Abstract
Increased cGMP-specific phosphodiesterase (PDE5) activity in renal inner medullary collecting duct (IMCD) cells contributes to resistance to atrial natriuretic peptide (ANP) and the excessive sodium retention seen in experimental nephrotic syndrome and liver cirrhosis. Normal pregnancy is also accompanied by sodium retention and plasma volume expansion, and pregnant rats are resistant to the natriuretic action of ANP. The authors investigated a possible role of increased renal PDE5 activity in the physiologic sodium retention of normal rat pregnancy. The natriuresis and increased urinary cGMP excretion (UcGMPV) evoked by acute volume expansion (a measure of renal responsiveness to endogeneous ANP) was blunted in 16-d pregnant versus virgin rats, despite equivalent increases in circulating ANP in pregnants and virgins. The ANP-dependent cGMP accumulation in isolated IMCD cells from pregnants was blunted versus virgins and restored by the PDE5-selective antagonist DMPPO (10−7 mol/L). PDE5 activity in vitro and PDE5 protein abundance in IMCD were greater in pregnants. Four days postpartum, volume expansion natriuresis, UcGMPV, and PDE5 protein levels in IMCD cell homogenates had returned to virgin values. These results demonstrate that normal rat pregnancy leads to in vivo and in vitro renal resistance to ANP, in association with heightened activity of the cGMP-specific PDE5 in IMCD. This may contribute to the physiologic sodium retention of normal pregnancy.
Normal pregnancy is characterized by marked maternal hemodynamic changes, including a profound plasma volume expansion. In women, a maximum increment of approximately 50% occurs; in the pregnant rat, an increase of 80 to 100% is normal (1-4). This large plasma volume expansion represents an “optimal, physiologic” response; in women, failure to volume expand is associated with poor reproductive performance and is also a feature of preeclampsia (1-3). The plasma volume expansion results from a slow, cumulative net renal sodium retention, the mechanism of which is unclear because there are many conflicting signals to the kidney in pregnancy. For example, large increases occur in circulating angiotensin and aldosterone levels, ureteral pressure increases and systemic BP falls, all of which will promote net sodium retention. On the other hand, several natriuretic systems are also activated, including the large (30 to 50%) increase in GFR, high progesterone levels that exert a marked antimineralocorticoid action, increased plasma atrial natriuretic peptide (ANP) concentration, increased renal production of nitric oxide (NO), and decreased renal Na,K-ATPase abundance and activity, (3-7).
In the case of ANP, there is evidence of a selective renal resistance to the natriuretic actions of ANP in normal rat pregnancy (8), which could contribute to a “permissive” renal sodium retention and plasma volume expansion. Renal resistance to ANP-mediated natriuresis coupled with cumulative sodium retention and volume expansion are also seen in several pathologic conditions, namely, congestive heart failure, nephrotic syndrome, and liver cirrhosis. ANP signals via guanosine-3’,5’-cyclic monophosphate (cGMP) and increased renal activity of the cGMP-specific phosphodiesterase (PDE5) plays a major role in mediating this resistance in experimental nephrotic syndrome and severe liver disease caused by common bile duct ligation (CBDL) in the rat (9-12). Increased PDE5 activity has also been observed in a canine model of congestive heart failure (13), raising the possibility that in these pathologic sodium-retaining conditions, renal resistance to ANP may occur via the common mechanism of an increase in PDE5 activity, which would blunt ANP signaling.
Because of the similarities in renal and systemic hemodynamics between these states of pathologic volume expansion and normal pregnancy, the present experiments were conducted to test the hypothesis that increased renal activity of PDE5 occurs during normal rat pregnancy.
Materials and Methods
We studied 96 female Sprague-Dawley rats weighing 240 to 280 g purchased from Harlan Sprague-Dawley (Indianapolis, IN). Rats were maintained at constant temperature and humidity with 12-h:12-h light-dark cycles and given free access to standard laboratory chow and tap water. The experimental protocols were reviewed and approved by the Committee on Animal Research of the University of California San Francisco. One week after arrival, two female rats were housed with one male and a vaginal wash was taken each morning to test for the presence of sperm. When sperm was detected this was designated as day 1 of pregnancy. Gestation lasts 22 d in the rat (4). A separate group of pregnant rats was allowed to deliver and subsequently studied on day 4 postpartum; unmated, age-matched females were used as the virgin control group.
Acute Volume Expansion
On the day of the experiment, the 16-d pregnant rats, virgin control rats, or rats at 4 d postpartum were anesthetized with Inactin (100 mg/kg intraperitoneally), and catheters were placed in the jugular vein, carotid artery, and bladder. All rats received an intravenous infusion of 0.9% saline at 2.4 ml/h. After 1 h of equilibration, a blood sample (100 μl) was obtained, and urine was collected for three 10-min intervals. After control measurements an intravenous infusion of isotonic saline was given (2% of body weight over 5 min) to produce an acute volume expansion (VE). Urine samples were obtained for three successive 10-min periods after VE. Mean arterial pressure (MAP) was monitored throughout the experiment by means of a Statham P23id BP transducer attached to a Grass Model 7D polygraph (Grass-Telefactor, West Warwick, RI). A 5-ml blood sample was harvested at the end of the experiment into a chilled Vacutainer tube (Becton-Dickinson Co., Rutherford, NJ) containing EDTA and aprotinin, 500 KIU. The blood sample was immediately centrifuged at 4°C, and the plasma was decanted and stored at −80°C until assayed for ANP concentration. Urine flow rate was determined gravimetrically, and urine sodium concentration measured by flame photometry (Model 943, Instrumentation Laboratories, Lexington, MA). Sodium excretion (UNaV) was calculated as the product of the two. Urinary cGMP concentration was determined in unextracted urine using a commercially available kit (Amersham, Piscataway, NJ). Before the assay, samples were acetylated according to the manufacturer’s instructions. Plasma samples underwent extraction through SepPak C18 cartridges (Water Associates, Milford, MA). Eluates were lyophilized, reconstituted in assay buffer, and assayed for plasma ANP concentration using a commercial kit (Peninsula Laboratories Inc., Belmont, CA).
ANP-Dependent cGMP Accumulation in Inner Medullary Collecting Duct (IMCD) Cells
IMCD cells from rat kidneys were isolated by collagenase digestion according to the method of Zeidel et al. (14) as performed in our laboratory (9-12). Aliquots of freshly prepared IMCD cells were suspended in 350 μl of buffer containing the following: NaCl, 124 mmol/L; KCl, 5 mmol/L; CaCl2, 1 mmol/L; MgSO4, 0.4 mmol/L; Na2 HPO4, 1 mmol/L; N-hydroethylpiperazine-N1–2 ethanesulfonic acid (HEPES), 50 mmol/L; and glucose, 7.5 mmol/L; pH 7.4. Cells were preincubated for 10 min at 37°C in a shaking water bath. In some preparations, the PDE5 inhibitor DMPPO 10−7 mol/L was included. Incubation was started by adding synthetic ANP (Peninsula Laboratories, Belmont, CA) at a concentration of 10−9 to 10−6 mol/L and terminated after 10 min by adding 750 μl of ice-cold TCA. The precipitated protein was sedimented by centrifugation, and the pellet was dissolved in 1 N NaOH and assayed for protein concentration by the Bradford dye-binding method (Bio-Rad Laboratories, Hercules, CA). The supernatant fluid was extracted four times with five volumes of water-saturated ethyl ether to remove the TCA before being evaporated to dryness under a stream of air and stored at −80°C until assayed for cGMP content. For the cGMP assay, samples were dissolved in 50 mmol/L sodium acetate buffer, pH 6.2, and 100-μl aliquots were assayed with a commercial kit (Amersham, Piscataway, NJ) after acetylation according to the manufacturer’s instructions. Results of duplicate determinations were averaged and expressed as femtomoles accumulated per 10-min incubation per milligram of protein.
ANP Binding Activity in IMCD Cells
Freshly dispersed IMCD cells were resuspended in 400 μl of phosphate-buffered saline (PBS). The binding reaction was run in 200 μl of Dulbecco Modified Eagle’s Medium H21, 0.2% bovine serum albumin (BSA), 10 mM HEPES buffer, pH 7.6. Varying concentrations of 125I-ANP (3 × 10−12 to 3 × 10−9 mol/L), and phosphoramidon (8 μg/ml; a neutral endopeptidase inhibitor that prevents ANP degradation) were used in the presence or absence of 3 × 10−6 mol/L unlabeled ANP. Reactions were initiated by the addition of 10 μl of the cell suspension and run for 30 min at 37°C. Cells were pelleted, resuspended, washed with PBS four times at 4°C, repelleted, and lysed in 1 ml 1 N NaOH. A 900-μl aliquot was taken for counting. Nonspecific binding was determined from the radioactive counts in the presence of 3 × 10−6 mol/L cold ANP, and the value was subtracted from total counts. Scatchard analysis yielded a straight line, consistent with a single homogeneous class of receptors. Protein concentration was determined by the Coomassie blue reagent (Pierce Biochemicals, Rockford, IL).
PDE Assay
Fresh IMCD cells were homogenized in a glass-Teflon homogenizer. The homogenizing medium had the following composition: sucrose, 250 mmol/L; Tris,10; 2-(morpholino) ethanesulfonic acid, 10 mmol/L; MgCl2, 5 mmol/L; ethylenediaminetetraacetic acid (EDTA), 1 mmol/L; egtazic acid (EGTA),1 mmol/L; and mercaptoethanol, 5 mmol/L; pH 7.4. The solution also contained 0.1 μmol/L phenylmethylsulfonyl fluoride (PMSF), 0.1 μmol/L leupeptin, and 0.1 μmol/L pepstatin. Homogenization (1:3 wet wt/vol), and all other procedures were conducted at 4°C. The homogenate was centrifuged for 60 min at 100,000 × g, and the supernatant was used for the PDE assay.
The PDE5 assay (total volume, 125 μl) was modified from Turko, Francis, and Corbin (15). The incubation mixture contained the following: MOPS, 40 mmol/L (pH 7.5); EGTA, 0.8 mmol/L; magnesium acetate, 15 mmol/L; and 0.5 μmol/L [3H]-cGMP (specific activity 444 GBq/mmol) and 0.2 mg/ml BSA. The PDE activity in aliquots incubated without Ca2+ and calmodulin was determined as basal PDE5 activity (16). In some preparations, the PDE5 inhibitor DMPPO 10−7 mol/L was included. Reactions were started by the addition of 25 μl of the diluted homogenate, followed by incubation for 15 min at 30°C. Heating at 95°C for 3 min stopped the reaction. After cooling, 10 μl of a solution of 10 mg/ml nucleotidase as crotalus atox venom were added followed by a 20 min incubation at 30°C. Nucleoside products were separated from unreacted nucleotides using a DEAE Sephadex A-25 column equilibrated with 20 mmol/L Tris-HCl buffer, pH 6.8. The column effluent was counted for [3H] activity in a liquid scintillation counter (Parkard Instruments, Chicago, IL).
Immunoblotting
We measured PDE5 protein content in kidneys of virgin and pregnant rats. The kidneys were separated into cortex and medulla. The cortex was diced into very small pieces with a razor, while medulla was processed for IMCD cells as described above. The cortical tissue fragments and IMCD cells were added to cold lysis buffer (3 ml/g tissue of RIPA buffer; Santa Cruz Biotechnologies, Santa Cruz, CA) containing 1× PBS, 1% igepal CA-630, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS) to which 100 μg/ml PMSF and 45 μg/ml aprotinin were then added. The suspension was homogenized using a polytron at 0°C and centrifuged at 10,000 × g at 4°C for 10 min. The supernatant was recentrifuged, and the second supernatant was measured for protein content using the dye-binding method of Bradford (Bio-Rad Laboratories, Hercules, CA) and stored at −20°C. Aliquots (40 μg protein) were size fractionated on precast 4 to 20% gradient SDS-polyacrylamide gels in an Amersham SE 280 vertical gel electrophoresis unit at 40 mA for 1 h and electrotransferred to nitrocellulose membranes. The membranes were then blocked with 5% dried milk in PBS, pH 7.5, containing 0.1% Tween 20 for 1 h at room temperature, rinsed, and incubated for 1 h with the primary antiserum, raised against bovine lung PDE5 (Chemicon International Inc., Temecula, CA) at a dilution of 1:1000. After rinsing, the membrane was next incubated with the horseradish peroxidase-conjugated donkey anti-rabbit secondary antibody diluted 1:1000, for 30 min (RPN 2108 ECL kit; Amersham, Arlington Heights, IL). The chemiluminescent signal was detected on x-ray film and quantitated by densitometric scanning.
Statistical Analyses
Experimental results are expressed as means ±SEM. The t tests for paired or unpaired data were used to assess differences within and between groups, respectively, and one-way and repeated-measures ANOVA with the Bonferroni post hoc test were used for multiple comparisons among and within groups. A P value < 0.05 was taken to indicate a significant difference.
Materials
All compounds were purchased from Sigma Chemical Co., St. Louis, MO, except as noted.
Results
As shown in Figure 1A, acute VE (intravenous 0.9% saline infusion, 2% body weight over 5 min) led to a brisk increase in UNaV in virgin rats that was maximal in the second 10-min urine collection period. This is similar to our published results in male rats (9-12). In pregnant rats, the increase in UNaV in response to the administered saline was markedly blunted when compared with virgins, and the natriuretic response to VE was restored by 4 d postpartum. The responses were paralleled by changes in UcGMPV, a marker of the renal actions of ANP. The blunted natriuretic response to acute volume expansion in pregnant rats was accompanied by a reduced rate of UcGMPV compared with virgin and 4 d postpartum rats (Figure 1B). MAP did not differ during control and after VE in the different groups (virgin, 109 ± 4 to 113 ± 5; pregnant, 111 ± 7 to 115 ± 5; postpartum, 110 ± 6 to 114 ± 4 mmHg; n = 6/group; P = NS for all). Figure 2 presents data on the effect of VE on the plasma concentration of immunoreactive ANP at the end of the 30-min experimental period. Baseline plasma ANP was not significantly different among the three groups and in response to VE plasma ANP concentrations increased to an equivalent extent in virgin, pregnant, and postpartum rats. Thus, pregnant rats underwent a similar increase in plasma ANP concentration after VE, although demonstrating blunted VE natriuresis, thereby confirming resistance to ANP-mediated natriuresis in pregnancy. Measurement of ANP binding activity in IMCD cells from virgin and pregnant rats as described in Materials and Methods revealed no difference in either the affinity (Kd) (virgin, 5.5 ± 0.7 × 10−11; pregnant, 6.5 ± 0.8 × 10−11 mol/L; P = NS) or maximum binding capacity (Bmax) (virgin, 2.8 ± 0.9 × 10−14; pregnant, 3.8 ± 1.0 × 10−14 mol/mg protein; n = 5/group; P = NS) in IMCD cells between virgin and pregnant rats, suggesting that the renal resistance to ANP in pregnancy is due to postreceptor changes.
Figure 1.
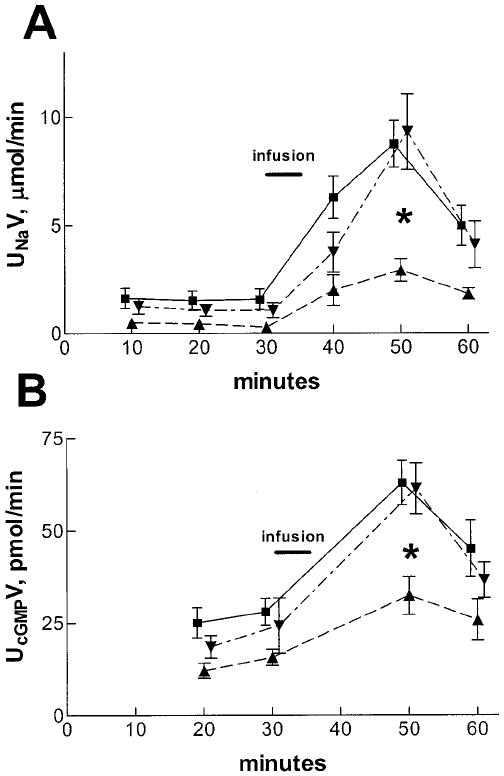
(A) Urinary sodium excretion (UNaV) after volume expansion with intravenous saline infusion (2% body weight over 5 min, indicated by the bar) in 12 virgin (■), 6 pregnant (▲), and 11 postpartum (▼) rats. The infusion caused UNaV to increase in all groups. However, the natriuresis was blunted in the pregnant rats compared to the virgin and postpartum groups (*P < 0.01). (B) Urinary cGMP excretion (UcGMPV) in 6 rats from each group. UcGMPV was also blunted in the pregnant rats compared with the other two groups after the volume expansion (P < 0.01).
Figure 2.
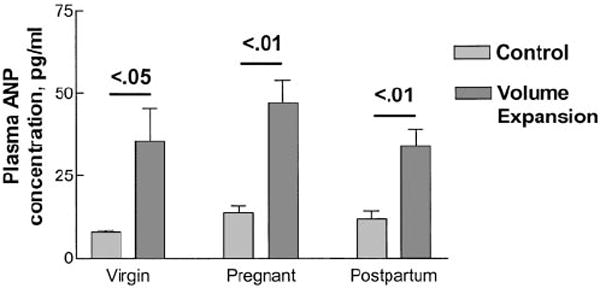
Plasma atrial natriuretic peptide (ANP) concentration in the three groups during hydropenia (control) and after volume expansion. Plasma ANP concentration did not differ significantly in control and rose equivalently after volume expansion in the three groups. n = 6 animals in each group.
Accumulation of cGMP by isolated IMCD cells from both virgin and pregnant rat kidneys increased in a dose-dependent manner in response to added ANP (Figure 3). However, IMCD cells from kidneys of pregnant rats exhibited significantly blunted cGMP accumulation compared with IMCD cells from virgins. This difference in response was abolished in the presence of the selective and potent PDE5 inhibitor DMPPO (17) (10−7 mol/L). As shown in Figure 4, the basal PDE activity of IMCD cells from pregnant rats was more than twice as high as that of cells from virgin rats; importantly, this increased PDE activity of IMCD cells from pregnant rats could be effectively inhibited by the PDE5-selective inhibitor DMPPO at 10−7 mol/L. In the samples from five pregnant rats in which paired measurements were available, PDE5 activity fell from 206 ± 18 pmol/mg per min without DMPPO to 106 ± 12 pmol/mg per min in the presence of the inhibitor (P = 0.0063). These observations suggest that increased PDE5 activity of IMCD cells from pregnant rat kidneys is responsible for the resistance to the natriuretic action of ANP during rat pregnancy. This possibility is supported by the results of the Western blots shown in Figure 5, which demonstrate that the PDE5 antiserum detected a single protein of molecular weight of approximately 98 kD, the predicted size of rat PDE5. PDE5 protein abundance in the cytosol of IMCD cells from pregnant rat kidneys was significantly greater than that of IMCD cells from virgin or postpartum rats. No difference in PDE5 protein was observed in cortical homogenates from these rats.
Figure 3.
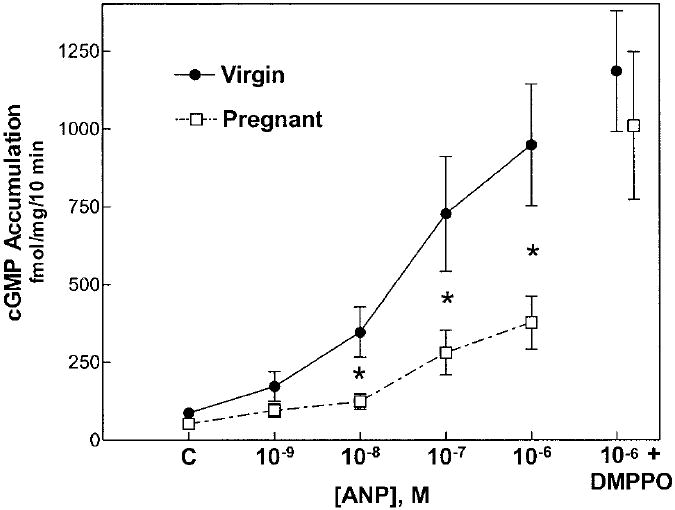
ANP-dependent cGMP accumulation by isolated inner medullary collecting duct (IMCD) cells from kidneys of virgin (solid circles) and pregnant (open squares) rats. ANP led to a dose-dependent increase in cGMP accumulation in both groups; however, the IMCD cells from pregnant rat kidneys displayed a markedly blunted response at ANP concentrations, 10−8 M and higher (*P < 0.05, virgin versus pregnant). This blunted response was corrected by addition of the PDE5-selective inhibitor DMPPO (10−7 M). Data are means ± SEM of six preparations in each group; C, control value.
Figure 4.
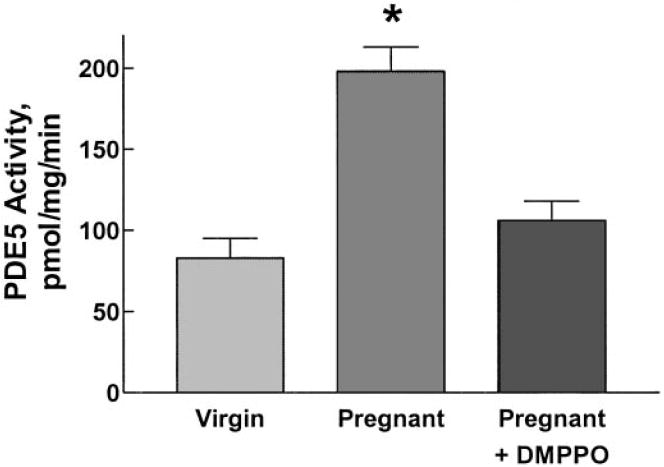
PDE5 activity in IMCD cell homogenates of virgin and pregnant rats. Activity was more than twice as great in homogenates from pregnant compared to virgin rats. This increase was almost completely inhibited by the PDE5-selective inhibitor DMPPO (10−7 M). Data are means ± SEM of values from six virgin rats and seven pregnant rats; PDE5 activity from five of the latter preparations was measured in the presence of DMPPO (see text).
Figure 5.
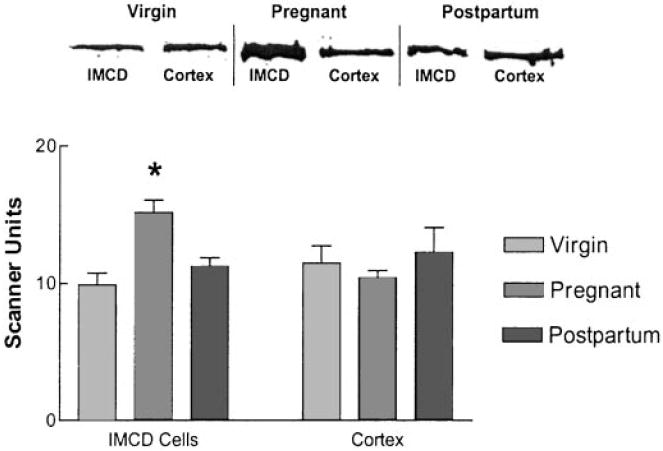
Immunoblotting of PDE5 protein abundance in cortical and IMCD cell homogenates from virgin, pregnant, and postpartum rat kidneys using a commercial antiserum to bovine lung PDE5. (Top) Representative immunoblot showing band intensity in IMCD cell but not cortical homogenates to be greater in samples from pregnant compared to virgin or postpartum rats; the product detected was of the expected size for rat PDE5 (98 kD). (Bottom) Scanning densitometry of immunoblots from 11 virgin, 11 pregnant, and 6 postpartum rats. PDE5 protein abundance was roughly 50% greater in IMCD cell homogenates from pregnant versus virgin or postpartum rat kidneys (*P < 0.05, one-way ANOVA), while no difference was detected in cortical homogenates. Data are means ± SEM.
Discussion
The main, novel finding of this study is that increased PDE5 activity and protein abundance occur selectively in the renal inner medulla in normal rat pregnancy. This correlates with the blunted ability of ANP (1) to stimulate cGMP accumulation in vitro, and (2) to increase UNaV and UcGMPV in vivo, in the normal pregnant rat. This localized renal resistance to the natriuretic actions of cGMP may play an important role in the physiologic extracellular fluid and plasma volume expansion of normal pregnancy.
Volume homeostasis in normal pregnancy remains an enigma. The massive, cumulative gestational volume expansion requires continual net renal sodium retention, yet many natriuretic as well as antinatriuretic signals are activated (3,4). In the case of ANP, however, the kidney becomes selectively resistant to the natriuretic actions of the hormone. The natriuretic response to administered ANP is markedly blunted in the pregnant rat versus the virgin, despite equivalent circulating levels (8); in the present study, we provide evidence of a similar response to VE stimulated, endogenous ANP. There were no differences in the renal hemodynamic or depressor responses to infused ANP in the pregnant rat that could explain the decreased natriuresis, suggesting that ANP signaling mediating these vascular responses is intact, but that the direct tubular actions of ANP are diminished in pregnancy (8). In the present study, we observed a selective blunting in ANP-dependent cGMP accumulation by IMCD cells from pregnant rats but no change in ANP receptor number or binding affinity, suggesting that a postreceptor event is responsible. Unchanged ANP receptors (NPR-A; the membrane-bound guanylyl cyclase) have also been reported in inner medulla of late pregnant sheep (18). In contrast, Omer et al. (19) reported downregulation of NPR-A in papilla and reduced receptor-coupled cGMP production in pregnant rats. This may reflect the difference in preparations used; our studies were carried out with freshly isolated, intact IMCD cells, whereas Omer et al. (19) used papillae that were homogenized and stored at −70°C.
There are other reasons to suspect that the blunted natriuretic response to ANP of normal pregnancy is a postreceptor event. Nitric oxide (NO) also signals via cGMP (by stimulation of the soluble guanylyl cyclase), and acetylcholine (Ach)-mediated natriuresis in the rat is predominantly NO-mediated. The natriuretic response to low-dose Ach is attenuated in the late pregnant rat, and this is not attributable to hemodynamic changes (20). Pressure natriuresis (increase in sodium excretion secondary to an abrupt rise in BP) is dependent on an intact renal NO system (21), and we have previously reported that pressure natriuresis is blunted in the late pregnant rat (22), an observation subsequently confirmed by Khraibi (23). In aggregate, these findings suggest that there is a ubiquitous blunting of the various natriuretic systems that signal via cGMP, rather than effects on system-specific receptors, since ANP and NO signal via different guanylyl cyclases. The same conclusion was drawn in a study of renal ANP resistance in a model of experimental nephrotic syndrome (9).
The activity of cyclic nucleotides is terminated by their degradation by phosphodiesterases (PDE), a family of enzymes that are classified on the basis of substrate affinity (cAMP versus cGMP), their response to pharmacologic inhibitors, and molecular cloning characteristics (24,25). At present, 11 family members have been recognized, most consisting of more than one gene. Since most gene products undergo tissue-specific alternative splicing, there is great variability in individual PDE isozymes according to location. Studies in dissected nephron segments and cultured cells show that cGMP hydrolysis is carried out by PDE1 and PDE5 in both glomeruli and IMCD (16,26). We have focused on the cGMP-specific PDE5 because of its involvement in the ANP resistance in states of pathologic sodium retention (9-12). Our present data strongly suggest that a localized increase in PDE5 protein abundance and enzyme activity takes place in IMCD cells in normal pregnancy. That PDE5 could be involved in the blunted ANP signaling was suggested from our finding that the selective PDE5 inhibitor DMPPO (17) corrected the blunted ANP-dependent cGMP accumulation of IMCD cells from pregnant rat kidneys in vitro. Furthermore, the increased PDE5 activity in cytosol of IMCD cells from pregnant rat kidneys was completely inhibited by DMPPO. In aggregate, these pharmacologic, biochemical, and immunoblotting data strongly implicate heightened protein expression and activity of PDE5 as a major component of the renal resistance to ANP occurring in normal rat pregnancy. The preservation of the hemodynamic actions of ANP (8) and the similar values of MAP observed in the different groups of our study, argue against heightened PDE5 activity in vascular tissue during pregnancy, and, coupled with the similar levels of PDE5 protein in cortical homogenates of virgin and pregnant rats (Figure 5), suggest that this response is confined to IMCD cells.
Our studies do not completely rule out a role for the other PDE family members with affinity for cGMP, namely PDE9, PDE10, and PDE11, because these isoforms are also found in kidney (27-30). However, PDE9 was poorly inhibited by the PDE5 inhibitors sildenafil and zaprinast (27,30). Similarly, PDE10 and PDE11 also were only weakly inhibited by PDE5 inhibitors (28,30). Although, the actions of DMPPO against these enzymes have not been examined, these PDE family subtypes would not appear to play a key role in renal ANP resistance in normal rat pregnancy.
Studies in postpartum rats indicated that the gestational adaptations in renal sodium handling were largely reversed 4 d after parturition. The full VE natriuresis was restored, as was blunted cGMP excretion, and, although we did not measure PDE5 activity, PDE5 protein levels in cytosol of IMCD cells were similar to virgins. These temporal relationships support a causal role of the increase in enzyme activity and protein levels in the blunted VE natriuresis and ANP resistance of pregnancy, and indicate that the signal(s) occurring in pregnancy responsible for these changes in IMCD cells have resolved soon after delivery. It will be important in future studies to define the time course, both of the onset of increased PDE5 activity during pregnancy and its resolution in the postpartum period.
In summary, the characteristics of renal resistance to ANP in normal rat pregnancy described here closely parallel those we have reported in experimental nephrotic syndrome and liver cirrhosis (9-12). Similar features of renal ANP resistance have also been described in salt-depleted rats (31,32). In pregnancy and salt depletion, this mechanism is physiologic and permits the plasma volume expansion required in pregnancy for normal intrauterine growth, and it provides a defense against further salt loss in salt-depleted animals. However, in nephrotic syndrome and cirrhosis of the liver, pathologic sodium retention is produced, which results in the development of edema and ascites.
Acknowledgments
This work was supported by a basic research grant # FY96–0081 from the March of Dimes Birth Defects Foundation and by NIH RO1 HD41751 to Dr. Baylis and grant RO1 DK58812 from the National Institutes of Health to Dr. Humphreys.
Footnotes
Portions of this work were presented at the World Congress of Nephrology, September 2001, San Francisco, CA, and have appeared in abstract form (J Am Soc Nephrol 12: 574A, 2001).
References
- 1.Chesley LC, Lindheimer MD. Renal hemodynamics and intra-vascular volume in normal and hypertensive pregnancy. In: Rubin PC, editor. Handbook of Hypertension. Amsterdam: Elsevier; 1988. pp. 38–65. [Google Scholar]
- 2.DeSwiet M. The physiology of normal pregnancy. In: Rubin PC, editor. Handbook of Hypertension. Amsterdam: Elsevier; 1988. pp. 1–9. [Google Scholar]
- 3.Lindheimer MD, Katz AI. Renal physiology and disease in pregnancy. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. New York: Raven Press; 1992. pp. 3371–3432. [Google Scholar]
- 4.Baylis C. Glomerular filtration and volume regulation in gravid animal models. Baillieres Clin Obstet Gynaecol. 1994;8:235–264. doi: 10.1016/s0950-3552(05)80320-7. [DOI] [PubMed] [Google Scholar]
- 5.Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest. 1995;96:482–490. doi: 10.1172/JCI118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santmyire BR, Baylis C. Isoform specific changes in kidney NOS activity during rat pregnancy. J Am Soc Nephrol. 1998;9:346A. Abstract. [Google Scholar]
- 7.Mahaney J, Felton C, Taylor D, Fleming W, Kong JQ, Baylis C. Renal cortical Na+-K+-ATPase activity and abundance is decreased in normal pregnant rats. Am J Physiol. 1998;275:F812–F817. doi: 10.1152/ajprenal.1998.275.5.F812. [DOI] [PubMed] [Google Scholar]
- 8.Masilamani S, Castro L, Baylis C. Pregnant rats are refractory to the natriuretic actions of atrial natriuretic peptide. Am J Physiol. 1994;267:R1611–R1616. doi: 10.1152/ajpregu.1994.267.6.R1611. [DOI] [PubMed] [Google Scholar]
- 9.Valentin JP, Qiu C, Muldowney WP, Ying WZ, Gardner DG, Humphreys MH. Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest. 1992;90:1302–1312. doi: 10.1172/JCI115995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valentin JP, Ying WZ, Sechi LA, Ling KT, Qiu C, Couser WG, Humphreys MH. Phosphodiesterase inhibitors correct resistance to natriuretic peptides in rats with Heymann nephritis. J Am Soc Nephrol. 1996;7:582–593. doi: 10.1681/ASN.V74582. [DOI] [PubMed] [Google Scholar]
- 11.Ni X, Cheng Y, Cao L, Gardner DG, Humphreys MH. Mechanisms contributing to renal resistance to atrial natriuretic peptide in rats with common bile-duct ligation. J Am Soc Nephrol. 1996;7:2110–2118. doi: 10.1681/ASN.V7102110. [DOI] [PubMed] [Google Scholar]
- 12.Ni XP, Safai M, Gardner DG, Humphreys MH. Increased cGMP phosphodiesterase activity mediates renal resistance to ANP in rats with bile duct ligation. Kidney Int. 2001;59:1264–1273. doi: 10.1046/j.1523-1755.2001.0590041264.x. [DOI] [PubMed] [Google Scholar]
- 13.Supaporn T, Sandberg SM, Borgeson DD, Heublein DM, Luchner A, Wei CM, Dousa TP, Burnett JC., Jr Blunted cGMP response to agonists and enhanced glomerular cyclic 3’,5’- nucleotide phosphodiesterase activities in experimental congestive heart failure. Kidney Int. 1996;50:1718–1725. doi: 10.1038/ki.1996.491. [DOI] [PubMed] [Google Scholar]
- 14.Zeidel ML, Silva P, Brenner BM, Seifter JL. cGMP mediates effects of atrial peptides on medullary collecting duct cells. Am J Physiol. 1987;252:F551–F559. doi: 10.1152/ajprenal.1987.252.3.F551. [DOI] [PubMed] [Google Scholar]
- 15.Turko IV, Francis SH, Corbin JD. Hydropathic analysis and mutagenesis of the catalytic domain of the cGMP-binding cGMP-specific phosphodiesterase (PDE5). cGMP versus cAMP substrate selectivity. Biochemistry. 1998;37:4200–4205. doi: 10.1021/bi972448r. [DOI] [PubMed] [Google Scholar]
- 16.Matousovic K, Tsuboi Y, Walker H, Grande JP, Dousa TP. Inhibitors of cyclic nucleotide phosphodiesterase isozymes block renal tubular cell proliferation induced by folic acid. J Lab Clin Med. 1997;130:487–495. doi: 10.1016/s0022-2143(97)90125-6. [DOI] [PubMed] [Google Scholar]
- 17.Coste H, Grondin P. Characterization of a novel potent and specific inhibitor of type V phosphodiesterase. Biochem Pharmacol. 1995;50:1577–1585. doi: 10.1016/0006-2952(95)02031-4. [DOI] [PubMed] [Google Scholar]
- 18.Mukaddam-Daher S, Gutkowska J, Tremblay J, Dam TV, Quillen EW., Jr Regulation of renal atrial natriuretic peptide receptors in pregnant sheep. Endocrinology. 1995;136:4565–4571. doi: 10.1210/endo.136.10.7664678. [DOI] [PubMed] [Google Scholar]
- 19.Omer S, Vaillancourt P, Peri KG, Varma DR, Mulay S. Down-regulation of renal atrial natriuretic factor receptors and receptor mRNAs during rat pregnancy. Am J Physiol. 1997;272:F87–F93. doi: 10.1152/ajprenal.1997.272.1.F87. [DOI] [PubMed] [Google Scholar]
- 20.Baylis C, Zhang XZ, Engels K. Selective blunting of the natriuretic response to acetylcholine (Ach) during pregnancy in the rat. J Am Soc Nephrol. 2000;11:24A. Abstract. [Google Scholar]
- 21.Majid DS, Williams A, Navar LG. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol. 1993;264:F79–F87. doi: 10.1152/ajprenal.1993.264.1.F79. [DOI] [PubMed] [Google Scholar]
- 22.Masilamani S, Hobbs GR, Baylis C. The acute pressure natriuresis response blunted and the blood pressure response reset in the normal pregnant rat. Am J Obstet Gynecol. 1998;179:486–491. doi: 10.1016/s0002-9378(98)70384-9. [DOI] [PubMed] [Google Scholar]
- 23.Khraibi A. Renal interstitial hydrostatic pressure and pressure natriuresis in pregnant rats. Am J Physiol. 2000;279:F353–F357. doi: 10.1152/ajprenal.2000.279.2.F353. [DOI] [PubMed] [Google Scholar]
- 24.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 25.Dousa TP. Cyclic-3’,5’-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 1999;55:29–62. doi: 10.1046/j.1523-1755.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- 26.Homma S, Gapstur SM, Coffey A, Valtin H, Dousa TP. Role of cAMP-phosphodiesterase isozymes in pathogenesis of murine nephrogenic diabetes insipidus. Am J Physiol. 1991;261:F345–F353. doi: 10.1152/ajprenal.1991.261.2.F345. [DOI] [PubMed] [Google Scholar]
- 27.Soderling SH, Bayuga SJ, Beavo JA. Identification and characterization of a novel family of cyclic nucleotide phosphodiesterases. J Biol Chem. 1998;273:15553–15558. doi: 10.1074/jbc.273.25.15553. [DOI] [PubMed] [Google Scholar]
- 28.Soderling SH, Bayuga SJ, Beavo JA. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc Natl Acad Sci USA. 1999;96:7071–7076. doi: 10.1073/pnas.96.12.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fawcett L, Baxendale R, Stacey P, McGrouther C, Harrow I, Soderling S, Hetman J, Beavo JA, Phillips SC. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci USA. 2000;97:3702–3707. doi: 10.1073/pnas.050585197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher DA, Smith JF, Pillar JS, St Denis SH, Cheng JB. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J Biol Chem. 1998;273:15559–15564. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- 31.Veress AT, Honrath U, Chong CK, Sonnenberg H. Renal resistance to ANF in salt-depleted rats is independent of sympathetic or ANG-aldosterone systems. Am J Physiol. 1997;272:F545–F550. doi: 10.1152/ajprenal.1997.272.4.F545. [DOI] [PubMed] [Google Scholar]
- 32.Kalinowski L, Szczepanska-Konkel M, Pawelczyk T, Bizon D, Angielski S. Inhibition of cGMP-phosphodiesterase restores the glomerular effects of atrial natriuretic factor in low sodium diet rats. Renal Physiol Biochem. 1995;18:254–266. doi: 10.1159/000173923. [DOI] [PubMed] [Google Scholar]


