, Quigley HA, et al. Pattern-evoked potentials and optic nerve fiber loss in monocular laser-induced glaucoma. Invest Ophthalmol Vis Sci. 1989;30:897–907. [PubMed] [Google Scholar]
- 12.Trimarchi C, Biral G, Domenici L, et al. The flash- and pattern-electroretinogram generators in the cat: a pharmacological approach. Clin Vis Sci. 1990;6:19–24. [Google Scholar]
- 13.Viswanathan S, Frishman LJ, Robson JG, et al. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1124–36. [PubMed] [Google Scholar]
- 14.Colotto A, Falsini B, Salgarello T, et al. Transiently raised intraocular pressure reveals pattern electroretinogram losses in ocular hypertension. Invest Ophthalmol Vis Sci. 1996;37:2663–70. [PubMed] [Google Scholar]
- 15.Kothe AC, Lovasik JV. A parametric evaluation of retinal vascular perfusion pressure and visual neural function in man. Electroencephalogr Clin Neurophysiol. 1990;75:185–99. doi: 10.1016/0013-4694(90)90172-g. [DOI] [PubMed] [Google Scholar]
- 16.Kremmer S, Tolksdorf-Kremmer A, Stodtmeister R. Simultaneous registration of VECP and pattern ERG during artificially raised intraocular pressure [in German] Ophthalmologica. 1995;209:233–41. doi: 10.1159/000310622. [DOI] [PubMed] [Google Scholar]
- 17.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111:161–8. doi: 10.1016/j.ophtha.2003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ventura LM, Porciatti V, Ishida K, et al. PERG abnormality and glaucoma. Ophthalmology. doi: 10.1016/j.ophtha.2004.07.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedland BR, Mallonee J, Anderson DR. Short-term dose response characteristics of acetazolamide in man. Arch Ophthalmol. 1977;95:1809–12. doi: 10.1001/archopht.1977.04450100111014. [DOI] [PubMed] [Google Scholar]
- 20.Bach M, Hawlina M, Holder GE, et al. Standard for pattern electroretinography. Doc Ophthalmol. 2000;101:11–8. doi: 10.1023/a:1002732114721. [DOI] [PubMed] [Google Scholar]
- 21.Jacobi PC, Walter P, Brunner R, Krieglstein GK. Reproducibility and intraindividual variability of the pattern electroretinogram. Ger J Ophthalmol. 1994;3:216–9. [PubMed] [Google Scholar]
- 22.Peachey NS, Sokol S, Moskowitz A. Recording the contralateral PERG: effect of different electrodes. Invest Ophthalmol Vis Sci. 1983;24:1514–6. [PubMed] [Google Scholar]
- 23.Seiple WH, Siegel IM. Recording the pattern electroretinogram: a cautionary note. Invest Ophthalmol Vis Sci. 1983;24:796–8. [PubMed] [Google Scholar]
- 24.Chen CS, Hood DC, Zhang X, et al. Repeat reliability of the multifocal visual evoked potential in normal and glaucomatous eyes. J Glaucoma. 2003;12:399–408. doi: 10.1097/00061198-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Katz J. Two eyes or one? The data analyst’s dilemma. Ophthalmic Surg. 1988;19:585–9. [PubMed] [Google Scholar]
- 26.Nesher R, Trick GL, Kass MA, Gordon MO. Steady-state pattern electroretinogram following long term unilateral administration of timolol to ocular hypertensive subjects. Doc Ophthalmol. 1990;75:101–9. doi: 10.1007/BF00146546. [DOI] [PubMed] [Google Scholar]
- 27.Falsini B, Colotto A, Porciatti V, Porrello G. Follow-up study with pattern ERG in ocular hypertension and glaucoma patients under timolol maleate treatment. Clin Vis Sci. 1992;7:341–7. [Google Scholar]
- 28.Colotto A, Salgarello T, Giudiceandrea A, et al. Pattern electroretinogram in treated ocular hypertension: a cross-sectional study after timolol maleate therapy. Ophthalmic Res. 1995;27:168–77. doi: 10.1159/000267663. [DOI] [PubMed] [Google Scholar]
- 29.Papst N, Bopp M, Schnaudigel OE. The pattern evoked electroretinogram associated with elevated intraocular pressure. Graefes Arch Clin Exp Ophthalmol. 1984;222:34–7. doi: 10.1007/BF02133775. [DOI] [PubMed] [Google Scholar]
- 30.Spadea L, D’Andrea D, Blasi MA, Balestrazzi E. Evaluation of electrofunctional data following argon-laser trabeculoplasty in primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 1991;229:414–7. doi: 10.1007/BF00166302. [DOI] [PubMed] [Google Scholar]
- 31.Spadea L, Giuffre I, Bianco G, Balestrazzi E. PERG and P-VEP after surgical trabeculectomy in primary open-angle glaucoma. Eur J Ophthalmol. 1995;5:92–5. doi: 10.1177/112067219500500205. [DOI] [PubMed] [Google Scholar]
- 32.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–64. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 33.Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987;105:1544–9. doi: 10.1001/archopht.1987.01060110090039. [DOI] [PubMed] [Google Scholar]
- 34.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 35.Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 36.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–40. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 37.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]