Abstract
Traumatic brain injury (TBI) features deficits often ameliorated by dopamine (DA) agonists. We have previously shown deficits in striatal DA neurotransmission, using fast scan cyclic voltammetry (FSCV), after controlled cortical impact (CCI) injury that are reversed after daily treatment with the DA uptake inhibitor Methylphenidate (MPH). The goal of this study was to determine how a single dose of MPH (5 mg/kg) induces changes in basal DA and metabolite levels and with electrically evoked overflow (EO) DA in the striatum of CCI rats. MPH induced changes in EO DA after a two-week daily pretreatment regime with MPH was also assessed. There were no baseline differences in basal DA or metabolite levels. MPH injection significantly increased basal [DA] output in dialysates for control but not injured rats. Also, MPH injection increased striatal peak EO [DA] to a lesser degree in CCI (176% of baseline) versus control rats (233% of baseline). However, daily pretreatment with MPH resulted in CCI rats having a comparable increase in EO [DA] after MPH injection when compared to controls. The findings further support the concept that daily MPH therapy restores striatal DA neurotransmission after CCI.
Keywords: controlled cortical impact, electrochemistry, voltammetry, microdialysis, dopamine transporter, methylphenidate
INTRODUCTION
Altered neurotransmission after traumatic brain injury (TBI) adversely impacts cognition and behavior. As we have demonstrated, the controlled cortical impact (CCI) injury model of TBI induces chronic alterations in striatal dopamine (DA) system protein expression, including increased tyrosine hydroxylase (Yan et al. 2007), a key protein for DA synthesis, and altered DA transporter (DAT) expression, the primary protein required for reuptake of DA into presynaptic terminals (Wagner et al. 2005a,b). Importantly, striatal DA transmission, assessed with fast scan cyclic voltammetry (FSCV) and a stimulated release paradigm, is also impaired after CCI, where evoked overflow as well as release and reuptake kinetics was impaired. (Wagner et al. 2005a, 2009). DA levels are elevated in humans and rats early after TBI (Wagner et al. 2007a; McIntosh et al. 1994; Massucci et al. 2004). However, these elevations are transient and limited to the first days after injury (McIntosh et al. 1994; Massucci et al. 2004).
Neurostimulants, including the DA reuptake inhibitor methylphenidate (MPH), improve cognition and learning in animal models (Kline et al. 2000; Wagner et al. 2007) and in clinical studies (Warden et al. 2006). These findings implicate DA systems with TBI induced cognitive and behavioral deficits frequently observed after TBI. Moreover, we found that daily treatment with MPH after CCI can restore DA neurotransmission measured with FSCV during MFB stimulation, establishing a neurobiological basis for MPH's therapeutic value (Wagner et al. 2009).
In naïve animals, a single dose of MPH significantly increases DA output in striatal dialysates when measured using microdialysis with high performance liquid chromatography (HPLC) (Huff and Davies 2002; Kuczenski and Segal 1997). MPH also rapidly upregulates transcription factor expression, including the ubiquitous transcription factor c-fos (Graybiel et al. 1990; Young et al. 1991). Regional increases in c-fos and other transcription factors have been reported in the early hours early following experimental TBI (Dash et al. 1995; Yang et al. 1994; Natale et al. 2003). We have found that both striatal c-fos and striatal neurotransmission are diminished 2 weeks after CCI. However, 2 weeks of daily treatment regime with MPH restored striatal c-fos and DA neurotransmission to near normal levels (Wagner et al. 2009). Decreased constitutive c-fos levels after CCI lead us to hypothesize that CCI may diminish the ability to augment neurotransmission in response to MPH injection. Given the neurorestorative response of daily MPH therapy on evoked DA responses and striatal c-fos levels after CCI, we further hypothesized that daily treatment with MPH after CCI may reverse this decrement.
As such, the purpose of this study was to assess the effects of a single dose of MPH on DA transmission, as measured in basal dialysates and evoked overflow (EO) DA in the CCI model of TBI. We measured changes in basal [DA] and the common DA metabolites, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), with microdialysis and HPLC. Additionally, we measured changes in electrically evoked striatal [DA] overflow following a single dose of MPH using FSCV and electrical stimulation of the medial forebrain bundle (MFB). Lastly, we determined the effect of daily MPH therapy on subsequent striatal evoked [DA] responses to MPH. We demonstrate that the effect of a single dose of MPH on basal DA and evoked DA responses are impaired two weeks after CCI. However, a single dose of MPH increased evoked DA release to near-normal levels following daily treatment regime of MPH
Materials and Methods
Animals
Young adult male, Sprague-Dawley rats (Hilltop Laboratories, Scottsdale, PA, USA), weighing 275-300g at the time of injury or sham surgery, were used in accordance with the regulations of the University of Pittsburgh's Institutional Animal Care and Use Committee. Sixty-eight rats received CCI or sham surgery or were naïve to surgery. The CCI injury device (Pittsburgh Precision Instruments, Inc. Pittsburgh, PA) used for this study has been described previously (Dixon et al. 1991). Under isoflurane anesthesia (4% isoflurane and a 2:1 N2O/O2 initially, followed by 1-1.5% isoflurane), animals were placed in a stereotaxic frame, and a craniotomy was performed over the left parietal cortex between lambda and bregma and approximately 2 mm lateral to the central suture. The exposed dura was then struck to a depth of 2.7-2.9 mm with an impact velocity of 4 m/s by the CCI device as previously described (Wagner et al. 2005a,b, 2007, 2009; Massucci et al. 2004). After application of the CCI, the bone flap from the craniotomy site was not replaced. The scalp was sutured, and each animal was returned to their home cage after recovery. Rats receiving sham surgery received all procedures except the impact.
Two weeks after either CCI or sham surgery, we assessed changes in basal striatal DA output with MPH administration by using microdialysis with HPLC for n=24 rats (experiment 1). We used CCI and naïve rats (n=12) to assess MPH induced changes in evoked extracellular DA with electrical stimulation of the MFB using fast scan cyclic voltammetry (FSCV) (experiment 2). An additional 32 rats were used to determine the effect of daily MPH pretreatment on subsequent evoked DA overflow occurring after MPH injection (experiment 3). Intraperitoneal injection of 5mg/kg was administered for single injection MPH challenge dose (Sigma St. Louis, MO). For pretreatment, daily intraperitoneal injections of either MPH (5mg/kg) (Sigma St. Louis, MO) or normal saline vehicle were administered beginning one day after surgery for CCI rats. Both CCI and naïve rats received daily injections for 14 days prior to voltammetry experiments. Daily dosing with 5 mg/kg MPH has been shown to improve behavior in male rats and restore DA EO presynaptic release and clearance parameters in CCI rats, with relatively small behavioral effects on motor activation and stereotypy (Wagner et al., 2007b). For both microdialysis and FSCV experiments, rats were initially re-anesthetized with chloral hydrate 400 mg/kg (initial) and 0.1 mg/kg atropine via intraperitoneal (i.p.) injection. Rats received additional injections of 50mg/kg chloral hydrate as needed to maintain a surgical level of anesthesia. A homeothermic blanket (Harvard Apparatus) was used to maintain core body temperature at 37°C for both microdialysis and FSCV procedures.
Microdialysis and HPLC
3 mm membrane length microdialysis probes (Scipro, Sweden) were stereotactically placed in the striatal hemisphere ipsilateral to the CCI using the stereotaxic flat skull coordinates (Paxinos and Watson 1998) [anteroposterior (AP), 1.7mm; mediolateral (ML), 3.0mm; and dorsoventral (DV), 6.4mm)] after anesthesia induction. Microdialysis probes were perfused with artificial cerebrospinal fluid (ACSF) with an ACSF composition in mM: 1.2 CaCl2, 2.0 Na2HPO4, 1.0 MgCl2, 2.7 KCl, 145 NaCl, pH 7.4) and using an ACSF flow rate of 2.0 μl/min. Probes were allowed to equilibrate for 60 minutes before beginning sample collection. After probe equilibration, three baseline microdialysate samples were taken, each over a 20 minute sampling period. At the conclusion of baseline sampling, rats received MPH (5mg/kg) (Sigma St. Louis, MO) or normal saline vehicle via intraperitoneal injection. After injection, six additional samples were collected, each over a 20 minute sampling period. At the conclusion of each collection period, DA, DOPAC, and HVA were measured from the fresh microdialysate samples collected using HPLC with electrochemical detection.
DA concentrations were detected by HPLC with electrochemical detection (ESA, Chelmsford, MA). The HPLC system consisted of a 582 pump and a 542 autosampler with cooler and CoulArray Detector (with two four-channel analytical cells). Analytes were separated on a C18 column (MC-150 Column--3mm × 15cm, ESA,. Chelmsford, MA) maintained at 31°C with a mobile phase flow of 0.6 mL/min. Eight serial coulometric electrodes, maintained at 31°C and with applied potentials from -120 to +300 mV in 60mV increments, were used for the measurement of DA and metabolites. The mobile phase was purchased commercially (MD-TM; ESA, ) to minimize variability.
Study sample concentrations were compared with injected standards and were determined using CoulArray® for Windows®32 software. Standards were also used to confirm a linear detector response for DA spiked solutions across a concentration range of the study samples. Data for each analyte are presented in nanomolar concentrations [nM] and as a percent change from baseline analyte concentration. To calculate analyte concentrations from microdialysates, standard samples were prepared in ACSF using known concentrations of DA, DOPAC, and HVA (Sigma, St. Louis, MO). After each in vivo study, probes were removed from the brain and equilibrated in ACSF at a flow rate of 2.0ul/min for 20 minutes. 40 μl aliquots of ACSF fluid spiked with standard solutions of analyte were collected over this 20 minute time epoch.
Fast Scan Cyclic Voltammetry
Carbon Fiber Microelectrodes (7μm diameter and 400μmm length) were fabricated and coated with Nafion as previously described (Wagner et al 2005a). Microelectrodes were placed stereotactically into the striatal hemisphere using flat skull coordinates [1.7 mm anterior/posterior (AP), 2.0 mm medial/lateral (ML), and -4.5 mm dorsal/ventral (DV)] (Paxinos and Watson 1998). A bipolar stimulating electrode (MS301-1, Plastics One, Roanoke, VA, USA) was also lowered into the medial forebrain bundle (MFB) at the stereotaxic coordinates of -4.0 mm AP, 1.7 mm ML, and -7.6 mm DV (Paxinos and Watson 1998) in the hemisphere ipsilateral to the injury site. A salt bridge was formed by placing an Ag/AgCl reference electrode in direct contact with the dura mater contralateral to the injury site.
The MFB was stimulated at a frequency of 60 Hz for 2 s every 10 minutes with a 280 μA biphasic constant current pulse and a 2 ms pulse width. The stimulating electrode was gradually lowered and the MFB stimulated until a stable baseline DA response was achieved. A stable baseline DA response in the rat was considered to be a signal that did not differ by more than 10% over at least 3 stimulations. Once a stable baseline stimulation response was achieved, 5 mg/kg MPH or saline was delivered via intraperitoneal injection. The evoked signal was then monitored every 10 minutes for 50-60 minutes after drug administration.
The specific process by which DA was detected using FSCV has been previously described (Lu et al. 1998; Wagner et al. 2005a, 2009). FSCV was performed with a computer-controlled potentiostat (EI-400; Ensman Instruments, Bloomington, IN, U.S.A.). The applied potential was held at 0mV vs. Ag/AgCl between voltammetric scans. Each scan was comprised of linear potential sweeps to 1,000 mV, then to -500mV, and back to 0 mV vs. Ag/AgCl at a rate of 300 V/s. Scans were repeated at 100-ms intervals during in vivo experiments. The presence of DA was determined by visually inspecting the background-subtracted cyclic voltammograms. The maximum concentration of EO DA overflow was taken as the maximum change in DA FSCV signal following MFB stimulation. Voltammetric currents were converted to [DA] concentration in micromolar (μM) through post-study calibration of the working electrode following removal from the brain and using standard samples prepared in ACSF with known concentrations of DA. The maximum EO [DA] achieved with three stable consecutive MFB stimulations (within 10% of each other) prior to MPH/Saline injection was obtained by averaging the peak [DA] across the three stimulations. The EO [DA] from each stimulus response obtained after MPH/Saline administration is reported as a percent of baseline EO DA.
Data Analysis
Statistical analyses were performed using SPSS 15.0 for Windows. Means and standard error of the mean (SEM) are reported. Independent t-tests were performed to examine injury related differences in basal [DA], [DOPAC], and [HVA] levels from striatal microdialysates as well as injury related differences in baseline EO [DA]. Relative changes in [DA] and [metabolite] over time, as well as FSCV data, were assessed using repeated measures analysis of variance (RMANOVA) and Fisher's least significant difference (LSD) to assess post-hoc pair-wise comparisons. To compare MPH induced changes in striatal DA neurotransmission with MFB stimulation across a similar baseline evoked DA response between injury and naïve groups in FSCV experiments, analyses were limited to those studies in which baseline DA evoked overflow was between 3-15μM. Single factor ANOVA with Fisher's LSD was used for both microdialysis and FSCV data to compare group effects at specific time points from MPH/saline challenge. A p-value ≤0.05 was considered significant for all analyses.
RESULTS
CCI Affects Basal DA and Metabolite Levels after MPH Injection
Experiment 1
CCI alone did not significantly alter DA, DOPAC, or HVA levels in striatal dialysate. Basal [DA] was 2.9+/-0.5 nM for CCI rats and 2.1+/-0.4 nM for sham rats. Basal [DOPAC] was 680.8+/-147.8 nM for CCI rats and 611.6+/-89.1nM for sham rats. Basal [HVA] was (602.5+/-101.7 nM for CCI rats vs. 509.7+/-56.1 nM for sham rats.
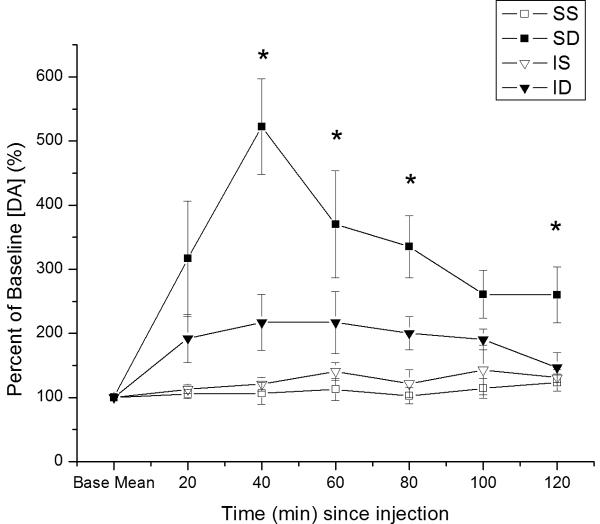
There were significant main effects of group (F=4.6; p<0.02), time (F=5.2; p<0.0001), and a group*time interaction (F=2.5; p=0.003) on relative changes in basal DA levels after MPH administration. MPH significantly altered relative DA output in striatal dialysates in sham rats over time compared to CCI groups and shams treated with vehicle (Figure 1a). Pair-wise, post-hoc comparisons show that shams treated with MPH had significantly higher DA output compared to saline vehicle groups over the time course evaluated (p<0.01 all comparisons). CCI rats treated with MPH were also statistically different from sham rats treated with MPH (p=0.03), reflecting the modest increases in [DA] noted after MPH administration for this group. Post-hoc analysis also showed that CCI rats treated with MPH were not significantly different from vehicle treated groups across the time course studied. Significant differences between groups occurred beginning at 40 minutes post-injection (p≤0.05 all comparisons), with the exception of 100 minutes, where a trend (p<0.07) was noted between groups. In each case, sham MPH rats, but not CCI MPH rats, were different from vehicle groups (p<0.05 all comparisons).
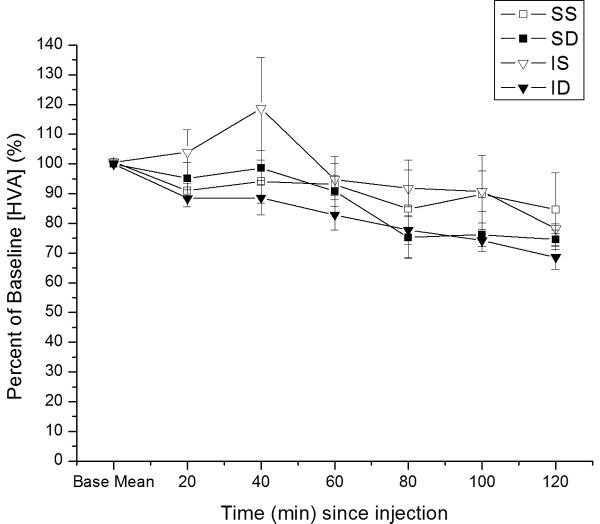
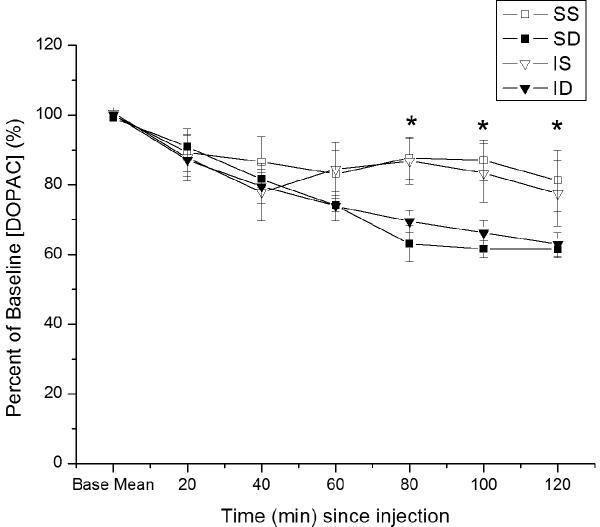
Figure 1.
Time dependent relative changes in extracellular [analyte] with 5 mg/kg MPH vs. saline injection. Values reported at each time point as percent of baseline values ± SEM. A) Relative changes in basal DA levels over time. Group differences in basal DA levels at 40, 60, 80, and 120 minutes post-injection (*p≤0.05 all comparisons for overall group effect with ANOVA at each time point). B) Relative changes in basal HVA levels over time. C). Relative changes in basal DOPAC levels over time. Group differences for [DOPAC] levels beginning 80 minutes after injection (*p≤0.05 all comparisons). (IS=CCI plus vehicle; ID=CCI plus MPH; SS=Sham plus vehicle; SD=Sham plus MPH).
Overall, there were significant main effects for group (F=2.6; p=0.08), time (F=34.6; p<0.0001), and a group*time interaction (F=3.3; p<0.0001) on relative changes in basal [DOPAC] after MPH vs. vehicle administration over time. [DOPAC] decreased over time in MPH treated groups (figure 1b). Statistically significant differences between groups for [DOPAC] occurred beginning 80 minutes after injection (p≤0.05 all comparisons). Here, MPH treated groups had declining DOPAC levels compared to saline vehicle groups at these later points post-injection. Post-hoc pair-wise comparisons show that saline treated shams had significantly higher DOPAC levels than MPH treated groups 80-120 minutes post MPH injection (p<0.04 all comparisons). Saline treated CCI rats had significantly higher DOPAC levels compared to compared to MPH groups at 80-100 minutes post injection (p<0.05) all comparisons, and a trend was noted at 120 minutes (p<0.09).
There was a significant main effect of time (F=12.3; p<0.0001) on relative changes in basal [HVA] after MPH vs. vehicle administration, but no significant group effect or group*time interaction. [HVA] decreased over time. (figure 1c). After 120 minutes from injection, the largest decrease was observed in CCI rats treated with MPH (68.6%+/-4.1% of baseline), and the smallest decrease was observed in saline treated sham rats (84.6%+/-12.4% of baseline). No significant differences between groups for [HVA] levels at any individual time point evaluated.
CCI Affects Evoked DA Overflow with Single Dose MPH
Experiment 2
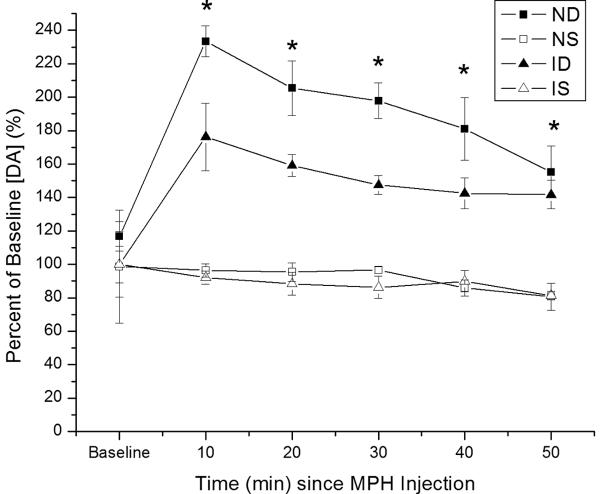
The goal for this experiment was to compare MPH induced changes in striatal DA neurotransmission with MFB stimulation across a similar baseline evoked DA response between injury and naïve groups using FSCV. There were no significant differences in the baseline EO [DA] across CCI and naïve groups (5.6+/-0.7μM vs. 6.1+/-1.5μM). Overall, there was a main effect for group (F=41.8; p<0.0001), time (F=7.9; p<0.0.0001), and a group*time interaction (F=3.4; p<0.001) on electrically EO of DA measured using FSCV. There were larger relative increases in EO DA over time for naïve rats treated with MPH than for other groups (Figure 2). Pair-wise comparisons show that naïve MPH rats had significantly larger changes in EO DA over time compared to both the saline groups (p<0.001 all comparisons). CCI MPH rats also had significantly higher EO DA compared to both vehicle groups (p<0.001 all comparisons), but they still had a smaller increase in evoked DA compared to MPH treated naïves (p=0.005). There were significant group differences in evoked DA overflow beginning as early as 10 minutes post-injection and lasting through the 50 minute study period (p<0.001 all comparisons). In all cases except the 50 minute time point, naïve MPH rats had a significantly larger increase in EO DA compared to CCI MPH rats as well as compared to saline vehicle treated groups (p<0.05 all comparisons). At 50 minutes post-injection, pair-wise comparisons show that MPH groups were not significantly different from one another (p=0.35) but were still both significantly different from saline groups (p=0.003).
Figure 2.
Time dependent relative changes in electrically evoked overflow DA with 5 mg/kg MPH vs. saline injection. Values reported at each time point as percent of baseline values ± SEM. Percent change in evoked overflow DA over time for injury and naïve groups. Group differences in evoked overflow at each time point post-injection (*p<0.001 all comparisons for overall group effect with ANOVA at each time point). (IS=CCI plus vehicle; ID=CCI plus MPH; NS=Naïve plus vehicle; ND=Naïve plus MPH.)
Daily Therapy with MPH after CCI Enhances DA Overflow with Single Dose MPH
Experiment 3
FSCV studies in this experiment were analyzed similar to that described in experiment 2. There were no significant differences in the baseline EO [DA] across injury/pretreatment groups [7.8+/-1.1 μM (CCI-saline pretreatment); 6.1+/-0.8 μM (CCI-MPH pretreatment); 5.8+/-0.9 μM (Naïve-saline pretreatment) 7.2+/-1.0 μ M (Naïve-MPH pretreatment)]. Analyses with all eight experimental groups (see figure 3A) shows that overall there was a main effect for group (F=6.7; p<0.0001), time (F=26.5; p<0.0001), and group*time interaction (F=4.5; p<0.0001). Similar to experiment 2, pair-wise comparisons show that CCI rats pretreated with saline had a significantly smaller change in EO DA over time after a single dose of MPH compared naïve rats pretreated with saline (p<0.05). However, CCI rats pretreated with MPH had a significantly larger change in EO DA after a single dose of MPH than CCI rats pretreated with saline (p=0.007). In addition, CCI rats pretreated with MPH had a similar increase in EO DA after MPH injection compared to that observed in either naïve group. There were significant group differences in EO DA across at each time point after MPH/saline challenge (p<0.001 all comparisons). Although there was a trend at 30 minutes (p=0.08), CCI rats pretreated with saline had significantly smaller changes in EO DA beginning 40 minutes after MPH injection compared to naïve rats pretreated with saline (p<0.05 all comparisons). CCI rats pretreated with saline had significantly smaller changes in EO DA at each time point after injection with MPH than CCI rats pretreated with MPH (p<0.02 all comparisons).
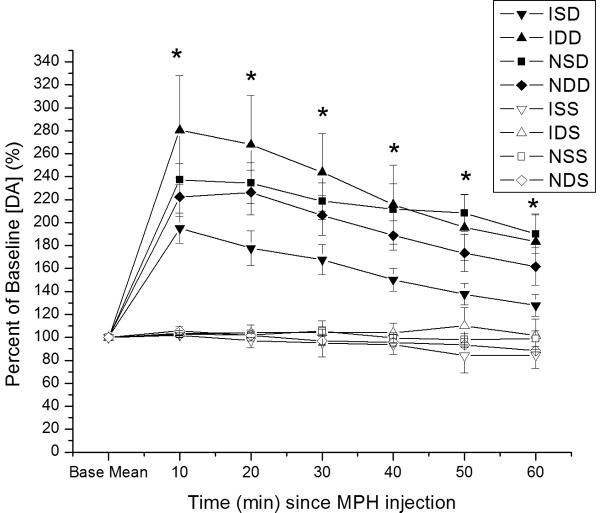
Figure 3.
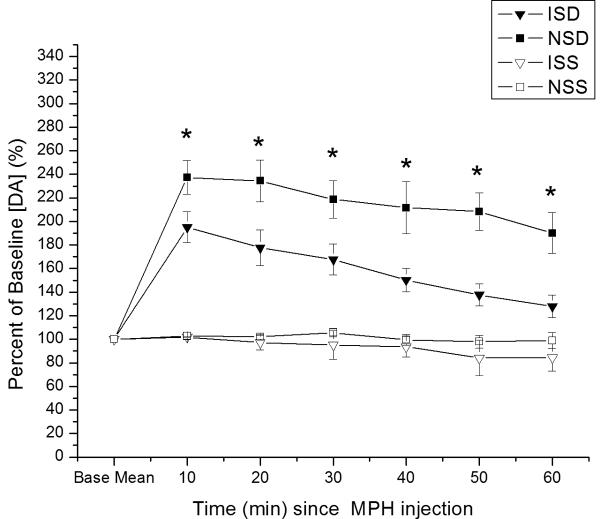
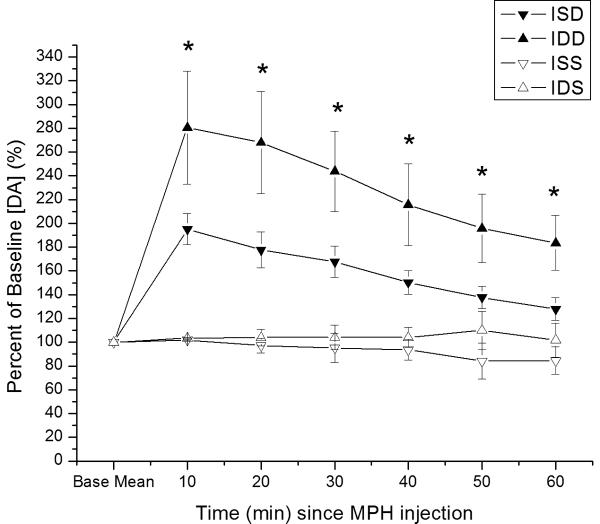
Time dependent relative changes in electrically evoked overflow DA with 5 mg/kg MPH vs. saline injection challenge. Experimental groups pretreated with either saline or MPH 5mg/kg. Values reported at each time point as percent of baseline values ± SEM. A). All eight experimental groups plotted as percent change in evoked DA overflow vs. time post MPH injection. Group differences in evoked overflow at each time point post-injection (*p<0.0001 all comparisons for overall group effect with ANOVA at each time point). B) Percent change in evoked DA overflow over time for injury and naïve groups pretreated with saline. Group differences in evoked overflow at each time points post-injection (*p<0.0001 all comparisons for overall group effect with ANOVA at each time point) C) Percent change in evoked overflow over time for injury groups. Group differences between groups at all time points post-injection (*p<0.04 all comparisons for overall group effect with ANOVA at each time point) (ISD=CCI with saline pretreatment and MPH challenge; IDD=CCI with MPH pretreatment and MPH challenge; NSD=Naïve with saline pretreatment and MPH challenge; NDD=Naïve with MPH pretreatment and MPH challenge; ISS=CCI with saline pretreatment and saline challenge; IDS=CCI with MPH pretreatment and saline challenge; NSS=Naïve with saline pretreatment and saline challenge; NDS=Naive with MPH pretreatment and saline challenge.)
When assessing only CCI and naïve groups pretreated with saline (see figure 3b), there was a significant effect of group (F=19.3; p<0.0001), time (F=20.9; p<0.0001) and a group*time interaction (F=7.6; p<0.0001). Pair-wise analysis shows that CCI rats pretreated with saline had significantly higher EO [DA] after MPH injection than vehicle treated groups, but EO [DA] after MPH was significantly lower in saline pretreated CCI rats compared to naïve rats pretreated with saline (p<0.005 all comparisons). Significant group differences occurred at each time point (p<0.001 all comparisons), with pair-wise analyses showing significantly smaller changes in EO [DA] with CCI compared to naïve rats after MPH injection (p<0.03 all comparisons). When assessing only CCI groups pretreated with either saline or MPH (figure 3c), there was a significant group (F=4.8; p<0.02), time (F=8.2; p<0.0001) and group*time interaction (F=3.5; p<0.0001). Post hoc analysis shows that CCI rats pretreated with MPH had greater changes in EO [DA] after MPH injection compared to saline pretreated CCI rats after MPH injection and compared to saline/MPH pretreated CCI rats receiving a single vehicle injection (p<0.04 all comparisons). There were significant differences in EO for groups at each time point (p<0.04 all comparisons), with pair-wise comparisons showing that CCI rats pretreated with MPH had a larger response to MPH injection than saline pretreated CCI rats at each time post MPH injection (p<0.05 all comparisons).
DISCUSSION
CCI causes damage to the striatum (Dunn-Meynell and Levin 1997; Tong et al. 2002), a subcortical structure heavily populated with dopaminergic neuron projections. Previous studies implicate DA systems in mediating cognitive deficits after TBI through alterations in striatal DA protein expression and DA neurotransmission (Wagner et al. 2005a,b), and treatment with neurostimulants like MPH is linked with improvements in cognitive function as well as restoration of striatal DA neurotransmission (Wagner et al. 2007, 2009; Warden et al. 2006). While low doses of stimulant have more subtle effects (Arnsten 2006), acute response to injection with various dopamine transporter inhibitors is typically characterized by elevations in extracellular dopamine, changes in monoaminergic transporter function, behavioral activation, and many molecular changes (Kuzcinski and Segal 1997; Huff and Davies 2002; Wagner et al. 2005a, 2007; Zetterstrom et al. 1988; Greco and Garris 2003; Jedynak et al. 2002; Hope et al. 2006; Arnsten 2006). Further, repeated exposure to these neurostimulants, including MPH, has been linked with more prolonged neuroadaptive changes (Brandon and Steiner 2003; Chase et al. 2003; Hope et al. 1992; Hawkin et al. 2004; Lin et al. 1996; Yano and Steiner 2005; Shaham and Hope 2005). Given the importance of DA systems in TBI and the influence that both acute and daily neurostimulant exposure has on behavior and DA neurotransmission, this study focused on how experimental TBI affects the typical immediate neurostimulant response on neurotransmission observed after MPH injection. The ability of a daily pretreatment regime with MPH to change the striatal evoked DA response that occurs after MPH injection was also tested. Our results show that CCI impairs the ability of nigrostriatal DA systems to augment both basal and electrically evoked extracellular DA levels after a neurostimulant challenge. Importantly, daily pretreatment with MPH after CCI reverses this impairment. The effect of chronic MPH pretreatment on changes in evoked DA with a single dose of MPH suggests that MPH is acting beyond a simple DA replacement therapy and may be affecting many aspects of neuronal function and signaling pathways to impact neurotransmission.
When measuring changes in [DA] output in striatal dialysates after MPH injection, peak [DA] occurred about 40 minutes after MPH injection for shams, which is consistent with previous observations and with the rapid absorption and metabolism of MPH (Huff and Davies 2002; Kuczenski and Segal 1997). Two hours after MPH administration, DA levels returned toward baseline, also indicating short half-life of MPH in rats (Kuczenski and Segal 2005). In experiment 1, MPH administration resulted in a smaller change in [DA] output in CCI rats compared to sham, which suggests impaired striatal DA function after TBI.
DA and metabolite levels in the striatal microdialysates were similar between injury groups and controls and similar to that found in other studies (Smith et al. 2005; Sadri-Vakili et al. 2008; Abin-Carriquiry et al. 2008; Gagnaire et al. 2006; Lonjon et al. 2004). These findings are consistent with other studies suggesting that only a transient elevation in basal striatal tissue DA levels occurs after CCI (McIntosh et al. 1994; Massucci et al. 2004). Despite no difference in pre-drug DA levels in experiment 1, response to MPH injection was injury specific and consistent with later voltammetry experiments assessing evoked DA, indicating that CCI has influenced how the striatum responds to pharmacological stimuli. However, given discrepancies in basal DA levels when using microdialysis versus FSCV (Kulgina et al. 2001; Borland and Michael 2004; Smith et al. 2005) and the impact of local tissue injury from microdialysis probes on basal DA levels (Borland et al. 2005), more work is warranted using FSCV to determine how CCI impacts basal [DA] in the striatum.
DOPAC is a major product of DA that is produced directly via monoamine oxidase (MAO). Alternatively, HVA is a principal metabolite of DA after its conversion by Catechol-O-methyl transferase (COMT) to 3-methyltyrosine (3-MT) or its conversion to DOPAC by MAO (Oechsner et al. 2002). There was a time dependent trend for decreased DOPAC with MPH administration, which implies a possible role for MPH in decreasing DA metabolism/degradation via oxidative pathways. MPH appears to have little effect on DA metabolism via o-methylation pathways since HVA levels were not affected after MPH administration. Other reports suggest that MPH does not impact extracellular HVA levels except at higher doses (e.g. 30mg/kg or greater). However, these same reports also conclude that DOPAC is also not significantly influenced by MPH administration (Kuczenski and Segal 1997; Zetterstrom et al. 1988; Butcher et al. 1991). Since metabolism from DA to DOPAC involves intraneuronal MAO activity (Youdim and Riederer 2004), DAT blockade and decreased reuptake of DA into cells may be one mechanism by which DOPAC levels could decrease over time in MPH treated groups. The localization of COMT in extra-neuronal and post-synaptic locations (Kaakkola et al. 1987) may be one reason why HVA levels are not affected by MPH challenge.
Findings from experiment 2 were consistent with that noted for experiment 1, where MPH administration resulted in a smaller increase in evoked DA overflow in CCI rats compared to controls. Changes in EO DA were immediate and lasted through the time course studied. The effects of CCI in diminishing evoked DA response to a single dose of MPH were replicated in experiment 3. However, a two week pretreatment course with MPH in CCI rats restored evoked DA levels observed with a single dose of MPH to that typically observed in naïve rats. This result is consistent with previous work showing that daily MPH therapy restores EO and DA clearance kinetics after CCI (Wagner et al. 2009).
The molecular mechanisms by which MPH exerts its restorative effects on striatal function after CCI are not yet well defined. CCI does result in decreased total striatal DAT expression chronically after injury, but there is an increase in membrane bound DAT in synaptosomes (Wagner et al. 2005a,b, 2009). However, transient alterations in DAT expression or trafficking acutely after MPH injection or during MFB stimulation for CCI rats may contribute to smaller changes observed with both basal and evoked DA. Our previous work demonstrates that DAT expression does not change after a daily MPH treatment regime in CCI rats. However, despite no changes in DAT expression with daily MPH therapy, robust changes occur with evoked DA and DA clearance with CCI and daily MPH treatment (Wagner et al. 2009). These findings suggest that daily MPH therapy induces functional changes in DAT or differences in DAT trafficking that then help restore DA transmission after CCI. Functional DAT changes associated with MPH pretreatment may be involved with the increased evoked DA overflow observed in experiment 3 with CCI rats after a single MPH injection.
Interestingly, striatal DA release is also impaired with CCI (Wagner et al. 2005a), and daily MPH therapy eliminates decrements in evoked DA release when administered for two weeks after CCI (Wagner et al. 2009). MPH can influence vesicular trafficking, increase the amount of DA stored per vesicle, and alter D2DR mediated presynaptic autofeedback, such that DA release is enhanced (Fleckenstein et al. 2008; Volz et al. 2007, 2008). It is possible that daily MPH treatment after CCI may influence the amount of DA released with MFB stimulation after MPH injection through some of these mechanisms.
Previous results suggest that, when using a maximal stimulating paradigm, CCI results in less evoked DA overflow. However, our approach for the current voltammetry experiments was to establish a comparable baseline stimulus response between injury groups in order to evaluate MPH challenge response under similar baseline conditions while avoiding the confounding factor of differing baseline responses. As such, we obtained a baseline stimulus response during the study of at least 15 nA before administering MPH or vehicle. Further, we restricted our data analysis post-hoc to studies where baseline evoked [DA] ranged between 3-15μM. This range encompassed the nearly all of the studies performed, and baseline evoked DA levels were similar across treatment groups and across experiments. This consistency in baseline performance allowed us to determine that the change in EO DA response with MPH injection is TBI specific. Although the molecular mechanisms of how EO DA after MPH injection is normalized with MPH pretreatment for CCI rats is not tested in this study, it is possible that CCI related decreases in constitutive striatal c-fos, and the large increases in c-fos after daily MPH therapy shown in previous work (Wagner et al. 2009), may play a role in this process.
Interestingly, evoked DA overflow with MPH challenge was not significantly different in naïve rats regardless of MPH or saline pretreatment. This result is also similar to previous work assessing daily MPH therapy in CCI vs. control groups (Wagner et al. 2009). Although behavioral sensitization can occur with repeated exposure to some stimulants (Hope et al. 2006), daily treatment with neurostimulants has been reported to attenuate upregulation of transcription factor expression compared to that typically observed when administering neurostimulant challenge to drug naïve rats (Chase et al. 2003; Hope et al. 1992). As such, diminished cell signaling and gene expression responses may occur in naive groups with daily MPH exposure in an effort to achieve relative maintenance of DA homeostasis. A loss of homeostatic potential after TBI may facilitate the neuroadaptive effects of daily MPH therapy on DA neurotransmission after MPH injection.
In conclusion, CCI results in a decreased ability to augment DA neurotransmission after injection with MPH. However, a daily pretreatment regime with MPH restores MPH induced augmentation of DA neurotransmission. This work has implications for MPH effects on recovery after TBI. Future studies are required to determine the molecular substrates underlying CCI and stimulant induced changes in DA striatal neurotransmission. Additionally, further study of kinetic changes with MPH challenge in CCI, as well as regional variability with MPH effects on DA transmission in the context of CCI, are warranted.
Acknowledgements
This work was supported by NIH K08HD40833 (AKW), R01NS40125 (CED), R21 NS057348 (AKW & ACM). Special thanks to Xiecheng Ma for her technical support on this study.
Abbreviations
- TBI
Traumatic brain injury
- DA
Dopamine
- MPH
Methylphenidate
- CCI
controlled cortical impact
- MFB
Medial forebrain bundle
- FSCV
Fast scan cyclic voltammetry
- DAT
DA transporter
- ACSF
artificial cerebrospinal fluid
- HPLC
High performance liquid chromatography
- DA
Dopamine
- DOPAC
dihydroxyphenylacetic acid
- HVA
homovanillic acid
- AP
anterior/posterior
- ML
medial/lateral
- DV
dorsal/ventral
- RMANOVA
repeated measures analysis of variance
- SEM
standard error of the mean
- LSD
least significant difference
- EO
evoked overflow
- MAO
monoamine oxidase
- COMT
Catechol-O-methyl transferase
- 3-MT
3-methyltyrosine
REFERENCES
- Abin-Carriquiry JA, Costa G, Urbanavicius J, Cassels BK, Rebolledo-Fuentes M, Wonnacott S, Dajas F. In vivo modulation of dopaminergic nigrostriatal pathways by cytisine derivatives: implications for Parkinson's Disease. Eur. J. Pharmacol. 2008;589:80–84. doi: 10.1016/j.ejphar.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. Review. [DOI] [PubMed] [Google Scholar]
- Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J. Neurochem. 2004;91:220–229. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J. Neurosci. Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Steiner H. Repeated methylphenidate treatment in adolescent rats alters gene regulation in the striatum. Eur. J. Neurosci. 2003;18:1584–1592. doi: 10.1046/j.1460-9568.2003.02892.x. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Liptrot J, Aburthnott GW. Characterisation of methylphenidate and nomifensine induced dopamine release in rat striatum using in vivo brain microdialysis. Neurosci. Lett. 1991;122:245–248. doi: 10.1016/0304-3940(91)90869-u. [DOI] [PubMed] [Google Scholar]
- Chase TD, Brown RE, Carrey N, Wilkinson M. Daily methylphenidate administration attenuates c-fos expression in the striatum of prepubertal rats. Neuroreport. 2003;14:769–772. doi: 10.1097/00001756-200304150-00022. [DOI] [PubMed] [Google Scholar]
- Dash PK, Moore AN, Dixon CE. Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J. Neurosci. 1995;15:2030–2039. doi: 10.1523/JNEUROSCI.15-03-02030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaqhmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Levin BE. Histological markers of neuronal, axonal and astrocytic changes after lateral rigid impact traumatic brain injury. Brain Res. 1997;761:25–41. doi: 10.1016/s0006-8993(97)00210-2. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR. Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: Neurotoxic and therapeutic implications. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.07.002. [Epub ahead of print] doi:10.1016/j.neuropharm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnaire F, Chalansonnet M, Carabin N, Micillino JC. Effects of subchronic exposure to styrene on the extracellular and tissue levels of dopamine, serotonin and their metabolites in rat brain. Arch. Toxicol. 2006;80:703–712. doi: 10.1007/s00204-006-0083-3. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc. Natl. Acad. Sci. USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco PG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur. J. Pharmacol. 2003;479:117–125. doi: 10.1016/j.ejphar.2003.08.062. Review. [DOI] [PubMed] [Google Scholar]
- Hawken CM, Brown RE, Carrey N, Wilkinson M. Long-term methylphenidate treatment down-regulates c-fos in the striatum of male CD-1 mice. Neuroreport. 2004;15:1045–1048. doi: 10.1097/00001756-200404290-00022. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc. Natl. Acad. Sci. USA. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ. Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur. J. Neurosci. 2006;24:867–875. doi: 10.1111/j.1460-9568.2006.04969.x. [DOI] [PubMed] [Google Scholar]
- Huff JK, Davies MI. Microdialysis monitoring of methylphenidate in blood and brain correlated with changes in dopamine and rat activity. J. Pharm. Biomed. Anal. 2002;29:767–777. doi: 10.1016/s0731-7085(02)00196-6. [DOI] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacological Reviews. 1995;47:133–178. Review. [PubMed] [Google Scholar]
- Jedynak JP, Ali SF, Haycock JW, Hope BT. Acute administration of cocaine regulates the phosphorylation of serine-19, -31 and -40 in tyrosine hydroxylase. J. Neurochem. 2002;82:382–388. doi: 10.1046/j.1471-4159.2002.00982.x. [DOI] [PubMed] [Google Scholar]
- Kaakkola S, Männistö PT, Nissinen E. Striatal membrane-bound and soluble catechol-O-methyl-transferase after selective neuronal lesions in the rat. J. Neural Transm. 1987;69:221–228. doi: 10.1007/BF01244343. [DOI] [PubMed] [Google Scholar]
- Kline AE, Yan HQ, Bao J, Marion DW, Dixon CE. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci. Lett. 2000;280:163–166. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J. Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol. Psychiatry. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. Review. [DOI] [PubMed] [Google Scholar]
- Kulgina NV, Zigmond MJ, Michael AC. Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience. 2001;19:121–128. doi: 10.1016/s0306-4522(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc. Natl. Acad. Sci. USA. 1996;93:14128–14133. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonjon M, Quentien MH, Risso JJ, Michiels JF, Carre E, Rostain JC, Darbin O. Alteration of striatal dopaminergic function induced by glioma development: a microdialysis and immunohistological study in the rat striatum. Neurosci. Lett. 2004;354:131–134. doi: 10.1016/j.neulet.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lu Y, Peters JL, Michael AC. Direct comparison of the response of voltammetry and microdialysis to electrically evoked release of striatal dopamine. J. Neurochem. 1998;70:584–593. doi: 10.1046/j.1471-4159.1998.70020584.x. [DOI] [PubMed] [Google Scholar]
- Massucci JL, Kline AE, Ma X, Zafonte RD, Dixon CE. Time dependent alterations in dopamine tissue levels and metabolism after experimental traumatic brain injury in rats. Neurosci. Lett. 2004;372:127–131. doi: 10.1016/j.neulet.2004.09.026. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Yu T, Gennarelli TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J. Neurochem. 1994;63:1426–1433. doi: 10.1046/j.1471-4159.1994.63041426.x. [DOI] [PubMed] [Google Scholar]
- Natale JE, Ahmed F, Cernak I, Stoica B, Faden AI. Gene expression profile changes are commonly modulated across models and species after traumatic brain injury. J. Neurotrauma. 2003;20:907–927. doi: 10.1089/089771503770195777. [DOI] [PubMed] [Google Scholar]
- Oechsner M, Strauss J, Stuerenberg HJ. COMT-inhibition increases serum levels of dihydroxyphenylacetic acid (DOPAC) in patients with advanced Parkinson's disease. J. Neural Transm. 2002;109:69–75. doi: 10.1007/s702-002-8237-z. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Sadri-Vakili G, Janis GC, Pierce RC, Gibbs TT, Farb DH. Nanomolar concentrations of pregnenolone sulfate enhance striatal dopamine overflow in vivo. J. Pharmacol. Exp. Ther. 2008;327:840–845. doi: 10.1124/jpet.108.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat. Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. Review. [DOI] [PubMed] [Google Scholar]
- Smith AD, Kozlowski DA, Bohn MC, Zigmond MJ. Effect of AdGDNF on dopaminergic neurotransmission in the striatum of 6-OHDA-treated rats. Exp. Neurol. 2005;193:420–426. doi: 10.1016/j.expneurol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Tong W, Igarashi T, Ferriero DM, Noble LJ. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp. Neurol. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, King JL, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate administration alters vesicular monoamine transporter-2 function in cytoplasmic and membrane associated vesicles. J. Pharmacol. Exp. Ther. 2007;323:738–745. doi: 10.1124/jpet.107.126888. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, Rowley SD, Hanson GR, Fleckenstein AE. Methylphenidate induced increases in vesicular dopamine sequestration and dopamine release in the striatum: the role of muscarinic and D2 receptors. J. Pharmacol. Exp. Ther. 2008;327:161–167. doi: 10.1124/jpet.108.139386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J. Neurochem. 2005a;95:457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Chen X, Kline AE, Li Y, Zafonte RD, Dixon CE. Gender and environmental enrichment impact dopamine transporter expression after experimental traumatic brain injury. Exp. Neurol. 2005b;195:475–483. doi: 10.1016/j.expneurol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Ren D, Conley Y, Ma X, Kerr ME, Zafonte RD, Puccio AM, Marion DW, Dixon CE. Gender and Genetic Associations with CSF Dopamine and Metabolite Production after Severe TBI. J Neurosurg. 2007a;106:537–547. doi: 10.3171/jns.2007.106.4.538. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Ren D, Willard LA, Wenger MK, Zafonte RD, Dixon CE. Gender associations with chronic methylphenidate treatment and behavioral performance following experimental TBI. Behav. Brain Res. 2007b;181:200–209. doi: 10.1016/j.bbr.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Drewencki L, Chen X, Santos FR, Khan AS, Harun R, Torres G, Michael AC, Dixon CE. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J. Neurochem. 2009;108:986–997. doi: 10.1111/j.1471-4159.2008.05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J. Neurotrauma. 2006;23:1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Ma X, Chen X, Li Y, Shao L, Dixon CE. Delayed increase of tyrosine hydroxylase expression in rat nigrostriatal system after traumatic brain injury. Brain Res. 2007;1134:171–179. doi: 10.1016/j.brainres.2006.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Mu XS, Xue JJ, Whitson J, Salminen A, Dixon CE, Liu PK, Hayes RL. Increased expression of c-fos mRNA and AP-1 transcription factors after cortical impact injury in rats. Brain Res. 1994;664:141–147. doi: 10.1016/0006-8993(94)91964-x. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Topography of methylphenidate (ritalin)-induced gene regulation in the striatum: differential effects on c-fos, substance P and opioid peptides. Neuropsychopharmacology. 2005;30:901–915. doi: 10.1038/sj.npp.1300613. [DOI] [PubMed] [Google Scholar]
- Youdim M, Riederer PF. A review of the mechanisms and role of monoamine oxidase inhibitors in Parkinson's disease. Neurology. 2004;63:S32–S35. doi: 10.1212/wnl.63.7_suppl_2.s32. [DOI] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fosimmunoreactive proteins via dopaminergic D1 receptors. Proc. Natl. Acad. Sci. USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterström T, Sharp T, Collin AK, Ungerstedt U. In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. Eur. J. Pharmacol. 1988;148:327–334. doi: 10.1016/0014-2999(88)90110-0. [DOI] [PubMed] [Google Scholar]