Abstract
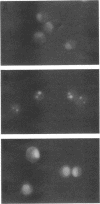
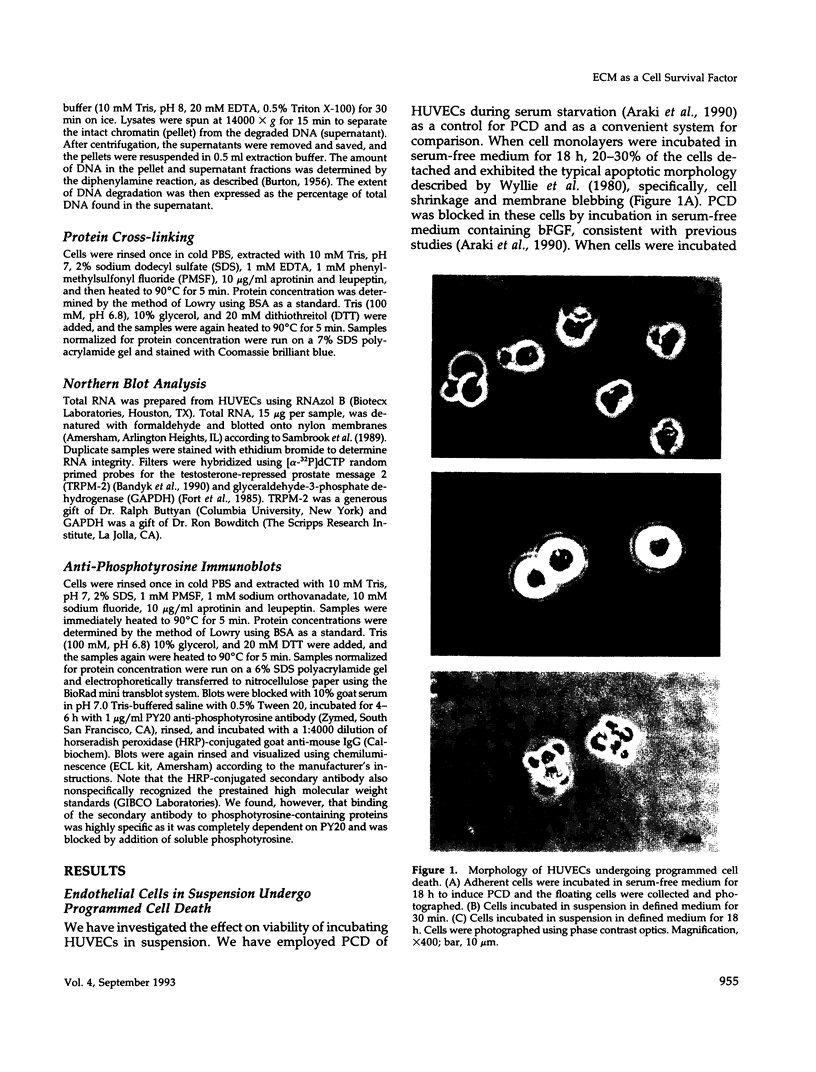
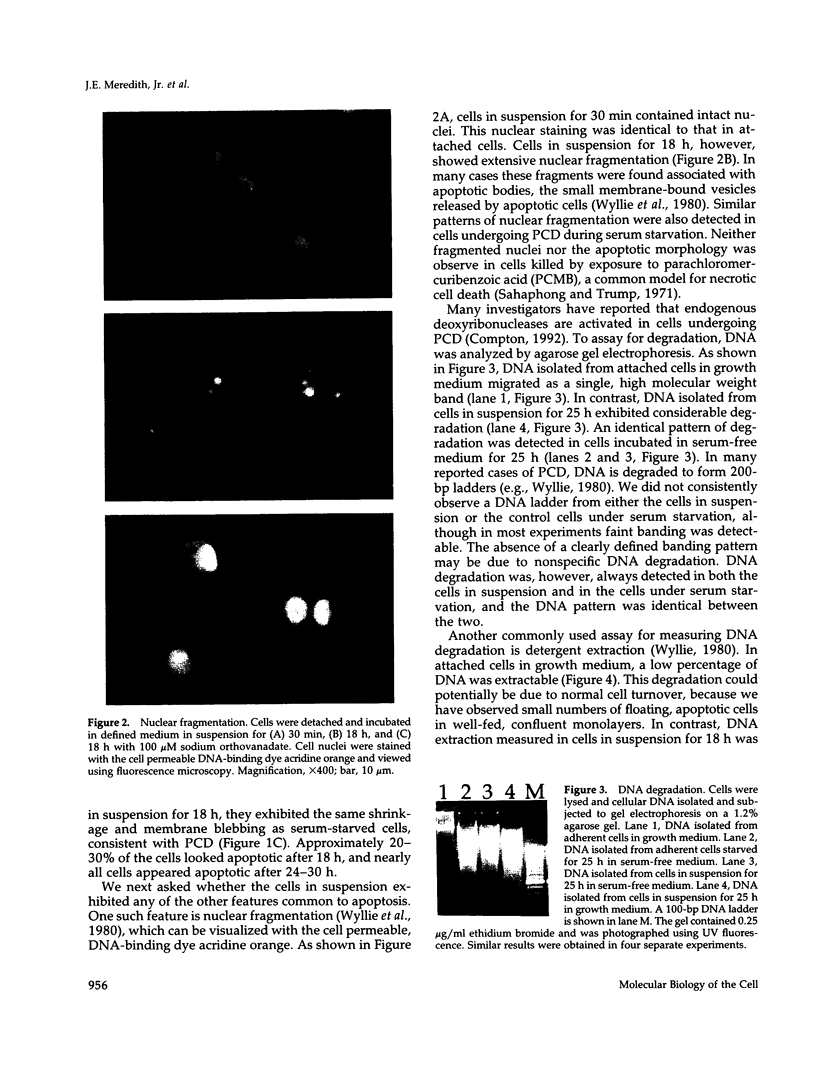
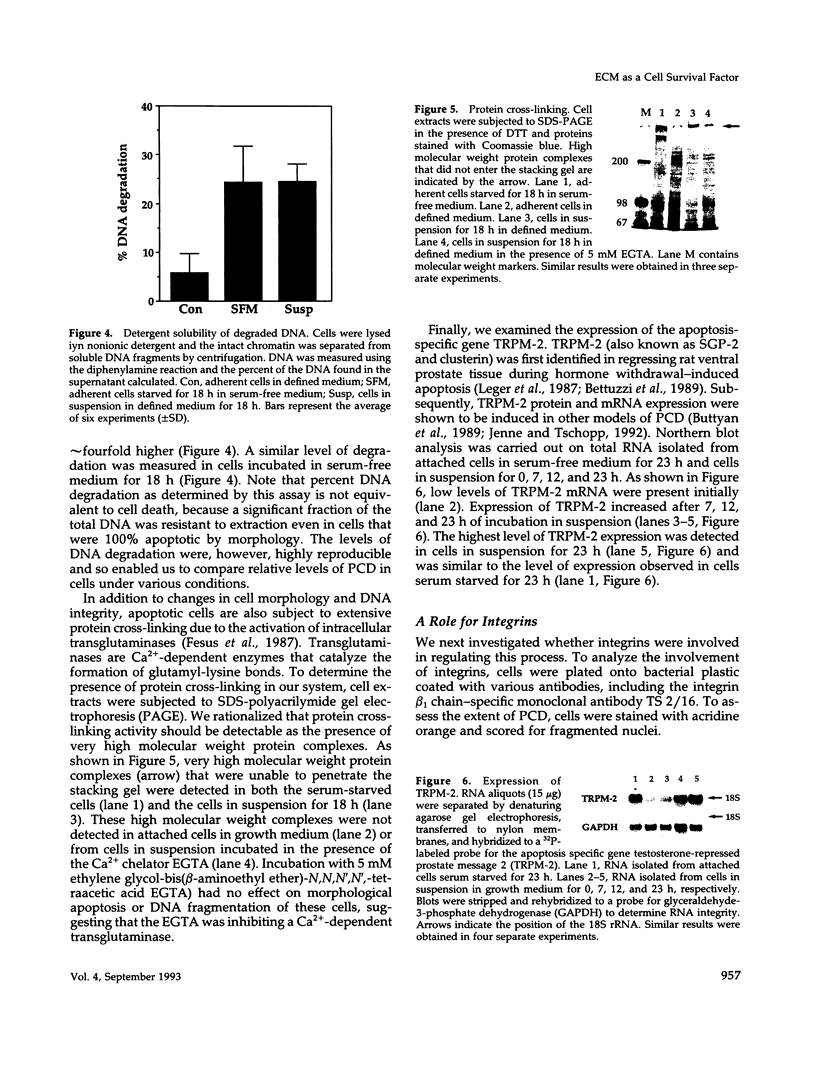
Programmed cell death (PCD) or apoptosis is a naturally occurring cell suicide pathway induced in a variety of cell types. In many cases, PCD is induced by the withdrawal of specific hormones or growth factors that function as survival factors. In this study, we have investigated the potential role of the extracellular matrix (ECM) as a cell survival factor. Our results indicate that in the absence of any ECM interactions, human endothelial cells rapidly undergo PCD, as determined by cell morphology, nuclei fragmentation, DNA degradation, protein cross-linking, and the expression of the PCD-specific gene TRPM-2. PCD was blocked by plating cells on an immobilized integrin beta 1 antibody but not by antibodies to either the class I histocompatibility antigen (HLA) or vascular cell adhesion molecule-1 (VCAM-1), suggesting that integrin-mediated signals were required for maintaining cell viability. Treatment of the cells in suspension with the tyrosine phosphatase inhibitor sodium orthovanadate also blocked PCD. When other cell types were examined, some, but not all, underwent rapid cell death when deprived of adhesion to the ECM. These results suggest that in addition to regulating cell growth and differentiation, the ECM also functions as a survival factor for many cell types.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Shimada Y., Kaji K., Hayashi H. Apoptosis of vascular endothelial cells by fibroblast growth factor deprivation. Biochem Biophys Res Commun. 1990 May 16;168(3):1194–1200. doi: 10.1016/0006-291x(90)91155-l. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyk M. G., Sawczuk I. S., Olsson C. A., Katz A. E., Buttyan R. Characterization of the products of a gene expressed during androgen-programmed cell death and their potential use as a marker of urogenital injury. J Urol. 1990 Feb;143(2):407–413. doi: 10.1016/s0022-5347(17)39975-5. [DOI] [PubMed] [Google Scholar]
- Bardon S., Vignon F., Montcourrier P., Rochefort H. Steroid receptor-mediated cytotoxicity of an antiestrogen and an antiprogestin in breast cancer cells. Cancer Res. 1987 Mar 1;47(5):1441–1448. [PubMed] [Google Scholar]
- Bettuzzi S., Hiipakka R. A., Gilna P., Liao S. T. Identification of an androgen-repressed mRNA in rat ventral prostate as coding for sulphated glycoprotein 2 by cDNA cloning and sequence analysis. Biochem J. 1989 Jan 1;257(1):293–296. doi: 10.1042/bj2570293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton M. M. A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992 Sep;11(2):105–119. doi: 10.1007/BF00048058. [DOI] [PubMed] [Google Scholar]
- Daniels K., Solursh M. Modulation of chondrogenesis by the cytoskeleton and extracellular matrix. J Cell Sci. 1991 Oct;100(Pt 2):249–254. doi: 10.1242/jcs.100.2.249. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Ernsberger U., Edgar D., Rohrer H. The survival of early chick sympathetic neurons in vitro is dependent on a suitable substrate but independent of NGF. Dev Biol. 1989 Oct;135(2):250–262. doi: 10.1016/0012-1606(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Fesus L., Thomazy V., Falus A. Induction and activation of tissue transglutaminase during programmed cell death. FEBS Lett. 1987 Nov 16;224(1):104–108. doi: 10.1016/0014-5793(87)80430-1. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Clusterin: the intriguing guises of a widely expressed glycoprotein. Trends Biochem Sci. 1992 Apr;17(4):154–159. doi: 10.1016/0968-0004(92)90325-4. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheim C., Barde Y. A., Thoenen H., Le Douarin N. M. In vivo effect of brain-derived neurotrophic factor on the survival of developing dorsal root ganglion cells. EMBO J. 1987 Oct;6(10):2871–2873. doi: 10.1002/j.1460-2075.1987.tb02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988 Feb;122(2):552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- Léger J. G., Montpetit M. L., Tenniswood M. P. Characterization and cloning of androgen-repressed mRNAs from rat ventral prostate. Biochem Biophys Res Commun. 1987 Aug 31;147(1):196–203. doi: 10.1016/s0006-291x(87)80106-7. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J. 1989 May;3(7):1843–1849. doi: 10.1096/fasebj.3.7.2497041. [DOI] [PubMed] [Google Scholar]
- Miekka S. I., Ingham K. C., Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1982 Jul 1;27(1):1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Rotello R. J., Hocker M. B., Gerschenson L. E. Biochemical evidence for programmed cell death in rabbit uterine epithelium. Am J Pathol. 1989 Mar;134(3):491–495. [PMC free article] [PubMed] [Google Scholar]
- Sahaphong S., Trump B. F. Studies of cellular injury in isolated kidney tubules of the flounder. V. Effects of inhibiting sulfhydryl groups of plasma membrane with the organic mercurials PCMB (parachloromercuribenzoate) and PCMB (parachloromercuribenzenesulfonate). Am J Pathol. 1971 May;63(2):277–298. [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A. Signaling by integrins: implications for tumorigenesis. Cancer Res. 1993 Apr 1;53(7):1503–1506. [PubMed] [Google Scholar]
- Schwartz M. A. Transmembrane signalling by integrins. Trends Cell Biol. 1992 Oct;2(10):304–308. doi: 10.1016/0962-8924(92)90120-c. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S. Lymphocyte interactions with extracellular matrix. FASEB J. 1991 Jun;5(9):2292–2299. doi: 10.1096/fasebj.5.9.1860621. [DOI] [PubMed] [Google Scholar]
- Strange R., Li F., Saurer S., Burkhardt A., Friis R. R. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992 May;115(1):49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Talhouk R. S., Bissell M. J., Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992 Sep;118(5):1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk R. S., Chin J. R., Unemori E. N., Werb Z., Bissell M. J. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991 Jun;112(2):439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]