Abstract
Peroxisome Proliferator-Activated Receptor (PPAR) α, the molecular target for fibrates used to treat dyslipidemia, exerts pleiotropic effects on vascular cells. In vascular smooth muscle cells (VSMCs), we have previously demonstrated that PPARα activation suppresses G1→S cell cycle progression by targeting the cyclin-dependent kinase inhibitor p16INK4a (p16). In the present study, we demonstrate that this inhibition of VSMC proliferation by PPARα is mediated through a p16-dependent suppression of telomerase activity, which has been implicated in key cellular functions including proliferation. PPARα activation inhibited mitogen-induced telomerase activity by repressing the catalytic subunit telomerase reverse transcriptase (TERT) through negative cross-talk with an E2F-1-dependent trans-activation of the TERT promoter. This trans-repression involved the recruitment of the retinoblastoma (RB) family proteins p107 and p130 to the TERT promoter resulting in impaired E2F-1 binding, an effect which was dependent on p16. The inhibition of cell proliferation by PPARα activation was lost in VSMC following TERT overexpression or knock-down, pointing to a key role of telomerase as a target for the antiproliferative effects of PPARα. Finally, we demonstrate that PPARα agonists suppress telomerase activation during the proliferative response following vascular injury indicating that these findings are applicable in vivo. In concert, these results demonstrate that the anti-proliferative effects of PPARα in VSMCs depend on the suppression of telomerase activity by targeting the p16/RB/E2F transcriptional cascade.
Keywords: PPARα, Telomerase, Smooth muscle cells, transcriptional regulation, p16INK4a
INTRODUCTION
Proliferation of vascular smooth muscle cells (VSMCs) contributes to atherosclerosis development and constitutes a primary mechanism resulting in postangioplasty restenosis, vein graft failure and transplant vasculopathy.1 Mitogenic growth factors secreted during vascular injury converge into the cell cycle as the final common signaling pathway regulating the proliferative response of VSMCs.1 G1→S cell cycle progression is regulated by the retinoblastoma (RB)/E2F pathway, which links growth-regulatory pathways to a transcriptional program required for DNA synthesis.2 In quiescent cells, the RB family of proteins, which include pRB, p107 and p130, binds to E2F transcription factors and inhibits their transcriptional activity.2 In response to growth factors, cyclin-dependent kinase (CDK)-cyclin complexes are activated to hyperphosphorylate pRB and thereby induce the release of E2F allowing transcription of S phase genes.3 CDK-inhibitors (CDKI), including p16INK4a (p16), impinge upon this pathway by inhibiting the activity of cyclin-CDK complexes providing a second layer of regulation.3
While DNA replication is regulated through the cell cycle machinery, emerging evidence indicates that telomerase activation may affect cell proliferation by maintaining telomere function.4 Telomeres, the DNA TTAGGG repeat sequences at the ends of eukaryotic chromosomes, are stabilized by telomerase to serve as protective capping and to prevent cellular senescence.5 Telomerase activity in telomerase-deficient cells can be restored by ectopic expression of the telomerase reverse transcriptase (TERT) indicating that TERT confers the catalytic activity and is the limiting factor for telomerase activation.6 In vitro, TERT is tightly regulated by mitogenic stimuli and required for VSMC proliferation.7–9 In animal models, telomerase deficiency reduces atherosclerosis and neointima formation,10, 11 indicating that telomerase may serve as a novel pharmacological target for the treatment of vascular diseases.11 However, although telomerase activity has been linked to VSMC proliferation,7–9 the mechanisms feeding into the transcriptional pathways that determine telomerase regulation in VSMCs remain to be investigated.
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily12, 13. PPARα mediates the hypolipidemic effects of fibrates, which are clinically utilized to treat dyslipidemia in patients at cardiovascular risk.12, 13 In addition to their metabolic efficacy, recent evidence has demonstrated that PPARα prevents intimal hyperplasia through direct pleiotropic effects on the vascular wall.12 Our previous work provided evidence that PPARα inhibits VSMC proliferation by inducing the expression of the CDKI p16.14 In the present study, we identify telomerase as the key downstream target for the antiproliferative effects of PPARα and demonstrate that PPARα interferes with a previously unrecognized p16/pRB/E2F-dependent transcriptional activation of telomerase in VSMCs.
MATERIALS AND METHODS
For the detailed description of Materials, please see the online data supplement.
Cell culture
Rat aortic VSMCs (rVSMCs) were cultured as described.9 Primary human coronary artery VSMCs (hVSMCs) were obtained from Lonza (Allendale, NJ, USA) and cultured using the VSMC Growth Medium 2 Bullet Kit as directed. In all experiments, hVSMCs of passage three to eight were subjected to G0/G1 phase synchronization by starvation for three days in medium supplemented with 0.4% FBS and devoid of any growth factors. Cells were pretreated for 24h with the indicated doses of PPARα ligand (fenofibric acid (FA), GW7647, gemfibrozil (GF), or Wy 14,643) or vehicle (Me2SO)) prior to stimulation of cell proliferation with growth medium (GM) containing 5% FBS and growth factors (hFGF, hEGF, and insulin as directed). When hVSMCs were harvested at 72h, the medium supplemented with PPARα agonists or DMSO was changed after 36h. Cell cycle distribution was determined by propidium iodide staining of DNA and flow cytometry analysis as described.14 Cell proliferation was assessed by cell counting using a hematocytometer. For all data shown, individual experiments were repeated at least three times in duplicates with different lots or preparations of VSMCs. For Western blot analyses, the graphical analysis represents results from three independent experiments and quantification by densitometry.
Adenoviral infection
hVSMCs (1×105 cells/6-well plates) were infected with Ad-GFP, Ad-GFP/TERT or Ad-E2F-1 at the indicated plaque forming units (PFU)/cell in 0.4% FBS. Transduction efficiency was above 94 % in cells infected with Ad-GFP as verified by FACS analysis. Infection of cells with Ad-GFP/PPARα has been described previously.14
siRNA analyses
Serum-deprived hVSMCs were transfected for 4h in Opti-MEM (Invitrogen, Carlsbad, CA) using the indicated quantities of siRNAs and either 16 μl (for 60-mm2 plate) or 8.4 μl (for 6-well plates) jetSI reagent (Polyplus Transfection, Illkirch, France). After transfection, 0.4%-FBS starvation medium supplemented with the indicated PPARα ligand or vehicle (Me2SO) was added overnight. FBS and growth factors were then added to achieve the same final concentration as in the routine medium.
Telomeric Repeat Amplification Protocol Assay
Telomerase activity was analyzed using a PCR based assay (TeloTAGGG Telomerase PCR ELISA Plus; Roche Applied Sciences) as described.9 Three μg of whole-cell protein was used for the elongation/amplification, and telomerase activity was quantified by ELISA according to the manufacturer’s instructions.
Reverse transcription and quantitative PCR
Transcript levels of target genes were assessed by quantitative real-time RT-PCR as previously described14 using an iCycler (Bio-Rad, Hercules, CA), SYBR Green I system (Bio-Rad, Hercules, CA), and the specific primers indicated in the supplementary information (Online Table I). mRNA levels of TATA box binding protein (hTBP) and the transcription factor mTFIIB were quantified simultaneously as house keeping gene in human and murine cells, respectively.
Western blotting
Western Blotting was performed as described14 following isolation of nuclear proteins using the NE-PER™ kit (Pierce, Rockford, IL, USA).
Transient transfection assays
rVSMCs plated in 24-well plates at a density of 5×104 cells/well were transfected for 4 to 6h in OptiMEM using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) at a ratio of 2 μl Lipofectamine 2000/0.8 μg of DNA. Luciferase activities were analyzed using the Dual-Luciferase Reporter Assay system (Promega, Madison, WI). Transfection efficiency was normalized to renilla luciferase activities generated by cotransfection of 0.1 μg/well pGL4.74[hRluc/TK] (pRL-Tk, Promega, Madison, WI).
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed using an assay kit (Millipore, Billerica, MA) and 10 μg of antibody for the immunoprecipitation as described.14 One percent of DNA/protein extract precleared with protein G agarose was utilized as non-precipitated genomic DNA (Input). Extracted DNA was amplified by real-time PCR with an annealing temperature of 54°C, 200 nM primer pairs that cover the proximal E2F1-binding element in the human TERT promoter (Online Table I), 40% pure protein/DNA-immunoprecipitated sample, and 1 mg HotStart-IT binding protein (USB, Cleveland, OH). Negative controls include PCRs performed with hTERTE2F1 oligonucleotides following immunoprecipitation with nonimmune IgG or with primers covering a distal region of TERT gene localized at -899/-723 of E2F1 element (hTERT control, Online Table I, data not shown).
Endothelial denudation injury
Eight to 12 week-old male Sv/129 mice were fed a standard chow diet (diet no. 7012, Harlan Teklad, Madison, WI) supplemented with fenofibrate (FF) or gemfibrozil (GF) for one week prior to endothelial denudation injury of the femoral artery. Guide wire injuries were performed on the right femoral artery as previously described while sham surgery without injury was performed on the contralateral left side.9 Carotid artery wire injuries were performed as described.14 Mice were euthanized two days after injury for isolation of liver and vascular tissues. Telomerase activities were analyzed in arterial tissues by ELISA as described above. The institutional animal care and use committee at the University of Kentucky approved all procedures on the mice.
Statistical Analysis
Differences between different groups were assessed by One-Way-Anova for in vitro assays and Kruskall-Wallis for in vivo analyses of telomerase activities in femoral arteries followed by Tukey tests. P values <0.05 were considered to be statistically significant.
RESULTS
PPARα agonists inhibit mitogen-induced telomerase activity and TERT gene expression
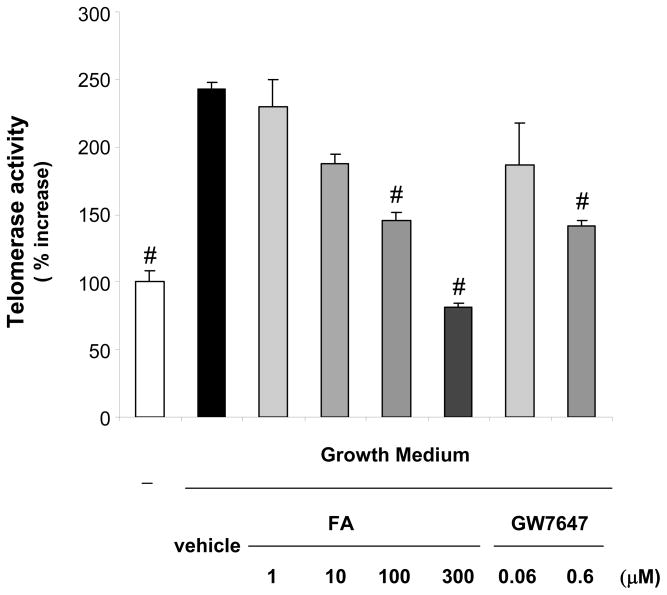
To investigate the regulation of telomerase activity by PPARα ligands, mitogen-induced telomerase activity was analyzed in hVSMCs treated with different PPARα agonists, including the clinically used FA and GW7647, a PPARα agonist with high affinity and selectivity for the receptor.15 Consistent with previous reports,8, 9 telomerase activity was induced following mitogenic stimulation of hVSMCs (Fig. 1). Pretreatment with either FA or GW7647 resulted in a dose-dependent inhibition of mitogen-induced telomerase activation. Similar results were obtained using other PPARα ligands including gemfibrozil (300 μM) or Wy14,643 (100 μM) (data not shown).
Figure 1. PPARα ligands inhibit mitogen-induced telomerase activity in hVSMCs.
hVSMCs were treated with vehicle (Me2SO) or the indicated doses of the PPARα ligands FA or GW7647. Cells were analyzed for telomerase activity 72h after stimulation with growth medium. Data are presented as mean ± SEM percent increase over quiescent cells (n=3; # P < 0.05 vs. vehicle + GM).
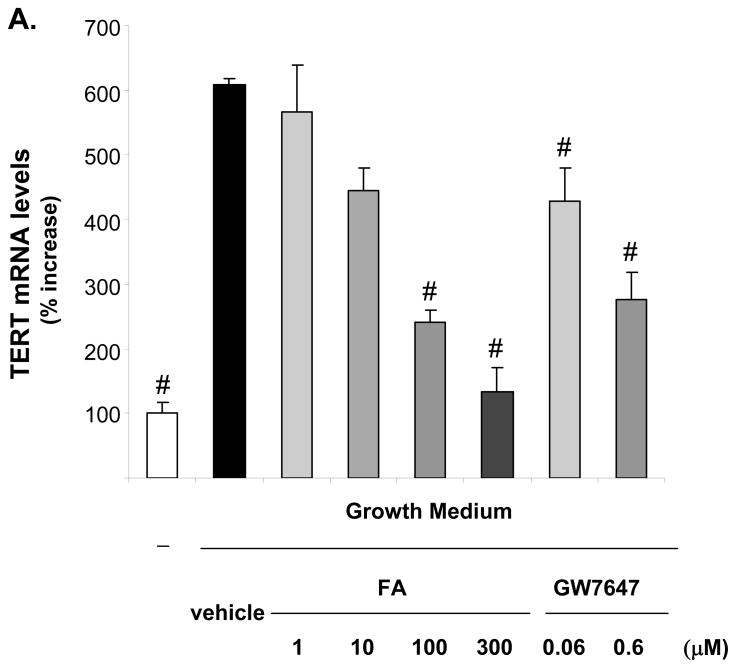
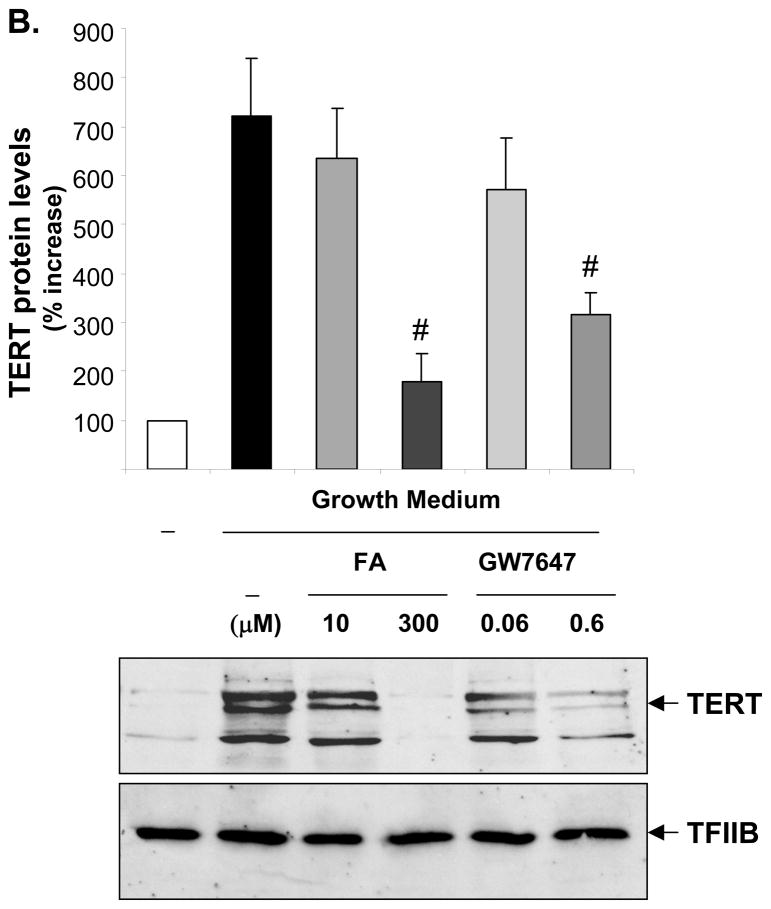
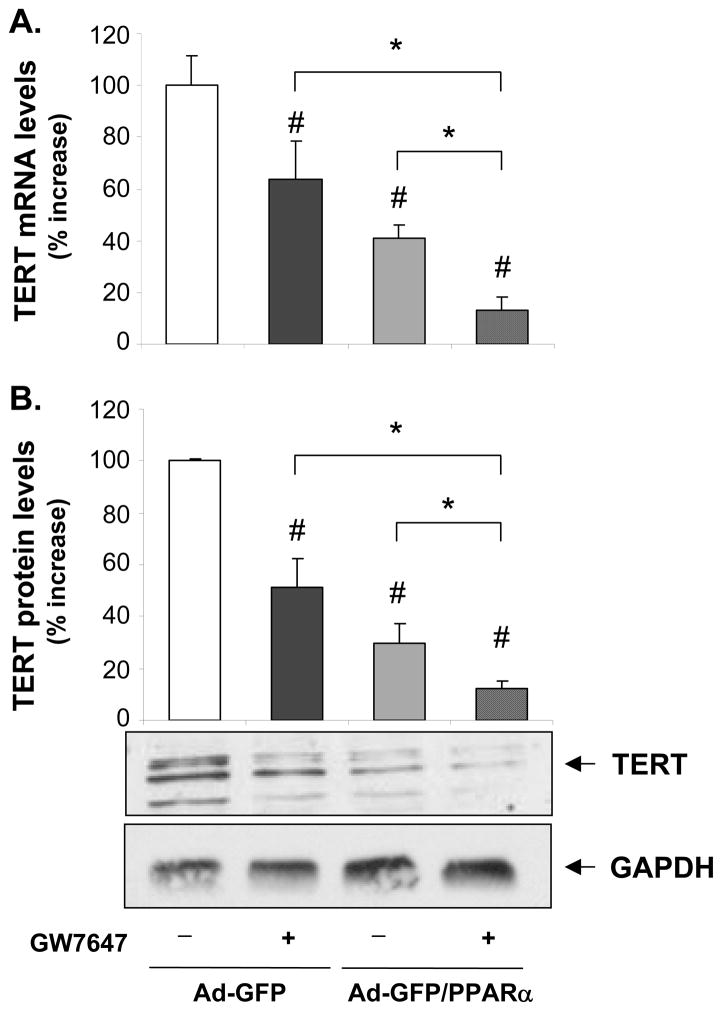
TERT confers the catalytic activity of telomerase,6 and we recently demonstrated that other components of telomerase including the telomerase RNA component and the telomerase-associated protein 1 are not regulated in VSMCs.9 Since it has been suggested that TERT transcription represents the rate-limiting step for its expression,6 we next focused our analyses on the regulation of TERT gene expression by ligand-activated PPARα. As depicted in Fig. 2, mitogen-induced TERT mRNA and protein expression were dose-dependently repressed by the PPARα ligands FA or GW7647 over a concentration range known to activate the receptor.15, 16 Similarly, adenoviral overexpression of PPARα suppressed TERT expression, an effect that was even further pronounced in the presence of GW7647 (Fig. 3). In concert, these data suggest that ligand-dependent PPARα activation inhibits telomerase activity by repressing mitogen-induced TERT transcription.
Figure 2. PPARα activation inhibits mitogen-induced TERT expression.
Quiescent hVSMCs were treated with vehicle or the indicated doses of FA or GW7647 and harvested 72h after incubation in growth medium. A, Quantitative real-time RT-PCR data of TERT mRNA expression levels is presented as mean ± SEM (n=3, # P < 0.05 vs. vehicle + GM). B, Western blot analysis of TERT and TFIIB (used for control of protein loading) expression in hVSMC nuclear protein extracts. The upper panel represents the mean ± SEM of TERT/TFIIB protein levels quantified by densitometry (n=3, # P < 0.05 vs. vehicle + GM). The autoradiograms shown are representative of three independently performed experiments.
Figure 3. Ligand-activated PPARα decreases TERT mRNA and protein expression levels.
hVSMCs were infected with Ad-GFP or Ad-GFP/PPARα adenoviral constructs and treated with vehicle or GW7647 (600 nM) for 12h. A, Quantitative RT-PCR analysis of TERT mRNA expression. B, Western blot for TERT and GAPDH (to assess equal protein loading) expression in whole-cell protein extracts. Values are expressed as mean ± SEM (n=3) relative to vehicle-treated Ad-GFP-infected cells. The blot represents a typical result of three independent experiments.
The PPARα/p16 pathway represses TERT transcription and telomerase activity through inhibition of E2F-1-dependent trans-activation of the TERT promoter
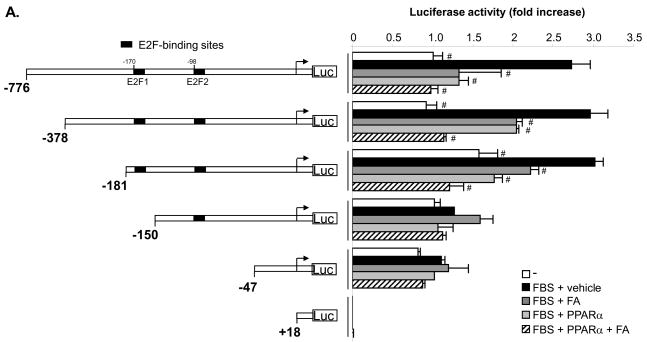
To identify the regulatory elements in the TERT promoter that confer the repression by PPARα, we performed transient transfection experiments in rVSMCs using 5′-deletion series of a luciferase vector driven by the human TERT promoter region spanning from -776 to +18 bp from the transcription initiation site (Fig. 4A). rVSMCs were cotransfected with a pSG5-PPARα expression vector or control plasmid and subjected to mitogenic stimulation in the presence of FA. Mitogen-induced transcriptional activity of the -776 bp TERT promoter was significantly repressed by overexpression of PPARα or treatment with FA (Fig. 4A). PPARα-dependent repression of TERT promoter activity was maintained upon 5′-deletion to -181 bp. In contrast, mitogenic induction and repression of TERT promoter activity by PPARα were lost upon further deletion to -150 or -47 bp, indicating that a 31 bp promoter region between -181 and -150 bp confers the repression of TERT promoter activity by PPARα.
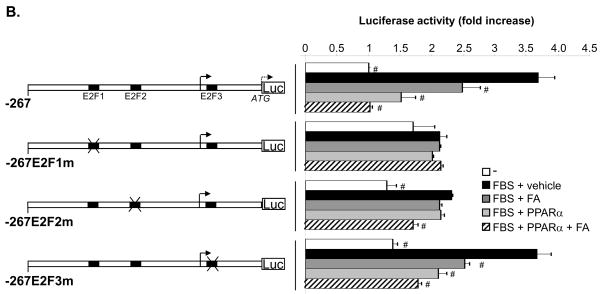
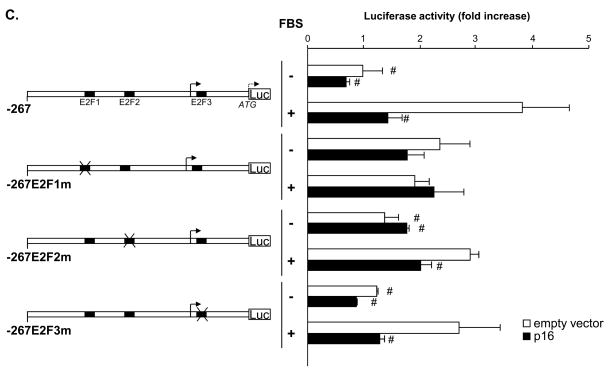
Figure 4. Activation of the PPARα/p16 pathway suppresses TERT transcription by inhibiting E2F-1-dependent trans-activation of the proximal TERT promoter.
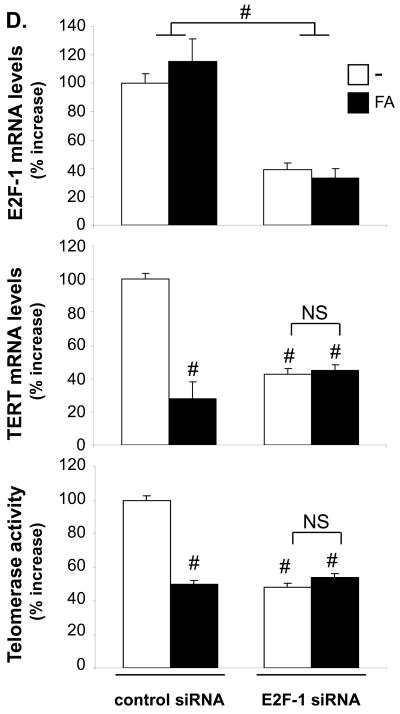
A–C, pGL3 luciferase reporter constructs driven by the indicated TERT promoter fragments (0.4μg) were cotransfected with the pSG5-PPARα (A–B) or pcDNA3-p16 (C) expression vectors or their corresponding empty control vectors (0.3 μg). A–B, Cells were incubated in fetal bovine lipoprotein-deficient serum in the presence of FA (300 μM) or vehicle. C, Cells were cultured in the presence (20%) or absence (0.4%) of FBS. After 24h, cells were harvested and luciferase activities analyzed. The nucleotide positions in the TERT promoter are indicated relative to the transcription start site. Data are expressed as normalized luciferase activity and presented as mean ± SEM fold increase over -776TERTLuc (A) or -267TERTLuc (B and C) activities in quiescent cells (# P < 0.05 vs. vehicle + FBS (A and B) or control vector + FBS (C)). D, hVSMCs were transfected with E2F-1 siRNA or non-silencing control siRNA (2 μg) and treated with FA (300 μM) or vehicle. Cells were harvested 36h after mitogen stimulation. E2F-1 and TERT mRNA expression or telomerase activities levels are expressed as mean ± SEM percent relative to control-transfected vehicle-treated cells (# P < 0.05).
Sequence analysis of this TERT promoter region did not reveal the presence of a PPAR-response element (PPRE), suggesting an indirect mechanism responsible for the repression of the TERT promoter by PPARα. Previous reports have identified three E2F-binding sites in the proximal TERT promoter located at -170/-174, -98/-94 and +9/+14 from the transcription initiation site referred to as E2F1, E2F2, and E2F3, respectively.17, 18 To further analyze their role for the regulation of the promoter, rVSMCs were transfected with a luciferase construct driven by the wild-type TERT promoter region from -267 to the immediate upstream part of the translation start codon (-267TERTLuc) or the same region bearing mutations of the E2F sites as indicated in (Fig. 4B). Consistent with our observations using 5′-deletion, promoter activity of the wild-type construct was repressed by PPARα and FA. Interestingly, both the mitogen-induced TERT promoter activity and the inhibitory effect of PPARα activation were abolished upon mutation of the E2F1 site (-267E2F1mTERTLuc), which is located within the -181 to -150 bp region. In contrast, the repression by ligand-activated PPARα was maintained in the construct -267E2F2mTERTLuc, although the mitogenic induction was slightly decreased (P<0.05 vs. -267E2F1m). Finally, mutation of the third E2F-binding site in the TERT promoter (-267E2F3m) did not affect the transcriptional regulation.
Since PPARα agonists inhibit S phase entry by trans-activating the promoter of the gene encoding the CDKI p16,14 we next analyzed the effects of p16 on TERT promoter activity. As depicted in Fig. 4C, overexpression of p16 completely suppressed mitogen-induced transcriptional activity of the wild-type -267TERTLuc construct to a level observed in quiescent rVSMCs. Similarly as observed following PPARα activation in Fig. 4B, this p16-mediated repression of the TERT promoter was dependent on a functional E2F1 site located at -170/-174. Therefore, PPARα-induced p16 expression provides a likely mechanism involved in the repression of TERT transcription by PPARα ligands.
To confirm the role of E2F-1 in the PPARα-dependent regulation of telomerase activation, we employed siRNA-mediated knock-down of E2F-1 in hVSMCs (Fig. 4D). E2F-1 knock-down decreased TERT mRNA expression and telomerase activity while complementary results were obtained upon overexpression of E2F-1 in quiescent hVSMCs (Online Figure I). However, in cells transfected with E2F-1 siRNA, the ability of the PPARα ligand to repress TERT transcription and inhibit telomerase activity was lost. In concert, these experiments suggest that the PPARα/p16 pathway inhibits mitogen-induced TERT expression and telomerase activity through negative cross-talk with an E2F-1-dependent trans-activation of the -170/-174 site in the TERT promoter.
PPARα modulates transcriptional complexes formed with E2F-1 and pRB proteins at the proximal TERT gene promoter
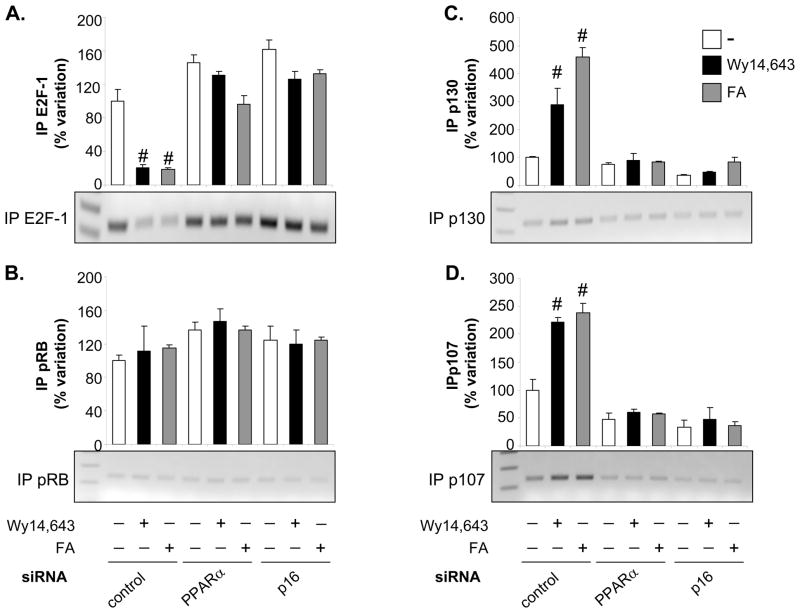
We next performed ChIP assays using primer pairs that cover the E2F element in the proximal TERT promoter to determine the modulation of transcriptional complexes by PPARα activation at this site. Consistent with experiments presented in Fig. 4, we confirmed that ligand-induced PPARα activation inhibits E2F-1 binding to the TERT promoter (Fig. 5). Inhibition of E2F-1 binding by PPARα ligands was dependent on the presence of PPARα and p16 as the efficacy of the ligands to repress E2F-1 binding was decreased upon siRNA-mediated knockdown of either protein.
Figure 5. Activation of PPARα modulates transcriptional complexes formed with E2F-1 and pRB proteins on the proximal TERT gene promoter.
Serum-deprived hVSMCs were transfected in 60-mm2 plates with non-silencing control or siRNA directed against PPARα or p16 (5 μg). After transfection, the starvation medium was supplemented with vehicle (Me2SO), Wy14,643 (100 μM) or FA (300 μM) for 12h. Cells were harvested 6h after mitogen stimulation and chromatin was immunoprecipitated using antibodies raised against E2F-1 (A), underphosphorylated pRB (B), p130 (C), and p107 (D). For each antibody, the upper panel depicts quantification of immunoprecipitated chromatin by real-time PCR using primer pairs that cover the E2F1 site located at -170/-174 in the proximal TERT promoter. Cycle threshold (Ct) values were normalized to Ct values of input samples. Data is presented as mean ± SEM (# P < 0.05 vs. vehicle-treated cells) relative to control siRNA-transfected hVSMCs treated with vehicle arbitrarily set as 100%. The lower panel depicts ethidium-bromide–stained agarose gels of representative results obtained after 34 cycles. Real-time PCR results from input (E) and immunoprecipitation with non-immune IgG (F) after 34 cycles are shown as controls.
Since the trans-activation potential of E2F is repressed by the RB family of proteins,2 we next analyzed their occupancy at the E2F1 site in the TERT promoter. Treatment with Wy14,643 or FA resulted in the recruitment of the p107 and p130 proteins to the E2F1 site in control-transfected hVSMCs, an effect which was prevented by knock-down of PPARα or p16 (Fig. 5). In contrast, a modest interaction of pRB with the E2F1 site was not regulated by PPARα ligands. Complementary results were obtained in murine VSMCs, in which Wy14,643 induced the recruitment of p107 and p130 to the TERT promoter in wild-type cells, but not in PPARα- or p16-deficient mVSMCs (Online Figure II). Taken together, these data indicate that ligand-dependent activation of the PPARα/p16 pathway represses TERT transcription by recruiting p107 and p130 to the proximal TERT promoter, which alters the ability of E2F-1 to trans-activate the TERT promoter.
TERT mediates the growth-inhibitory effects of PPARα activation in VSMCs
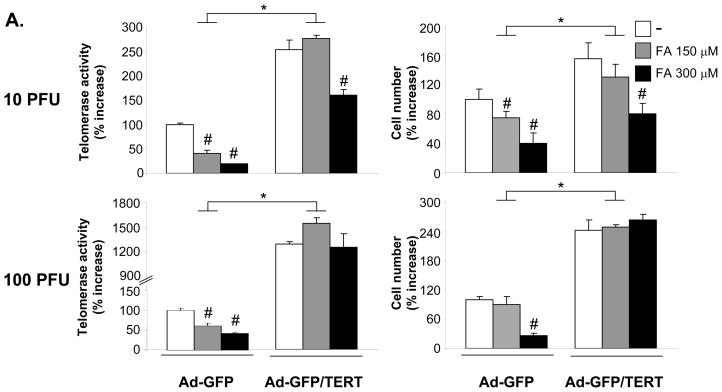
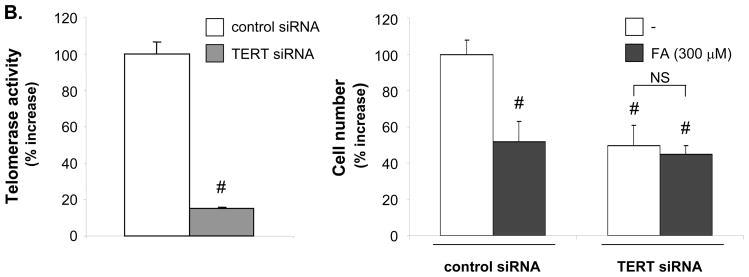
To investigate whether the inhibition of VSMC proliferation by PPARα is mediated by targeting TERT, we infected hVSMCs with adenoviral constructs co-expressing GFP and TERT (Ad-GFP/TERT) or overexpressing GFP alone (Ad-GFP) (Fig. 6A). Infection of hVSMCs with 10 and 100 PFU/cell of Ad-GFP/TERT resulted in a significant increase of telomerase activity and cell proliferation. Consistent with our previous study,14 the PPARα ligand FA dose-dependently inhibited hVSMC proliferation in cells infected with control Ad-GFP. However, this efficacy of FA to inhibit hVSMC growth was either partially or completely inhibited in hVSMCs infected with 10 or 100 PFU/cell, respectively. Overexpression of TERT further prevented the ability of FA to arrest hVSMCs in G0/G1 phase and inhibit S phase entry14 (Online Figure III). Conversely, knock-down of TERT expression decreased telomerase activity and cell proliferation while preventing the growth-inhibitory effect of FA (Fig. 6B). Collectively, these data demonstrate that activation of telomerase by overexpressing TERT induces VSMC proliferation and that TERT constitutes an important target for the inhibition of VSMC proliferation by PPARα.
Figure 6. Inhibition of VSMC proliferation by PPARα ligands is mediated via repression of TERT.
A, G0/G1-arrested hVSMCs were infected for 6h with the indicated doses of Ad-GFP or Ad-GFP/TERT (A) or transfected with TERT siRNA (2 μg) (B). Cells were pretreated with the indicated doses of FA and reincubated in routine medium for 72h (A) or 36h (B). Telomerase activity (left panel) and cell proliferation (right panel) are expressed as mean ± SEM percent increase over vehicle-treated cells (n=3, # P < 0.05 vs. vehicle; * P < 0.05 vs. Ad-GFP (A) or # P < 0.05 vs. control siRNA- vehicle-treated cells (B).
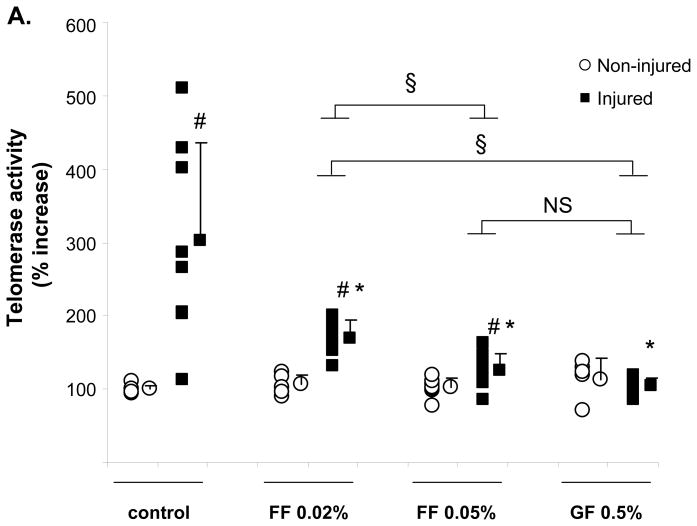
PPARα activation inhibits telomerase activation during the proliferative response underlying neointima formation in vivo
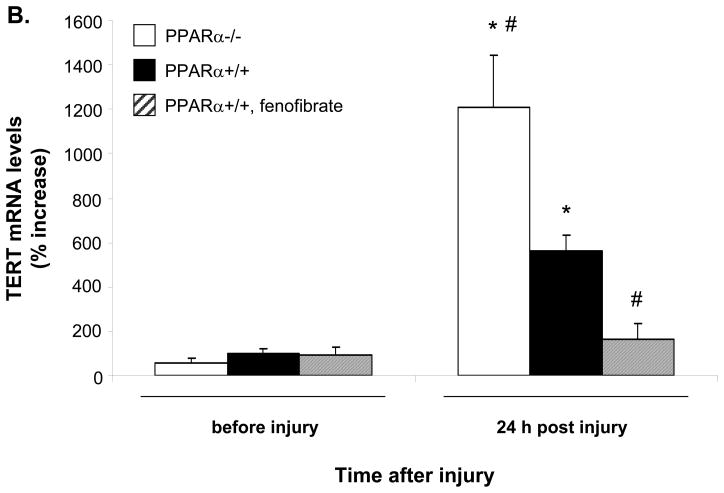
To finally determine whether the down-regulation of telomerase activity by PPARα ligands is applicable in vivo, we performed femoral artery denudation injuries in mice treated with fenofibrate (FF 0.02 %, 0.05 %) or gemfibrozil (GF 0.5 %). Using liver tissues from these mice, we verified that FF (0.05%) and GF (0.5%) significantly induced mRNA expression of the bona fide PPARα target gene acyl-CoA oxidase (ACO)19 to a similar extent (Online Figure IV). As reported in previous studies9, 20, we observed a significant induction of telomerase activity 48h after injury (Fig. 7A). Telomerase activation following injury was significantly decreased in mice treated with the PPARα agonists FF (FF 0.02 % 44.0%±7.5 inhibition, P=0.02; FF 0.05% 58.9%±7.5 inhibition, P=0.003) and GF (GF 0.5% 65.6%±3.6 inhibition, P<0.001). The ability of PPARα to repress TERT expression during the proliferative response was further addressed using a carotid injury mouse model14 (Fig. 7B). TERT mRNA levels were induced 24h after injury in carotid segments isolated from wild-type mice. This induction was augmented in tissues isolated from PPARα−/− mice and prevented by fenofibrate treatment in wild-type mice. Together, these data demonstrate that PPARα activation suppresses TERT expression and telomerase activation in response to vascular injury in vivo and point to an important role of TERT for the inhibition of neointimal VSMC proliferation by PPARα ligands.
Figure 7. PPARα activation inhibits TERT gene expression and telomerase activation during the proliferative response after vascular injury.
A, Sv/129 mice were fed a diet supplemented with fenofibrate (FF) 0.05% (n=8), FF 0.02% (n=6), or gemfibrozil (GF) 0.5% (n=7) for one week prior to endovascular femoral artery wire injury. Mice fed standard chow diet served as control group (n=8). Forty eight hours after sham surgery or arterial injury tissues were harvested and analyzed for telomerase activity. For each group, the individual values are indicated on the left and the mean ± SD is indicated on the right (# P < 0.05 vs. non-injured sham surgery, *P < 0.05 vs. injured control, § P < 0.05 comparing drug treatment groups as indicated). B, PPARα−/− or PPARα+/+ Sv/129 mice treated with FF 0.05% for one week were subjected to carotid artery injury. TERT mRNA levels were measured in the carotid segments harvested either before or 24h after injury. Data are expressed as mean ± SD (n=5 for each group) percent increase relative to the non-injured PPARα wild-type mice (* P < 0.05 vs. before injury of the same group, # P < 0.05 vs. PPARα wild-type mice).
DISCUSSION
Telomere maintenance is essential for cell proliferation and the primary mechanism preventing telomere attrition is through the action of telomerase.4 Earlier work has demonstrated that telomerase is activated in response to mitogenic stimulation of VSMCs and following vascular injury8–10. While these studies pointed to the control of telomerase activation as an important mechanism regulating VSMC proliferation7, 8, the transcriptional pathways underlying TERT expression and the mechanisms by which telomerase activation contributes to cell proliferation remain elusive. In the present study, both ectopic TERT expression and TERT siRNA approaches confirm a key role of telomerase activation in the control of VSMC proliferation and cell cycle progression. By exploiting the underlying transcriptional mechanisms governing TERT expression in VSMCs, we demonstrate that TERT transcription in VSMCs depends on a functional E2F site located at -174/-170 in the TERT promoter. Since E2F-1 is activated in the late G1 phase due to the dissociation from hyperphosphorylated pRB proteins,2 these results identify TERT as a bona-fide E2F-1 target gene and provide a molecular basis for the induction of TERT in response to mitogens.8, 9 Consistent with this notion, overexpression of E2F has recently been found to induce TERT transcription in somatic cells. 17, 18. Moreover, expression of the upstream tumor suppressor and CDKI p16, which attenuates VSMC proliferation and intimal hyperplasia as we previously demonstrated14, impinges upon TERT transcription and telomerase activation. Therefore, these observations support the concept that E2F released during cell cycle progression contributes to the activation of telomerase, which further augments cell proliferation during mitogenic stimulation.
The second key observation presented here demonstrates that PPARα activation in VSMCs inhibits TERT gene expression and telomerase activity in vitro and during the proliferative response underlying neointima formation in vivo. Overexpression of TERT in VSMCs reversed the anti-proliferative efficacy of PPARα ligands placing the inhibition of telomerase by PPARα in a central position for the growth-inhibitory effect of fibrates14. At the molecular level, the inhibition of TERT gene expression by ligand-activated PPARα involves the inhibition of E2F-1 binding and the assembly of the pocket proteins p107 and p130 at the proximal TERT promoter. In contrast, binding of hypophosphorylated pRB to the proximal E2F site was unaffected by the PPARα ligand, which is consistent with recent studies suggesting that p107 and p130, but not pRB, are involved in the trans-repression of E2F-responsive genes.21 Our studies further demonstrate that p16 is required for the recruitment of p107 and p130 to the TERT promoter and the inhibition of E2F-1 binding by PPARα ligands. Since p16 expression is trans-activated through direct PPARα binding to a degenerated DR1 PPRE,14 a model appears conceivable, in which PPARα-mediated telomerase repression requires p16 as an upstream transcriptional target gene (Fig. 8). Consistent with these data, recent studies suggest that gene repression at E2F sites through the recruitment of p107 and/or p130 mediates p16-induced growth arrest22, 23. Furthermore, in the context of VSMC proliferation, p107 and p130 have been implicated in mediating cell cycle arrest while p130 overexpression inhibits injury-induced neointima formation.24, 25 Considering this evidence and our observation that PPARα or p16 deficiency increase TERT expression and telomerase activity (Online Figure V), we infer that PPARα inhibits VSMC proliferation by repressing telomerase activity through a p16-dependent recruitment of p107 and p130 to the TERT promoter, an effect that will ultimately alter the ability of E2F-1 to trans-activate TERT transcription.
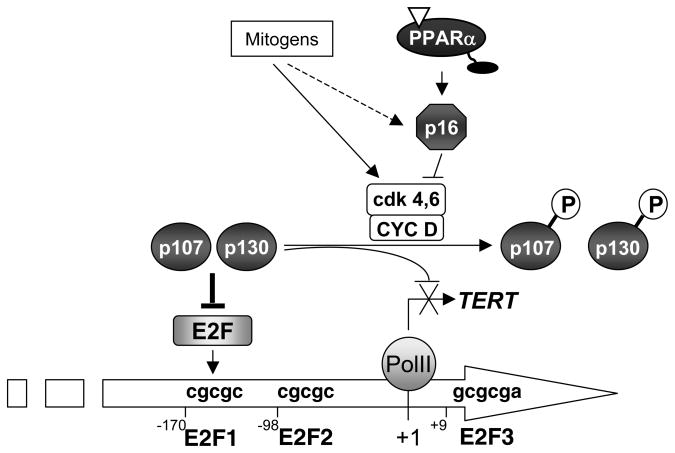
Figure 8. Model for the repression of the TERT promoter by PPARα.
Ligand-induced activation of PPARα prevents G1→S VSMC cell cycle progression and proliferation by transcriptionally increasing expression levels of the CDKI p16, an effect that represses the activity of cyclin D-cdk4/6 complexes to phosphorylate pocket proteins. The hypophosphorylated pRB proteins p107 and p130 are recruited to the proximal E2F-binding element of TERT gene promoter. The complexes formed with p107 and p130 induce active trans-repression of TERT promoter, thereby preventing the E2F-1-dependent trans-activation of the TERT promoter. This inhibition of TERT transcription will inhibit telomerase activity and thereby prevent VSMC proliferation.
VSMC proliferation is regulated through a complex network of interconnected pathways and transcriptional repression of the p16/RB/E2F pathway may not be the only means, by which PPARα mediates antiproliferative signals. The search for endogenous factors regulating VSMC proliferation has recently characterized several novel nodal proteins required for VSMC division and survival, including survivin1, 26, 27 and the pre-B-cell colony-enhancing factor (PBEF).28 Interestingly, survivin, a member of the mammalian “inhibitor of apoptosis” family, enhances telomerase activity in tumor cells by inducing TERT expression, is transcriptionally repressed by pRB/p130, and induced by E2F-dependent trans-activation in fibroblasts.29, 30 Considering this similarity of transcriptional regulation, PPARα may repress survivin transcription in VSMCs, which could ultimately alter telomerase activation through a yet to be defined molecular mechanism. Similarly, van der Veer et al. have recently reported that PBEF enhances VSMC survival and extends lifespan while its antagonism induces premature senescence.28, 31. Although, to date a link between PBEF and telomerase in the regulation of cell proliferation has not been reported, it is intriguing to speculate that both survivin and PBEF may induce telomerase to enhance cell survival and promote VSMC proliferation, a process that could be transcriptionally repressed by PPARα.
Clinically, the cardiovascular benefit of fibrates was initially attributed to their metabolic efficacy to improve dyslipidemia; however, recent studies suggest pleiotropic vascular effects including the inhibition of neointima formation12, 14. This study extends these observations and outlines repression of telomerase as previously unrecognized mechanism, by which PPARα activation inhibits vascular VSMC proliferation. Fibrates are often co-administered with statins and given that both drugs inhibit VSMC proliferation14, 32, combination therapy could be most effective in preventing cardiovascular complications driven by aberrant VSMC proliferation. Although statins have been reported to limit VSMC proliferation by inhibiting Rho32, we have previously demonstrated that the pleiotropic anti-inflammatory statin effects are mediated through PPARα33. Considering these findings and the remarkable similarity between the pleiotropic effects of statins and fibrates, it is intriguing to suggest a mechanistic link between both classes of drugs and further studies are warranted to assess their potential additive or synergistic effects on VSMC proliferation and characterize the involved mechanisms.
Supplementary Material
Acknowledgments
We thank Violeta Arsenescu for technical assistance in the isolation of femoral tissues and Dr. Carole Amant for performing carotid arterial injuries.
SOURCES OF FUNDING
This work was supported in part by the National Institutes of Health(RO1 HL084611 to D. B.) and by an American Diabetes Association Research Award (1 06-RA-17 to D.B.). F. Gizard was supported by a Fulbright Research Scholar Grant and a Postdoctoral Fellowship Grant from the American Heart Association (0725313B). T. Nomiyama and Y. Zhao were supported by Fellowship Grants from the American Heart Association (0725620B and 0815514D).
Footnotes
DISCLOSURES: None.
References
- 1.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 2.Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–67. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- 3.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 4.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 5.Chan SW, Blackburn EH. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene. 2002;21:553–563. doi: 10.1038/sj.onc.1205082. [DOI] [PubMed] [Google Scholar]
- 6.Poole JC, Andrews LG, Tollefsbol TO. Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT) Gene. 2001;269:1–12. doi: 10.1016/s0378-1119(01)00440-1. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Li H, Mu FT, Ebisui O, Funder JW, Liu JP. Telomerase activation causes vascular smooth muscle cell proliferation in genetic hypertension. FASEB J. 2002;16:96–98. doi: 10.1096/cj.01-0447fje. [DOI] [PubMed] [Google Scholar]
- 8.Minamino T, Kourembanas S. Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ Res. 2001;89:237–243. doi: 10.1161/hh1501.094267. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa D, Nomiyama T, Nakamachi T, Heywood EB, Stone JF, Berger JP, Law RE, Bruemmer D. Activation of peroxisome proliferator-activated receptor gamma suppresses telomerase activity in vascular smooth muscle cells. Circ Res. 2006;98:e50–59. doi: 10.1161/01.RES.0000218271.93076.c3. [DOI] [PubMed] [Google Scholar]
- 10.Minamino T, Komuro I. The role of telomerase activation in the regulation of vascular smooth muscle cell proliferation. Drug News Perspect. 2003;16:211–216. doi: 10.1358/dnp.2003.16.4.829332. [DOI] [PubMed] [Google Scholar]
- 11.Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ Res. 2006;99:1167–1180. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- 12.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glass CK. Going nuclear in metabolic and cardiovascular disease. J Clin Invest. 2006;116:556–560. doi: 10.1172/JCI27913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gizard F, Amant C, Barbier O, Bellosta S, Robillard R, Percevault F, Sevestre H, Krimpenfort P, Corsini A, Rochette J, Glineur C, Fruchart JC, Torpier G, Staels B. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J Clin Invest. 2005;115:3228–3238. doi: 10.1172/JCI22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown PJ, Stuart LW, Hurley KP, Lewis MC, Winegar DA, Wilson JG, Wilkison WO, Ittoop OR, Willson TM. Identification of a subtype selective human PPARalpha agonist through parallel-array synthesis. Bioorg Med Chem Lett. 2001;11:1225–1227. doi: 10.1016/s0960-894x(01)00188-3. [DOI] [PubMed] [Google Scholar]
- 16.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 17.Crowe DL, Nguyen DC, Tsang KJ, Kyo S. E2F-1 represses transcription of the human telomerase reverse transcriptase gene. Nucleic Acids Res. 2001;29:2789–2794. doi: 10.1093/nar/29.13.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Won J, Yim J, Kim TK. Opposing regulatory roles of E2F in human telomerase reverse transcriptase (hTERT) gene expression in human tumor and normal somatic cells. FASEB J. 2002;16:1943–1945. doi: 10.1096/fj.02-0311fje. [DOI] [PubMed] [Google Scholar]
- 19.Duez H, Lefebvre B, Poulain P, Torra IP, Percevault F, Luc G, Peters JM, Gonzalez FJ, Gineste R, Helleboid S, Dzavik V, Fruchart JC, Fievet C, Lefebvre P, Staels B. Regulation of human apoA-I by gemfibrozil and fenofibrate through selective peroxisome proliferator-activated receptor alpha modulation. Arterioscler Thromb Vasc Biol. 2005;25:585–591. doi: 10.1161/01.ATV.0000154140.73570.00. [DOI] [PubMed] [Google Scholar]
- 20.Torella D, Leosco D, Indolfi C, Curcio A, Coppola C, Ellison GM, Russo VG, Torella M, Volti GL, Rengo F, Chiariello M. Aging exacerbates negative remodeling and impairs endothelial regeneration after balloon injury. Am J Physiol Heart Circ Physiol. 2004;287:H2850–2860. doi: 10.1152/ajpheart.01119.2003. [DOI] [PubMed] [Google Scholar]
- 21.Rayman JB, Takahashi Y, Indjeian VB, Dannenberg JH, Catchpole S, Watson RJ, te Riele H, Dynlacht BD. E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex. Genes Dev. 2002;16:933–947. doi: 10.1101/gad.969202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang HS, Postigo AA, Dean DC. Active transcriptional repression by the Rb-E2F complex mediates G1 arrest triggered by p16INK4a, TGFbeta, and contact inhibition. Cell. 1999;97:53–61. doi: 10.1016/s0092-8674(00)80714-x. [DOI] [PubMed] [Google Scholar]
- 23.Bruce JL, Hurford RK, Jr, Classon M, Koh J, Dyson N. Requirements for cell cycle arrest by p16INK4a. Mol Cell. 2000;6:737–742. doi: 10.1016/s1097-2765(00)00072-1. [DOI] [PubMed] [Google Scholar]
- 24.Claudio PP, Fratta L, Farina F, Howard CM, Stassi G, Numata S, Pacilio C, Davis A, Lavitrano M, Volpe M, Wilson JM, Trimarco B, Giordano A, Condorelli G. Adenoviral RB2/p130 gene transfer inhibits smooth muscle cell proliferation and prevents restenosis after angioplasty. Circ Res. 1999;85:1032–1039. doi: 10.1161/01.res.85.11.1032. [DOI] [PubMed] [Google Scholar]
- 25.Sindermann JR, Smith J, Kobbert C, Plenz G, Skaletz-Rorowski A, Solomon JL, Fan L, March KL. Direct evidence for the importance of p130 in injury response and arterial remodeling following carotid artery ligation. Cardiovasc Res. 2002;54:676–683. doi: 10.1016/s0008-6363(02)00272-9. [DOI] [PubMed] [Google Scholar]
- 26.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conte MS, Altieri DC. Survivin regulation of vascular injury. Trends Cardiovasc Med. 2006;16:114–117. doi: 10.1016/j.tcm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 28.van der Veer E, Nong Z, O’Neil C, Urquhart B, Freeman D, Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- 29.Endoh T, Tsuji N, Asanuma K, Yagihashi A, Watanabe N. Survivin enhances telomerase activity via up-regulation of specificity protein 1- and c-Myc-mediated human telomerase reverse transcriptase gene transcription. Exp Cell Res. 2005;305:300–311. doi: 10.1016/j.yexcr.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y, Saavedra HI, Holloway MP, Leone G, Altura RA. Aberrant Regulation of Survivin by the RB/E2F Family of Proteins. J Biol Chem. 2004;279:40511–40520. doi: 10.1074/jbc.M404496200. [DOI] [PubMed] [Google Scholar]
- 31.van der Veer E, Ho C, O’Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of Human Cell Lifespan by Nicotinamide Phosphoribosyltransferase. J Biol Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- 32.Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA Reductase Inhibitors Attenuate Vascular Smooth Muscle Proliferation by Preventing Rho GTPase-induced Down-regulation of p27Kip1. J Biol Chem. 1999;274:21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.