Abstract
Bipolar disorder (BD) is a major medical and social burden, whose cause, pathophysiology and treatment are not agreed on. It is characterized by recurrent periods of mania and depression (Bipolar I) or of hypomania and depression (Bipolar II). Its inheritance is polygenic, with evidence of a neurotransmission imbalance and disease progression. Patients often take multiple agents concurrently, with incomplete therapeutic success, particularly with regard to depression. Suicide is common. Of the hypotheses regarding the action of mood stabilizers in BD, the “arachidonic acid (AA) cascade” hypothesis is presented in detail in this review. It is based on evidence that chronic administration of lithium, carbamazepine, sodium valproate, or lamotrigine to rats downregulated AA turnover in brain phospholipids, formation of prostaglandin E2, and/or expression of AA-cascade enzymes, including cytosolic phospholipase A2, cyclooxygenase-2 and/or acyl-CoA synthetase. The changes were selective for AA, since brain docosahexaenoic or palmitic acid metabolism, when measured, was unaffected, and topiramate, ineffective in BD, did not modify the rat brain AA cascade. Downregulation of the cascade by the mood stabilizers corresponded to inhibition of AA neurotransmission via dopaminergic D2-like and glutamatergic NMDA receptors. Unlike the mood stabilizers, antidepressants that increase switching of bipolar depression to mania upregulated the rat brain AA cascade. These observations suggest that the brain AA cascade is a common target of mood stabilizers, and that bipolar symptoms, particularly mania, are associated with an upregulated cascade and excess AA signaling via D2-like and NMDA receptors. This review presents ways to test these suggestions.
Keywords: bipolar disorder, brain, lithium, valproic, carbamazepine, lamotrigine, arachidonic acid, phospholipase A2, mood stabilizer, antidepressant, antipsychotic, mania, depression, rat, kinetics
2. Introduction
2.1. General background
Bipolar disorder (BD) is a major medical, social and economic burden worldwide, and its treatment represents a critical as yet unfulfilled challenge for psychiatry. The estimated lifetime cost of BD in the United States ranges from $11,720 for patients with a single manic episode, to $624,785 for patients with nonresponsive/chronic episodes (Begley et al., 2001). Symptoms appear on average at 22 years of age (Goodwin and Jamison, 2007), but the disease often is not diagnosed until 10 years later, with treatment delayed for an additional 10 years (Ghaemi, 2001). Even with intensive treatment in academic centers, BD therapy is inadequate and patients remain symptomatic for half of the year on average (Post et al., 1996).
BD is characterized by recurrent changes in mood. Bipolar I consists of cycles of mania and depression, Bipolar II of cycles of hypomania and depression. With rapid cycling (four or more episodes of depression or mania/hypomania or mixed episodes within a year), the disease has a poorer prognosis (Belmaker, 2004; DSM-IV, 1994). In Bipolar I, which afflicts 1.2 to 1.5% of the adult US population (Narrow et al., 2002), depression is 3-times more common than mania (Judd et al., 2002).
BD patients frequently have multiple medical illnesses in addition to their profound mood disturbances (Evans et al., 2005). Smoking and substance abuse are common (Begley et al., 2001). Obesity and diabetes often are caused by therapy. Sleep apnea and obsessive compulsive disorder can confound the presentation. The suicide rate is 5-17 fold higher than in the general population, attaining a lifetime risk of 10% to 20% (Bostwick and Pankratz, 2000).
There is no agreed upon neuropathology or pathophysiology of BD, and a generally accepted behavioral animal model for the disease does not exist (Cryan and Slattery, 2007; Kato et al., 2007). Some consider BD and unipolar major depressive disorder as part of an overlapping spectrum (Judd and Akiskal, 2003). However, in this review they will be treated as separate entities. BD and unipolar depression differ in their responsiveness to antidepressants (Ghaemi et al., 2004; Sachs et al., 2000), their clinical history including the presence of manic or hypomanic episodes in BD, clinical characteristics of depression (Benazzi, 2007), and family history. BD has a heritability of 86-90%, while major depression has a heritability of 48-75% (McGuffin et al., 2003). Over an 11-year follow-up period, however, 9% of unipolar depressed patients were diagnosed later as Bipolar II and 4% as Bipolar 1 because of the later appearance of hypomanic or manic episodes (Akiskal et al., 1995).
2.2. Heritability and genetics
More than two-thirds of BD probands have at least one close affected relative or a relative with unipolar major depression. Concordance rates in monozygotic and dizygotic twins are 40% and < 10%, respectively (Cardno et al., 1999; Kieseppa et al., 2004). Familial factors can determine responsiveness to lithium, the drug of choice for the disease (Alda, 1999; Grof et al., 2002).
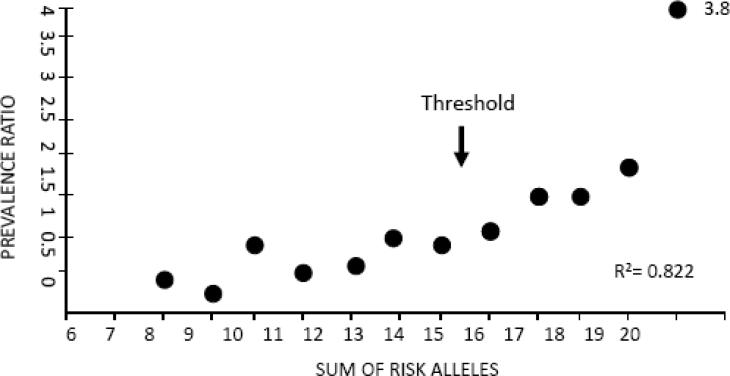
A recent genome-wide association study with replication, involving 550,000 single nucleotide polymorphisms, implicated 88 different risk alleles in BD (Baum et al., 2008) (Figure 1), consistent with a polygenic threshold inheritance (Falconer, 1967). A combination of more than 15 of any of these alleles significantly increased the risk for BD above the risk in the general population, whereas a combination of any 19 increased the risk 3.8-fold. Multiple risk alleles also were identified in other whole genome scans (Schumacher et al., 2005; Sklar et al., 2008; Wellcome Trust, 2007; Wigg et al., 2008; Willour et al., 2003). Comparisons showed, however, that the studies had poor replication of positive findings, typical of measurements having multiple poor sensitivity items and suggesting additional artifacts (Crow, 2007; Sklar et al., 2008). This limitation of this “bottom-up” approach makes it difficult to identify dysfunctional brain metabolic cascades characteristic of BD.
Figure 1. Prevalence ratio of bipolar disorder increases with risk allele burden.
A combination of more than any 15 of risk alleles significantly increases the risk for BD above the risk (1.0) in the general population, whereas a combination of any 19 increases the risk 3.8 fold. From (Baum et al., 2008).
Gene linkage and candidate gene association studies also have identified risk alleles for BD, but such studies compared to whole genome scans are considered underpowered and capable of revealing only part of the genetic disturbance (Baum et al., 2008). Many identified alleles are related to neurotransmission (Kato, 2007). They include the serotonergic (5-hydroxytryptamine, 5-HT) 5-HT2A receptor (Bonnier et al., 2002), the serotonin reuptake transporter (5-HTT) (Lasky-Su et al., 2005), the dopamine D2 receptor (Massat et al., 2002), the dopamine reuptake transporter (DAT) (Greenwood et al., 2006; Horschitz et al., 2005), N-methyl-D-aspartate (NMDA) receptor subunits (Martucci et al., 2006), G protein receptor kinase-3 (GRK-3) (Barrett et al., 2003), the L-type voltage-dependent calcium channel alpha 1C subunit (CACNA1C) (Sklar et al., 2008), and the ©-aminobutyric acid receptor (Craddock et al., 2008).
3. Proposed mechanisms of bipolar disorder
3.1. Neurotransmission imbalance
Clinical responses of BD patients to different centrally acting drugs have suggested that BD symptoms arise from an imbalance of neurotransmission, consisting of excessive dopaminergic and glutamatergic transmission and reduced cholinergic muscarinic transmission (Bunney and Garland-Bunney, 1987; Bymaster and Felder, 2002; Janowsky and Overstreet, 1995; Post et al., 1980). Thus, drugs that inhibit dopaminergic transmission, such as olanzapine and haloperidol, have an antimanic action in the disease (Bhana and Perry, 2001; Bymaster and Felder, 2002). In contrast, drugs that stimulate dopamine synthesis (levodopa), bind to dopamine receptors (bromocriptine), or reduce dopamine reuptake (amphetamine), can precipitate mania (Fisher et al., 1991; Peet and Peters, 1995; Sultzer and Cummings, 1989). The cholinesterase inhibitor, physostigmine, and the cholinergic agonist, RS86, have mood-depressing effects in bipolar mania, as well as in schizophrenic and healthy subjects (Berger et al., 1989). Consistent with increased glutamate signaling in BD, memantine (1-amino-3,5-dimethyl-adamantane), an NMDA receptor antagonist approved for treating moderate to severe Alzheimer disease (FDA, 2009), was found beneficial in bipolar depression (Davis et al., 1978; Janowsky and Overstreet, 1995; Teng and Demetrio, 2006), and Riluzole (2-amino-6-trifluoromethoxybenzothiazole), an inhibitor of glutamate release, was reported effective in combination with lithium in an open label trial in BD patients (Zarate et al., 2005).
3.2. Disease progression
Symptom worsening, cognitive decline and progressive brain atrophy have been reported in BD patients, suggesting disease progression. Cycles of mania and depression are reported to increase in frequency with disease duration, and to have shorter inter-episode intervals (Goodwin and Jamison, 2007; Kessing, 1999; Post, 1990). One study found that cognitive deficits were present regardless of mood state, with executive functioning, episodic memory, and sustained concentration most consistently impaired (Osuji and Cullum, 2005). Structural magnetic resonance (MR) imaging demonstrated significant thinning of the cortical mantle, widening of cortical sulci and dilatation of the lateral cerebral ventricles in BD patients, with evidence of atrophy progression depending on symptom recurrence (Coyle et al., 2006; Lyoo et al., 2006; Strakowski et al., 2002). Whether atrophy is a trait marker is not certain, since BD relatives, unlike relatives of schizophrenic patients, did not demonstrate brain atrophy in one study (McDonald et al., 2006). Atrophy, cognitive decline and symptom worsening also have been noted in schizophrenic patients (DeLisi, 2008; Nesvag et al., 2008).
3.2.1. Neuroinflammation and excitotoxicity
Neuroinflammation and excitotoxicity may contribute to progressive atrophy and disease worsening in BD. High levels of C-reactive protein and of pro-inflammatory cytokines have been identified in plasma from BD patients, suggesting a generalized inflammatory process. Postmortem frontal cortex from BD patients demonstrated elevated protein and mRNA levels of a number of inflammatory markers, interleukin (IL)-1β, the IL-1 receptor, inducible nitric oxide synthase, glial fibrillary acidic protein, and myeloid differentiation primary response gene 88 (Rao et al., In press), as well as apoptotic markers (Kim et al., 2008). MR spectroscopy demonstrated an elevated brain glutamate/glutamine ratio in adults with BD (Michael et al., 2003). Fewer NMDA receptors and reduced mRNA levels of NMDA receptor subunits NR1, NR2A and NR3A were reported on postmortem (Kim et al., 2007b), and densities of NMDA receptor-associated post-synaptic proteins, PSD-95 and SAP102, were reduced (Beneyto et al., 2007). These differences imply excess NMDA receptor function associated with excitotoxicity (Gascon et al., 2005).
4. Treatment of bipolar disorder
There is a clear need for improved treatment of BD. Currently approved agents used as monotherapy do not produce long-term responses in the majority of patients, and patient compliance is difficult to achieve (Post et al., 1996). For example, in a collated analysis of 2300 patients with acute mania who were given a placebo or any of 8 recognized “effective” agents as monotherapy, patients treated with only one agent experienced 45-60% positive symptom responses, compared with a 30% positive response to placebo (Bowden et al., 2005b). Without factoring in inadequate dosage and noncompliance in this study, only half of the already low fraction of drug responders may have had a “veritable” drug response.
BD patients often are treated with more than one agent in various doses and for various times, using combinations of mood stabilizers, antipsychotics, antidepressants and hypnotic benzodiazepines (Bowden, 2003). This approach is called “rational polypharmacy” (Post et al., 1996; Sachs, 1996), but often reduces to adding a drug for a specific symptom as it appears.
4.1. Mood stabilizers and atypical antipsychotics
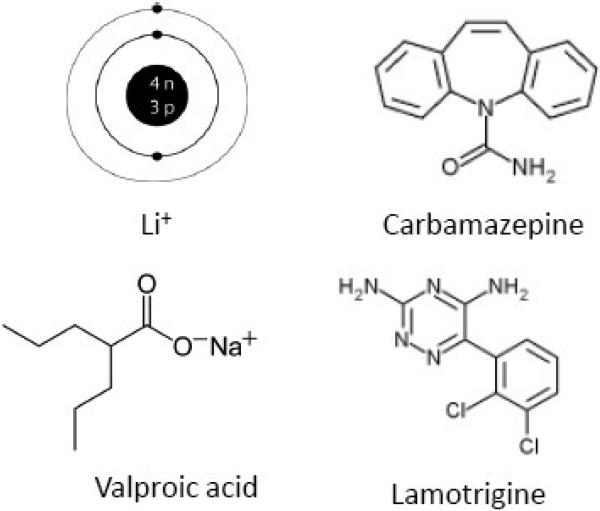
FDA-approved agents (Figure 2) for treating Bipolar I include the “mood-stabilizers”1 lithium (as carbonate or citrate salt, approved for mania and maintenance therapy), divalproex (Depakote®, containing sodium valproate and valproic acid (2-propylpentanoic acid) in 1:1 molar ratio; approved for mania), carbamazepine (Tegretol®, 5-carbamoyl-5H-dibenz[b,f]azepine, approved for mania), and lamotrigine (Lamictal®, 6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine, approved as prophylactic treatment of Bipolar I to delay time to occurrence of the different mood episodes) (Bowden et al., 2005b; FDA, 2009; Fountoulakis et al., 2008; Ketter et al., 2005). Topiramate (Topomax®, 2,3:4,5-Di-O-isopropylidene-β-D-fructopyranose sulfamate), an anticonvulsant suggested from initial observations to be effective in BD, was later found ineffective in four double blind trials (Kushner et al., 2006); it is discussed below in comparative studies with approved mood stabilizers.
Figure 2.
Chemical structures of FDA-approved mood stabilizers
Outcome comparisons among FDA-approved mood stabilizers as monotherapy have been reported (Bowden, 2003; Fountoulakis et al., 2008). For example, patients with mania accompanied by two or more depressive symptoms had greater improvement with valproate than with lithium, whereas patients with mania alone had equivalent responses of the order of 30-40% (Bowden, 1995; Swann et al., 1997). Valproate or lithium in acute mania and in maintenance studies was more effective than carbamazepine, with which recurrence occurred 50% more often (Kleindienst and Greil, 2000; Lerer et al., 1987; Vasudev et al., 2000).
Atypical antipsychotic agents that are FDA-approved for acute mania in BD are olanzapine (Zyprexia®), aripiprazole (Abilify®), chlorpromazine (Clozaril®), risperidone (Risperdal®), quetiapine (Seroquel®), and ziprasidone (Geodon®) (Bowden et al., 2005b; FDA, 2009; Ketter et al., 2005). In addition, quetiapine is approved for bipolar depression and maintenance therapy adjunctive to lithium or divalproex, and aripiprazole and olanzapine are approved for maintenance monotherapy in Bipolar I.
The atypical antipsychotics have a high affinity as antagonists for dopamine D2 receptors, and many also have high affinities as antagonists for serotonergic 5-HT2 receptors. Some also have affinities for adrenergic, histamine H1 and muscarinic M1 receptors (Kapur and Remington, 2001). No one drug has a clear advantage over the others for treating mania, but as a group they are less likely than conventional antipsychotics to cause troubling extrapyramidal symptoms (Bowden et al., 2005b; Ketter et al., 2005; Simpson, 2005; Yatham, 2005). The atypical antipsychotics are especially useful for rapidly dampening hyperactive motoric symptoms in bipolar mania, before lithium's effects are expressed (Bhana and Perry, 2001; Lieberman and Goodwin, 2004; Post and Calabrese, 2004).
4.2. Antidepressants and switching to mania
The treatment of bipolar mania with mood stabilizers has achieved some success, but treating the three-times more common bipolar depression remains problematic (Fountoulakis et al., 2005). Lamotrigine is of some help, as is fluoxetine (a selective serotonin reuptake inhibitor, SSRI) with olanzapine added, and quetiapine recently was approved as monotherapy (Section 4.1). Paroxetine and bupropion were not found useful as adjunctive therapy with mood stabilizers in bipolar depression (Bowden et al., 2005b; Sachs et al., 2007).
Some antidepressants increase the likelihood of “switching” to mania in depressed bipolar patients, when used as monotherapy or with mood stabilizers (Calabrese et al., 1999a; Post et al., 2006). Elevated switch rates are reported with broad spectrum tricyclic antidepressants that inhibit reuptake of norepinephrine and serotonin (Kleindienst and Greil, 2000), SSRIs and monoamine oxidase inhibitors, but not with bupropion, a norepinephrine and dopamine reuptake inhibitor and nicotinic antagonist (Leverich et al., 2006; Post et al., 2006). Switch rates are reduced if an antimanic mood stabilizer is given with the antidepressant (31.6% versus 84.2%) (Ghaemi et al., 2004).
4.3. Treatment side effects
4.3.1. Lithium in healthy volunteers
When administered to healthy volunteers, lithium caused lethargy, dysphoria, a loss of interest in interacting with others, mental confusion (Judd et al., 1977a), slowed performance on cognitive and motor tests (Judd et al., 1977b), and altered circadian rhythm, the most common effect being a delay in the phase of measured rhythms relative to an entraining light-dark cycle (Klemfuss, 1992). Lthium increased auditory and visual evoked responses in healthy volunteers (Hegerl et al., 1990; Ulrich et al., 1990).
4.3.2. Boxed warnings
Lithium, divalproex and carbamazepine have boxed warnings because of safety concerns. Lithium can be nephrotoxic and neurotoxic, whereas divalproex can produce hepatic and teratogenic effects and contribute to pancreatitis. Carbamazepine may produce adverse hematological effects. As a class, atypical antipsychotics have a boxed warning for sudden death in elderly patients. They increase risk for hyperglycemia, diabetes, and cardiovascular dysfunction, and often produce severe weight gain and sedation (Bowden et al., 2005b; Ketter et al., 2005; Simpson, 2005).
4.3.3. Potential neurotoxicity
In animal and cell models, mood stabilizers and antipsychotic agents used in BD were shown to be neuroprotective through a number of processes, including the reduction of apoptosis and stimulation of brain neurotrophic and growth factors (Section 8.) (Chang et al., 2009; Chen et al., 1999; Chuang, 2005; Gould et al., 2004b; Lieberman et al., 2008; Lu et al., 2004). Thus, neuroprotection may underlie some of their action, especially in view of evidence for apoptosis, neuroinflammation and excitotoxicity in the postmortem BD brain (Kim et al., 2008; Rao et al., In press).
On the other hand, as long-term therapy, mood stabilizers as well as antipsychotics may have neurotoxic effects. For example, chronic lithium administration caused neuronal apoptosis in rat brain (Gomez-Sintes and Lucas, 2008), and chronic exposure to atypical antipsychotics produced oxidative brain damage (Martins et al., 2008; Terry et al., 2003). Twelve to 27 months of exposure of macaque monkeys to haloperidol or olanzapine at therapeutically equivalent plasma concentrations caused widespread brain shrinkage and reduced astrocyte numbers in parietal gray matter (Dorph-Petersen et al., 2005; Konopaske et al., 2008).
5. The brain arachidonic acid cascade
“How can we argue that we are treating [...bipolar disorder...] at a fundamental, etiological level of the illness when we don't know what the chemical problem is, when our best guesses about how various mood stabilizers work are that they work differently from each other, and when we don't know how these proposed mechanisms might or might not be related?”
S. Sobo (Sobo, 1999)
We have yet to convert the disparate data about BD and the agents approved to treat it into a coherent understanding of disease mechanisms and drug targets. The limited efficacy of each of the approved mood stabilizers as monotherapy in bipolar mania (Bowden et al., 2005a; Ketter et al., 2001), the limited treatment of bipolar depression (Sachs et al., 2007), the many untoward side effects of approved agents, and the high morbidity, suicide rate and cost to society of BD, render understanding the disease and designing more appropriate treatments for it of the highest priority.
Many hypotheses have been proposed for the mechanisms of action of agents approved for treating BD, particularly the mood stabilizers. One, the “arachidonic acid (AA, 20:4n-6) cascade hypothesis,” asserts that these agents commonly alleviate BD symptoms, particularly bipolar mania, by downregulating brain AA metabolism (Chang et al., 1996; Chang et al., 1999; Rao et al., 2008; Rapoport and Bosetti, 2002). This hypothesis is elaborated in this review. Other hypotheses are considered briefly in Section 12. below.
5.1. Roles of arachidonic acid in brain
AA is an n-6 (omega-6) polyunsaturated fatty acid (PUFA) that is esterified predominantly in the stereospecifically numbered (sn)-2 position of brain membrane phospholipids, as is the n-3 PUFA, docosahexaenoic acid (DHA, 22:6n-3). Both PUFAs are derived from the diet, directly or by hepatic elongation of their nutritionally essential precursors, linoleic acid (18:2n-6) or α-linolenic acid (18:3n-3), respectively, since vertebrates cannot synthesize them or their precursors de novo from 2-carbon fragments, and the brain's elongation capacity is inconsequential (Demar et al., 2005; Holman, 1986; Igarashi et al., 2007).
When released by a phospholipase A2 (PLA2) from a phospholipid, AA and its eicosanoid metabolites have multiple biological effects, many of which are modulated by released DHA and its metabolites (Bazan, 2007; Contreras and Rapoport, 2002; Farooqui et al., 2007; Fitzpatrick and Soberman, 2001; Serhan, 2006; Shimizu and Wolfe, 1990). AA and its metabolites can influence multiple processes within brain (Piomelli, 1995), including neurotransmission (Axelrod, 1990; DeGeorge et al., 1991; Rapoport, 2003), membrane excitability (Xiao and Li, 1999), long term potentiation (McGahon et al., 1997), gene transcription (Sellmayer et al., 1997), membrane fluidity (Bazan, 2005), neurite outgrowth (Ikemoto et al., 1997), cerebral blood flow (Stefanovic et al., 2006), sleep, memory and behavior (Chen and Bazan, 2005; Fitzpatrick and Soberman, 2001; Huang et al., 2007). Many AA metabolites are considered to be pro-inflammatory, whereas DHA and its metabolites are considered anti-inflammatory (Bazan, 2007; Farooqui et al., 2007; Serhan, 2006). AA is released from synaptic membrane phospholipids during normal neurotransmission (Jones et al., 1996), whereas higher quantities are released, together with other fatty acids, during pathological processes such as neuroinflammation, excitotoxicity, ischemia, and convulsions. These high quantities of AA and of concurrently formed lyosphopholipids can cause neuronal damage by multiple mechanisms (Bazan et al., 1981; Chang et al., 2009; Contreras and Rapoport, 2002; Farooqui et al., 2007; Rabin et al., 1998; Rao et al., 2007c; Rao et al., 2007d; Rosenberger et al., 2004).
5.2. Receptors coupled to phospholipase A2 enzymes
Three major PLA2 enzyme classes in the mammalian brain mediate receptor-initiated release of AA or DHA as second messengers (Burke and Dennis, 2009): (1) AA-selective cytosolic cPLA2 (85 kDa, Type IVA), which requires < 1 mM Ca2+ and phosphatidylinositol-4,5-biphosphate for translocation to the membrane plus phosphorylation for its activation (Clark et al., 1991); (2) secretory sPLA2 (14 kDa, Type IIA), which requires a much higher Ca2+ concentration (20 mM) for activation; and (3) DHA-selective “Ca2+-independent” iPLA2 (88 kDa, Type VIA), which is considered “Ca2+-independent” from in vitro studies (Strokin et al., 2003), but can be activated by Ca2+ derived from intracellular stores such as the endoplasmic reticulum (Nowatzke et al., 1998; Rosa and Rapoport, 2009). cPLA2, which co-localizes with and is coupled to cyclooxygenase (COX)-2 at post-synaptic sites, co-evolved with COX-2. iPLA2 also is found at post-synaptic sites, as well as in astrocytes (Kaufmann et al., 1996; Ong et al., 1999; Ong et al., 2005; Sun et al., 2005; Tay et al., 1995). sPLA2 participates in neurotransmitter release from axonal terminals (Matsuzawa et al., 1996).
Table 1 identifies receptors that are coupled to activation of cPLA2 and/or sPLA2, so as to release AA from membrane phospholipids. Coupling occurs by complex mechanisms as yet incompletely understood, and can involve a G-protein, entry of extracellular Ca2+ into the cell (to activate cPLA2), or release of Ca2+ from intracellular stores (Balsinde et al., 1998; Clark et al., 1991; Ertley et al., 2007; Nowatzke et al., 1998; Rosa and Rapoport, 2009; Takano et al., 2000). Post-synaptic neuroreceptors mediate AA release during neurotransmission or, with excess glutamate availability, during excitotoxicity, whereas astrocytic cytokine receptors mediate AA release during neuroinflammation (Axelrod, 1990; Basselin et al., 2006a; DeGeorge et al., 1991; Rao et al., 2007e; Rapoport, 2003; Rosenberger et al., 2004).
Table 1.
Receptors coupled to PLA2 activation and arachidonic acid signaling
| Neuroreceptors | Subtype1 |
| –Coupled via G-proteins | |
| Cholinergic muscarinic M1,3,5(Bayon et al., 1997; DeGeorge et al., 1991) | |
| Dopaminergic D2-like(Bhattacharjee et al., 2005; Vial and Piomelli, 1995) | |
| Serotonergic 5-HT2A/2C(Felder et al., 1990; Qu et al., 2003) | |
| Adrenergic β2(Pavoine et al., 1999) | |
| Bradykinin β2(Prado et al., 2002) | |
| Metabotropic glutamatergic mGlur1α(Nitsch et al., 1997) | |
| –Ionotropic, coupled via Ca2+ | |
| NMDA(Basselin et al., 2006a; Weichel et al., 1999) | |
| AMPA(Gaudreault et al., 2004) | |
| Astrocytic Receptors | Subtype2 |
| TNFα(Luschen et al., 2000) | |
| IL-1β(Dinarello, 1988) | |
| Purigenic 2Y2(Sun et al., 2005) | |
References mainly for cPLA2
Activation of both cPLA2 and sPLA2
5.3. Quantifying brain fatty acid turnover
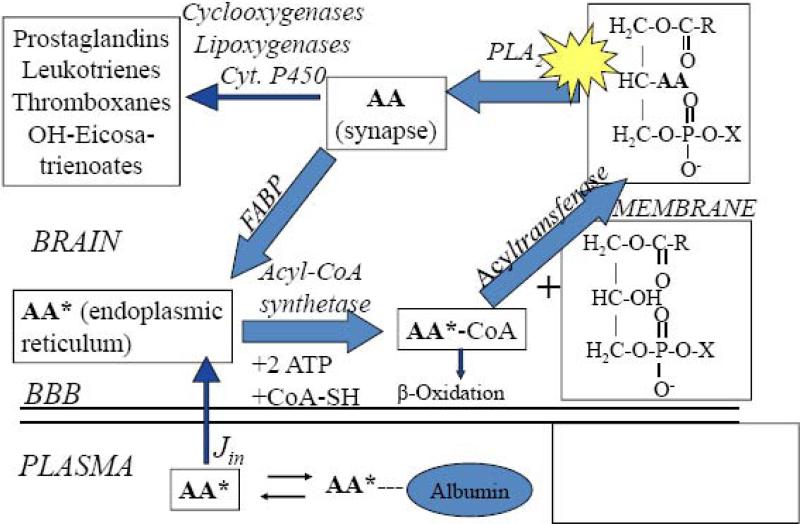
Figure 3 illustrates an outline of the brain AA cascade, when initiated by neuroreceptor-mediated activation of PLA2 at the post-synaptic membrane (Fitzpatrick and Soberman, 2001; Jones et al., 1996; Rapoport, 2001; Robinson et al., 1992; Shimizu and Wolfe, 1990). Comparable DHA and palmitic acid cascades have been described (Rapoport et al., 2007; Robinson et al., 1992; Sun and MacQuarrie, 1989). In unanesthetized rats, it has been shown that after AA is released from synaptic membrane phospholipid, a small fraction (about 4%) is rapidly metabolized to n-6 eicosanoids by COX, lipoxygenase (LOX) or cytochrome P450 epoxygenase enzymes. The remainder is recycled into membrane phospholipid via the brain arachidonoyl-CoA pool, with the help of acyl-CoA synthetase and acyltransferase enzymes and the consumption of 2 ATPs, or is lost by β-oxidation and other pathways. Since AA cannot be synthesized de novo in vertebrates, and its circulating precursor, linoleic acid, is almost entirely oxidized after entering the brain, the AA lost by metabolism is replaced by unesterified plasma AA, at a rate Jin (Eq. 2, Footnote 2) (DeMar et al., 2006a; Rapoport et al., 2001).
Figure 3. Model of brain arachidonic acid cascade initiated at synapse.
AA is liberated from the stereospecifically numbered (sn)-2 position of a phospholipid by activation (star) of phospholipase A2 (PLA2). A fraction is converted to bioactive eicosanoid, while the remainder diffuses to the endoplasmic reticulum bound to a fatty acid binding protein (FABP). There, it is converted to arachidonoyl-CoA by acyl-CoA synthetase with the consumption of 2 ATPs, then re-esterified into an available lysophospholipid by acyltransferase, or β-oxidized in mitochondria. AA in the endoplasmic reticulum exchanges freely with unesterified unbound AA in plasma, into which labeled AA (AA*) has been infused. Other metabolic pathways are not shown. Jin, the rate of incorporation of unesterified unlabeled AA into brain, equals the net rate of loss by metabolism, since AA cannot be synthesized de novo in brain. Adapted from (Rapoport and Bosetti, 2002).
The kinetics of the brain AA, DHA and palmitic acid cascades have been quantified in unanesthetized rodents by infusing the respective radiolabel intravenously for 5 min, so as to rapidly produce a constant plasma specific activity. At 5 min, the brain is exposed to high energy microwave radiation to stop enzyme activity, and then is subjected to chemical and radiotracer analyses. The resultant data are used to calculate coefficients of incorporation k*, rates of incorporation Jin, half-lives and turnover rates of the unlabeled fatty acid in whole brain and in individual brain phospholipids, by operational equations obtained from a kinetic model (Robinson et al., 1992).2 Regional values of k* and Jin also can be imaged by freezing rather than microwaving the brain, sectioning it in a cryostat, and subjecting the sections to quantitative autoradiography to determine regional radioactivity (Eqs. 1 and 2 in Footnote 2).
6. Mood stabilizers downregulate global brain arachidonic acid cascade
6.1. Global turnover rates
To compare central drug effects in rodents and humans, appropriate dose regiments should be chosen so as to produce clinically relevant plasma concentrations, since peripheral pharmacokinetics often differ between species. Table 2 summarizes reported clinical plasma therapeutic concentrations of the four FDA-approved mood stabilizers, and experimental plasma concentrations that have been used in rodent studies.
Table 2.
Therapeutic concentration ranges of FDA-approved mood stabilizers and topiramate, and concentrations used in experimental rat studies
| Drug | Therapeutic plasma concentrations in patients | Plasma concentrations achieved in rat infusion studies |
|---|---|---|
| mM | ||
| Lithium | 0.6-1.2(Camus et al., 2003; Eilers, 1995) | 0.73(Bosetti et al., 2002b; Chang et al., 1996) |
| Carbamazepine | 0.017 - 0.051(Bialer et al., 1998; Eilers, 1995) | 0.053(Ghelardoni et al., 2005) |
| Valproate | 0.20 - 1.5(Bowden et al., 1994; Eilers, 1995; Jacobsen, 1993) | 0.21(Chang et al., 2001) |
| Lamotrigine | 0.02 - 0.03(Doose et al., 2003) | 0.04(Hassel et al., 2001; Lee et al., 2007a) |
| Topiramate1 | 0.010 to 0.050(Doose and Streeter, 2002) | 0.018(Ghelardoni et al., 2005) |
Failed phase III trials(Kushner et al., 2006)
Turnover rates of AA, DHA and palmitic acid in brain phospholipids, as well as values of λ(Footnote 2) were measured in unanesthetized rats that had been fed a lithium-free diet for 6 weeks, or a LiCl-containing diet that produced a therapeutically relevant plasma lithium concentration (Table 2). As shown in Table 3, each of the three fatty acids had high turnover rates in rats on the control diet, consistent with their participation in active signaling processes that consume ATP (Purdon and Rapoport, 1998). Chronic lithium reduced AA turnover in brain phospholipids by about 80%, without significantly affecting DHA or palmitate turnover (Chang et al., 1996; Chang et al., 1999; Rapoport and Bosetti, 2002). In later studies, chronic sodium valproate and carbamazepine also were shown to reduce AA turnover without altering DHA turnover. In contrast, neither lamotrigine nor topiramate affected turnover of either fatty acid (Table 4), although lamotrigine did reduce AA incorporation coefficients k* in rat brain (Table 5 below) (Lee et al., 2008a).
Table 3.
Chronic lithium administration selectively reduces arachidonic acid turnover in rat brain phospholipids
| Fatty Acid | λ | Turnover Rate (% per hour) | ||
|---|---|---|---|---|
| PtdIns | PtdCho | PtdEtn | ||
| Arachidonic Acid (20:4n-6) | ||||
| Control | 0.04±0.00 | 15.3±0.4 | 18.3±0.6 | 1.1±0.1 |
| Lithium | 0.18±0.02#,** | 2.6±0.1** | 5.0±0.3** | 0.2±0.0** |
| Docosahexaenoic Acid (22:6n-3) | ||||
| Control | 0.03±0.00 | 17.7±1.7 | 3.1±0.4 | 1.2±0.1 |
| Lithium | 0.03±0.00 | 31.0±9.0 | 4.5±1.2 | 1.6±0.4 |
| Palmitic Acid (16:0) | ||||
| Control | 0.02±00 | 29.1±2.6 | 7.0±0.4 | 4.1±0.4 |
| Lithium | 0.02±00 | 26.0±1.2 | 5.1±0.3* | 3.5±0.1 |
1After (Chang et al., 1996; Chang et al., 1999; Rapoport and Bosetti, 2002)
Differs from control mean ± SEM (n = 5-6)
p < 0.05
p ± 0.01
LiCl or control diet was fed for 6 weeks, giving brain lithium concentration of 0.73 ± 0.03 mM.
An elevation in λ (Footnote 2) indicates that less AA is being provided by release from phospholipid than entry from plasma.
See Footnote 2 for definitions of λ and turnover rate.
PtdIns, phosphatidylinositol; PtdCho, cholineglycerophospholipid; PtdEtn, ethanolamineglycerophospholipid;
Table 4.
Baseline changes in whole brain arachidonic acid cascade markers after chronic administration of each of four mood stabilizers to rats
Abbreviations: AP, activator protein; GRE, glucocorticoid response element; Nc, no significant change; NF-κB, nuclear factor-kappa B; PEA, polyoma enhancer activator; cPLA2, cytosolic phospholipase A2; sPLA2, secretory PLA2; iPLA2, Ca2+-independent PLA2; COX, cyclooxygenase; LOX, lipoxygenase; mPGES, microsomal prostaglandin E synthase
Table 5.
Mood stabilizers modulate neuroreceptor signaling via arachidonic acid in unanesthetized ratsa
| Receptor Subtype | |||||
|---|---|---|---|---|---|
| |
|
NMDA |
D2-like |
M1,3,5 |
5-HT2A/C |
| Agonist used |
|
NMDA |
Quinpirole |
Arecoline |
DOI |
| Antagonist used |
|
MK-801 |
Raclopride or butaclamol |
Atropine |
Mianserin |
| Mood Stabilizer |
Baseline effect |
Effect on Response to Acute Agonist, k* for AA# |
|||
| Lithium |
↑ in auditory and visual areas(Basselin et al., 2005b) |
↓ (Basselin et al., 2006a) |
↓(Basselin et al., 2005a) |
↑(Basselin et al., 2003) |
↓ in auditory and visual areas(Basselin et al., 2005b) |
| Carbamazepine |
Nc(Basselin et al., 2008b) |
↓(Basselin et al., 2007a) |
↓(Basselin et al., 2008b) |
? |
? |
| Sodium valproate |
Nc(Basselin et al., 2008a) |
↓(Basselin et al., 2008a) |
? |
? |
? |
| Lamotrigine | ↓(Lee et al., 2007a) | ? | ? | ? | ? |
Each mood stabilizer was administered chronically to produce a plasma concentration therapeutically relevant to bipolar disorder (Table 1), and compared with chronic vehicle. Baseline effect on k* for AA (AA incorporation) was measured compared to chronic vehicle. Effect on responses to agonists shown were also measured and compared to response in saline injected animals. In saline-treated animals, responses to agonists could be blocked by antagonists shown in table. Nc, no significant change; DOI, (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride.
All but the lamotrigine measurements were obtained using quantitative autoradiographic imaging; bk* for whole brain phospholipid
6.2. Effects on cascade enzymes
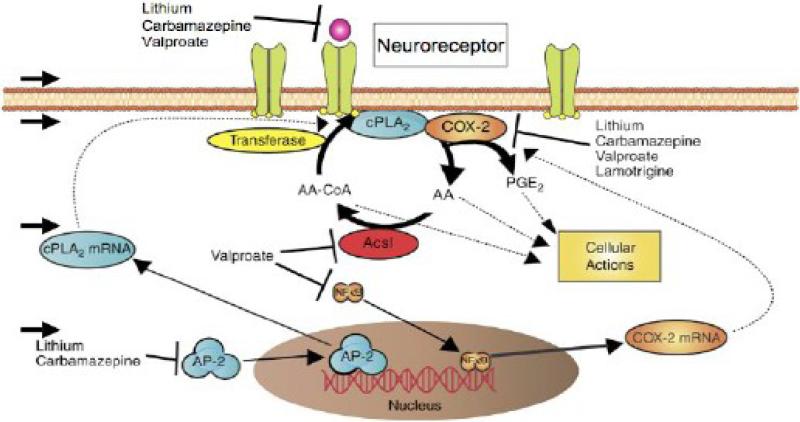
6.2.1. Phospholipases A2 and Acyl-CoA synthetase
Consistent with their reducing AA but not DHA turnover in rat brain phospholipids, chronic lithium and carbamazepine downregulated brain mRNA, protein and activity levels of AA-selective cPLA2, without altering expression of sPLA2 or DHA-selective iPLA2 (Table 4). Reduced expression of cPLA2 was associated with a reduced brain AA-CoA concentration, the sequential product of AA hydrolysis from phospholipid by the enzyme (Figure 3). Lithium's downregulation of cPLA2 was accompanied by reduced binding activity of subunits of a cPLA2 transcription factor, activator protein (AP)-2, which was attributed to decreased brain protein kinase (PK)Cα, PKCε and AA-dependent PKC activities (Rao et al., 2005). Chronic carbamazepine decreased AP-2 DNA-binding and cAMP-dependent PKA activity, as well as AP-2 phosphorylation (Rao et al., 2007b). Chronic sodium valproate did not alter expression of any of the three PLA2 enzymes or of AP-2 (Bosetti et al., 2003; Chang et al., 2001; Rao et al., 2005), but non-competitively inhibited a microsomal acyl-CoA synthetase that is selective for AA conversion to arachidonoyl-CoA (Figure 3) (Bazinet et al., 2006b). Chronic lamotrigine and topiramate did not change brain expression of cPLA2, sPLA2 or iPLA2 (Ghelardoni et al., 2005; Lee et al., 2008a).
6.2.2. Downstream oxidative enzymes
Once released from brain phospholipid, unesterified AA can be oxidized to bioactive eicosanoids (Figure 3) (Fitzpatrick and Soberman, 2001). Prostaglandin (PG) G2 is formed in first step in this oxidation, and then is converted to PGH2 by COX enzymes,. PGD2, PGE2, PGF2α and PGI2 may be produced from PGH2 via PG synthases, thromboxanes (TX) (e.g. TXB2) via TX synthases. In normal brain, PGE2 is produced preferentially via COX-2, TXB2 via COX-1 (Basselin et al., 2007c; Bosetti et al., 2004). Epoxyeicosatrienoic acids and hydroxyeicosatetraenoic acids also are formed via cytochrome P450 epoxygenase, whereas hydroxyperoxyeicosatetraenoic acids, leading to leukotrienes, hydroxyeicosatetraenoic acids, lipoxins and hepoxilins are produced via lipoxygenases (LOXs) and other enzymes (Bosetti, 2007).
The mood stabilizers approved for BD target many downstream AA metabolic processes. In unanesthetized rats, chronic lithium significantly reduced brain protein and activity of COX-2, as well as PGE2 concentration (Bosetti et al., 2002a), without changing expression of COX-1, 5-LOX, cytochrome P450 epoxygenase or microsomal PGE synthase-1 (mPGES-1) (Weerasinghe et al., 2004) (Table 4). Chronic lithium also elevated brain 17-OH-DHA, a precursor of anti-inflammatory resolvins and neuroprotectins derived from DHA via 12- and 15-LOX, in a rat model of neuroinflammation (Basselin et al., Unpublished observations; Serhan, 2006). Chronic carbamazepine reduced COX activity without changing the COX-1 or COX-2 protein level, and reduced brain concentrations of PGE2 and TXB2. Protein levels of 5-LOX and cytochrome P450 epoxygenase, and the concentration of the 5-LOX product, leukotriene B4 (LTB4), also were unaltered (Basselin et al., 2007a; Ghelardoni et al., 2004). Chronic sodium valproate reduced brain mRNA, protein and activity levels of both COX-1 and COX-2, and brain concentrations of TXB2 and PGE2, their respective preferred metabolites (Basselin et al., 2008a; Bosetti et al., 2003). Protein levels of 5-LOX and of cytochrome P450 epoxygenase and the concentration of LTB4 were unchanged. DNA binding activity of nuclear factor (NF)-κB, a COX-2 transcription factor, was decreased in relation to a decreased level of its p50 subunit (Rao et al., 2007a). Chronic lamotrigine decreased COX-2 protein and mRNA (Lee et al., 2008a), consistent with a reduction in k* for AA (Table 5 below) (Lee et al., 2007a), whereas chronic topiramate did not significantly change any measured marker (Ghelardoni et al., 2004; Ghelardoni et al., 2005).
7. Mood stabilizers may correct neurotransmission imbalance involving arachidonic acid
7.1. Neuroreceptor specificity
The fundamental assumption of neuropsychopharmacology is that cognition and behavior depend on neurotransmission involving different neurotransmitters, neurotransmitter transporters, neuroreceptors and second messengers (Cooper et al., 2003). Responses of BD patients to drugs other than mood stabilizers that act at specific neuroreceptors have suggested that bipolar symptoms arise from excessive dopaminergic and glutamatergic transmission, reduced cholinergic transmission, and disturbed serotonergic transmission (Section 3.1.). If this proposed neurotransmission imbalance involves AA, then mood stabilizers might be effective in BD by correcting the imbalance. This possibility was tested by using quantitative autoradiography to image regional brain AA signaling in unanesthetized rats that had been treated chronically with a clinically relevant dose of mood stabilizer and then acutely administered an agonist to relevant cPLA2-coupled neuroreceptors (Table 1), including cholinergic muscarinic M1,3,5, serotonergic 5-HT2A/2C, dopaminergic D2-like (D2, D3, D4) and glutamatergic NMDA receptors.
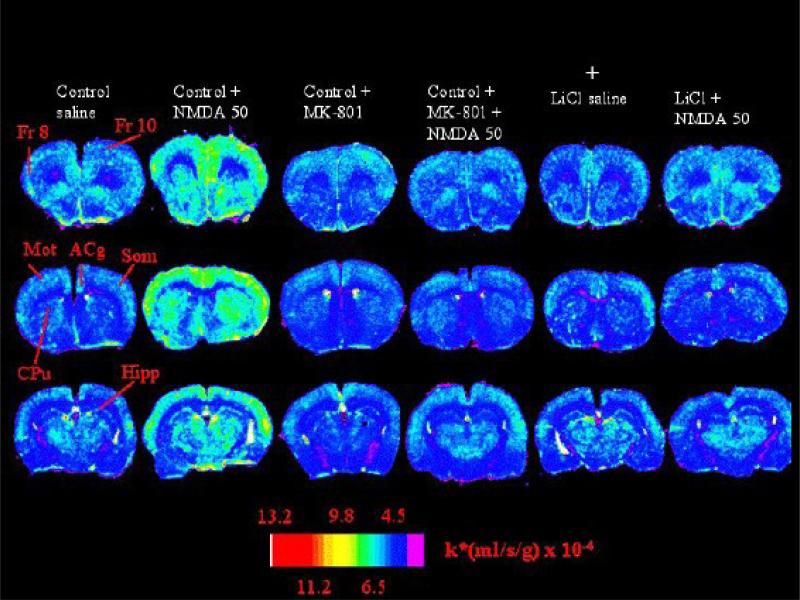
Results of one such study are illustrated in Figure 5, which presents color-coded autoradiographs of coronal brain sections from rats that had been injected i.p. acutely with NMDA (50 mg/kg) or vehicle, after being fed a LiCl-containing or control diet for 6 weeks (Basselin et al., 2006a). NMDA widely increased AA incorporation coefficients k* (Footnote 2, Eq. 1) in rats fed the control diet, but not in rats pretreated with the NMDA receptor antagonist, MK-801, or fed LiCl. MK-801 itself reduced k* for AA by 30% on average.
Figure 5. MK-801 and chronic lithium block NMDA stimulation of arachidonic acid signal in rat brain.
AA incorporation coefficients k* in autoradiographs of coronal brain sections. Two left columns: rats on a control diet, that had been injected i.p. with saline or 50 mg/kg NMDA. Two central columns: rats on a control diet injected with MK-801 or NMDA following MK-801; Two right columns: rats on a LiCl diet injected with saline or NMDA. NMDA increased k* in rats on the control diet, but not in rats pretreated with MK-801 or in rats fed the LiCl diet. Fr 8 and 10, frontal cortex 8 and 10; Mot, Som, motor and somatosensory cortex; Acg, anterior cingulate cortex; CPu, caudate putamen; Hipp, hippocampus. From (Basselin et al., 2006a).
Results from such imaging studies, summarized in Table 5, indicate that chronic mood stabilizers could correct the proposed neurotransmission imbalance of BD, if the imbalance involved AA as a second messenger. Chronic lithium, carbamazepine and valproate each blocked the k* increments caused by acute NMDA (Basselin et al., 2006a; Basselin et al., 2007a; Basselin et al., 2008b), and chronic lithium and carbamazepine blocked k* increments to the D2-like receptor agonist, quinpirole (Basselin et al., 2005a; Basselin et al., 2008b). These actions on D2-like and NMDA receptor signaling may be related to colocalization of the receptors in prefrontal cortex, striatum, and nucleus accumbens (Ikeda et al., 2003; Liu et al., 2006; Tarazi and Baldessarini, 1999; Tseng and O'Donnell, 2004). In addition, chronic lithium blocked responses to the 5-HT2A/2C receptor agonist (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI) in auditory and visual rat brain regions (Basselin et al., 2005b). Consistent with the imbalance hypothesis and with evidence that lithium reduces the convulsive threshold to cholinomimetics (Ormandy et al., 1991), chronic lithium augmented k* responses to the nonspecific muscarinic agonist, arecoline (Basselin et al., 2003). When measured, the direction of the changes in brain PGE2 and/or TXB2 concentrations following an agonist in rats treated with mood stabilizers corresponded to the direction of the changes in k* for AA.
Neither chronic carbamazepine nor valproate produced consistent changes in baseline (resting) k* for AA. On the other hand, chronic lithium increased baseline k* in rat auditory and visual cortical cortices, inferior and superior colliculi, and geniculate bodies (Basselin et al., 2003; Basselin et al., 2006b) (Table 5), as it did glucose metabolism in these areas (Basselin et al., 2006c). These effects may be related to lithium's ability to potentiate auditory and visual evoked responses in humans (Hegerl et al., 1990). Lithium's potentiation of M1,3,5 receptor signaling and suppression of D2-like receptor signaling involving AA in rat brain (Table 5) may explain the tremors and occasional extrapyramidal symptoms that it causes in patients (Ghadirian et al., 1996).
When measured by whole brain analysis rather than with quantitative autoradiography, chronic lamotrigine significantly decreased k* for AA into brain phospholipids by about 15% (Lee et al., 2007a) (Table 5).
7.1.1. Reconciling neurotransmitter-specific effects with global brain effects
Downregulation of the global brain AA cascade by the mood stabilizers (Section 6.) may largely represent downregulation of the cPLA2 activation and AA turnover associated with glutamatergic (e.g. NMDA) receptor signaling, since most brain synapses are excitatory and glutamatergic (Attwell and Laughlin, 2001; Raichle and Gusnard, 2002). Consistent with this interpretation, the NMDA receptor antagonist MK-801 reduced global AA incorporation by 30% in unanesthetized rats (Figure 5), whereas acutely administered antagonists to M1,3,5 receptors (atropine), D2-like receptors (butaclamol, raclopride) or 5-HT2A/2C receptors (mianserin) did not markedly decrease baseline AA incorporation into rat brain (Bhattacharjee et al., 2008; DeGeorge et al., 1991; Hayakawa et al., 2001; Qu et al., 2003).
7.2. Targeting neuroreceptors and their coupling machinery
Suppression of the AA cascade by mood stabilizers may occur at multiple levels: neuroreceptors, their coupling to cPLA2, AA cascade enzyme activities, or gene transcription (Figure 4). Thus, in addition to reducing expression of cPLA2 and activity of COX-2, mood stabilizers can directly modify NMDA receptor mechanisms. Lithium reduced NMDA receptor-mediated Ca2+ influx into cells by inhibiting NR2 phosphorylation (Hashimoto et al., 2003). Sodium valproate inhibited PKA and PKC, which phosphorylate the NMDA receptor (Du et al., 2004). It also reduced rat brain expression of NMDA receptor-interacting proteins, postsynaptic density protein PSD-95 and type II Ca2+/calmodulin-dependent protein kinase beta subunit (Bosetti et al., 2005), and inhibited histone deacetylase, which can deacetylate histones on the gene for the NMDA receptor transcription factor, specificity protein-1 (Sp1) (Bai and Kusiak, 1995; Phiel et al., 2001). Sodium valproate blocked induction of Fos and binding activity of AP-1 DNA, which modulates transcription of NMDA receptor subunit, NR2B (Qiang and Ticku, 2005), and it altered NMDA receptor levels in hippocampal neurons (Caldeira et al., 2007).
Figure 4. Mood stabilizers downregulate the rat brain arachidonic acid cascade during neurotransmission at different levels.
The first level is at the neuroreceptor itself, the second its coupling mechanism with cPLA2. cPLA2 also can be transcriptionally downregulated by drug action on its transcription factor, AP-2 (lithium and carbamazepine), whereas reincorporation of AA can be slowed by drug inhibition of an AA-selective acyl-CoA synthetase (valproate). These effects are correlated with reduced turnover of AA in membrane phospholipids. Drugs also can inhibit formation of PGE2 and/or TXB2 by downregulating activity and/or transcription of COX-2 (and expression of NF-κB) and COX-1 respectively. See Text and Table 4 for detailed effects. Adapted from (Rao et al., 2008).
With regard to G-protein coupled receptors, chronic lithium reduced brain levels of the Gαi1 and Gαi2 subunits of the inhibitory Gα protein that couples D2-like receptors to cPLA2 (Sidhu and Niznik, 2000), and guanosine-5'-triphosphate (GTP) binding to these subunits (Wang and Friedman, 1999). Chronic carbamazepine reduced D2 receptor density, D2-like receptor coupled Gαo/i, and D2-like receptor phosphorylation (Beutler et al., 2005; Montezinho et al., 2006). Chronic lithium and carbamazepine but not valproate increased levels in rat brain of GRK3, which is involved in homologous desensitization of agonist-activated G-protein coupled receptors (Ertley et al., 2007). GRK3 but not GRK4 is reduced in the postmortem BD brain (Rao et al., 2009).
8. Arachidonic acid cascade and neuroprotection
Mood stabilizers have been shown to be neuroprotective in animal and cellular models of neurodegeneration, excitotoxicity, stroke-ischemia-hypoxia, stress-induced neuronal loss, neuroinflammation, and immunological damage (Section 4.3.3.) (Chang et al., 2009; Chen et al., 1999; Chuang, 2004; Lee et al., 2000). Part of this neuroprotection might be due to suppression of an upregulated brain AA cascade, which can cause cell damage and behavioral changes (Basselin et al., 2007a; Basselin et al., 2007b; Bazan et al., 1995; Bosetti, 2007), or stimulation of formation of anti-inflammatory DHA metabolites (Basselin et al., Unpublished observations) (Section 6.2.2.). Chronic lithium attenuated upregulated AA metabolism in a rat model of neuroinflammation, as did chronic lithium, valproate and carbamazepine in a model of acute NMDA-induced excitotoxicity (Table 5) (Basselin et al., 2006a; Basselin et al., 2007b). The ability of each of the four approved mood stabilizers to increase rat brain expression of brain derived neurotrophic factor (BDNF) and of the anti-apoptotic factor, B-cell lymphoma-2 (Bcl-2), which are reduced in the BD brain (Dean et al., 2006), also may contribute to their neuroprotective action (Chang et al., 2009; Garrido et al., 2003; Rao et al., 2007d). These effects may be mediated by AA (Chang et al., 2009; Rao et al., 2007d).
9. Antidepressants, switching and the arachidonic acid cascade
Certain antidepressants, when given to BD depressed patients, increase the tendency to “switch” to mania (Section 4.1.). This limits therapy for bipolar depression, perhaps unduly in view of the controversy concerning “switching.” Increased switch rates are reported for imipramine and fluoxetine, but not for bupropion (Calabrese et al., 1999b; Leverich et al., 2006; Post et al., 2006).
These differences in switch rates correlate with the antidepressant effects on the rat brain AA cascade. Thus, chronic fluoxetine and imipramine, which increase switching, when given to rats to produce a therapeutically relevant plasma concentration (Table 6), increased AA turnover in brain phospholipids; brain cPLA2 expression (activity, protein, mRNA, and phosphorylation), and expression of the cPLA2 transcription factor subunit, AP-2α (Lee et al., 2007b; Lee et al., 2008 ; Rao et al., 2006). Chronic bupropion, which doesn't increase switching, did not alter any of these markers (Table 7).
Table 6.
Therapeutic plasma concentrations of antidepressants, and concentrations used in experimental rat studies
| Antidepressant | Clinical plasma concentrations | Plasma concentrations in rat infusion studies |
|---|---|---|
| mg/L | ||
| Fluoxetine1 | 150-500(Wille et al., 2008) | 314(Durand et al., 1999; Lee et al., 2007b) |
| Imipramine2 | 45-150(Wille et al., 2008) | 100 - 600(Daniel et al., 1981; Lee et al., 2007b) |
| Bupropion3 | 25-100(Wille et al., 2008) | 289 (max)(Lee et al., 2008 ; Suckow et al., 1986) |
N-Methyl-g-[4-(trifluoromethyl)phenoxy]benzenepropanamine.
(5-[3-(dimethylamino) propyl] -10,11-dihydro-5H-dibenz [b,f]-azepine monohydrochloride).
(±)-2-(tertbutylamino)-1-(3-chlorophenyl)propan-1-one
Table 7.
Brain arachidonic acid cascade markers following chronic administration of each of three antidepressants to rats
10. Animal models with an upregulated AA cascade
There is no generally accepted behavioral animal model for BD (Cryan and Slattery, 2007; Kato et al., 2007). Nevertheless, as FDA-approved mood stabilizers have shown to downregulate parameters of the brain AA cascade in normal rats, animal models having a pathologically upregulated brain AA cascade may help in drug discovery and in considering mechanisms relevant to BD. Table 8 characterizes five such models.
Table 8.
Animal models with an upregulated brain arachidonic acid cascade
| Parameter | Model | ||||
|---|---|---|---|---|---|
| Chronic i.p. NMDA in rat | 6-day cerebroventricular low-dose lipopolysaccharidebinfusion in rat | 15 weeks dietary n-3 PUFA deprivation in 20 day old rat | COX-2−/− knockout mouse | 5-HTT+/− or 5-HTT−/− knockout mouse | |
| AA turnoverc | ↑ | ↑ | ↑e | ||
| AA incorporatio n (k*or Jin)d | ↑ | ↑ | ↑e | ↑ | ↑ |
| CPLA2 IVA | ↑ | ↑ | ↑ | ↑ | ↑ |
| iPLA2 VIA | Nc | Nc | ↓ | Nc | |
| SPLA2 IIA | Nc | ↑, Nc | ↑ | ↑ | |
| COX-1 | Nc | ↓ | ↑ | ||
| COX-2 | Nc | ↑ | Absent | ||
| Total COX | ↑ | ↓ | |||
| 5-LOX | Nc | ||||
| mPGES-2 | ↓ | ||||
| AP-2 | ↑ | ||||
| PGE2 | ↑ | ↓ | ↓ | ||
| TXB2 | ↑ | ↓ | |||
| Altered Behavior | Yesa | Yes | Yes | ||
| References | (Lee et al., 2008b; Rao et al., 2007e) | (Basselin et al., 2007b; Lee et al., 2004; Richardson et al., 2005; Rosenberger et al., 2004) | (DeMar et al., 2006b; Igarashi, 2009; Rao et al., 2007c) | (Basselin et al., 2006c; Bosetti et al., 2004) | (Basselin et al., 2009; Murphy and Lesch, 2008) |
Measured after 1 month of infusion
0.5-1.0 ng/h
Measured by direct chemical analysis
Measured by direct chemical analysis and/or quantitative autoradiography
Turnover of AA elongation product, 22:5n-6, is increased
Empty boxes indicate that measurement was not performed
In one rat model, chronic administration of a subconvulsive dose (25 mg/kg i.p. daily for 21 days) of NMDA increased brain markers of excitotoxicity and neuroinflammation (Chang et al., 2008; Rao et al., 2007e), selectively upregulated AA turnover in brain phospholipid, and elevated expression of cPLA2 and AP-2. In a second rat model, 6 days of intracerebroventricular infusion of low dose lipopolysaccharide (0.5-1.0 ng/h) (Basselin et al., 2007b; Lee et al., 2004; Rosenberger et al., 2004) increased AA turnover and cPLA2 and sPLA2 activities in brain, whereas behavior was disturbed when measured after 1 month of infusion (Richardson et al., 2005). The brain AA cascade also was upregulated by feeding rats an n-3 PUFA deficient diet for 15 weeks. This dietary regimen decreased brain DHA by 30% and increased brain docosapentaenoic acid (DPA, 22:5n-6), an AA elongation product, by an equivalent amount. AA turnover was unaffected, whereas DPA turnover was increased (Contreras et al., 2001; Igarashi, 2009; Rao et al., 2007c). BDNF, cAMP response element binding protein (CREB) transcription factor activity, phosphorylated CREB (important in learning and memory) and p38 mitogen-activated protein kinase were reduced in brain (Rao et al., 2007d), and the rats showed aggression, depression and increased locomotion on behavioral tests (DeMar et al., 2006b).
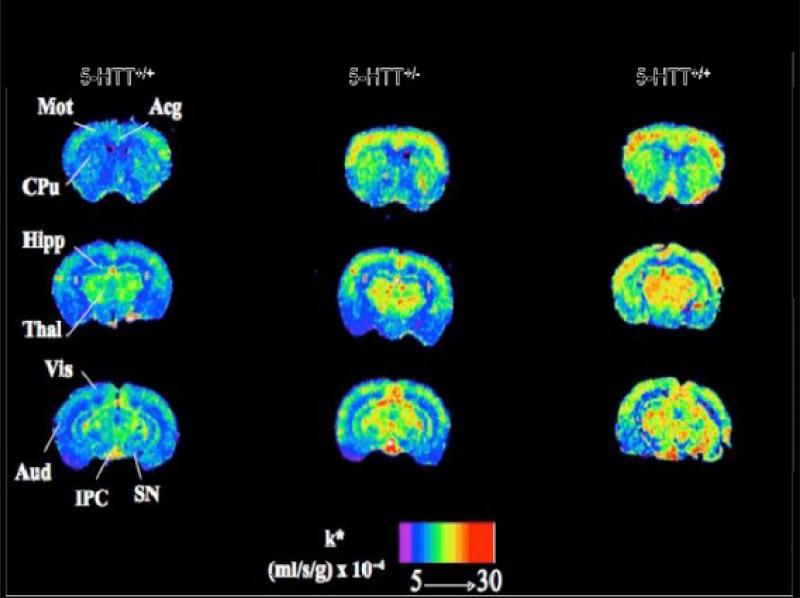
Two genetic mouse models also show upregulated AA incorporation into brain phospholipid. The COX-2 knockout mouse, which lacks the COX-2 gene from birth, also has increased cPLA2, sPLA2, and COX-1 expression in brain. The 5-HTT knockout mouse demonstrates BD-like behavioral disturbances (Murphy and Lesch, 2008) and increased brain cPLA2 activity (Basselin et al., 2009). Regional AA incorporation into brain is elevated by 20-70% in both heterozygous and homozygous knockouts (Figure 7), due to tonic activation of cPLA2-coupled 5-HT2A/2C receptors by elevated synaptic 5-HT concentrations. The 5-HTT+/− mouse is considered a model for humans having a short (S) compared with long (L) allele of the 5-HTT promoter, who are at increased risk for BD and other psychiatric diseases (Murphy and Lesch, 2008).
11. The arachidonic acid cascade in bipolar disorder patients
A small case-control trial reported increased serum PLA2 activity in BD patients (Noponen et al., 1993). Genetic mapping identified involvement of the sPLA2 gene (Jacobsen et al., 1999), but this was not replicated (Meira-Lima et al., 2003), cPLA2 was not implicated in other analyses (Dikeos et al., 2006; Pae et al., 2004). One study reported decreased cytosolic PGES in postmortem frontal and temporal cortices from BD patients, but not in patients medicated prior to death (Maida et al., 2006).
The postmortem BD compared with control frontal cortex is reported to have increased mRNA and protein levels of cPLA2, sPLA2 and COX-2 (but not of iPLA2), and increased AP-2 and NF-κB levels (Rao et al., 2007b), changes opposite in direction to those produced by mood stabilizers in the rat brain (Table 4). These changes are accompanied by elevated expression of markers of apoptosis, neuroinflammation and excitotoxicity (Kim et al., 2007a; Kim et al., 2008; Rao et al., In press), and may be caused by or related to these pathological processes (Chang et al., 2008; Dinarello, 1988; Rao et al., 2007e; Rosenberger et al., 2004) (Section 3.2).
Dietary n-3 PUFA deprivation in rats can upregulate brain AA metabolic markers and disturb AA - DHA interactions (Table 8) (Contreras and Rapoport, 2002; Igarashi, 2009; Rao et al., 2007c). Thus, reports that subjects on a diet low in DHA-containing marine products had an increased prevalence of BD (Noaghiul and Hibbeln, 2003), that dietary n-3 PUFA supplementation ameliorated BD symptoms (Frangou et al., 2006; Ross et al., 2007; Stoll et al., 1999), and that the DHA concentration in the postmortem BD brain was reduced by 30% (McNamara et al., 2008; Schwarz et al., 2008), would be consistent with an association between BD and an upregulated or otherwise dysfunctional brain AA cascade. However, other reports have not confirmed a positive effect of dietary n-3 PUFA supplementation or a decreased brain DHA in the postmortem brain from BD patients (Igarashi et al., Submitted; Keck et al., 2006) or suicide victims (Lalovic et al., 2007). The issue is far from settled.
12. Other proposed mechanisms of action of mood stabilizers
Many brain targets other than the AA cascade have been proposed for mood stabilizers. These include enzymes in which lithium competes for a magnesium binding site (e.g., inositol monophosphatase, inositol polyphosphate 1-phosphatase, glycogen synthase kinase-3 (GSK-3), fructose 1,6-bisphosphatase, bisphosphate nucleotidase, phosphoglucomutase); valproate-inhibitable enzymes (succinate semialdehyde dehydrogenase, succinate semialdehyde reductase, histone deacetylase); specific targets of carbamazepine (sodium channels, adenosine receptors, adenylate cyclase); signaling pathways involving PKC, cyclic AMP, or myo-inositol; synaptic mechanisms involving glutamate, GABA, G-proteins and GRKs (Du et al., 2007; Ertley et al., 2007; Li et al., 2002; Manji and Lenox, 2000); processes modulating apoptosis and neuroprotection, including the expression of brain growth and neurotrophic factors (Chen et al., 1999; Chuang, 2005; Gould et al., 2004b); and calcium currents and voltage gated sodium channels (Farber et al., 2002; Stefani et al., 1996). When given chronically to rodents, mood stabilizers altered brain mRNA levels of genes related to ion channel formation and transport, G-protein signaling, lipid, glucose and amino acid metabolism, transcription and translation, the phosphatidylinositide cycle, protein kinases, phosphatases, and apoptosis (Bosetti et al., 2002b; Bosetti et al., 2005; Chetcuti et al., 2006; McQuillin et al., 2007).
Among hypotheses regarding the action of mood stabilizers, the “myo-inositol depletion hypothesis” and the “GSK-3 inhibition hypothesis” continue to be explored experimentally and tested in the clinic (Eden Evins et al., 2006; Rowe et al., 2007). Ultimately, of course, which hypothesis if any is valid and could be used for drug discovery in BD will be determined empirically. Compared to the “AA cascade hypothesis,” however, neither the myo-inositol nor the GSK-3 inhibition hypothesis identifies one common action of each of the four FDA-approved mood stabilizers.
The “myo-inositol depletion hypothesis” is that the phosphatidylinositide cycle, which participates in neurotransmission and is initiated by the activation of phospholipase C, is the target of lithium (Berridge et al., 1982; Berridge et al., 1989; Hokin and Dixon, 1993; Lenox and Hahn, 2000). To maintain a normal cycle, the brain must resynthesize myo-inositol, which does not readily cross the blood-brain barrier (Barchas et al., 1994). In the cycle, inositol-1,4,5-trisphosphate is serially dephosphorylated to form myo-inositol, the last dephosphorylation step being mediated by inositol monophosphatase. Lithium is postulated to reduce myo-inositol formation within the cycle by inhibiting inositol monophosphatase, thereby interfering with phospholipase C-mediated neurotransmission events (Berridge et al., 1982; Berridge et al., 1989; Hokin and Dixon, 1993; Lenox and Hahn, 2000). Limitations to the “myoinositol depletion hypothesis” have been thoroughly summarized (Atack, 1996). The hypothesis does not appear to apply to carbamazepine or valproate. While lithium inhibited partially purified inositol monophosphatase activity in the low millimolar range, carbamazepine stimulated the enzyme starting in the low micromolar range, and valproate neither stimulated nor inhibited the enzyme over a wide concentration range (Vadnal and Parthasarathy, 1995) (see Table 2).
GSK-3 also is considered a target of mood stabilizers (Gould and Manji, 2005; Schloesser et al., 2008). GSK-3 modulates the Wnt and insulin signaling pathways, as well as neurotrophic signaling involving phosphoinositide-3 kinase, generally through downregulation (Jope, 2003). While data indicate a lithium action on GSK-3 at a therapeutically relevant concentration, this is not the case for valproate or carbamazepine. Thus, in primary neocortical neurons, GSK-3 was inhibited by 1 mM lithium but not by valproate up to a concentration > 100 mM, or by carbamazepine even at 0.5 mM (Di Daniel et al., 2006; Ryves et al., 2005), much higher concentrations than their respective therapeutic concentrations (Table 2). Nevertheless, chronic sodium valproate and lithium, but not carbamazepine, increased rat frontal cortex β-catenin, indirectly suggesting GSK-3 pathway modulating activity (Gould et al., 2004a).
13. Discussion
Following the discovery in 1949 of lithium's efficacy in BD (Cade, 1949), divalproex and carbamazepine were approved by the FDA for bipolar mania, and lamotrigine was approved for delaying the appearance of the different mood states. Lithium and divalproex also are recommended for maintenance therapy. Atypical antipsychotics are especially useful for acute mania. One, quetiapine, is approved for bipolar depression and maintenance therapy adjunctive to lithium or divalproex, and aripiprazole and olanzapine are approved for maintenance monotherapy in Bipolar I. Depression is 3 times more common than mania in BD, but depression is generally inadequately treated. Some antidepressants if administered as monotherapy or with mood stabilizers increase “switching” to mania.
Multiple risk alleles, each with a small individual contribution, are consistent with a polygenic threshold inheritance of BD. To date, however, genetic findings have shown poor replication and have not consistently identified defective brain cascades as likely therapeutic targets. In contrast, a “top down” approach based on identifying a common mechanism of action of agents that have been shown in controlled clinical trials to work or not to work in BD has been more informative, and is the focus of this review. Two hypotheses that have been generated by this approach are the “myo-inositol depletion” and “GSK-3 targeting” hypotheses. While each might explain lithium's mechanism of action, neither convincingly accounts for the actions of the other mood stabilizers or, moreover, why some antidepressants enhance switching from bipolar depression to mania.
The “AA cascade hypothesis,” which is closely considered in this review, identifies a common target of the four approved mood stabilizers as the brain AA cascade, and tentatively explains why some antidepressants increase switching of bipolar depression to mania. This hypothesis was derived largely from studies in unanesthetized rats chronically administered FDA-approved mood stabilizers, as well as the clinically-proven ineffective topiramate for comparison. Lithium, carbamazepine and sodium valproate were shown to downregulate AA turnover in brain phospholipids, without changing DHA or palmitic acid turnover. Lamotrigine reduced AA incorporation coefficients k* in brain phospholipids. The effect on AA turnover of lithium and carbamazepine was ascribed to reduced expression of AA-selective cPLA2 and of its AP-2 transcription factor, whereas valproate's effect was ascribed to its inhibition of an AA-selective microsomal acyl-CoA synthetase. Each of the four agents depressed rat brain COX-2 expression and, when measured, the concentration of the COX-2 - derived AA metabolite, PGE2. Topiramate, which had been proposed as a mood stabilizer based on initial trials, but later failed Phase III trials, did not alter any brain AA cascade marker. Thus, the AA cascade hypothesis corresponds to proven clinical efficacy of the tested drugs.
The AA cascade hypothesis is consistent with evidence that BD symptoms arise from excessive dopaminergic and glutamatergic but reduced cholinergic signaling, provided that the signaling uses AA as a second messenger. Thus, imaging in unanesthetized rats showed that lithium upregulated muscarinic cholinergic M1,3,5 receptor signaling involving AA; lithium and carbamazepine blocked D2-like receptor-initiated AA signaling; and lithium, valproate and carbamazepine each blocked NMDA receptor-initiated AA signaling (Table 5). The NMDA effects may explain much of the global effects of the mood stabilizers on the AA cascade, since most brain synapses are excitatory and glutamatergic (Attwell and Laughlin, 2001; Raichle and Gusnard, 2002).
More research is needed to examine disease progression and deterioration in BD, which only recently is being addressed. Progression may be a trait feature, but it also may be determined by factors such as diet, substance abuse, obesity, and bipolar disorder drugs. Postmortem studies indicate the presence of neuroinflammation, excitotoxicity and apoptosis in the BD brain, processes that can underlie progression (Kim et al., 2007a; Kim et al., 2008; Rao et al., In press). Chronic administration of atypical antipsychotics has been shown to cause atrophy and astrocyte loss in monkey brain, and chronic lithium produced neuronal death in rat brain (Section 4.3.). On the other hand, mood stabilizers and antipsychotics have been reported to be neuroprotective in animal models of neuroinflammation and excitotoxicity, in some cases by downregulating the brain AA cascade.
Observations that mood stabilizers selectivity downregulate the rat brain AA cascade at therapeutically relevant plasma concentrations, and that antidepressants that increase switching of bipolar depression to mania upregulate the cascade, suggest that an upregulated brain AA cascade contributes to BD symptoms, particularly bipolar mania. Cascade-suppressing drugs, as well as dietary n-3 PUFA supplementation (Section 11.) might be used to test the relation between disturbed behavior and disturbed AA metabolism in animal models with disturbances of both behavior and AA metabolism (Table 8). Additionally, cascade parameters can be measured in such pathological models, as well as in the normal rat, to screen for new and potentially clinically relevant therapeutic agents for BD. Based on this review and prior suggestions (Chang et al., 1996; Chang et al., 1999; Rapoport and Bosetti, 2002), one would predict efficacy in BD from NMDA transmission modifiers, inhibitors of cPLA2 or of COX-2 synthetase, such as non-steroidal anti-inflammatory agents including COX inhibitors and aspirin (which can acetylate COX-2 to form specific anti-inflammatory derivatives of AA and DHA) (Breitner, 2003; Farooqui et al., 2006; Ketterer et al., 1996; Rapoport and Bosetti, 2002; Serhan, 2006; Stolk et al., Submitted for publication), other promoters of brain DHA conversion to resolvins and neuroprotectins (Basselin et al., Unpublished observations; Serhan, 2006) including dietary n-3 PUFA supplementation, and inhibitors of AA-selective acyl-CoA synthetase, including valproate-like compounds (Bazinet et al., 2006b; Bialer and Yagen, 2007). These predictions could be tested by direct clinical trials or by analyzing relevant databases (Stolk et al., Submitted for publication).
In addition, the presence of an upregulated brain AA cascade in BD patients could be tested for directly by imaging regional brain AA incorporation parameters (k* and Jin) during manic, euthymic and depressive phases of the disease, with the help of positron emission tomography. Increased AA incorporation associated with neuroinflammation has been measured in this way in patients with Alzheimer disease (Esposito et al., 2007; Esposito et al., 2008; Giovacchini et al., 2004).
Figure 6. Coronal autoradiographs of brain showing effects of 5-HTT genotype on regional arachidonic acid incorporation coefficients k* in mice.
Acg, anterior cingulate cortex; Aud, auditory cortex; CPu, caudate-putamen; Hipp, hippocampus; IPC, interpeduncular nucleus; Mot, motor cortex; SN, substantia nigra; Thal, thalamus; Vis, visual cortex. Adapted from (Basselin et al., 2009).
14. Acknowledgements
This work was entirely supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. We thank Professor Donald S. Klein and Dr. Jakob Shimshoni for their helpful comments on this paper.
1.1. Abbreviations
- AA
arachidonic acid
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP
activator protein
- BD
bipolar disorder
- BDNF
brain derived neurotrophic factor
- Bcl-2
B-cell lymphoma-2
- COX
cyclooxygenase
- CREB
cAMP response element binding protein
- DAT
dopamine reuptake transporter
- DHA
docosahexaenoic acid
- DOI
(+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride
- FABP
fatty acid binding protein
- GRE
glucocorticoid response element
- GRK
G-protein receptor kinase
- GSK
glycogen synthase kinase
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HTT
serotonin reuptake transporter
- IL
interleukin
- 5-LOX
5-lipoxygenase
- LTB4
leukotriene B4
- MR
magnetic resonance
- NMDA
N-methyl-D-aspartic acid
- NF-κB
nuclear factor kappa B
- NR
NMDA receptor
- PEA
polyoma enhancer activator
- PGE2
prostaglandin E2
- PK
protein kinase
- mPGES
microsomal prostaglandin E synthase
- PLA2
phospholipase A2
- cPLA2
cytosolic PLA2
- iPLA2
calcium-independent PLA2
- sPLA2
secretory PLA2
- PUFA
polyunsaturated fatty acid
- sn
stereospecifically numbered
- SSRI
selective serotonin reuptake inhibitor
- TX
thromboxane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This term was introduced into the psychiatric vocabulary some 20 years ago, based on observations that lithium offered antimanic as well as antidepressant action. It was extended to the anticonvulsants, valproic acid, carbamazepine and lamotrigine, yet the first two have little efficacy against depression and lamotrigine shows little efficacy against mania Sobo, S., 1999. Mood stabilizers and mood swings: In search of a definition. Psychiatric times. 16.. The term “mood stabilizer” has since been modified to identify medication that decreases vulnerability to subsequent episodes of mania or depression and does not exacerbate the current episode or maintenance phase of treatment. Sachs, G.S., 1996. Bipolar mood disorder: practical strategies for acute and maintenance phase treatment. J Clin Psychopharmacol. 16, 32S-47S.
| (1) |
| (2) |
| (3) |
15. References
- Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Keller M, Warshaw M, Clayton P, Goodwin F. Switching from ‘unipolar’ to bipolar II. An 11-year prospective study of clinical and temperamental predictors in 559 patients. Arch Gen Psychiatry. 1995;52:114–23. doi: 10.1001/archpsyc.1995.03950140032004. [DOI] [PubMed] [Google Scholar]
- Alda M. Pharmacogenetics of lithium response in bipolar disorder. J. Psychiatry Neurosci. 1999;24:154–158. [PMC free article] [PubMed] [Google Scholar]
- Atack JR. Inositol monophosphatase, the putative therapeutic target for lithium. Brain Res Brain Res Rev. 1996;22:183–90. [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Receptor-mediated activation of phospholipase A2 and arachidonic acid release in signal transduction. Biochem Soc Trans. 1990;18:503–7. doi: 10.1042/bst0180503. [DOI] [PubMed] [Google Scholar]
- Bai G, Kusiak JW. Functional analysis of the proximal 5'-flanking region of the N-methyl-D-aspartate receptor subunit gene, NMDAR1. J Biol Chem. 1995;270:7737–44. doi: 10.1074/jbc.270.13.7737. [DOI] [PubMed] [Google Scholar]
- Balsinde J, Balboa MA, Dennis EA. Functional coupling between secretory phospholipase A2 and cyclooxygenase-2 and its regulation by cytosolic group IV phospholipase A2. Proc Natl Acad Sci U S A. 1998;95:7951–6. doi: 10.1073/pnas.95.14.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchas JD, Hamblin MW, Malenka RC. Biochemical hypotheses of mood and anxiety disorders. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. Basic Neurochemistry. Fifth Edition Raven Press; New York: 1994. pp. 979–1001. [Google Scholar]
- Barrett TB, Hauger RL, Kennedy JL, Sadovnick AD, Remick RA, Keck PE, McElroy SL, Alexander M, Shaw SH, Kelsoe JR. Evidence that a single nucleotide polymorphism in the promoter of the G protein receptor kinase 3 gene is associated with bipolar disorder. Mol Psychiatry. 2003;8:546–57. doi: 10.1038/sj.mp.4001268. [DOI] [PubMed] [Google Scholar]
- Basselin M, Chang L, Seemann R, Bell JM, Rapoport SI. Chronic lithium administration potentiates brain arachidonic acid signaling at rest and during cholinergic activation in awake rats. J Neurochem. 2003;85:1553–62. doi: 10.1046/j.1471-4159.2003.01811.x. [DOI] [PubMed] [Google Scholar]
- Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration to unanesthetized rats attenuates brain dopamine D2-like receptor-initiated signaling via arachidonic acid. Neuropsychopharmacology. 2005a;30:1064–75. doi: 10.1038/sj.npp.1300671. [DOI] [PubMed] [Google Scholar]
- Basselin M, Chang L, Seemann R, Bell JM, Rapoport SI. Chronic lithium administration to rats selectively modifies 5-HT2A/2C receptor-mediated brain signaling via arachidonic acid. Neuropsychopharmacology. 2005b;30:461–72. doi: 10.1038/sj.npp.1300611. [DOI] [PubMed] [Google Scholar]
- Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006a;31:1659–74. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]
- Basselin M, Chang L, Rapoport SI. Chronic lithium chloride administration to rats elevates glucose metabolism in wide areas of brain, while potentiating negative effects on metabolism of dopamine D(2)-like receptor stimulation. Psychopharmacology (Berl) 2006b;187:303–311. doi: 10.1007/s00213-006-0425-0. [DOI] [PubMed] [Google Scholar]
- Basselin M, Villacreses NE, Langenbach R, Ma K, Bell JM, Rapoport SI. Resting and arecoline-stimulated brain metabolism and signaling involving arachidonic acid are altered in the cyclooxygenase-2 knockout mouse. J Neurochem. 2006c;96:669–79. doi: 10.1111/j.1471-4159.2005.03612.x. [DOI] [PubMed] [Google Scholar]
- Basselin M, Villacreses NE, Chen M, Bell JM, Rapoport SI. Chronic carbamazepine administration reduces N-methyl-D-aspartate receptor-initiated signaling via arachidonic acid in rat brain. Biol Psychiatry. 2007a;62:934–43. doi: 10.1016/j.biopsych.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basselin M, Villacreses NE, Lee HJ, Bell JM, Rapoport SI. Chronic lithium administration attenuates up-regulated brain arachidonic acid metabolism in a rat model of neuroinflammation. J Neurochem. 2007b;102:761–72. doi: 10.1111/j.1471-4159.2007.04593.x. [DOI] [PubMed] [Google Scholar]
- Basselin M, Villacreses NE, Lee HJ, Bell JM, Rapoport SI. Flurbiprofen, a cyclooxygenase inhibitor, reduces the brain arachidonic acid signal in response to the cholinergic muscarinic agonist, arecoline, in awake rats. Neurochem Res. 2007c;32:1857–67. doi: 10.1007/s11064-007-9372-3. [DOI] [PubMed] [Google Scholar]
- Basselin M, Chang L, Chen M, Bell JM, Rapoport SI. Chronic administration of valproic acid reduces brain NMDA signaling via arachidonic acid in unanesthetized rats. Neurochem Res. 2008a;33:2229–40. doi: 10.1007/s11064-008-9700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basselin M, Chang L, Chen M, Bell JM, Rapoport SI. Chronic carbamazepine administration attenuates dopamine D2-like receptor-initiated signaling via arachidonic acid in rat brain. Neurochem Res. 2008b;33:1373–83. doi: 10.1007/s11064-008-9595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basselin M, Fox MA, Chang L, Bell JM, Greenstein D, Chen M, Murphy DL, Rapoport SI. Imaging elevated brain arachidonic acid signaling in unanesthetized serotonin transporter (5-HTT)-deficient mice. Neuropsychopharmacology. 2009;34:1695–709. doi: 10.1038/npp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basselin M, Chen M, Rapoport SI, Murphy RC, Farias SE. Lithium modifies brain arachidonic and docosahexaenoic metabolism in rat lipopolysaccharide models of neuroinflammation. Unpublished observations. [DOI] [PMC free article] [PubMed] [Retracted]
- Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nothen MM, Georgi A, Schumacher J, Schwarz M, Abou Jamra R, Hofels S, Propping P, Satagopan J, Detera-Wadleigh SD, Hardy J, McMahon FJ. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayon Y, Hernandez M, Alonso A, Nunez L, Garcia-Sancho J, Leslie C, Sanchez Crespo M, Nieto ML. Cytosolic phospholipase A2 is coupled to muscarinic receptors in the human astrocytoma cell line 1321N1: characterization of the transducing mechanism. Biochem. J. 1997;323:281–287. doi: 10.1042/bj3230281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Aveldano de Caldironi MI, Rodriguez de Turco EB. Rapid release of free arachidonic acid in the central nervous system due to stimulation. Prog. Lipid Res. 1981;20:523–529. doi: 10.1016/0163-7827(81)90092-8. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Rodriguez de Turco EB, Allan G. Mediators of injury in neurotrauma: intracellular signal transduction and gene expression. J Neurotrauma. 1995;12:791–814. doi: 10.1089/neu.1995.12.791. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Lipid signaling in neural plasticity, brain repair, and neuroprotection. Mol Neurobiol. 2005;32:89–103. doi: 10.1385/MN:32:1:089. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–41. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic valproate does not alter the kinetics of docosahexaenoic acid within brain phospholipids of the unanesthetized rat. Psychopharmacology (Berl) 2005;182:180–5. doi: 10.1007/s00213-005-0059-7. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic Acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol Psychiatry. 2006a;59:401–7. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Weis MT, Rapoport SI, Rosenberger TA. Valproic acid selectively inhibits conversion of arachidonic acid to arachidonoyl-CoA by brain microsomal long-chain fatty acyl-CoA synthetases: relevance to bipolar disorder. Psychopharmacology (Berl) 2006b;184:122–9. doi: 10.1007/s00213-005-0272-4. [DOI] [PubMed] [Google Scholar]
- Begley CE, Annegers JF, Swann AC, Lewis C, Coan S, Schnapp WB, Bryant-Comstock L. The lifetime cost of bipolar disorder in the US: an estimate for new cases in 1998. Pharmacoeconomics. 2001;19:483–95. doi: 10.2165/00019053-200119050-00004. [DOI] [PubMed] [Google Scholar]
- Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–86. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- Benazzi F. Bipolar II Disorder : Epidemiology, Diagnosis and Management. CNS Drugs. 2007;21:727–40. doi: 10.2165/00023210-200721090-00003. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Berger M, Riemann D, Hochli D, Spiegel R. The cholinergic rapid eye movement sleep induction test with RS-86. State or trait marker of depression? Arch Gen Psychiatry. 1989;46:421–8. doi: 10.1001/archpsyc.1989.01810050035006. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982;206:587–95. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–9. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Beutler AS, Li S, Nicol R, Walsh MJ. Carbamazepine is an inhibitor of histone deacetylases. Life Sci. 2005;76:3107–15. doi: 10.1016/j.lfs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Bhana N, Perry CM. Olanzapine: a review of its use in the treatment of bipolar I disorder. CNS Drugs. 2001;15:871–904. doi: 10.2165/00023210-200115110-00005. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee AK, Chang L, Lee HJ, Bazinet RP, Seemann R, Rapoport SI. D2 but not D1 dopamine receptor stimulation augments brain signaling involving arachidonic acid in unanesthetized rats. Psychopharmacology (Berl) 2005;180:735–42. doi: 10.1007/s00213-005-2208-4. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee AK, Chang L, White L, Bazinet RP, Rapoport SI. Imaging apomorphine stimulation of brain arachidonic acid signaling via D2-like receptors in unanesthetized rats. Psychopharmacology (Berl) 2008;197:557–66. doi: 10.1007/s00213-008-1073-3. [DOI] [PubMed] [Google Scholar]
- Bialer M, Levy RH, Perucca E. Does carbamazepine have a narrow therapeutic plasma concentration range? Ther Drug Monit. 1998;20:56–9. doi: 10.1097/00007691-199802000-00010. [DOI] [PubMed] [Google Scholar]
- Bialer M, Yagen B. Valproic Acid: second generation. Neurotherapeutics. 2007;4:130–7. doi: 10.1016/j.nurt.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnier B, Gorwood P, Hamon M, Sarfati Y, Boni C, Hardy-Bayle MC. Association of 5-HT(2A) receptor gene polymorphism with major affective disorders: the case of a subgroup of bipolar disorder with low suicide risk. Biol Psychiatry. 2002;51:762–5. doi: 10.1016/s0006-3223(01)01228-8. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Rintala J, Seemann R, Rosenberger TA, Contreras MA, Rapoport SI, Chang MC. Chronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E(2) concentration in rat brain. Mol Psychiatry. 2002a;7:845–50. doi: 10.1038/sj.mp.4001111. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Seemann R, Bell JM, Zahorchak R, Friedman E, Rapoport SI, Manickam P. Analysis of gene expression with cDNA microarrays in rat brain after 7 and 42 days of oral lithium administration. Brain Res Bull. 2002b;57:205–9. doi: 10.1016/s0361-9230(01)00744-4. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Weerasinghe GR, Rosenberger TA, Rapoport SI. Valproic acid down-regulates the conversion of arachidonic acid to eicosanoids via cyclooxygenase-1 and -2 in rat brain. J Neurochem. 2003;85:690–6. doi: 10.1046/j.1471-4159.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Langenbach R, Weerasinghe GR. Prostaglandin E2 and microsomal prostaglandin E synthase-2 expression are decreased in the cyclooxygenase-2-deficient mouse brain despite compensatory induction of cyclooxygenase-1 and Ca2+-dependent phospholipase A2. J Neurochem. 2004;91:1389–97. doi: 10.1111/j.1471-4159.2004.02829.x. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Bell JM, Manickam P. Microarray analysis of rat brain gene expression after chronic administration of sodium valproate. Brain Res Bull. 2005;65:331–8. doi: 10.1016/j.brainresbull.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J Neurochem. 2007;102:577–586. doi: 10.1111/j.1471-4159.2007.04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry. 2000;157:1925–32. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, Dilsaver SC, Davis JM, Rush AJ, Small JG, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. Jama. 1994;271:918–24. [PubMed] [Google Scholar]
- Bowden CL. Predictors of response to divalproex and lithium. J Clin Psychiatry. 1995;56(Suppl 3):25–30. [PubMed] [Google Scholar]
- Bowden CL. Acute and maintenance treatment with mood stabilizers. Int J Neuropsychopharmacol. 2003;6:269–75. doi: 10.1017/S1461145703003535. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Grunze H, Mullen J, Brecher M, Paulsson B, Jones M, Vagero M, Svensson K. A randomized, double-blind, placebo-controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. J Clin Psychiatry. 2005a;66:111–21. doi: 10.4088/jcp.v66n0116. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Ketter TA, Sachs GS, Thase ME. Focus on bipolar disorder treatment. J. Clin. Psychiatry. 2005b;66:1598–1609. doi: 10.4088/jcp.v66n1217. [DOI] [PubMed] [Google Scholar]
- Breitner JC. NSAIDs and Alzheimer's disease: how far to generalise from trials? Lancet Neurol. 2003;2:527. doi: 10.1016/s1474-4422(03)00498-8. [DOI] [PubMed] [Google Scholar]
- Bunney WEJ, Garland-Bunney BL. Mechanisms of action of lithium in affective illness: Basic and clinical implications. In: Meltzer HY, editor. Psychopharmacology: The Third Generation of Progress. Raven; New York: 1987. pp. 553–565. [Google Scholar]
- Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–42. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Felder CC. Role of the cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. Mol Psychiatry. 2002;7(Suppl 1):S57–63. doi: 10.1038/sj.mp.4001019. [DOI] [PubMed] [Google Scholar]
- Cade JF. Lithium salts in the treatment of psychotic excitement. Medical Journal of Australia. 1949;2:349–352. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999a;60:79–88. doi: 10.4088/jcp.v60n0203. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Rapport DJ, Kimmel SE, Shelton MD. Controlled trials in bipolar I depression: focus on switch rates and efficacy. Eur Neuropsychopharmacol. 1999b;9(Suppl 4):S109–12. doi: 10.1016/s0924-977x(99)00023-1. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–19. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Camus M, Hennere G, Baron G, Peytavin G, Massias L, Mentre F, Farinotti R. Comparison of lithium concentrations in red blood cells and plasma in samples collected for TDM, acute toxicity, or acute-on-chronic toxicity. Eur J Clin Pharmacol. 2003;59:583–7. doi: 10.1007/s00228-003-0670-7. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, Venturi P, Jones LA, Lewis SW, Sham PC, Gottesman II, Farmer AE, McGuffin P, Reveley AM, Murray RM. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch Gen Psychiatry. 1999;56:162–8. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- Chang MC, Grange E, Rabin O, Bell JM, Allen DD, Rapoport SI. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci Lett. 1996;220:171–4. doi: 10.1016/s0304-3940(96)13264-x. [DOI] [PubMed] [Google Scholar]
- Chang MC, Bell JM, Purdon AD, Chikhale EG, Grange E. Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochem Res. 1999;24:399–406. doi: 10.1023/a:1020989701330. [DOI] [PubMed] [Google Scholar]
- Chang MC, Contreras MA, Rosenberger TA, Rintala JJ, Bell JM, Rapoport SI. Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J Neurochem. 2001;77:796–803. doi: 10.1046/j.1471-4159.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem Res. 2008;33:2318–23. doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Rapoport SI, Rao JS. Chronic administration of mood stabilizers upregulates BDNF and bcl-2 expression levels in rat frontal cortex. Neurochem Res. 2009;34:536–41. doi: 10.1007/s11064-008-9817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bazan NG. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 2005;77:65–76. doi: 10.1016/j.prostaglandins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Chen G, Yuan P, Hawver DB, Potter WZ, Manji HK. Increase in AP-1 transcription factor DNA binding activity by valproic acid. Neuropsychopharmacology. 1997;16:238–45. doi: 10.1016/S0893-133X(96)00239-4. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72:1327–30. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Chetcuti A, Adams LJ, Mitchell PB, Schofield PR. Altered gene expression in mice treated with the mood stabilizer sodium valproate. Int J Neuropsychopharmacol. 2006;9:267–76. doi: 10.1017/S1461145705005717. [DOI] [PubMed] [Google Scholar]
- Chuang DM. Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Crit Rev Neurobiol. 2004;16:83–90. doi: 10.1615/critrevneurobiol.v16.i12.90. [DOI] [PubMed] [Google Scholar]
- Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci. 2005;1053:195–204. doi: 10.1196/annals.1344.018. [DOI] [PubMed] [Google Scholar]
- Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–51. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Contreras MA, Chang MC, Rosenberger TA, Greiner RS, Myers CS, Salem N, Jr., Rapoport SI. Chronic nutritional deprivation of n-3 alpha-linolenic acid does not affect n-6 arachidonic acid recycling within brain phospholipids of awake rats. J Neurochem. 2001;79:1090–9. doi: 10.1046/j.1471-4159.2001.00658.x. [DOI] [PubMed] [Google Scholar]
- Contreras MA, Rapoport SI. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr. Opin. Lipidol. 2002;13:267–272. doi: 10.1097/00041433-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. Oxford University Press; Oxford: 2003. [Google Scholar]
- Coyle TR, Kochunov P, Patel RD, Nery FG, Lancaster JL, Mangin JF, Riviere D, Pillow DR, Davis GJ, Nicoletti MA, Serap Monkul E, Fox PT, Soares JC. Cortical sulci and bipolar disorder. Neuroreport. 2006;17:1739–42. doi: 10.1097/01.wnr.0000239957.53072.f0. [DOI] [PubMed] [Google Scholar]
- Craddock N, Jones L, Jones IR, Kirov G, Green EK, Grozeva D, Moskvina V, Nikolov I, Hamshere ML, Vukcevic D, Caesar S, Gordon-Smith K, Fraser C, Russell E, Norton N, Breen G, St Clair D, Collier DA, Young AH, Ferrier IN, Farmer A, McGuffin P, Holmans PA, Donnelly P, Owen MJ, O'Donovan MC. Strong genetic evidence for a selective influence of GABA(A) receptors on a component of the bipolar disorder phenotype. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.66. (E Pub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ. How and why genetic linkage has not solved the problem of psychosis: review and hypothesis. Am J Psychiatry. 2007;164:13–21. doi: 10.1176/ajp.2007.164.1.13. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: Recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Damberg M, Ekblom J, Oreland L. Chronic pharmacological treatment with certain antidepressants alters the expression and DNA-binding activity of transcription factor AP-2. Life Sci. 2000;68:669–78. doi: 10.1016/s0024-3205(00)00969-3. [DOI] [PubMed] [Google Scholar]
- Daniel W, Adamus A, Melzacka M, Szymura J, Vetulani J. Cerebral pharmacokinetics of imipramine in rats after single and multiple dosages. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:209–13. doi: 10.1007/BF00503818. [DOI] [PubMed] [Google Scholar]
- Davis KL, Berger PA, Hollister LE, Defraites E. Physostigmine in mania. Arch Gen Psychiatry. 1978;35:119–22. doi: 10.1001/archpsyc.1978.01770250121012. [DOI] [PubMed] [Google Scholar]
- Dean B, Gray L, Scarr E. Regionally specific changes in levels of cortical S100beta in bipolar 1 disorder but not schizophrenia. Aust N Z J Psychiatry. 2006;40:217–24. doi: 10.1080/j.1440-1614.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- DeGeorge JJ, Nariai T, Yamazaki S, Williams WM, Rapoport SI. Arecoline-stimulated brain incorporation of intravenously administered fatty acids in unanesthetized rats. J Neurochem. 1991;56:352–5. doi: 10.1111/j.1471-4159.1991.tb02603.x. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull. 2008;34:312–21. doi: 10.1093/schbul/sbm164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demar JC, Jr., Ma K, Chang L, Bell JM, Rapoport SI. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem. 2005;94:1063–76. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr., Lee HJ, Ma K, Chang L, Bell JM, Rapoport SI, Bazinet RP. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta. 2006a;1761:1050–9. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr., Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006b;47:172–80. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- Di Daniel E, Cheng L, Maycox PR, Mudge AW. The common inositol-reversible effect of mood stabilizers on neurons does not involve GSK3 inhibition, myo-inositol-1-phosphate synthase or the sodium-dependent myoinositol transporters. Mol Cell Neurosci. 2006:27–36. doi: 10.1016/j.mcn.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Dikeos DG, Papadimitriou GN, Souery D, Del-Favero J, Massat I, Blackwood D, Cichon S, Daskalopoulou E, Ivezic S, Kaneva R, Karadima G, Lorenzi C, Milanova V, Muir W, Nothen M, Oruc L, Rietschel M, Serretti A, Van Broeckhoven C, Soldatos CR, Stefanis CN, Mendlewicz J. Lack of genetic association between the phospholipase A2 gene and bipolar mood disorder in a European multicentre case-control study. Psychiatr Genet. 2006;16:169–71. doi: 10.1097/01.ypg.0000218615.19892.86. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1. Ann N Y Acad Sci. 1988;546:122–32. doi: 10.1111/j.1749-6632.1988.tb21627.x. [DOI] [PubMed] [Google Scholar]
- Doose DR, Streeter AJ. Topiramate - chemistry, biotransformation and pharmacokinetics. In: Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. Lippincott, Williams and Wilkins; Philadelphia: 2002. pp. 727–734. [Google Scholar]
- Doose DR, Brodie MJ, Wilson EA, Chadwick D, Oxbury J, Berry DJ, Schwabe S, Bialer M. Topiramate and lamotrigine pharmacokinetics during repetitive monotherapy and combination therapy in epilepsy patients. Epilepsia. 2003;44:917–22. doi: 10.1046/j.1528-1157.2003.64402.x. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–61. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- DSM-IV . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, Einat H, Manji HK. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24:6578–89. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA, Jr., Manji HK. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormede P, Chaouloff F. Effects of repeated fluoxetine on anxiety-related behaviours, central serotonergic systems, and the corticotropic axis axis in SHR and WKY rats. Neuropharmacology. 1999;38:893–907. doi: 10.1016/s0028-3908(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Eden Evins A, Demopulos C, Yovel I, Culhane M, Ogutha J, Grandin LD, Nierenberg AA, Sachs GS. Inositol augmentation of lithium or valproate for bipolar depression. Bipolar Disord. 2006;8:168–74. doi: 10.1111/j.1399-5618.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- Eilers R. Therapeutic drug monitoring for the treatment of psychiatric disorders. Clinical use and cost effectiveness. Clin Pharmacokinet. 1995;29:442–50. doi: 10.2165/00003088-199529060-00005. [DOI] [PubMed] [Google Scholar]
- Ertley RN, Bazinet RP, Lee HJ, Rapoport SI, Rao JS. Chronic treatment with mood stabilizers increases membrane GRK3 in rat frontal cortex. Biol Psychiatry. 2007;61:246–9. doi: 10.1016/j.biopsych.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Esposito G, Giovacchini G, Der M, Liow JS, Bhattacharjee AK, Ma K, Herscovitch P, Channing M, Eckelman WC, Hallett M, Carson RE, Rapoport SI. Imaging signal transduction via arachidonic acid in the human brain during visual stimulation, by means of positron emission tomography. Neuroimage. 2007;34:1342–51. doi: 10.1016/j.neuroimage.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M, Hallett M, Herscovitch P, Eckelman WC, Carson RE, Rapoport SI. Imaging neuroinflammation in Alzheimer's disease with radiolabeled arachidonic acid and PET. J Nucl Med. 2008;49:1414–21. doi: 10.2967/jnumed.107.049619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr., Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–89. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Falconer DS. The inheritance of liability to diseases with variable age of onset, with particular reference to diabetes mellitus. Ann Hum Genet. 1967;31:1–20. doi: 10.1111/j.1469-1809.1967.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Farber NB, Jiang XP, Heinkel C, Nemmers B. Antiepileptic drugs and agents that inhibit voltage-gated sodium channels prevent NMDA antagonist neurotoxicity. Mol Psychiatry. 2002;7:726–33. doi: 10.1038/sj.mp.4001087. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- FDA [May 27];FDA approved drug products. 2009 http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci U S A. 1990;87:2187–91. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G, Pelonero AL, Ferguson C. Mania precipitated by prednisone and bromocriptine. Gen Hosp Psychiatry. 1991;13:345–6. doi: 10.1016/0163-8343(91)90041-t. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F, Soberman R. Regulated formation of eicosanoids. J. Clin. Invest. 2001;107:1347–1351. doi: 10.1172/JCI13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountoulakis KN, Vieta E, Sanchez-Moreno J, Kaprinis SG, Goikolea JM, Kaprinis GS. Treatment guidelines for bipolar disorder: a critical review. J Affect Disord. 2005;86:1–10. doi: 10.1016/j.jad.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Grunze H, Panagiotidis P, Kaprinis G. Treatment of bipolar depression: an update. J Affect Disord. 2008;109:21–34. doi: 10.1016/j.jad.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- Frechilla D, Otano A, Del Rio J. Effect of chronic antidepressant treatment on transcription factor binding activity in rat hippocampus and frontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:787–802. doi: 10.1016/s0278-5846(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Garrido R, Springer JE, Hennig B, Toborek M. Apoptosis of spinal cord neurons by preventing depletion nicotine attenuates arachidonic acid-induced of neurotrophic factors. J Neurotrauma. 2003;20:1201–13. doi: 10.1089/089771503322584628. [DOI] [PubMed] [Google Scholar]
- Gascon S, Deogracias R, Sobrado M, Roda JM, Renart J, Rodriguez-Pena A, Diaz-Guerra M. Transcription of the NR1 subunit of the N-methyl-D-aspartate receptor is down-regulated by excitotoxic stimulation and cerebral ischemia. J Biol Chem. 2005;280:35018–27. doi: 10.1074/jbc.M504108200. [DOI] [PubMed] [Google Scholar]
- Gaudreault SB, Chabot C, Gratton JP, Poirier J. The caveolin scaffolding domain modifies 2-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor binding properties by inhibiting phospholipase A2 activity. J Biol Chem. 2004;279:356–62. doi: 10.1074/jbc.M304777200. [DOI] [PubMed] [Google Scholar]
- Ghadirian AM, Annable L, Belanger MC, Chouinard G. A cross-sectional study of parkinsonism and tardive dyskinesia in lithium-treated affective disordered patients. J Clin Psychiatry. 1996;57:22–8. [PubMed] [Google Scholar]
- Ghaemi N. Bipolar Disorder. 2001 http://www.familyaware.org/expertprofiles/drghaemi4.asp#diagnosed.
- Ghaemi SN, Rosenquist KJ, Ko JY, Baldassano CF, Kontos NJ, Baldessarini RJ. Antidepressant treatment in bipolar versus unipolar depression. Am J Psychiatry. 2004;161:163–5. doi: 10.1176/appi.ajp.161.1.163. [DOI] [PubMed] [Google Scholar]
- Ghelardoni S, Tomita YA, Bell JM, Rapoport SI, Bosetti F. Chronic carbamazepine selectively downregulates cytosolic phospholipase A2 expression and cyclooxygenase activity in rat brain. Biol Psychiatry. 2004;56:248–54. doi: 10.1016/j.biopsych.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Ghelardoni S, Bazinet RP, Rapoport SI, Bosetti F. Topiramate does not alter expression in rat brain of enzymes of arachidonic acid metabolism. Psychopharmacology (Berl) 2005;180:523–9. doi: 10.1007/s00213-005-2189-3. [DOI] [PubMed] [Google Scholar]
- Giovacchini G, Lerner A, Toczek MT, Fraser C, Ma K, DeMar JC, Herscovitch P, Eckelman WC, Rapoport SI, Carson RE. Brain incorporation of [11C]arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J Nucl Med. 2004;45:1471–9. [PubMed] [Google Scholar]
- Gomez-Sintes R, Lucas JJ. Exploring cellular and molecular mechanism underlying the neurological side effects of lithium therapy. Soc Neurosci Abst. 2008;56.20 [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. Oxford University Press; New York: 2007. [Google Scholar]
- Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004a;29:32–8. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry. 2004b;9:734–55. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–37. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry. 2006;11:125–33. 115. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, O'Donovan C, Alda M. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63:942–7. doi: 10.4088/jcp.v63n1013. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Fujimaki K, Jeong MR, Christ L, Chuang de M. Lithium-induced inhibition of Src tyrosine kinase in rat cerebral cortical neurons: a role in neuroprotection against N-methyl-D-aspartate receptor-mediated excitotoxicity. FEBS Lett. 2003;538:145–8. doi: 10.1016/s0014-5793(03)00167-4. [DOI] [PubMed] [Google Scholar]
- Hassel B, Tauboll E, Gjerstad L. Chronic lamotrigine treatment increases rat hippocampal GABA shunt activity and elevates cerebral taurine levels. Epilepsy Res. 2001;43:153–63. doi: 10.1016/s0920-1211(00)00196-0. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Chang MC, Rapoport SI, Appel NM. Selective dopamine receptor stimulation differentially affects [3H]arachidonic acid incorporation, a surrogate marker for phospholipase A2-mediated neurotransmitter signal transduction, in a rodent model of Parkinson's disease. J Pharmacol Exp Ther. 2001;296:1074–84. [PubMed] [Google Scholar]
- Hegerl U, Herrmann WM, Ulrich G, Muller-Oerlinghausen B. Effects of lithium on auditory evoked potentials in healthy subjects. Biol. Psychiatry. 1990;27:555–560. doi: 10.1016/0006-3223(90)90449-c. [DOI] [PubMed] [Google Scholar]
- Hokin LE, Dixon JF. The phosphoinositide signaling system. I. Historical background. II. Effects of lithium on the accumulation of second messenger inositol 1,4,5-trisphosphate in brain cortex slices. Prog. Brain Res. 1993;98:309–315. doi: 10.1016/s0079-6123(08)62413-9. [DOI] [PubMed] [Google Scholar]
- Holman RT. Control of polyunsaturated acids in tissue lipids. J Am Coll Nutr. 1986;5:183–211. doi: 10.1080/07315724.1986.10720125. [DOI] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Lau T, Rietschel M, Schloss P. A dopamine transporter mutation associated with bipolar affective disorder causes inhibition of transporter cell surface expression. Mol Psychiatry. 2005;10:1104–9. doi: 10.1038/sj.mp.4001730. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol. 2007;7:33–8. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–70. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- Igarashi M. 2009. Unpublished.
- Igarashi M, Ma K, Gao F, Kim HW, Greenstein D, Rapoport SI, Rao JS. No evidence of docosahexaenoic acid deficit in bipolar disorder brain. Submitted.
- Ikeda H, Akiyama G, Fujii Y, Minowa R, Koshikawa N, Cools AR. Role of AMPA and NMDA receptors in the nucleus accumbens shell in turning behaviour of rats: interaction with dopamine receptors. Neuropharmacology. 2003;44:81–7. doi: 10.1016/s0028-3908(02)00334-9. [DOI] [PubMed] [Google Scholar]
- Ikemoto A, Kobayashi T, Watanabe S, Okuyama H. Membrane fatty acid modifications of PC12 cells by arachidonate or docosahexaenoate affect neurite outgrowth but not norepinephrine release. Neurochem Res. 1997;22:671–8. doi: 10.1023/a:1027393724676. [DOI] [PubMed] [Google Scholar]
- Jacobsen FM. Low-dose valproate: a new treatment for cyclothymia, mild rapid cycling disorders, and premenstrual syndrome. J Clin Psychiatry. 1993;54:229–34. [PubMed] [Google Scholar]
- Jacobsen NJ, Franks EK, Owen MJ, Craddock NJ. Mutational analysis of phospholipase A2A: a positional candidate susceptibility gene for bipolar disorder. Mol Psychiatry. 1999;4:274–9. doi: 10.1038/sj.mp.4000476. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Overstreet DH. The role of acetylcholine mechanisms in mood disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology. The Fourth Generation of Progress. Raven; New York: 1995. pp. 945–956. [Google Scholar]
- Jones CR, Arai T, Bell JM, Rapoport SI. Preferential in vivo incorporation of [3H]arachidonic acid from blood into rat brain synaptosomal fractions before and after cholinergic stimulation. J. Neurochem. 1996;67:822–829. doi: 10.1046/j.1471-4159.1996.67020822.x. [DOI] [PubMed] [Google Scholar]
- Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24:441–3. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- Judd LL, Hubbard B, Janowsky DS, Huey LY, Attewell PA. The effect of lithium carbonate on affect, mood, and personality of normal subjects. Arch Gen Psychiatry. 1977a;34:346–51. doi: 10.1001/archpsyc.1977.01770150104012. [DOI] [PubMed] [Google Scholar]
- Judd LL, Hubbard B, Janowsky DS, Huey LY, Takahashi KI. The effect of lithium carbonate on the cognitive functions of normal subjects. Arch Gen Psychiatry. 1977b;34:355–7. doi: 10.1001/archpsyc.1977.01770150113013. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–7. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73:123–31. doi: 10.1016/s0165-0327(02)00332-4. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med. 2001;52:503–17. doi: 10.1146/annurev.med.52.1.503. [DOI] [PubMed] [Google Scholar]
- Kato T. Molecular genetics of bipolar disorder and depression. Psychiatry Clin Neurosci. 2007;61:3–19. doi: 10.1111/j.1440-1819.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- Kato T, Kubota M, Kasahara T. Animal models of bipolar disorder. Neurosci Biobehav Rev. 2007;31:832–842. doi: 10.1016/j.neubiorev.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2317–21. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck PE, Jr., Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA, Altshuler LL, Kupka R, Nolen WA, Leverich GS, Denicoff KD, Grunze H, Duan N, Post RM. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60:1020–2. doi: 10.1016/j.biopsych.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Kessing LV. The effect of the first manic episode in affective disorder: a case register study of hospitalised episodes. J Affect Disord. 1999;53:233–9. doi: 10.1016/s0165-0327(98)00126-8. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, Willis MW, Danielson A, Frye MA, Herscovitch P, Post RM. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Wang PW, Nowakowska C, editors. Treatment of acute mania in bipolar disorder. American Psychiatric Publishing; Washington, DC: 2005. [Google Scholar]
- Ketterer MW, Brymer J, Rhoads K, Kraft P, Lovallo WR. Is aspirin, as used for antithrombosis, an emotion-modulating agent? J Psychosom Res. 1996;40:53–8. doi: 10.1016/0022-3999(95)00524-2. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–21. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- Kim H-W, Lee HJ, Rapoport SI, Rao JS. Hyperglutamatergic state in postmortem frontal cortex of bipolar disorder patients. Soc. Neurosci. Abstr. 2007a;707.4/Z3 [Google Scholar]
- Kim HW, Rapoport SI, Rao JS. Increased expression of apoptotic factors in postmortem brain from bipolar disorder patients. Soc Neurosci Abst. 2008;414.4 [Google Scholar]
- Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007b;104:91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Kleindienst N, Greil W. Differential efficacy of lithium and carbamazepine in the prophylaxis of bipolar disorder: results of the MAP study. Neuropsychobiology. 2000;42(Suppl 1):2–10. doi: 10.1159/000054844. [DOI] [PubMed] [Google Scholar]
- Klemfuss H. Rhythms and the pharmacology of lithium. Pharmacol Ther. 1992;56:53–78. doi: 10.1016/0163-7258(92)90037-z. [DOI] [PubMed] [Google Scholar]
- Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759–65. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner SF, Khan A, Lane R, Olson WH. Topiramate monotherapy in the management of acute mania: results of four double-blind placebo-controlled trials. Bipolar Disord. 2006;8:15–27. doi: 10.1111/j.1399-5618.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- Lalovic A, Levy E, Canetti L, Sequeira A, Montoudis A, Turecki G. Fatty acid composition in postmortem brains of people who completed suicide. J Psychiatry Neurosci. 2007;32:363–70. [PMC free article] [PubMed] [Google Scholar]
- Lasky-Su JA, Faraone SV, Glatt SJ, Tsuang MT. Meta-analysis of the association between two polymorphisms in the serotonin transporter gene and affective disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;133:110–5. doi: 10.1002/ajmg.b.30104. [DOI] [PubMed] [Google Scholar]
- Lee H, Villacreses NE, Rapoport SI, Rosenberger TA. In vivo imaging detects a transient increase in brain arachidonic acid metabolism: a potential marker of neuroinflammation. J Neurochem. 2004;91:936–45. doi: 10.1111/j.1471-4159.2004.02786.x. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Rao JS, Chang L, Rapoport SI, Bazinet RP. Chronic lamotrigine does not alter the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat: implications for the treatment of bipolar disorder. Psychopharmacology (Berl) 2007a;193:467–74. doi: 10.1007/s00213-007-0803-2. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Rao JS, Ertley RN, Chang L, Rapoport SI, Bazinet RP. Chronic fluoxetine increases cytosolic phospholipase A(2) activity and arachidonic acid turnover in brain phospholipids of the unanesthetized rat. Psychopharmacology (Berl) 2007b;190:103–15. doi: 10.1007/s00213-006-0582-1. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Ertley RN, Rapoport SI, Bazinet RP, Rao JS. Chronic administration of lamotrigine downregulates COX-2 mRNA and protein in rat frontal cortex. Neurochem Res. 2008a;33:861–6. doi: 10.1007/s11064-007-9526-3. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Rao JS, Chang L, Rapoport SI, Bazinet RP. Chronic N-methyl-D-aspartate administration increases the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat. J Lipid Res. 2008b;49:162–8. doi: 10.1194/jlr.M700406-JLR200. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Rao JS, Chang L, Rapoport SI, Kim HW. Chronic imipramine but not bupropion increases arachidonic acid signaling in rat brain: is this related to ‘switching’ in bipolar disorder? Mol Psychiatry. 2008 doi: 10.1038/mp.2008.117. E Pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000;106:723–31. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenox RH, Hahn CG. Overview of the mechanism of action of lithium in the brain. J. Clin. Psychiatry. 2000;61(Suppl 9):5–15. [PubMed] [Google Scholar]
- Lerer B, Moore N, Meyendorff E, Cho SR, Gershon S. Carbamazepine versus lithium in mania: a double-blind study. J Clin Psychiatry. 1987;48:89–93. [PubMed] [Google Scholar]
- Leverich GS, Altshuler LL, Frye MA, Suppes T, McElroy SL, Keck PE, Jr., Kupka RW, Denicoff KD, Nolen WA, Grunze H, Martinez MI, Post RM. Risk of switch in mood polarity to hypomania or mania in patients with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry. 2006;163:232–9. doi: 10.1176/appi.ajp.163.2.232. [DOI] [PubMed] [Google Scholar]
- Li X, Ketter TA, Frye MA. Synaptic, intracellular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders? J Affect Disord. 2002;69:1–14. doi: 10.1016/s0165-0327(00)00361-x. [DOI] [PubMed] [Google Scholar]
- Lieberman DZ, Goodwin FK. Use of olanzapine in the treatment of bipolar I disorder. Expert Rev Neurother. 2004;4:759–67. doi: 10.1586/14737175.4.5.759. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, Aprille JR, Dwyer DS, Li XM, Mahadik SP, Duman RS, Porter JH, Modica-Napolitano JS, Newton SS, Csernansky JG. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar N, Fibuch E, Haines M, Neve K, Liu F, Xiong Z, Wang J. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Lu XH, Bradley RJ, Dwyer DS. Olanzapine produces trophic effects in vitro and stimulates phosphorylation of Akt/PKB, ERK1/2, and the mitogen-activated protein kinase p38. Brain Res. 2004;1011:58–68. doi: 10.1016/j.brainres.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Luschen S, Adam D, Ussat S, Kreder D, Schneider-Brachert W, Kronke M, Adam-Klages S. Activation of ERK1/2 and cPLA(2) by the p55 TNF receptor occurs independently of FAN. Biochem Biophys Res Commun. 2000;274:506–12. doi: 10.1006/bbrc.2000.3173. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, Kim N, Dunner DL, Renshaw PF. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Maida ME, Hurley SD, Daeschner JA, Moore AH, Kerry O'banion M. Cytosolic prostaglandin E(2) synthase (cPGES) expression is decreased in discrete cortical regions in psychiatric disease. Brain Res. 2006 doi: 10.1016/j.brainres.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry. 2000;48:518–30. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]
- Martins MR, Petronilho FC, Gomes KM, Dal-Pizzol F, Streck EL, Quevedo J. Antipsychotic-induced oxidative stress in rat brain. Neurotox Res. 2008;13:63–9. doi: 10.1007/BF03033368. [DOI] [PubMed] [Google Scholar]
- Martucci L, Wong AH, De Luca V, Likhodi O, Wong GW, King N, Kennedy JL. N-methyl-d-aspartate receptor NR2B subunit gene GRIN2B in schizophrenia and bipolar disorder: Polymorphisms and mRNA levels. Schizophr Res. 2006;84:214–21. doi: 10.1016/j.schres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Massat I, Souery D, Del-Favero J, Van Gestel S, Serretti A, Macciardi F, Smeraldi E, Kaneva R, Adolfsson R, Nylander PO, Blackwood D, Muir W, Papadimitriou GN, Dikeos D, Oruc L, Segman RH, Ivezic S, Aschauer H, Ackenheil M, Fuchshuber S, Dam H, Jakovljevic M, Peltonen L, Hilger C, Hentges F, Staner L, Milanova V, Jazin E, Lerer B, Van Broeckhoven C, Mendlewicz J. Positive association of dopamine D2 receptor polymorphism with bipolar affective disorder in a European Multicenter Association Study of affective disorders. Am J Med Genet. 2002;114:177–85. [PubMed] [Google Scholar]
- Matsuzawa A, Murakami M, Atsumi G, Imai K, Prados P, Inoue K, Kudo I. Release of secretory phospholipase A2 from rat neuronal cells and its possible function in the regulation of catecholamine secretion. Biochem J. 1996;318(Pt 2):701–9. doi: 10.1042/bj3180701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, Bramon E, Filbey F, Quraishi S, Walshe M, Murray RM. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163:478–87. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- McGahon B, Clements MP, Lynch MA. The ability of aged rats to sustain long-term potentiation is restored when the age-related decrease in membrane arachidonic acid concentration is reversed. Neuroscience. 1997;81:9–16. doi: 10.1016/s0306-4522(97)00116-4. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn CG, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–99. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillin A, Rizig M, Gurling HM. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet Genomics. 2007;17:605–17. doi: 10.1097/FPC.0b013e328011b5b2. [DOI] [PubMed] [Google Scholar]
- Meira-Lima I, Jardim D, Junqueira R, Ikenaga E, Vallada H. Allelic association study between phospholipase A2 genes and bipolar affective disorder. Bipolar Disord. 2003;5:295–9. doi: 10.1034/j.1399-5618.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Gossling M, Arolt V, Heindel W, Pfleiderer B. Acute mania is accompanied by elevated glutamate/glutamine levels within the left dorsolateral prefrontal cortex. Psychopharmacology (Berl) 2003;168:344–6. doi: 10.1007/s00213-003-1440-z. [DOI] [PubMed] [Google Scholar]
- Montezinho LP, Castro MM, Duarte CB, Penschuck S, Geraldes CF, Mork A. The interaction between dopamine D2-like and beta-adrenergic receptors in the prefrontal cortex is altered by mood-stabilizing agents. J Neurochem. 2006;96:1336–48. doi: 10.1111/j.1471-4159.2005.03654.x. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59:115–23. doi: 10.1001/archpsyc.59.2.115. [DOI] [PubMed] [Google Scholar]
- Nesvag R, Lawyer G, Varnas K, Fjell AM, Walhovd KB, Frigessi A, Jonsson EG, Agartz I. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Deng A, Wurtman RJ, Growdon JH. Metabotropic glutamate receptor subtype mGluR1alpha stimulates the secretion of the amyloid beta-protein precursor ectodomain. J Neurochem. 1997;69:704–12. doi: 10.1046/j.1471-4159.1997.69020704.x. [DOI] [PubMed] [Google Scholar]
- Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–7. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- Noponen M, Sanfilipo M, Samanich K, Ryer H, Ko G, Angrist B, Wolkin A, Duncan E, Rotrosen J. Elevated PLA2 activity in schizophrenics and other psychiatric patients. Biol Psychiatry. 1993;34:641–9. doi: 10.1016/0006-3223(93)90157-9. [DOI] [PubMed] [Google Scholar]
- Nowatzke W, Ramanadham S, Ma Z, Hsu FF, Bohrer A, Turk J. Mass spectrometric evidence that agents that cause loss of Ca2+ from intracellular compartments induce hydrolysis of arachidonic acid from pancreatic islet membrane phospholipids by a mechanism that does not require a rise in cytosolic Ca2+ concentration. Endocrinology. 1998;139:4073–85. doi: 10.1210/endo.139.10.6225. [DOI] [PubMed] [Google Scholar]
- Ong WY, Sandhya TL, Horrocks LA, Farooqui AA. Distribution of cytoplasmic phospholipase A2 in the normal rat brain. J. Hirnforsch. 1999;39:391–400. [PubMed] [Google Scholar]
- Ong WY, Yeo JF, Ling SF, Farooqui AA. Distribution of calcium-independent phospholipase A2 (iPLA 2) in monkey brain. J Neurocytol. 2005;34:447–58. doi: 10.1007/s11068-006-8730-4. [DOI] [PubMed] [Google Scholar]
- Ormandy GC, Song L, Jope RS. Analysis of the convulsant-potentiating effects of lithium in rats. Exp. Neurol. 1991;111:356–361. doi: 10.1016/0014-4886(91)90103-j. [DOI] [PubMed] [Google Scholar]
- Osuji IJ, Cullum CM. Cognition in bipolar disorder. Psychiatr Clin North Am. 2005;28:427–41. doi: 10.1016/j.psc.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Chuang D-M. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J. Neurochem. 1997;69:2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- Pae CU, Yu HS, Kim JJ, Lee CU, Lee SJ, Lee KU, Jun TY, Paik IH, Serretti A, Lee C. BanI polymorphism of the cytosolic phospholipase A2 gene and mood disorders in the Korean population. Neuropsychobiology. 2004;49:185–8. doi: 10.1159/000077364. [DOI] [PubMed] [Google Scholar]
- Pavoine C, Magne S, Sauvadet A, Pecker F. Evidence for a beta2-adrenergic/arachidonic acid pathway in ventricular cardiomyocytes. Regulation by the beta1-adrenergic/camp pathway. J. Biol. Chem. 1999;274:628–637. doi: 10.1074/jbc.274.2.628. [DOI] [PubMed] [Google Scholar]
- Peet M, Peters S. Drug-induced mania. Drug Saf. 1995;12:146–53. doi: 10.2165/00002018-199512020-00007. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–41. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Piomelli D. Arachidonic acid. In: Bloom FE, Kupfer DE, editors. Psychopharmacology: The Fourth Generation of Progress. Raven; New York: 1995. pp. 595–607. [Google Scholar]
- Post RM, Jimerson DC, Bunney WE, Jr., Goodwin FK. Dopamine and mania: behavioral and biochemical effects of the dopamine receptor blocker pimozide. Psychopharmacology (Berl) 1980;67:297–305. doi: 10.1007/BF00431272. [DOI] [PubMed] [Google Scholar]
- Post RM. Sensitization and kindling perspectives for the course of affective illness: toward a new treatment with the anticonvulsant carbamazepine. Pharmacopsychiatry. 1990;23:3–17. doi: 10.1055/s-2007-1014476. [DOI] [PubMed] [Google Scholar]
- Post RM, Ketter TA, Pazzaglia PJ, Denicoff K, George MS, Callahan A, Leverich G, Frye M. Rational polypharmacy in the bipolar affective disorders. Epilepsy Res Suppl. 1996;11:153–80. [PubMed] [Google Scholar]
- Post RM, Calabrese JR. Bipolar depression: the role of atypical antipsychotics. Expert Rev Neurother. 2004;4:S27–33. doi: 10.1586/14737175.4.6.S27. [DOI] [PubMed] [Google Scholar]
- Post RM, Altshuler LL, Leverich GS, Frye MA, Nolen WA, Kupka RW, Suppes T, McElroy S, Keck PE, Denicoff KD, Grunze H, Walden J, Kitchen CM, Mintz J. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry. 2006;189:124–31. doi: 10.1192/bjp.bp.105.013045. [DOI] [PubMed] [Google Scholar]
- Prado GN, Taylor L, Zhou X, Ricupero D, Mierke DF, Polgar P. Mechanisms regulating the expression, self-maintenance, and signaling-function of the bradykinin B2 and B1 receptors. J. Cell. Physiol. 2002;193:275–286. doi: 10.1002/jcp.10175. [DOI] [PubMed] [Google Scholar]
- Purdon AD, Rapoport SI. Energy requirements for two aspects of phospholipid metabolism in mammalian brain. Biochem J. 1998;335(Pt 2):313–8. doi: 10.1042/bj3350313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Ticku MK. Role of AP-1 in ethanol-induced N-methyl-D-aspartate receptor 2B subunit gene up-regulation in mouse cortical neurons. J Neurochem. 2005;95:1332–41. doi: 10.1111/j.1471-4159.2005.03464.x. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chang L, Klaff J, Balbo A, Rapoport SI. Imaging brain phospholipase A2 activation in awake rat in response to 5-HT2A/2C agonist, (+−)-2,5-dimethoxy-4-iodophenyl-2-aminopropane (DOI). Neuropsychopharmacology. 2003;28:244–252. doi: 10.1038/sj.npp.1300022. [DOI] [PubMed] [Google Scholar]
- Rabin O, Chang MC, Grange E, Bell J, Rapoport SI, Deutsch J, Purdon AD. Selective acceleration of arachidonic acid reincorporation into brain membrane phospholipid following transient ischemia in awake gerbil. J. Neurochem. 1998;70:325–334. doi: 10.1046/j.1471-4159.1998.70010325.x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proc Natl Acad Sci U S A. 2002;99:10237–9. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Rapoport SI, Bosetti F. Decrease in the AP-2 DNA-binding activity and in the protein expression of AP-2 alpha and AP-2 beta in frontal cortex of rats treated with lithium for 6 weeks. Neuropsychopharmacology. 2005;30:2006–13. doi: 10.1038/sj.npp.1300740. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, Lee HJ, Rapoport SI, Bazinet RP. Chronic fluoxetine upregulates activity, protein and mRNA levels of cytosolic phospholipase A2 in rat frontal cortex. Pharmacogenomics J. 2006;6:413–20. doi: 10.1038/sj.tpj.6500391. [DOI] [PubMed] [Google Scholar]
- Rao JS, Bazinet RP, Rapoport SI, Lee HJ. Chronic treatment of rats with sodium valproate downregulates frontal cortex NF-kappaB DNA binding activity and COX-2 mRNA. Bipolar Disord. 2007a;9:513–20. doi: 10.1111/j.1399-5618.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- Rao JS, Bazinet RP, Rapoport SI, Lee HJ. Chronic administration of carbamazepine down-regulates AP-2 DNA-binding activity and AP-2alpha protein expression in rat frontal cortex. Biol Psychiatry. 2007b;61:154–61. doi: 10.1016/j.biopsych.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, DeMar JC, Jr., Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007c;12:151–7. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr., Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007d;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, Rapoport SI, Bazinet RP, Lee HJ. Chronic NMDA administration to rats up-regulates frontal cortex cytosolic phospholipase A2 and its transcription factor, activator protein-2. J Neurochem. 2007e;102:1918–27. doi: 10.1111/j.1471-4159.2007.04648.x. [DOI] [PubMed] [Google Scholar]
- Rao JS, Lee HJ, Rapoport SI, Bazinet RP. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol Psychiatry. 2008;13:585–96. doi: 10.1038/mp.2008.31. [DOI] [PubMed] [Google Scholar]
- Rao JS, Rapoport SI, Kim HW. Decreased GRK3 but not GRK2 expression in frontal cortex from bipolar disorder patients. Int J Neuropsychopharmacol. 2009:1–10. doi: 10.1017/S146114570900025X. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rao JS, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. doi: 10.1038/mp.2009.47. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI. In vivo fatty acid incorporation into brain phospholipids in relation to plasma availability, signal transduction and membrane remodeling. J. Mol. Neurosci. 2001;16:243–261. doi: 10.1385/JMN:16:2-3:243. [DOI] [PubMed] [Google Scholar]
- Rapoport SI, Chang MCJ, Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J. Lipid Res. 2001;42:678–685. [PubMed] [Google Scholar]
- Rapoport SI, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry. 2002;59:592–6. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. In vivo approaches to quantifying and imaging brain arachidonic and docosahexaenoic acid metabolism. J Pediatr. 2003;143:S26–34. doi: 10.1067/s0022-3476(03)00399-8. [DOI] [PubMed] [Google Scholar]
- Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77:251–61. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RL, Kim EM, Gardiner T, O'Hare E. Chronic intracerebroventricular infusion of lipopolysaccharide: effects of ibuprofen treatment and behavioural and histopathological correlates. Behav Pharmacol. 2005;16:531–41. doi: 10.1097/01.fbp.0000179278.03868.96. [DOI] [PubMed] [Google Scholar]
- Rintala J, Seemann R, Chandrasekaran K, Rosenberger TA, Chang L, Contreras MA, Rapoport SI, Chang MC. 85 kDa cytosolic phospholipase A2 is a target for chronic lithium in rat brain. Neuroreport. 1999;10:3887–3890. doi: 10.1097/00001756-199912160-00030. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- Rosa AO, Rapoport SI. Intracellular- and extracellular-derived Ca(2+) influence phospholipase A(2)-mediated fatty acid release from brain phospholipids. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbalip.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem. 2004;88:1168–78. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21. doi: 10.1186/1476-511X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev. 2007;31:920–31. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryves WJ, Dalton EC, Harwood AJ, Williams RS. GSK-3 activity in neocortical cells is inhibited by lithium but not carbamazepine or valproic acid. Bipolar Disord. 2005;7:260–5. doi: 10.1111/j.1399-5618.2005.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs GS. Bipolar mood disorder: practical strategies for acute and maintenance phase treatment. J Clin Psychopharmacol. 1996;16:32S–47S. doi: 10.1097/00004714-199604001-00005. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Koslow CL, Ghaemi SN. The treatment of bipolar depression. Bipolar Disord. 2000;2:256–60. doi: 10.1034/j.1399-5618.2000.20306.x. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, Friedman ES, Bowden CL, Fossey MD, Ostacher MJ, Ketter TA, Patel J, Hauser P, Rapport D, Martinez JM, Allen MH, Miklowitz DJ, Otto MW, Dennehy EB, Thase ME. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–22. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:110–33. doi: 10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Kaneva R, Jamra RA, Diaz GO, Ohlraun S, Milanova V, Lee YA, Rivas F, Mayoral F, Fuerst R, Flaquer A, Windemuth C, Gay E, Sanz S, Gonzalez MJ, Gil S, Cabaleiro F, del Rio F, Perez F, Haro J, Kostov C, Chorbov V, Nikolova-Hill A, Stoyanova V, Onchev G, Kremensky I, Strauch K, Schulze TG, Nurnberg P, Gaebel W, Klimke A, Auburger G, Wienker TF, Kalaydjieva L, Propping P, Cichon S, Jablensky A, Rietschel M, Nothen MM. Genomewide scan and fine-mapping linkage studies in four European samples with bipolar affective disorder suggest a new susceptibility locus on chromosome 1p35-p36 and provides further evidence of loci on chromosome 4q31 and 6q24. Am J Hum Genet. 2005;77:1102–11. doi: 10.1086/498619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, Prabakaran S, Whitfield P, Major H, Leweke FM, Koethe D, McKenna P, Bahn S. High Throughput Lipidomic Profiling of Schizophrenia and Bipolar Disorder Brain Tissue Reveals Alterations of Free Fatty Acids, Phosphatidylcholines, and Ceramides. J Proteome Res. 2008;7:4266–4277. doi: 10.1021/pr800188y. [DOI] [PubMed] [Google Scholar]
- Sellmayer A, Danesch U, Weber PC. Modulation of the expression of early genes by polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 1997;57:353–7. doi: 10.1016/s0952-3278(97)90410-5. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Novel chemical mediators in the resolution of inflammation: resolvins and protectins. Anesthesiol Clin. 2006;24:341–64. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Niznik HB. Coupling of dopamine receptor subtypes to multiple and diverse G proteins. Int J Dev Neurosci. 2000;18:669–77. doi: 10.1016/s0736-5748(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Simpson GM. Atypical antipsychotics and the burden of disease. Am J Manag Care. 2005;11:S235–41. [PubMed] [Google Scholar]
- Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, Nimgaonkar VL, McQueen MB, Faraone SV, Kirby A, de Bakker PI, Ogdie MN, Thase ME, Sachs GS, Todd-Brown K, Gabriel SB, Sougnez C, Gates C, Blumenstiel B, Defelice M, Ardlie KG, Franklin J, Muir WJ, McGhee KA, Macintyre DJ, McLean A, Vanbeck M, McQuillin A, Bass NJ, Robinson M, Lawrence J, Anjorin A, Curtis D, Scolnick EM, Daly MJ, Blackwood DH, Gurling HM, Purcell SM. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;3:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobo S. Mood stabilizers and mood swings: In search of a definition. Psychiatric times. 1999:16. [Google Scholar]
- Stefani A, Spadoni F, Siniscalchi A, Bernardi G. Lamotrigine inhibits Ca2+ currents in cortical neurons: functional implications. Eur J Pharmacol. 1996;307:113–6. doi: 10.1016/0014-2999(96)00265-8. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Bosetti F, Silva AC. Modulatory role of cyclooxygenase-2 in cerebrovascular coupling. Neuroimage. 2006;32:23–32. doi: 10.1016/j.neuroimage.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Stolk P, Souverein PC, Wilting I, Leufkens HGM, Klein DF, Rapoport SI, Heerdink ER. Low dose aspirin appears beneficial in bipolar disorder patients who are taking lithium: a pharmacoepidemiological study. Submitted for publication.
- Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–12. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, Shear P, Adler CM. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am J Psychiatry. 2002;159:1841–7. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+. Br J Pharmacol. 2003;139:1014–22. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckow RF, Smith TM, Perumal AS, Cooper TB. Pharmacokinetics of bupropion and metabolites in plasma and brain of rats, mice, and guinea pigs. Drug Metab Dispos. 1986;14:692–7. [PubMed] [Google Scholar]
- Sultzer DL, Cummings JL. Drug-induced mania--causative agents, clinical characteristics and management. A retrospective analysis of the literature. Med Toxicol Adverse Drug Exp. 1989;4:127–43. doi: 10.1007/BF03259908. [DOI] [PubMed] [Google Scholar]
- Sun GY, MacQuarrie RA. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann N Y Acad Sci. 1989;559:37–55. doi: 10.1111/j.1749-6632.1989.tb22597.x. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Yu S, Wood WG, Gonzalez FA, Simonyi A, Sun AY, Weisman GA. Phospholipase A2 in astrocytes: responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Mol Neurobiol. 2005;31:27–41. doi: 10.1385/MN:31:1-3:027. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bowden CL, Morris D, Calabrese JR, Petty F, Small J, Dilsaver SC, Davis JM. Depression during mania. Treatment response to lithium or divalproex. Arch Gen Psychiatry. 1997;54:37–42. doi: 10.1001/archpsyc.1997.01830130041008. [DOI] [PubMed] [Google Scholar]
- Takano T, Panesar M, Papillon J, Cybulsky AV. Cyclooxygenases-1 and 2 couple to cytosolic but not group IIA phospholipase A2 in COS-1 cells. Prostaglandins Other Lipid Mediat. 2000;60:15–26. doi: 10.1016/s0090-6980(99)00033-7. [DOI] [PubMed] [Google Scholar]
- Tamura T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. The effects of antidepressant drug treatments on activator protein-1 binding activity in the rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:375–81. doi: 10.1016/s0278-5846(01)00286-x. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Regional localization of dopamine and ionotropic glutamate receptor subtypes in striatolimbic brain regions. J Neurosci Res. 1999;55:401–10. doi: 10.1002/(SICI)1097-4547(19990215)55:4<401::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tay A, Simon JS, Squire J, Hamel K, Jacob HJ, Skorecki K. Cytosolic phospholipase A2 gene in human and rat: chromosomal localization and polymorphic markers. Genomics. 1995;26:138–41. doi: 10.1016/0888-7543(95)80093-2. [DOI] [PubMed] [Google Scholar]
- Teng CT, Demetrio FN. Memantine may acutely improve cognition and have a mood stabilizing effect in treatment-resistant bipolar disorder. Rev Bras Psiquiatr. 2006;28:252–4. doi: 10.1590/s1516-44462006000300020. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Hill WD, Parikh V, Waller JL, Evans DR, Mahadik SP. Differential effects of haloperidol, risperidone, and clozapine exposure on cholinergic markers and spatial learning performance in rats. Neuropsychopharmacology. 2003;28:300–9. doi: 10.1038/sj.npp.1300039. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–9. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich G, Herrmann WM, Hegerl U, Muller-Oerlinghausen B. Effect of lithium on the dynamics of electroencephalographic vigilance in healthy subjects. J Affect Disord. 1990;20:19–25. doi: 10.1016/0165-0327(90)90046-b. [DOI] [PubMed] [Google Scholar]
- Vadnal R, Parthasarathy R. Myo-inositol monophosphatase: diverse effects of lithium, carbamazepine, and valproate. Neuropsychopharmacology. 1995;12:277–85. doi: 10.1016/0893-133X(94)00088-H. [DOI] [PubMed] [Google Scholar]
- Vasudev K, Goswami U, Kohli K. Carbamazepine and valproate monotherapy: feasibility, relative safety and efficacy, and therapeutic drug monitoring in manic disorder. Psychopharmacology (Berl) 2000;150:15–23. doi: 10.1007/s002130000380. [DOI] [PubMed] [Google Scholar]
- Vial D, Piomelli D. Dopamine D2 receptors potentiate arachidonate release via activation of cytosolic, arachidonate-specific phospholipase A2. J Neurochem. 1995;64:2765–72. doi: 10.1046/j.1471-4159.1995.64062765.x. [DOI] [PubMed] [Google Scholar]
- Wang H-Y, Friedman E. Effects of lithium on receptor-mediated activation of G proteins in rat brain cortical membranes. Neuropharmacology. 1999;38:403–414. doi: 10.1016/s0028-3908(98)00197-x. [DOI] [PubMed] [Google Scholar]
- Weerasinghe GR, Rapoport SI, Bosetti F. The effect of chronic lithium on arachidonic acid release and metabolism in rat brain does not involve secretory phospholipase A2 or lipoxygenase/cytochrome P450 pathways. Brain Res Bull. 2004;63:485–9. doi: 10.1016/j.brainresbull.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Weichel O, Hilgert M, Chatterjee SS, Lehr M, Klein J. Bilobalide, a constituent of Ginkgo biloba, inhibits NMDA-induced phospholipase A2 activation and phospholipid breakdown in rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:609–15. doi: 10.1007/s002109900131. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigg K, Feng Y, Gomez L, Kiss E, Kapornai K, Tamas Z, Mayer L, Baji I, Daroczi G, Benak I, Osvath VK, Dombovari E, Kaczvinszk E, Besnyo M, Gadoros J, King N, Szekely J, Kovacs M, Vetro A, Kennedy JL, Barr CL. Genome scan in sibling pairs with juvenile-onset mood disorders: Evidence for linkage to 13q and Xq. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30883. [DOI] [PubMed] [Google Scholar]
- Wille SM, Cooreman SG, Neels HM, Lambert WE. Relevant issues in the monitoring and the toxicology of antidepressants. Crit Rev Clin Lab Sci. 2008;45:25–89. doi: 10.1080/10408360701713112. [DOI] [PubMed] [Google Scholar]
- Willour VL, Zandi PP, Huo Y, Diggs TL, Chellis JL, MacKinnon DF, Simpson SG, McMahon FJ, Potash JB, Gershon ES, Reich T, Foroud T, Nurnberger JI, Jr., DePaulo JR, Jr., McInnis MG. Genome scan of the fifty-six bipolar pedigrees from the NIMH genetics initiative replication sample: chromosomes 4, 7, 9, 18, 19, 20, and 21. Am J Med Genet B Neuropsychiatr Genet. 2003;121B:21–7. doi: 10.1002/ajmg.b.20051. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Li X. Polyunsaturated fatty acids modify mouse hippocampal neuronal excitability during excitotoxic or convulsant stimulation. Brain Res. 1999;846:112–21. doi: 10.1016/s0006-8993(99)01997-6. [DOI] [PubMed] [Google Scholar]
- Yatham LN. Translating knowledge of genetics and pharmacology into improving everyday practice. Bipolar Disord. 2005;7(Suppl 4):13–20. doi: 10.1111/j.1399-5618.2005.00211.x. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005;57:430–2. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]