Abstract
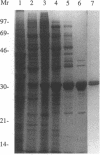
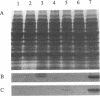
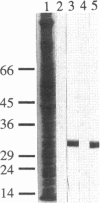
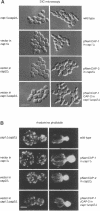
We cloned and analyzed two genes, cap-1 and cap-2, which encode the alpha and beta subunits of Caenorhabditis elegans capping protein (CP). The nematode CP subunits are 55% (cap-1) and 66% (cap-2) identical to the chicken CP subunits and 32% (cap-1) and 48% (cap-2) identical to the yeast CP subunits. Purified nematode CP made by expression of both subunits in yeast is functionally similar to chicken skeletal muscle CP in two different actin polymerization assays. The abnormal cell morphology and disorganized actin cytoskeleton of yeast CP null mutants are restored to wild-type by expression of the nematode CP subunits. Expression of the nematode CP alpha or beta subunit is sufficient to restore viability to yeast cap1 sac6 or cap2 sac6 double mutants, respectively. Therefore, despite evolution of the nematode actin cytoskeleton to a state far more complex than that of yeast, one important component can function in both organisms.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. E., Botstein D., Drubin D. G. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature. 1991 Dec 5;354(6352):404–408. doi: 10.1038/354404a0. [DOI] [PubMed] [Google Scholar]
- Adams A. E., Cooper J. A., Drubin D. G. Unexpected combinations of null mutations in genes encoding the actin cytoskeleton are lethal in yeast. Mol Biol Cell. 1993 May;4(5):459–468. doi: 10.1091/mbc.4.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amatruda J. F., Cannon J. F., Tatchell K., Hug C., Cooper J. A. Disruption of the actin cytoskeleton in yeast capping protein mutants. Nature. 1990 Mar 22;344(6264):352–354. doi: 10.1038/344352a0. [DOI] [PubMed] [Google Scholar]
- Amatruda J. F., Cooper J. A. Purification, characterization, and immunofluorescence localization of Saccharomyces cerevisiae capping protein. J Cell Biol. 1992 Jun;117(5):1067–1076. doi: 10.1083/jcb.117.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda J. F., Gattermeir D. J., Karpova T. S., Cooper J. A. Effects of null mutations and overexpression of capping protein on morphogenesis, actin distribution and polarized secretion in yeast. J Cell Biol. 1992 Dec;119(5):1151–1162. doi: 10.1083/jcb.119.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbauer T., Kleinschmidt J. A., Walsh M. J., Weiner O. H., Franke W. W. Identification of a widespread nuclear actin binding protein. Nature. 1989 Dec 14;342(6251):822–825. doi: 10.1038/342822a0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., Helfman D. M., Hemmingsen S. M. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature. 1992 Nov 5;360(6399):84–87. doi: 10.1038/360084a0. [DOI] [PubMed] [Google Scholar]
- Barstead R. J., Waterston R. H. The basal component of the nematode dense-body is vinculin. J Biol Chem. 1989 Jun 15;264(17):10177–10185. [PubMed] [Google Scholar]
- Bowdish K., Tang Y., Hicks J. B., Hilvert D. Yeast expression of a catalytic antibody with chorismate mutase activity. J Biol Chem. 1991 Jun 25;266(18):11901–11908. [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. E., Heiss S. G., Mermall V., Cooper J. A. Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry. 1989 Oct 17;28(21):8506–8514. doi: 10.1021/bi00447a036. [DOI] [PubMed] [Google Scholar]
- Caldwell J. E., Waddle J. A., Cooper J. A., Hollands J. A., Casella S. J., Casella J. F. cDNAs encoding the beta subunit of cap Z, the actin-capping protein of the Z line of muscle. J Biol Chem. 1989 Jul 25;264(21):12648–12652. [PubMed] [Google Scholar]
- Casella J. F., Casella S. J., Hollands J. A., Caldwell J. E., Cooper J. A. Isolation and characterization of cDNA encoding the alpha subunit of Cap Z(36/32), an actin-capping protein from the Z line of skeletal muscle. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5800–5804. doi: 10.1073/pnas.86.15.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella J. F., Craig S. W., Maack D. J., Brown A. E. Cap Z(36/32), a barbed end actin-capping protein, is a component of the Z-line of skeletal muscle. J Cell Biol. 1987 Jul;105(1):371–379. doi: 10.1083/jcb.105.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Walker S. B., Pollard T. D. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J Muscle Res Cell Motil. 1983 Apr;4(2):253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- Dear S., Staden R. A sequence assembly and editing program for efficient management of large projects. Nucleic Acids Res. 1991 Jul 25;19(14):3907–3911. doi: 10.1093/nar/19.14.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Ashman L. K. The use of alkaline phosphatase-conjugated anti-immunoglobulin with immunoblots for determining the specificity of monoclonal antibodies to protein mixtures. Methods Enzymol. 1986;121:497–509. doi: 10.1016/0076-6879(86)21050-2. [DOI] [PubMed] [Google Scholar]
- Fire A., Albertson D., Harrison S. W., Moerman D. G. Production of antisense RNA leads to effective and specific inhibition of gene expression in C. elegans muscle. Development. 1991 Oct;113(2):503–514. doi: 10.1242/dev.113.2.503. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 May;77(5):2546–2550. doi: 10.1073/pnas.77.5.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann H., Noegel A. A., Eckerskorn C., Rapp S., Schleicher M. Ca2+-independent F-actin capping proteins. Cap 32/34, a capping protein from Dictyostelium discoideum, does not share sequence homologies with known actin-binding proteins. J Biol Chem. 1989 Jul 25;264(21):12639–12647. [PubMed] [Google Scholar]
- Helms C., Dutchik J. E., Olson M. V. A lambda DNA protocol based on purification of phage on DEAE-cellulose. Methods Enzymol. 1987;153:69–82. doi: 10.1016/0076-6879(87)53048-8. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R., Brenner S., Hodgkin J., Herman R. K. A uniform genetic nomenclature for the nematode Caenorhabditis elegans. Mol Gen Genet. 1979 Sep;175(2):129–133. doi: 10.1007/BF00425528. [DOI] [PubMed] [Google Scholar]
- Hug C., Miller T. M., Torres M. A., Casella J. F., Cooper J. A. Identification and characterization of an actin-binding site of CapZ. J Cell Biol. 1992 Feb;116(4):923–931. doi: 10.1083/jcb.116.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Moriyama K., Matsumoto S., Kawasaki H., Nishida E., Yahara I. Isolation of a yeast essential gene, COF1, that encodes a homologue of mammalian cofilin, a low-M(r) actin-binding and depolymerizing protein. Gene. 1993 Feb 14;124(1):115–120. doi: 10.1016/0378-1119(93)90770-4. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson R., Aspenström P., Byström A. S. A chicken beta-actin gene can complement a disruption of the Saccharomyces cerevisiae ACT1 gene. Mol Cell Biol. 1991 Jan;11(1):213–217. doi: 10.1128/mcb.11.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Wild M., Rosenzweig B., Hirsh D. Wild-type and mutant actin genes in Caenorhabditis elegans. J Mol Biol. 1989 Aug 5;208(3):381–392. doi: 10.1016/0022-2836(89)90503-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moerman D. G., Benian G. M., Barstead R. J., Schriefer L. A., Waterston R. H. Identification and intracellular localization of the unc-22 gene product of Caenorhabditis elegans. Genes Dev. 1988 Jan;2(1):93–105. doi: 10.1101/gad.2.1.93. [DOI] [PubMed] [Google Scholar]
- Moerman D. G., Benian G. M., Waterston R. H. Molecular cloning of the muscle gene unc-22 in Caenorhabditis elegans by Tc1 transposon tagging. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2579–2583. doi: 10.1073/pnas.83.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Methods to characterize actin filament networks. Methods Enzymol. 1982;85(Pt B):211–233. doi: 10.1016/0076-6879(82)85022-2. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A., Mooseker M. S., Cooper J. A. Localization of capping protein in chicken epithelial cells by immunofluorescence and biochemical fractionation. J Cell Biol. 1992 Jul;118(2):335–346. doi: 10.1083/jcb.118.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]