Abstract
Our previous studies indicate that reduction of lipocalin 2 (mouse 24p3) expression by either anti-sense or siRNA approaches strongly reduces the overgrowth of BCR-ABL + mouse myeloid 32D in marrow and spleen of NOD/SCID mice. In this study, we used the mouse bone marrow transplant model to further explore the role of 24p3 in BCR-ABL-induced leukemia. Consistent with our previous findings, when using non-irradiated mice as recipient, donor marrow cells expressing BCR-ABL but lacking 24p3 did not cause leukemia or any disease after 75 days, whereas all mice receiving wild type BCR-ABL donor cells died with CML-like disease. An agar clone of the BCR-ABL + human CML cell line K562 (C5) that secretes relatively high levels of lipocalin 2 (human NGAL) induced suppression of hematopoiesis in spleen and marrow of mice, leading to early death in contrast to parental K562 or K562 clone (C6) expressing low amounts of NGAL. Compared withK562 cells, overexpressing NGAL in K562 led to a higher apoptosis rate and an atrophy phenotype in the spleen of the inoculated mice. Plasma from both leukemic mice and CML patients showed elevated lipocalin 2 levels compared with healthy individuals. Moreover, we found that a primary stable cell line from wild-type mouse marrow cells expressing BCR-ABL caused solid tumors in nude mice whereas a similar BCR-ABL + cell line from 24p3 null mice did not. These findings demonstrate that lipocalin 2 has at least two functions related to tumorigenesis, one involving apoptosis induction of normal hematopoietic cells and the other being tissue invasion by leukemia cells.
Keywords: lipocalin 2, CML, tumorigenesis, BCR-ABL, apoptosis
Introduction
In the progression of chronic myelogenous leukemia (CML), leukemic cells gradually replace the normal marrow cells and overpopulate the spleen and liver, resulting in a high number of white blood cells in the peripheral blood and anemia in the patients. The driving force for most CML patients is the BCR-ABL oncoprotein, which perturbs the balance of cell growth and cell death in normal hematopoietic cells and controls their malignant properties (Groffen et al., 1984; Ben-Neriah et al., 1986).
Our previous experiments with BCR-ABL + mouse hematopoietic cells (32Dp210) indicate that the infiltration of marrow and spleen tissues by leukemia cells is caused by the persistent expression and secretion of a small glycoprotein lipocalin 2 (mouse 24p3), which is induced by BCR-ABL (Lin et al., 2005). The tyrosine kinase activity of BCR-ABL is required for 24p3 induction. Importantly, we showed that 24p3 induces apoptosis in normal hematopoietic cells but not in BCR-ABL + leukemia cells (32Dp210 cells). In a mouse model, we found that BCR-ABL + cells with lowered expression of 24p3 have increased levels of normal hematopoiesis and reduced invasion of leukemia cells in marrow and spleen tissues (Lin et al., 2005).
Lipocalin 2 belongs to the lipocalin family that involves in a variety of physiological activities (Flower, 1996). Lipocalin 2 is an important factor in innate immune responses. Mice lacking lipocalin 2 (mouse 24p3) develop normally but show hypersensitivity to bacteria infection (Flo et al., 2004; Berger et al., 2006). Lipocalin 2 binds to an iron siderophore (Goetz et al., 2002) that is required for the growth of bacteria. Lipocalin 2 is also implicated in kidney development and renal injury (Yang et al., 2002). When withdrawing IL-3 from in vitro cultured mouse cytokine-dependent hematopoietic cells, the transcription and secretion of lipocalin 2 (mouse 24p3) is highly elevated and the conditioned medium (CM) derived from these cells causes apoptosis in certain hematopoietic cell types including primary hematopoietic cells (Devireddy et al., 2001). Importantly, high levels of lipocalin 2 are associated with other types of cancers such as breast cancer (Fernandez et al., 2005), pancreatic cancer (Furutani et al., 1998), ovarian cancer (Lim et al., 2007) and esophageal cancer (Zhang et al., 2007). Human lipocalin 2 (NGAL) was originally purified as a component complexed with metalloproteinase MMP-9 (Kjeldsen et al., 1993), which is involved in the invasive behavior of many types of cancers (Egeblad and Werb, 2002). It was shown that the presence of lipocalin 2 stabilizes MMP-9 activity (Yan et al., 2001). Importantly, a recent study showed that inhibition of MMP-9 activity reduces the invasive behavior in BCR-ABL + cells (Nieborowska-Skorska et al., 2006).
In our earlier study, agar clones derived from human CML cell line K562 induced a leukemia syndrome involving suppression of normal hematopoiesis in marrow and reduction in spleen size, displaying a ‘wasting’ syndrome in non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice (Lin et al., 2001). These experiments suggested that these clones of K562 cells might secrete factors that inhibit proliferation and/or survival of normal hematopoietic cells. Later we found that these clones express high level of lipocalin 2 (human NGAL) compared with the parental cells.
We have shown that mice inoculated with 32Dp210 with lowered 24p3 expression display a much more normal level of hematopoiesis in bone marrow and spleen compared with the mice receiving 32Dp210 cells. However, these mice developed ascites that is composed of BCR-ABL + leukemia cells (Lin et al., 2005), which might be due to an incomplete removal of 24p3 expression. Therefore, we want to examine the effect on BCR-ABL-induced leukemia formation when 24p3 is completely eliminated from leukemia cells. In this report, we used the bone marrow transplant model to test this possibility. We found that marrow cells from 24p3 null mice transduced with BCR-ABL did not cause leukemia after transplanting into non-irradiated wild-type (WT) mice whereas all the mice receiving WT BCR-ABL + donor cells developed a CML-like disease with high levels of 24p3 in the peripheral blood. Using a primary BCR-ABL + marrow cell line lacking 24p3, we found that 24p3 is also required for solid tumor formation. Injection of NOD/SCID mice with an agar clone of K562 cells (C5) that expresses high levels of NGAL-induced severe suppression of hematopoiesis, resulting in early death of mice. In contrast, mice injected with K562 clones that express low levels of NGAL (C6) did notshow the severe suppression of hematopoiesis. Pathological analysis indicates that nude mice receiving K562 overexpressing NGAL showed decreased cell numbers in spleen, correlating with higher apoptosis rate measured by caspase 3 activity. Plasma from blood and marrow of CML patients contained elevated NGAL levels, which correlated with the level of disease.
Results
24p3 expression is required for BCR-ABL induced leukemia
We employed the mouse bone marrow transplant model (Daley et al., 1990; Pear et al., 1998; Li et al., 1999) to examine the effects of the complete lack of 24p3 on BCR-ABL-induced myeloid leukemia (Supplementary Figure 1A and B). 5-FU-treated marrow cells from either WT or 24p3 null mice served as donor cells. The donor cells were infected with retrovirus encoding BCR-ABL (b3a2) and the green fluorescent protein (GFP) before intravenous (i.v.) injection into syngeneic WT recipient mice (C57BL/6). All of the transplant experiments were conducted with a single preparation of MigR1-BCR-ABL/GFP virus to ensure the consistency of the infection efficiency.
The marrow transplant procedures typically involve sublethal irradiation of the recipients for rapid engraftment of leukemic cells. However, sublethal irradiation will eliminate most of the native cells in bone marrow and spleen. Under this setting, we reasoned that BCR-ABL + cells would not require 24p3 to populate in bone marrow environment. In addition, the number of donor cells is an important factor for the timing and efficacy of engraftment and disease. To test the effect of BCR-ABL + donor cells lacking 24p3 on leukemia induction, we performed bone marrow transplant experiments under three conditions.
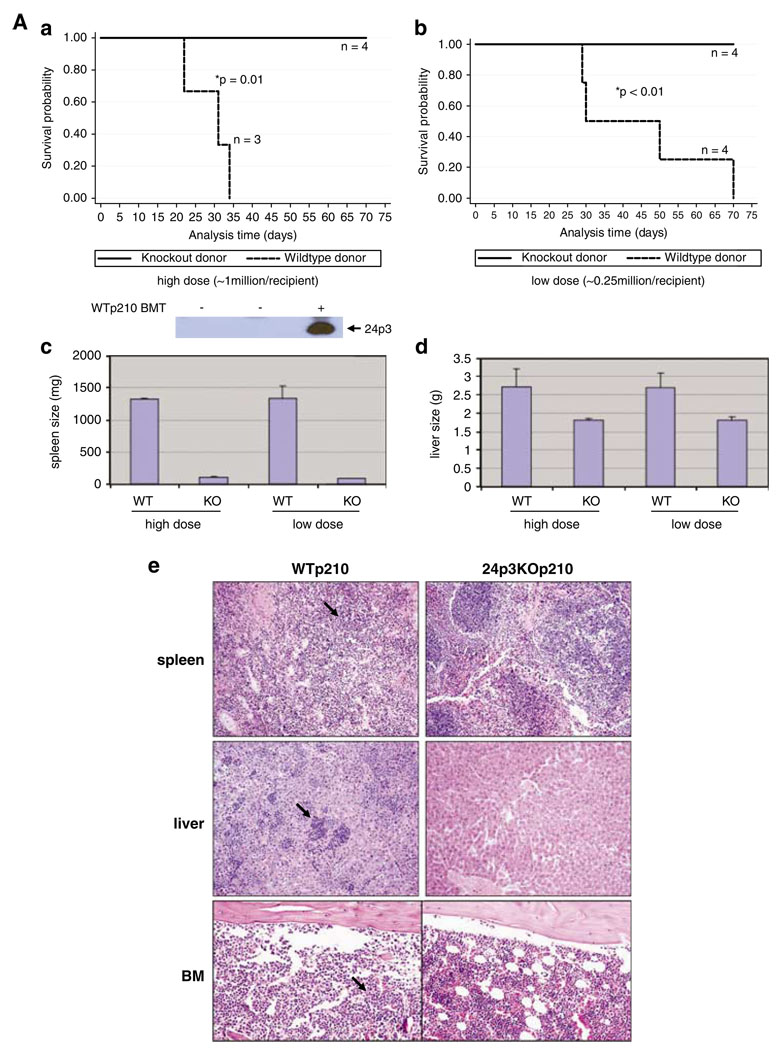
In the first set of experiments we did not precondition the mice with irradiation to maintain healthy marrow and spleen tissues so as to compare the effects of donor cells lacking 24p3 expression to those expressing 24p3 on leukemia formation. We inoculated the recipient mice with two doses of donor cells lacking 24p3 (Supplementary Figure 1C) infected with the BCR-ABL retrovirus. The results of these transplant studies (Figure 1Aa and b) showed that either a low dose (0.25 × 106 cells) or a high dose (1 × 106 cells) of BCR-ABL + donor cells from 24p3 null mice did not cause detectable disease, and no deaths were observed in the non-irradiated recipients over a 75-day period. Upon euthanization, these mice showed similar size and morphology of spleen and liver compared with normal mice (spleen 90–120 mg, liver 1.6–1.8 g) (Figure 1Ac–e). We also were unable to detect any GFP+ cells in bone marrow (data not shown) and normal bone marrow structure was maintained in these mice (Figure 1Ae). In contrast, all recipient mice (n = 7) receiving either dose of WT donor cells infected with BCR-ABL retrovirus developed CML-like disease with enlarged spleen and liver with the average size of 1300 mg and 2.6 g, respectively (Figure 1Aa–d) and infiltration of leukemic cells in spleen, liver and bone marrow (Figure 1Ae). Itis important to note that the blood of leukemic mice contained high amount of 24p3 protein whereas it was undetectable in that of healthy mice (Figure 1A, inset below panel a). Thus, when spleen and bone marrow remain intact in the recipient mice, our data indicated that 24p3 expression is absolutely required to transplant BCR-ABL-induced leukemia.
Figure 1.
24p3 is required for BCR-ABL-mediated leukemia induction in the mouse BMT model. (A) BCR-ABL + marrow cells from 24p3KO mice are unable to induce disease in non-irradiated recipient mice. (a) Survival of non-irradiated recipient mice injected with 1 × 106 BCR-ABL + donor cells from either WT or 24p3KO mice (*P = 0.01). The panel below panel a is an anti-24p3 western blot of mouse plasma from normal mice (labeled ‘−’), and mice with disease (labeled ‘+’). (b) Survival of non-irradiated mice injected with 0.25 × 106 BCR-ABL + donor cells from either WT or 24p3 KO mice (*P < 0.01). (c) The average spleen weight of mice shown in panels a and b; the error bars represent s.e.m. (d) The average liver weight from mice shown in panels a and b; the error bars represents s.e.m. (e) H&E staining of tissues from mice shown in panels a and b ( × 400). Arrow points to tumor cells. (B) BCR-ABL + marrow cells from 24p3KO mice cause minor disease in irradiated recipient mice injected with a low dose of donor cells. (a) Survival of irradiated recipient mice (600 cGy) injected with 0.3 × 106 donor cells from either WT mice or 24p3KO mice (*P < 0.01). (b) The percentage of green fluorescent protein (GFP+) cells in bone marrow from the mice shown in panel a. (c) The average spleen weight of the mice shown in panel a. (C) Transplantation of a high dose of BCR-ABL + marrow cells induces leukemia in irradiated recipient mice using donor cells from WT and 24p3KO mice. (a) Survival curves of BCR-ABL-transplanted mice. (b) Average spleen weight from mice shown in panel a. (c) Histology of spleen and bone marrow of mice shown in panel a ( × 400). Error bars represent s.e.m.
We reason that the requirement for 24p3 for leukemia induction by BCR-ABL expression might be reduced when using irradiated mice as recipients. In this case, we pre-conditioned the recipient mice with one-time irradiation of 600 cGy on the injection day. When given a low dose of donor cells lacking 24p3 (0.3 × 106 cells), one in eight recipients died (Figure 1Ba). The rest of the mice lived beyond 50 days before euthanization. At this point, three mice from this group had enlarged spleens and infiltration of GFP+ cells to an average of 20% in bone marrow (Figure 1Bb and c). Histology analysis of spleen, liver and BM showed the variation in the level of leukemia disease among these mice (Supplementary Figure 2). As expected, under the same experimental conditions, WT BCR-ABL + donor cells caused the death in all eight recipients within 40 days (Figure 1Ba). Enlarged spleens and liver were observed (Figure 1Bc and data not shown) and more than 70% GFP+ cells were detected in the marrow (Figure 1Bb). Histology analysis indicated a vigorous leukemia in these mice (Supplementary Figure 2).
We found that BCR-ABL-mediated leukemia is independent of 24p3 function when a high dose of donor cells (1 × 106) was given to irradiated recipients. Under this condition, mice injected with WT donor cells (n = 4) all died on day 17 with average spleen size of 300 mg (Figure 1Ca and b) and high infiltration of GFP+ cells in bone marrow, spleen and liver (data not shown). Mice receiving BCR-ABL + donor cells lacking 24p3 (n = 5) died between day 16 and day 20. These mice also showed enlarged spleen (average 390 mg) and infiltration of leukemic cells in bone marrow and spleen (Figure 1Cb and c). Taken together, our bone marrow transplant experiments under different conditions showed quite clearly that 24p3 secretion by BCR-ABL marrow cells is only required when there are intact and healthy marrow and spleen tissues (Figure 1A) or when there are sufficient marrow/spleen cells that are readily available to out-grow the leukemia cells (Figure 1B).
24p3 is required for BCR-ABL-induced solid tumor growth in mice
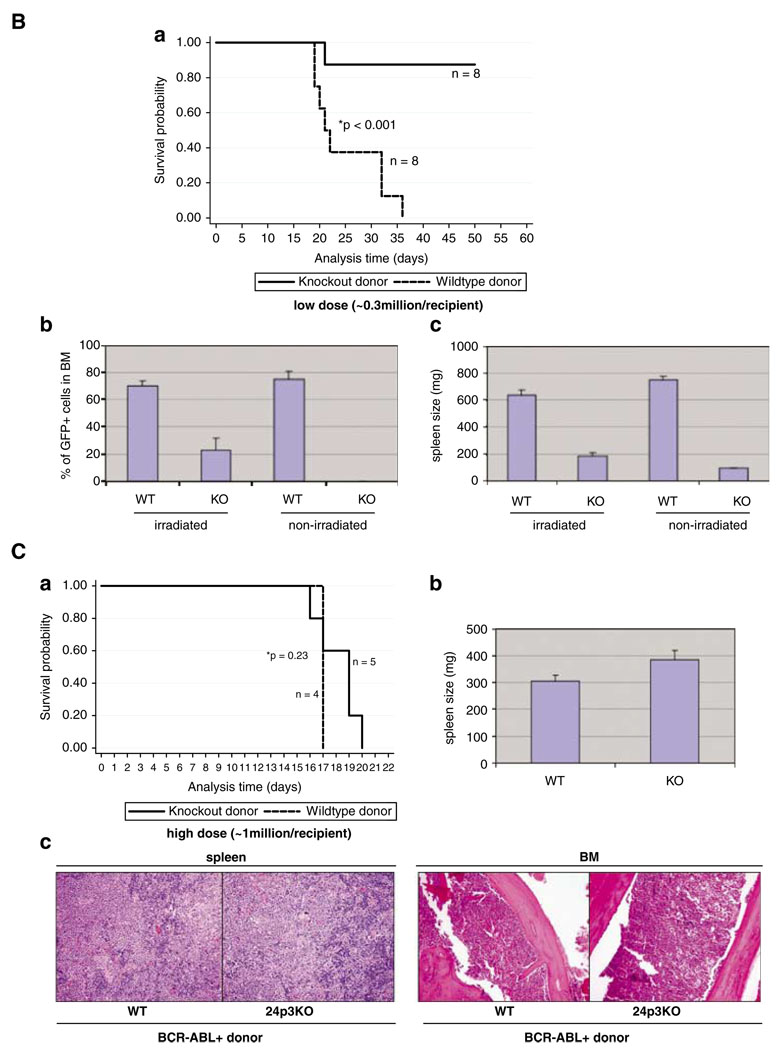
We were able to generate a stable BCR-ABL + cell line from marrow cells lacking 24p3 (24p3KOp210, Supplementary Figure 3). It was derived from 24p3(−/−) donor cells, as in the bone marrow transplant experiments and the retroviral-infected cells were subjected to selection in the absence of growth factors. A similar BCR-ABL + cell line from WT donor cells was also generated and was used as control (WTp210, Supplementary Figure 3). These two cell lines showed similar growth kinetic in vitro (data not shown).
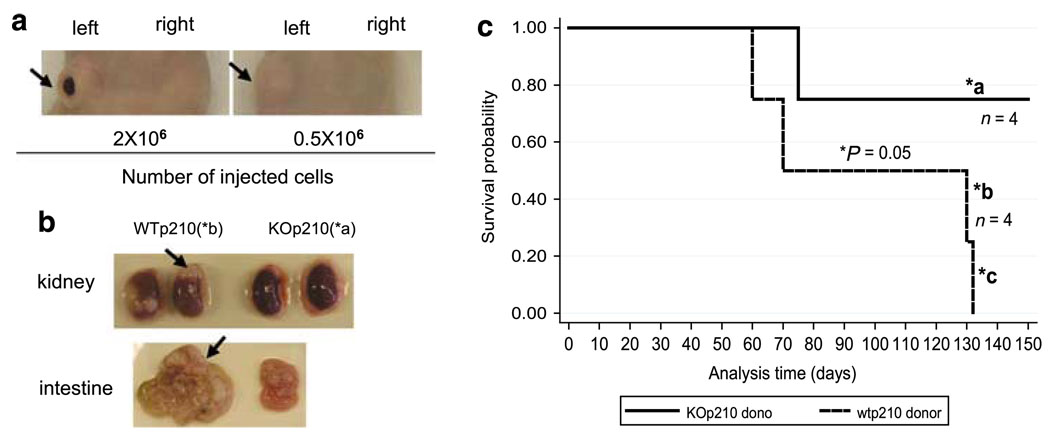
Following subcutaneous (s.c.) injection of these cells into nude mice, we found that solid tumor formed only on the site injected with WTp210 cells after 4 months of inoculation (Figure 2A, Supplementary Figure 4C). No tumor was detected at the site injected with 24p3KOp210 cells (Figure 2a). Furthermore, i.v. injection of these cells showed that BCR-ABL + cells from 24p3KO mice caused no significant disease, although one of four mice died of unknown causes (Figure 2c). In contrast, WTp210 cell caused death in all four injected mice (Figure 2b). After 4 months, mice receiving WTp210 cells showed visible weight loss compared with mice receiving 24p3KOp210 (26 vs 31 g, Supplementary Figure 4E). One terminally ill mouse that received i.v. WTp210 cells showed solid tumors in the kidneys and intestine (Figure 2c, Supplementary Figure 4A) and infiltration of leukemia cells in the bone marrow (Supplementary Figure 4A). The solid tumors in the kidney were composed of >80% GFP + cells indicating their BCR-ABL + origin (data not shown). The same mice also showed signs for anemia, as its red blood cell population and hematocrit were only half compared with that in the mice receiving 24p3KOp210 cells, and it also showed in increased population of myeloid lineage cells (Supplementary Figure 4D). Another mouse receiving WTp210 cells through i.v. died with enlarged spleen with invasion of leukemic cells in spleen and liver (Supplementary Figure 4B).
Figure 2.
24p3 expression is required for solid tumor formation. (a) BCR-ABL + marrow cells from 24p3KO mice do not cause solid tumors when s.c. inoculated in nude mice. Mice were injected with either 0.5 × 106 or 2 × 106 of WTp210 cells (left flank) and 24p3KOp210 cells (right flank). (b) Kidney and intestinal tumors observed in mice injected with WTp210 cells (*b) but not 24p3KOp210 cells (*a). Arrow points to tumor site. (c) Survival curves of irradiated nude mice (250 cGy) i.v. injected with 5 × 106 cells (*P = 0.05, *a, *b, *c mark for individual mice).
Agar clones of CML cell line K562 with high amounts of NGAL suppress hematopoiesis
We have previously shown that agar clones of K562 cells were active in inducing a ‘wasting’ syndrome with shrinking spleens in NOD/SCID mice (Lin et al., 2001). Interestingly, we found that these more active agar clones secreted higher levels of lipocalin 2 (human NGAL) compared with uncloned K562 cells (Supplementary Figure 5A), suggesting a role of NGAL in blocking normal hematopoiesis.
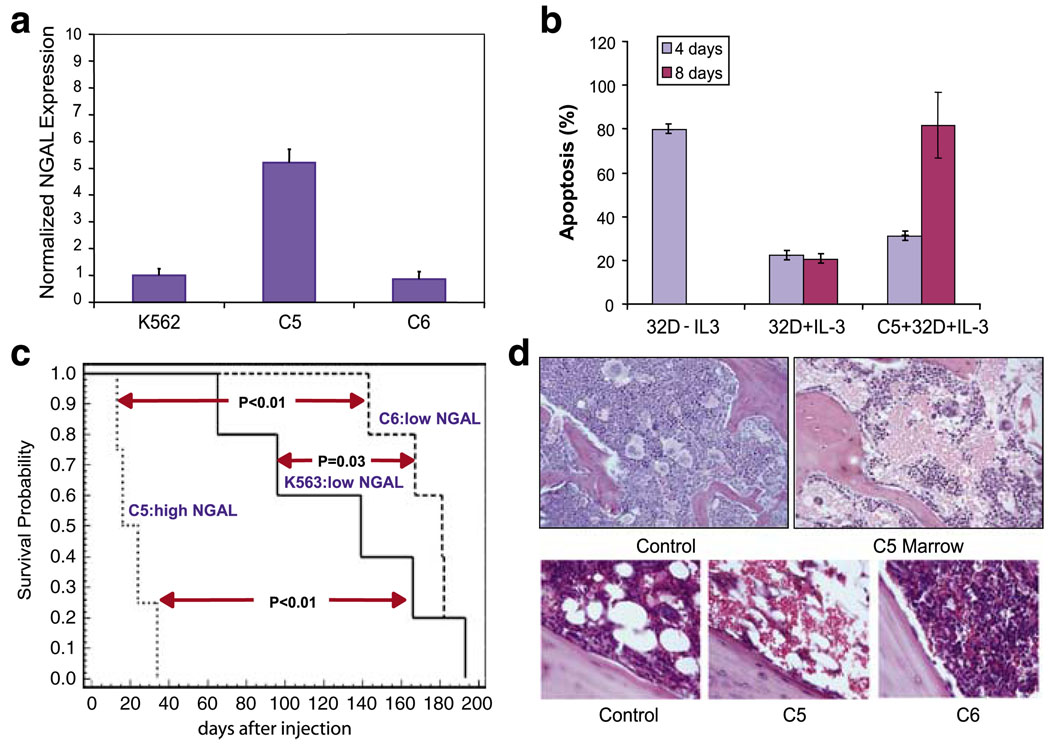
We screened a number of agar clones of K562 cells to identify clones that expressed relatively high levels of NGAL (Figure 3a). One such clone (C5) expressed five times higher levels of NGAL than either K562 cells or another agar clone (C6) (Figure 3a). We found that normal mouse hematopoietic cells (32D cells) underwent cell death in the presence of C5 clones, as C5 cells induced apoptosis in ~80% of 32D cells after co-culture for 8 days compared with only ~20% cell death in 32D cells in the absence of C5 clone (Figure 3b). In addition, we found that CM derived from COS1 transfected with NGAL-induced apoptosis in primary bone marrow cells at a higher rate compared with CM from COS1 cells transfected with vector alone (Supplementary Figure 5B).
Figure 3.
Agar clones of the chronic myelogenous leukemia (CML) cell line K562 induce suppression of hematopoiesis in bone marrow of NOD/SCID mice. (a) Human lipocalin 2 (NGAL) transcripts in agar clones C5 and C6 of K562 measured by quantitative RT–PCR. NGAL transcripts were normalized to levels of c-ABL transcripts. (b) Co-culture of C5 K562 cells with myeloid mouse blast cells (32D cells) grown in IL-3 (0.1 ng/ml) induced apoptosis measured by Annexin V/PI staining. 32D withdrawal of IL-3 as positive control. (c) Survival curves of irradiated (250 cGy) NOD/SCID mice injected with K562, C5 and C6 cells (1 × 106). (d) Top panels, H&E staining of normal marrow (not irradiated) and a marrow from a C5-injected mouse 18 days after injection ( × 100). Bottom panels ( × 400), (left) normal marrow from a mouse at 18 days after irradiation; (middle) marrow from a mouse injected with C5 cells at 18 days after irradiation; (right) marrow from a mouse injected with C6 cells at 18 days after irradiation. Error bars represent s.e.m.
Injection of these clones into irradiated NOD/SCID mice showed that the C5 clone caused early death in four of four mice but injection of the C6 clone caused a slower developing disease much like K562 cells (Figure 3c). Similar to what we observed with the K clones in the previous study (Lin et al., 2001), the C5-injected mice exhibited small spleen and atrophy in spleen and bone marrow (Figure 3d), indicating suppression of hematopoiesis. The C6-injected mice had marrow resembling extramedullary leukemia, and survival was much longer (Figures 3c and d, bottom right).
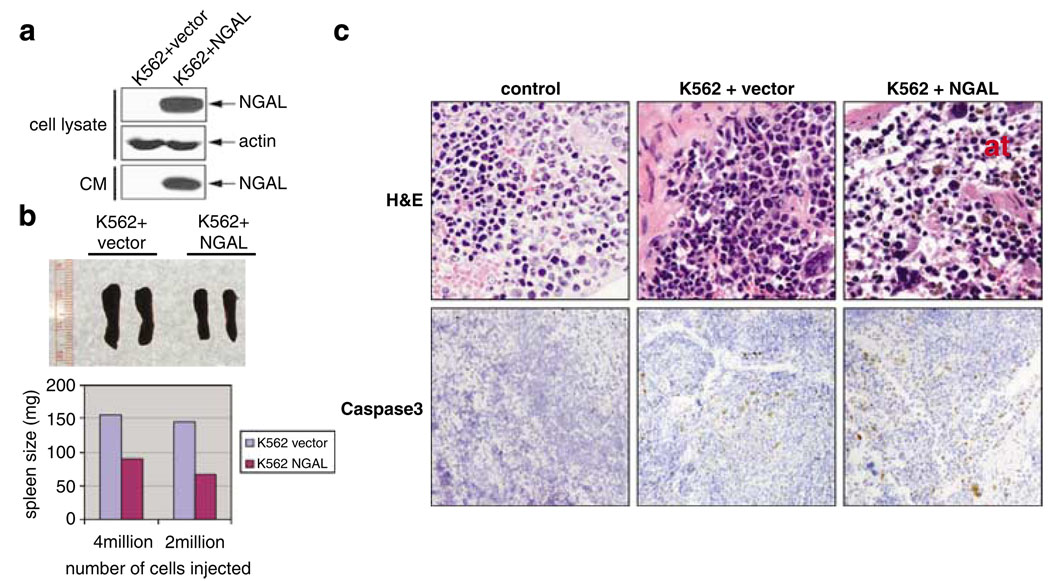
To confirm that NGAL was the cause of suppression of hematopoiesis, we i.v. injected either K562 cells or K562 cells overexpressing NGAL (K562 + NGAL) (Figure 4a) into irradiated nude mice. The mice were euthanized 45 days post inoculation. In either case, we found that the spleens from the mice receiving K562 + NGAL were smaller (90 and 60 mg) compared with mice receiving vector-transfected K562 cells (average of 150 mg) (Figure 4b). Compared with irradiated control mice (Figure 4c, upper left), we found that the spleen from K562-inoculated mice showed ‘space’ between the cells (Figure 4c, upper middle), which was more dramatic in the spleen from mice inoculated with K562 + NGAL cells (Figure 4c, upper right). In addition, we observed an almost complete disappearance of myeloid progenitor cells (stained as light-blue nucleus) near the capsule region of spleen in K562 + NGAL-inoculated mice and a decreased cell number in the overall spleen structure (Figure 4c, upper right), similar to the ‘atrophy’ phenotype we observed with the K562 clones with high NGAL expression (Figure 3d). The spleen tissues of mice inoculated with either K562 or K562 + NGAL cells displayed increased apoptotic rate, as indicated by the presence of cleaved caspase 3 (Figure 4c, bottom middle and right) compared with the irradiated control (Figure 4c, bottom left), suggesting the atrophy in the spleens of these mice was a result of the increased apoptosis. Taken together, our results suggest that NGAL plays a primary role in suppressing of hematopoiesis in these mice, most likely by inducing apoptosis within normal hematopoietic cells.
Figure 4.
Overexpression of human lipocalin 2 (NGAL) in K562 causes suppression of hematopoiesis. (a) Western blot showing overexpression of NGAL in K562 cells. (b) Irradiated mice (250 cGy) receiving K562 cells overexpressing NGAL have reduced spleens. Lower panel is the quantitation of the spleen weight. (c) Top panel, H&E staining of spleens from normal mice (irradiated) (left), mice injected with K562 transfected with vector alone (middle) and mice injected with K562 overexpressing NGAL (left) at 45 days post cell inoculation. ‘at’ marks atrophy. Bottom panel is the immunohistochemical staining of cleaved caspase 3 (brown spots).
CML patients with active disease contain elevated levels of NGAL
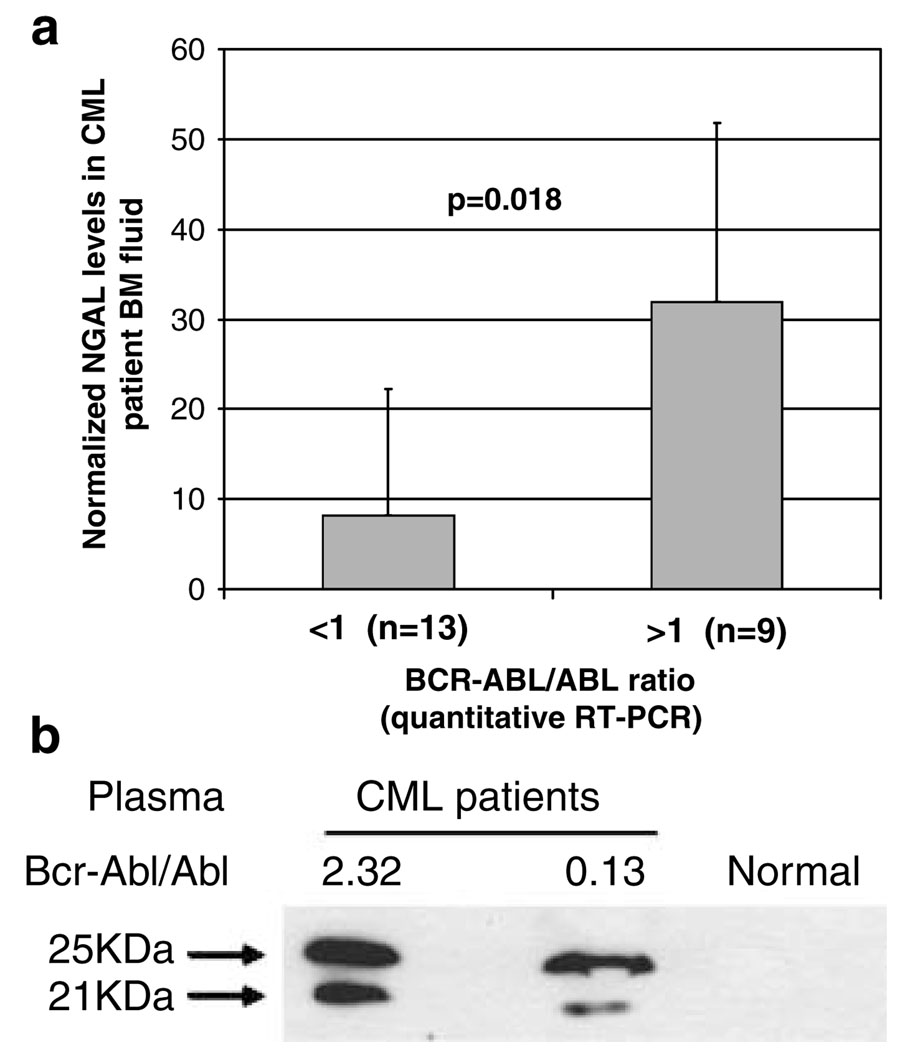
As the plasma from leukemia mice contained a high level of 24p3 (Figure 1A, inset), we examined the NGAL levels in either peripheral blood or BM fluid from CML patients with active disease. Quantitative RT–PCR was used to determine the level of active disease in CML patients by comparing the ratio of BCR-ABL to total ABL transcripts (Guo et al., 2002). We examined NGAL expression in BM fluid from 22 CML patients using western blotting (Figure 5a). We summarized the data according to BCR-ABL/ABL ratio, with >1 being more active disease. Under this categorization, we found that high NGAL expression in CML patients was dependent on the BCR-ABL/ABL ratio (P < 0.02). Increased NGAL expression is also shown in blood plasma from CML patients compared with a healthy individual (Figure 5b). Interestingly, we detected two forms of NGAL proteins, one migrating at about 24–25 kDa and the other about 21 kDa in plasma from CML patients (Figure 5b). The biological significance of the two forms is not understood but we note that the two forms of NGAL were detected in CM of COS1 cells transfected with NGAL separated on 16% SDS–PAGE (Supplementary Figure 6). We found that the two forms are not the result of the differences in glycosylation, as mutating the NGAL glycosylation site (N65 to Q) (Rudd et al., 1999) still retained two forms (Supplementary Figure 6).
Figure 5.
Plasma from chronic myelogenous leukemia (CML) patients with active disease contain elevated level of human lipocalin 2 (NGAL). (a) Summary of NGAL expression in 22 CML patients. 10 µl of BM fluid was analysed on 15% SDS–PAGE followed by blotting with anti-NGAL antibody. NGAL level was normalized by standard recombinant NGAL run in the same gel. The level of disease was estimated by quantitative RT–PCR on the same sample as expressed as the ratio of BCR-ABL to total ABL transcripts (Guo et al., 2002). Error bars represent s.e.m. (b). Western blotting of blood plasma samples from CML patients shows two bands of NGAL. The ratio of BCR-ABL/ABL transcripts is shown above the bands.
Discussion
Our studies showed that lipocalin 2 expression by BCR-ABL + donor bone marrow cells obtained from mice was absolutely required for leukemia induction in recipient mice with intact marrow and spleen tissues. If irradiation was used at a dose that depletes marrow and spleen tissues of viable hematopoietic cells, BCR-ABL + donor marrow lacking lipocalin 2 caused either a lethal leukemia or a moderate disease, depending on the dose of donor cells. Thus, when marrow and spleen tissues are intact and are actively growing, BCR-ABL + leukemia cells cannot efficiently invade these hematopoietic tissues unless they secrete sufficient levels of lipocalin 2. These findings confirm our earlier findings performed with BCR-ABL + mouse hematopoietic cells, which show that reduction of 24p3 expression in these cells largely prevents invasion of marrow and spleen tissues (Lin et al., 2005). One major difference was observed in that study compared with findings using BCR-ABL + marrow cells from 24p3KO mice in bone marrow transplants. We detected no disease in nonirradiated mice transplanted with BCR-ABL + marrow cells from 24p3 KO mice. In the 24p3 anti-sense or knockdown studies, mice died because of liver invasion and/or ascites formation, probably caused by residual levels of 24p3 secretion by the 24p3 knockdown BCR-ABL + cells. Based on our findings using mouse bone marrow transplant system as a model to study CML, we think that using non-irradiated mice as recipient will mimic more closely the microenvironment seen in humans who develop CML.
One surprising finding is that lipocalin 2 is also required for solid tumor formation induced by BCR-ABL. We showed that the established primary WTp210 cells were able to form solid tumor when inoculated into nude mice (s.c. or i.v.) but not 24p3KOp210 cells, suggesting that lipocalin 2 is also necessary for tissue invasion. Lipocalin 2 expression is highly elevated in many types of solid cancers (Furutani et al., 1998; Fernandez et al., 2005; Lim et al., 2007; Zhang et al., 2007), and its level was significantly elevated in the transplanted mice with disease in our studies. Rather than being a marker for tumor events, our results strongly argue that the increased expression of lipocalin 2 in these tumor types is required for tumor formation and expansion.
The role of lipocalin 2 in solid tumor formation might be linked with the fact that it stabilizes MMP-9 enzymatic activity (Yan et al., 2001). As a protease targeting substrates involved in organization of extra-cellular matrix, MMP-9 has been implicated in cancer formation and metastasis (Egeblad and Werb, 2002). Understanding the effects of the interaction between lipocalin 2 and MMP-9 may reveal a role of lipocalin 2 in tumor migration and invasion.
Our current study showed that an agar clone from K562 with high NGAL expression (C5) led to early death and atrophy in spleen and bone marrow, showing severe suppression of hematopoiesis. A similar ‘atrophy’ phenotype was observed in nude mice inoculated with K562 overexpressing NGAL, showing fewer numbers of hematopoietic cells in the spleen compared with normal. It is interesting to note that we observed a higher rate of apoptosis in spleen tissue derived from mice inoculated with K562 overexpressing NGAL than K562 cells expressing vector alone, indicating that atrophy in spleen tissues was caused by apoptosis of hematopoietic cells due to NGAL expression. In this regard, we observed an almost complete depletion of myeloid progenitor cells near the capsule region of the spleen derived from mice receiving K562 overexpressing NGAL as determined by histology analysis. The same spleen also showed signs of degeneration in erythroid lineages. Co-culture of the C5 clone of K562 cells induced apoptosis in mouse hematopoietic 32D target cells. We also showed that CM derived from COS1 transfected with NGAL-induced cell death in primary bone marrow cells. Our published findings concerning the suppression of hematopoiesis by 24p3, which is secreted by BCR-ABL + mouse 32D cells, indicate that the erythroid lineage is severely depressed in the spleens and marrow of leukemic mice (Lin et al., 2005). Blocking expression of 24p3 caused a strong recovery of erythroid lineage cells (TER119 +) in the spleen and marrow of these leukemic mice (Lin et al., 2005). Others have shown that NGAL functions as a negative regulator of red blood cell production in mouse and human systems (Miharada et al., 2005, 2007). They found that NGAL is transcribed not only in mature blood cells (granulocytes, macrophages and erythroid lineages) but also in hematopoietic stem/progenitor cells but transcription of NGAL RNA does not necessarily correlate with NGAL secretion as the mechanism involved is unknown. In culture systems purified NGAL was shown to induce apoptosis in human umbilical cord blood hematopoietic cells. Their studies indicate that the level of normal red blood cells is regulated by NGAL apoptotic effects on certain populations of erythroid progenitor cells. We propose that in CML patients, high levels of NGAL circulating in the blood and marrow compartment cause induction of apoptosis of erythroid progenitor cells and lead to a severe anemia. These events are being further investigated.
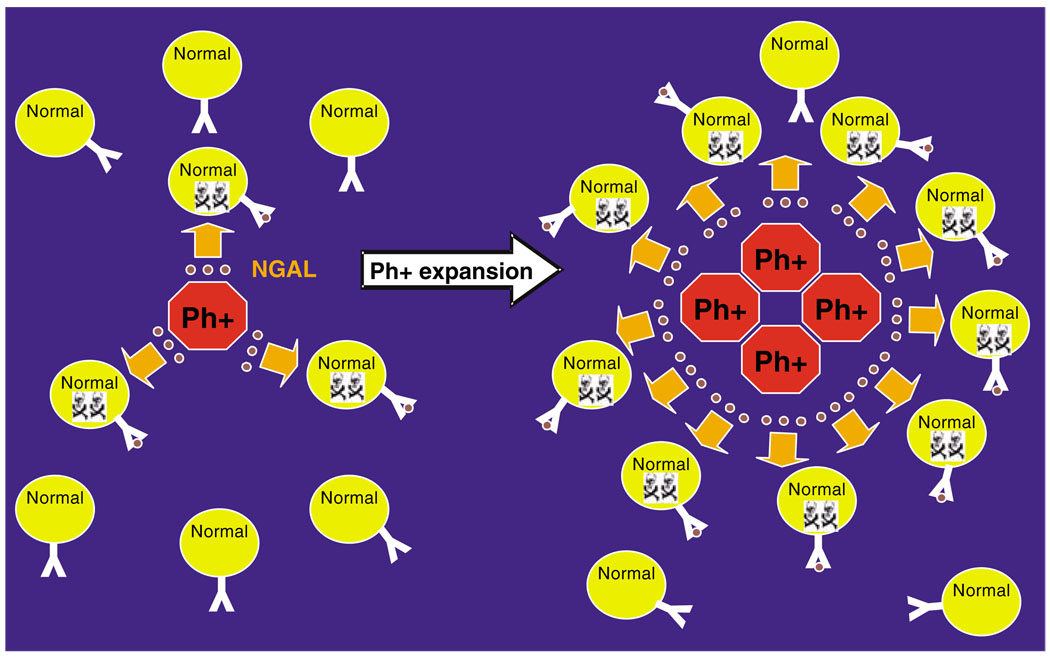
As reported in the studies of Devireddy et al. (2005) 24p3 causes apoptosis by mechanism involving 24p3 entry in cells through the 24p3 receptor and depleting the targeted cell of iron, leading to apoptosis. 24p3 and NGAL bind organic iron and represent a first line of defense against bacterial pathogens (Goetz et al., 2002; Flo et al., 2004; Berger et al., 2006). The 24p3 receptor is lacking in BCR-ABL + cells (Devireddy et al., 2005), which is consistent with our findings that BCR-ABL + cells are resistant to the effects of 24p3 and NGAL (Lin et al., 2005). An iron-deficient form of 24p3 is responsible for the apoptotic effects of 24p3, as the internalized 24p3 sequesters iron and is exported from the target cell thereby causing iron depletion and apoptosis by a mechanism involving activation of pro-apoptotic Bim (Devireddy et al., 2005). This lead us to propose that in the early stages of CML, the Philadelphia chromosome+ (Ph+) cell is able to expand in the bone marrow by a stepwise process mediated by NGAL-induced apoptosis of normal hematopoietic cells but not leukemia cells followed by expansion of the leukemia cells in the space created by dead or dying normal hematopoietic cells (Figure 6).
Figure 6.
A model for the role of human lipocalin 2 (NGAL) in the expansion of BCR-ABL + cells within the marrow at early stage chronic myelogenous leukemia (CML) patients. NGAL is illustrated by the brown round circles; The Y like structure on the surface of normal hematopoietic cells represents the receptor for NGAL. The skull and cross bone indicates dying normal hematopoietic cells; Ph+ marks Philadelphia chromosome-positive cells expressing BCR-ABL.
In conclusion, our findings suggest that there are two activities associated with lipocalin 2 (24p3/NGAL) in tumorigenesis, with one relating to apoptosis induction of normal hematopoietic cells and the other facilitating tissue invasion to form solid tumors. Interestingly, we detected two different forms of lipocalin 2 in CML patients and in cell culture studies (Figure 5 and Supplementary Figure 6). Further studies are needed to address how structural changes in the lipocalin 2 affect its biological functions. Nevertheless, our findings suggest that approaches to inhibit NGAL function within blood by any means will likely have a role in CML therapy in the future.
Materials and methods
Mouse bone marrow transplant experiments
Donor bone marrow cells were derived from age-matched WT (C57B/7 at8 weeks) and 24p3 null mice (kindly provided by Dr Aderem). Donor cells were prepared by harvesting bone marrow cells from mice treated with 5-fluorouracil (5-FU, 200 mg/kg) for 4 days. After stimulating in complete bone marrow medium (CBM) containing Iscove’s MDM, 15% inactivated FBS, 0.1% BSA, 25 µm β-mercaptoethanol (ME), penicillin/streptomycin, 5% WEHI-conditioned medium, 10 ng/ml recombinant mouse IL-3, 20 ng/ml recombinant mouse IL-6, 100 ng/ml recombinant mouse stem cell factor, the collected bone marrow cells from femur (2 million/femur) were infected with retroviral stocks containing MigR1-BCR-ABL-GFP (viral titer >1 × 106) at 1:1 (v/v) ratio in the presence of 8 µg/ml of polybrene. To increase transduction efficiency, virus and cells were cosedimented at 1000 g for 90 min at room temperature. After overnight incubation at 37 °C, the medium and viral mixture was replaced with new CBM early the next morning and viral infection was repeated for 6 h. After the second transduction, the bone marrow cells were incubated at 37 °C for 4 h before the medium was replaced with new CBM. The infected bone marrow cells were injected i.v. into either irradiated (600 cGy) or non-irradiated mice.
Analysis of transplanted mice
Diseased mice were euthanized and blood was collected by cardio puncture. Bone marrow, spleen and liver tissues were collected and the infiltration of BCR-ABL + cell marked with GFP was assessed by fluorescent microscopy and FACS analysis. Fixed tissues section (4 µ were analysed by hematoxylin and eosin (H&E) staining and immunohistochemistry using antibody against mouse cleaved caspase 3 (Cell Signaling no. 9661).
Establishment of primary marrow BCR-ABL + cell lines from donor mice
BCR-ABL-transduced bone marrow cells from either WT or 24p3 null mice that are used for the mouse bone marrow transplant experiments were also cultured in vitro in CBM for another 3 days before subjected to selection by gradually reducing growth factors in the culture medium (RPMI + 10% fetal bovine serum with 1:10, 1:20, 1:30, 1:40 dilution of CBM) over a period of weeks. The established stable BCR-ABL + cell lines from either WT or 24p3 null mice were cultured in RPMI + 10% fetal bovine serum before use. RT–PCR or genomic PCR was used to verify the genotype of the cell lines using the following primer sets:
BCR-ABL (RT–PCR): 5′-AGAACCTGAGAGCCAGAAGCAAC-3′ and 5′-TCAGGAACGTGTAACTCTTGCC G-3′; Actin (RT–PCR): 5′-GCTGGAAGGTGGACAGTG AG-3′ and 5′-ATGGATGACGATATCGCTGC-3′; 24p3 (genomic PCR): 5′-GCTCTACCTCTCATTTCTTGCAGT TC-3′ and 5′-TGCATTGGTCTGTAGGAGAGAAAG-3′.
Selecting soft-agar colonies with high NGAL expression
Human myeloid leukemia cells K562 were plated on soft-agar plates. RT–PCR was used to identify colonies with high NGAL expression compared with parental K562 cells using the following primer set: NGAL-F (5′-GTGAGCACCAACTACAACCAGCAT-3′) and NGAL-R (5′-AGTTTCGAAGTCAGCTCCTTGGTT-3′). BCR-ABL PCR primers are the same as mentioned above.
Nude mice experiments
For nude mice experiments WT or 24p3 knockout marrow cells stably expressing BCR-ABL were washed once with PBS and resuspended in PBS before injection. In all 5 or 10 million cells were i.v. injected into BALB/c nude mice irradiated at 250 cGy. About0.5 or 2 million cells were subcutaneously inoculated on the back of the recipient mice. For NOD/SCID mice experiments, 1 × 106 K562 cells, C5 K562 and C6 K562 cells were injected into irradiated mice as described (Lin et al., 2001).
Detecting NGAL in CML patient samples by western blotting
A total of 5–20 µl of plasma of either BM fluid or blood from CML patients was separated on 15% SDS–PAGE and blotted with NGAL antibody. The BCR-ABL/ABL ratios were performed by standard quantitative RT–PCR assays on peripheral blood using primers as described (Guo et al., 2002).
Statistical analysis
The P-values of differences between the Kaplan–Meier survival curves was determined by use of the Stata10 software for calculating the log-rank test values, and the results were confirmed by Tarone–Ware test and the Peto-Peto test. Values obtained by the various tests were very similar.
Supplementary Material
Acknowledgements
We also want to thank Dr Bastinaella Perazzona for critically reading the paper. This research was supported in part by a grant from NIH (PO1 CA49639).
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986;233:212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:1834–1839. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–1305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–834. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res. 2005;11:5390–5395. doi: 10.1158/1078-0432.CCR-04-2391. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318(Part 1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani M, Arii S, Mizumoto M, Kato M, Imamura M. Identification of a neutrophil gelatinase-associated lipocalin mRNA in human pancreatic cancers using a modified signal sequence trap method. Cancer Lett. 1998;122:209–214. doi: 10.1016/s0304-3835(97)00391-1. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacterio-static agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Groffen J, Stephenson JR, Heisterkamp N, De Klein A, Bartram CR, Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984;36:93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Guo JQ, Lin H, Kantarjian H, Talpaz M, Champlin R, Andreeff M, et al. Comparison of competitive-nested PCR and real-time PCR in detecting BCR-ABL fusion transcripts in chronic myeloid leukemia patients. Leukemia. 2002;16:2447–2453. doi: 10.1038/sj.leu.2402730. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- Li S, Ilaria RL, Jr, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Ahmed N, Borregaard N, Riley C, Wafai R, Thompson EW, et al. Neutrophil gelatinase-associated lipocalin (NGAL) an early-screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. Int J Cancer. 2007;120:2426–2434. doi: 10.1002/ijc.22352. [DOI] [PubMed] [Google Scholar]
- Lin F, Monaco G, Sun T, Liu J, Lin H, Stephens C, et al. BCR gene expression blocks Bcr-Abl induced pathogenicity in a mouse model. Oncogene. 2001;20:1873–1881. doi: 10.1038/sj.onc.1204409. [DOI] [PubMed] [Google Scholar]
- Lin H, Monaco G, Sun T, Ling X, Stephens C, Xie S, et al. Bcr-Abl-mediated suppression of normal hematopoiesis in leukemia. Oncogene. 2005;24:3246–3256. doi: 10.1038/sj.onc.1208500. [DOI] [PubMed] [Google Scholar]
- Miharada K, Hiroyama T, Sudo K, Danjo I, Nagasawa T, Nakamura Y. Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. J Cell Physiol. 2007;215:526–537. doi: 10.1002/jcp.21334. [DOI] [PubMed] [Google Scholar]
- Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. FASEB J. 2005;19:1881–1883. doi: 10.1096/fj.05-3809fje. [DOI] [PubMed] [Google Scholar]
- Nieborowska-Skorska M, Hoser G, Rink L, Malecki M, Kossev P, Wasik MA, et al. Id1 transcription inhibitor-matrix metalloproteinase 9 axis enhances invasiveness of the breakpoint cluster region/abelson tyrosine kinase-transformed leukemia cells. Cancer Res. 2006;66:4108–4116. doi: 10.1158/0008-5472.CAN-05-1584. [DOI] [PubMed] [Google Scholar]
- Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, et al. Efficientand rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- Rudd PM, Mattu TS, Masure S, Bratt T, Van den Steen PE, Wormald MR, et al. Glycosylation of natural human neutrophil gelatinase B and neutrophil gelatinase B-associated lipocalin. Biochemistry. 1999;38:13937–13950. doi: 10.1021/bi991162e. [DOI] [PubMed] [Google Scholar]
- Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xu L, Xiao D, Xie J, Zeng H, Wang Z, et al. Upregulation of neutrophil gelatinase-associated lipocalin in oesophageal squamous cell carcinoma: significantcorrelat ion with cell differentiation and tumour invasion. J Clin Pathol. 2007;60:555–561. doi: 10.1136/jcp.2006.039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.