Abstract
We have shown that Caenorhabditis elegans lacking the PCM-1 protein repair L-isoaspartyl methyltransferase are more sensitive to oxidative stress than wild-type nematodes. Exposure to the redox-cycling quinone juglone upon exit from dauer diapause results in defective egg laying (Egl phenotype) in the pcm-1 mutants only. Treatment with paraquat, a redox-cycling dipyridyl, causes a more severe developmental delay at the second larval stage in pcm-1 mutants than in wild-type nematodes. Finally, exposure to homocysteine and homocysteine thiolactone, molecules that can induce oxidative stress via distinct mechanisms, results in a more pronounced delay in development at the first larval stage in pcm-1 mutants than in wild-type animals. Homocysteine treatment also induced the Egl phenotype in mutant but not wild-type nematodes. All of the effects of these agents were reversed upon addition of vitamin C, indicating that the developmental delay and egg-laying defects result from oxidative stress. Furthermore, we have demonstrated that a mutation in the gene encoding the insulin-like receptor DAF-2 suppresses the Egl phenotype in pcm-1 mutants treated with juglone. Our results support a role of PCM-1 in the cellular responses mediated by the DAF-2 insulin-like signaling pathway in C. elegans for optimal protection against oxidative stress.
Keywords: oxidative stress, Caenorhabditis elegans, protein L-isoaspartyl O-methyltransferase (pcm-1), delay in development, egg-laying defect (Egl)
1. Introduction
Reactive oxygen species are produced as by-products of normal metabolism and can directly damage proteins and other cellular components. Cells protect themselves with a variety of antioxidants and detoxification enzymes (Halliwell and Gutteridge, 2007). The expression of these enzymes in response to oxidative stress in Caenorhabditis elegans is primarily initiated by the insulin-like signaling pathway mediated by the DAF-2 receptor (Honda and Honda, 1999; Gami and Wolkow, 2006; Murakami, 2007). Down-regulation of the DAF-2 pathway up-regulates the activity of DAF-16, a transcription factor that promotes the expression of anti-oxidant enzymes (Ogg et al., 1997; Braeckman et al., 2001; Murphy et al., 2003; McElwee et al., 2003; Chávez et al., 2007). Mutations in the pathway can give up to a ten-fold increase in lifespan (Ayyadevara et al., 2008). In addition to the DAF-2 pathway, the mitogen-activated protein kinase (MAPK) pathway also responds to environmental stress and regulates development and lifespan by interacting with DAF-16 (Wolf et al., 2007). C. elegans mutants that are hypersensitive to the increased generation of reactive oxygen species exhibit premature aging (Yanase et al., 2002). For example, the mev-1 mutant, which lacks a functional cytochrome b subunit of complex II in the electron transport chain, has a shorter lifespan in comparison to its wild-type counterpart (Ishii et al., 1993; Yamamoto et al., 1996).
Another type of protein damage during aging occurs by the non-oxidative deamidation and isomerization of asparaginyl and aspartyl residues. This damage can be recognized by the protein repair L-isoaspartyl O-methyltransferase, which initiates the conversion of isomerized aspartyl residues to normal L-aspartyl residues (Clarke, 2003). Interestingly, oxidative damage can increase the load of isomerization damage in cells. For example, increased levels of L-isoaspartyl residues are found in erythrocyte hemoglobin (O’Connor et al., 1988) and membrane proteins (Ingrosso et al., 2000) exposed to oxidative stress, as well as in erythrocytes from glucose-6-phosphate dehydrogenase deficient-patients that are more susceptible to oxidative damage (Ingrosso et al., 2002). Additionally, radiation-induced reactive oxygen species were found to induce protein isomerization in cultured human cells that was reversed with antioxidants (D’Angelo et al., 2005). Furthermore, endothelial cells lacking the protein repair methyltransferase are more susceptible to apoptosis induced by hydrogen peroxide treatment, whereas cells overexpressing the enzyme are more resistant (Cimmino et al., 2008). Finally, proteins in mouse brain that most rapidly accumulate isoaspartyl damage can also be prone to oxidative damage (Zhu et al., 2006). Hence, a connection between oxidative stress and protein isomerization damage and repair has been proposed. We were thus interested in understanding the convergence of the oxidative stress response and protein isomerization/repair pathways.
In C. elegans, the protein L-isoaspartyl O-methyltransferase enzyme is encoded by the pcm-1 gene. Pcm-1 deletion mutant C. elegans show normal adult lifespan and no defects in development or fecundity when grown under standard conditions (Kagan et al., 1997; Banfield et al., 2008). However, mutant nematodes are at a competitive disadvantage when co-cultured with wild-type animals (Kagan et al., 1997). Significant effects have been seen in pcm-1 mutant nematodes under environmental stress: the survival of L1 larvae under starvation conditions is reduced in the mutants (Gomez et al., 2007). Pcm-1 mutant nematodes also show defects in the dauer phase (Banfield et al., 2008; Gomez et al., 2008). Under unfavorable conditions, such as low food supply and oxidative stress, C. elegans enter dauer diapause, an alternate third larval stage in which nematodes are more resistant to environmental stress; as dauer larvae, they have SDS-resistant cuticles and enhanced expression of enzymes involved in resistance to oxidative stress (Cassada and Russell, 1975; McElwee et al., 2003). C. elegans lacking pcm-1 tend to form fewer dauer larvae (Banfield et al., 2008) and have a reduced lifespan in the dauer stage (Kagan et al., 1997; Banfield et al. 2008; Gomez et al., 2008). In addition, pcm-1 dauer larvae show a reduction in autophagy (Gomez et al., 2007). Interestingly, the pcm-1 mutant defect in dauer survival is reversed when the daf-2 gene is also mutated (Banfield et al., 2008). Finally, PCM-1 overexpression leads to adult lifespan extension in C. elegans (Banfield et al., 2008) and Drosophila (Chavous et al., 2001). These results suggest an interplay between PCM-1 activity and DAF-2 signaling and their joint participation in the regulation of lifespan and resistance to stress.
In this paper, we show that pcm-1 mutant C. elegans are more sensitive to oxidative stress than their wild-type counterparts. Following exposure to the redox-cycling agent juglone, only pcm-1 mutant dauer larvae entered the adult stage with a defect in egg laying. Nematodes containing a mutation in both pcm-1 and insulin-like receptor daf-2 genes no longer displayed the Egl phenotype. The bipyridyl compound paraquat induced a delay in development in wild-type and pcm-1 nematodes that is most prominent at the second larval stage in the mutant nematodes. Upon exposure to homocysteine, an amino acid derivative that both inhibits methylation reactions and induces oxidative stress, wild-type and pcm-1 mutant C. elegans underwent a delay in development that was more severe in the absence of PCM-1. All of the phenotypes that developed as a result from treatment with these three oxidizing agents were prevented upon addition of the antioxidant vitamin C, suggesting that the effects were oxidative stress-induced. We hypothesize that PCM-1 may function to oppose the actions of the DAF-2 mediated pathway not only to enhance survival under unfavorable conditions, but also to enhance resistance to oxidative stress.
2. Experimental procedures
2.1. Worm strains and culture conditions
Standard procedures used to maintain C. elegans strains were adapted from Sulston and Hodgkin (1988). M9 media and S media were prepared as described (Lewis and Fleming, 1995). The N2 strain was obtained from the Caenorhabditis Genetics Center (St. Paul, MN). The pcm-1 (qa201) mutant lacks exons 2–5 of the pcm-1 gene and alternative exon 1 from the partially overlapping C10F3.4 gene (Fig. 1; Kagan et al., 1997; www.wormbase.org). This allele was backcrossed eight times into an N2 background (Banfield et al., 2008). The pcm-1(tm363) strain was a gift from Shohei Mitani at Tokyo Women’s Medical University in Tokyo and lacks exons 1–2 of the pcm-1 gene and regions of the first intron of the C10F3.4 gene (www.wormbase.org). The C10F3.4 deletion mutant tm2679 lacks exons 6–9 of the C10F3.4 gene and was also a gift from Shohei Mitani at Tokyo Women’s Medical University. daf-2 mutants in linkage group III include daf-2(m41); daf-2(e1370);pcm-1(qa201); and daf-2(m596);pcm-1(qa201). Two transgenic strains (PL51 and PL54; Banfield et al., 2008) were also used. Strain PL51 expresses a plasmid encoding PCM-1 in a pcm-1 mutant background, resulting in an approximate seven-fold overexpression of the methyltransferase over wild-type N2 worms grown in liquid culture (Supplemental Table S1). Strain PL54 contains a similar plasmid with two mutated residues in the PCM-1 active site (G88V G90V) and demonstrates no methyltransferase activity (Supplemental Table S1). Daf-2 strains were maintained at 15 °C or 20°C on nematode growth medium plates streaked with OP50 Escherichia coli (NGM+OP50); other strains were similarly maintained but at 20 °C (Sulston and Hodgkin, 1988).
Fig. 1. Genetic organization of the C. elegans PCM-1 region.
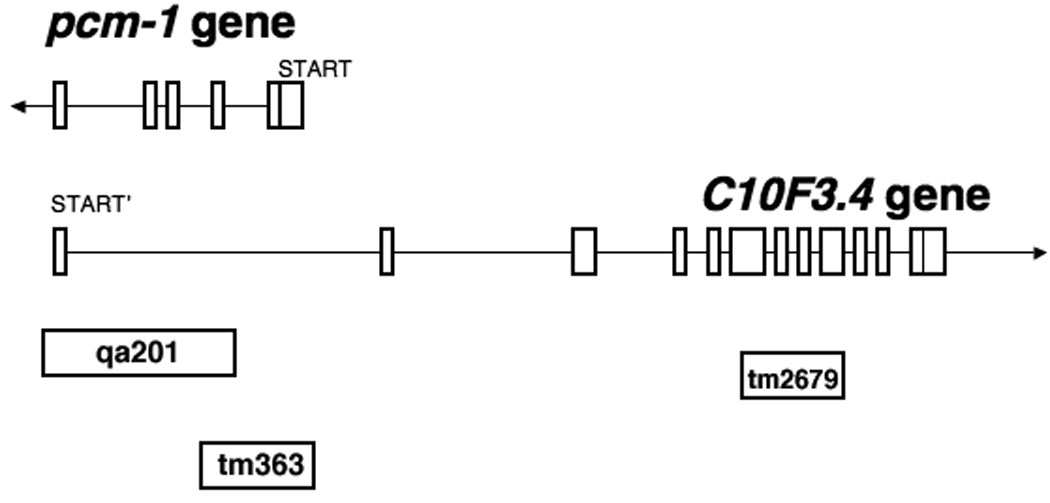
Gene map for C. elegans pcm-1 and the partially overlapping C10F3.4 gene. Exons are shown by boxes; introns by lines. The regions deleted in each mutant are indicated; the direction of transcription is shown with an arrow at the end of the transcript.
2.2. Preparation of eggs and dauer larvae
Individual preparations of about 100 eggs were made by mixing approximately 20 egg-laying adults in 0.7 ml of M9 medium, 0.25 ml of 5.6 % sodium hypochlorite, and 0.05 ml of 10 M sodium hydroxide for 2 min at room temperature. The eggs were pelleted by centrifugation and resuspended in 0.1 ml of S medium and were then immediately plated on 60 mm plastic Petri dishes containing about 7 ml of NGM agar that had been previously streaked with 1 mg of E. coli OP50 cells (wet weight) and incubated for 24 h at room temperature. For preparation of dauer larvae, eggs were transferred to 35 mm plates as described above but that were seeded with half the number of E. coli cells and that also contained pheromone. Pheromone was prepared by organic extraction of S media used to grow large cultures of nematodes that had exhausted their OP50 food source (Golden and Riddle, 1984). Plates were incubated for 72 h at 20 °C. Dauer larvae were manually picked for transfer either to plates containing paraquat or homocysteine derivatives or to drops containing juglone (see below).
2.3. Determination of developmental stage
The larval stage was determined by monitoring the development of the vulva under a dissecting microscope and was confirmed by body length (Lewis and Fleming, 1995). The Egl phenotype was determined by observing the distinguishing features of a swollen abdomen containing unhatched eggs coupled with decreased locomotion (Trent et al., 1983, Bastiani and Mendel 2006). Dauer larvae were characterized by their body length and width, decreased motility, absence of pharyngeal activity, and dark shaded abdomens (Lewis and Fleming, 1995).
2.4. Treatment of eggs and dauer larvae with juglone
Eggs (about 100) were plated on 60 mm NGM+OP50 plates containing 236 µM juglone at 20 °C and were scored for survival on an hourly basis by their ability to advance to L1 larvae. For the effects on dauer larvae, eggs (about 100) were transferred to five freshly-made 35 mm NGM+OP50 pheromone plates. The plates were sealed with Parafilm and incubated at 20 °C for 72 h when dauers had formed. For each strain, a group of 50 dauers was manually transferred to a 30 µl drop of S medium on the lid of the plate that either contained no addition, 236 µM juglone, 0.5 mM or 1.0 mM L-vitamin C, or combinations of juglone and vitamin C. Juglone (5-hydroxy-1,4-naphthoquinone, Sigma Chemicals # H47003) was made as a 10 mM stock in ethanol; vitamin C (L-ascorbic acid) was a Fluka product (# 95209). The dauer larvae were allowed to incubate in the drop for 90 min at room temperature and were subsequently transferred to two 60 mm NGM+OP50 plates using glass capillary tubes. Dauer larval survival was monitored after 24 h, 48 hr, and 72 hr by their response to mechanical stimulation. The presence of the egg-laying defect (Egl) was determined after plating on NGM+OP50 plates.
2.5. Treatment with paraquat
Paraquat (methyl viologen, Sigma Chemicals # 856177) and/or vitamin C were added to liquid NGM agar just prior to pouring on 60 mm plates. After the agar solidified, plates were streaked with 1 mg wet weight of OP50 E. coli cells and were incubated overnight at room temperature. The following day, approximately 100 eggs were added to the plate which was then sealed with Parafilm and incubated at 25 °C for 48 h before determination of the developmental state. Adult survival was determined as described in Supplemental Table S2.
2.6. Treatment with homocysteine and homocysteine derivatives
DL-Homocysteine was obtained from Sigma Chemicals (# H4628), DL-homocysteine thiolactone hydrochloride was obtained from ICN Biomedicals (# 101976), and DL-homocystine was obtained from Sigma Chemicals (# H0501). These derivatives and/or vitamin C were added to liquid NGM agar just prior to pouring on 60 mm plates. After the agar solidified, plates were streaked with 1 mg (wet weight) of OP50 E. Coli cells and were incubated overnight at room temperature. The following day, approximately 100 eggs were added to the plate which was then sealed with Parafilm and incubated at 25 °C for 48 h before determination of the developmental state. Adult survival was determined as described in Supplemental Table S2.
3. Results
3.1. The oxidizing agent juglone induces Egl egg-laying defects in pcm-1 mutant dauer larvae
Juglone is a quinone that is taken up by cells and reduced to the semiquinone radical by NAD(P)H flavoenzymes such as cytochrome P450. The semiquinone radical can react with molecular oxygen to form superoxide anion and to reform the quinone for additional cycles of superoxide generation and diversion of reduced nicotinamides from their role in maintaining reduced glutathione. In addition, the semiquinone can reduce iron from Fe3+ to Fe2+, a species that can subsequently react with hydrogen peroxide (formed from superoxide by the dismutase) to generate destructive hydroxyl radicals via Fenton chemistry (Halliwell and Gutteridge, 2007). In C. elegans studies, juglone has been used to select for oxidative stress-resistant mutants, partially because it can penetrate the cuticle structure (De Castro et al., 2004). Because of the links between oxidative damage and protein isomerization damage, we were interested in asking if nematodes lacking the PCM-1 protein repair L-isoaspartyl methyltransferase might be particularly sensitive to juglone treatment.
Two distinct pcm-1 deletion strains, qa201 and tm363, were used in the oxidative stress experiments. The pcm-1 deletion strain qa201 lacks exons 2–5 of the pcm-1 gene, whereas pcm-1 deletion mutant tm363 lacks exons 1–2 (Fig. 1). No protein L-isoaspartyl methyltransferase activity is detectable in either mutant strain (Supplemental Table S1). The pcm-1 deletion mutants qa201 and tm363 also lack portions of the overlapping gene C10F3.4; specifically, pcm-1 deletion mutants qa201 and tm363 lack C10F3.4 exon 1 and C10F3.4 intronic regions, respectively (Fig. 1). To determine if oxidative stress phenotypes were specific to the pcm-1 deletion, the C10F3.4 deletion strain tm2679, lacking exons 6–9, was also tested.
Initially, young adults from the two pcm-1 deletion strains were treated with 236 µM juglone in liquid culture or on NGM plates containing juglone using methods described previously (Cypser and Johnson, 2002; De Castro et al., 2004). We observed no difference in the survival of the qa201 or the tm363 pcm-1 mutants and the wild type N2 nematodes under these conditions (Supplemental Table S2). We also tested the ability of eggs from each of these strains to undergo larval development in both liquid cultures containing juglone and juglone-containing (236 µM) NGM+OP50 plates. However, under our conditions, eggs from wild-type and pcm-1 mutant strains did not hatch to first stage larvae (L1) animals.
We then examined whether dauer larvae from each strain could withstand juglone treatment. Dauer larvae from both wild-type and pcm-1 mutant C. elegans strains were treated with 236 µM juglone for 90 min and were monitored for development and changes in morphology after transfer to plates containing a food source and lacking juglone. Under these conditions, wild-type dauer larvae remained healthy, developed normally, and laid eggs that developed into healthy young larvae. However, the pcm-1 mutant dauer larvae were affected (Fig. 2). As shown in Table 1, upon exposure to juglone, approximately 28% and 24% of the of the pcm-1 (qa201) and pcm-1 (tm363) dauer larvae developed into defective egg-laying adults. The egg-laying phenotype (Egl) is characterized by reduced motility, egg retention, and a swollen abdomen containing late-developed larvae (Trent et al., 1983; Desai and Horvitz, 1989; Bastiani and Mendel, 2006). The juglone treated pcm-1 mutant worms displayed all of these phenotypes, as well as an abnormal pharynx and intestine (Fig. 2). Additionally, the adult nematodes retain eggs that have developed past the stage at which they are normally expelled through the vulva; as a consequence, the egg-laying defective animals appear more transparent than normal gravid adults (Fig. 2; Desai and Horvitz, 1989).
Fig. 2.
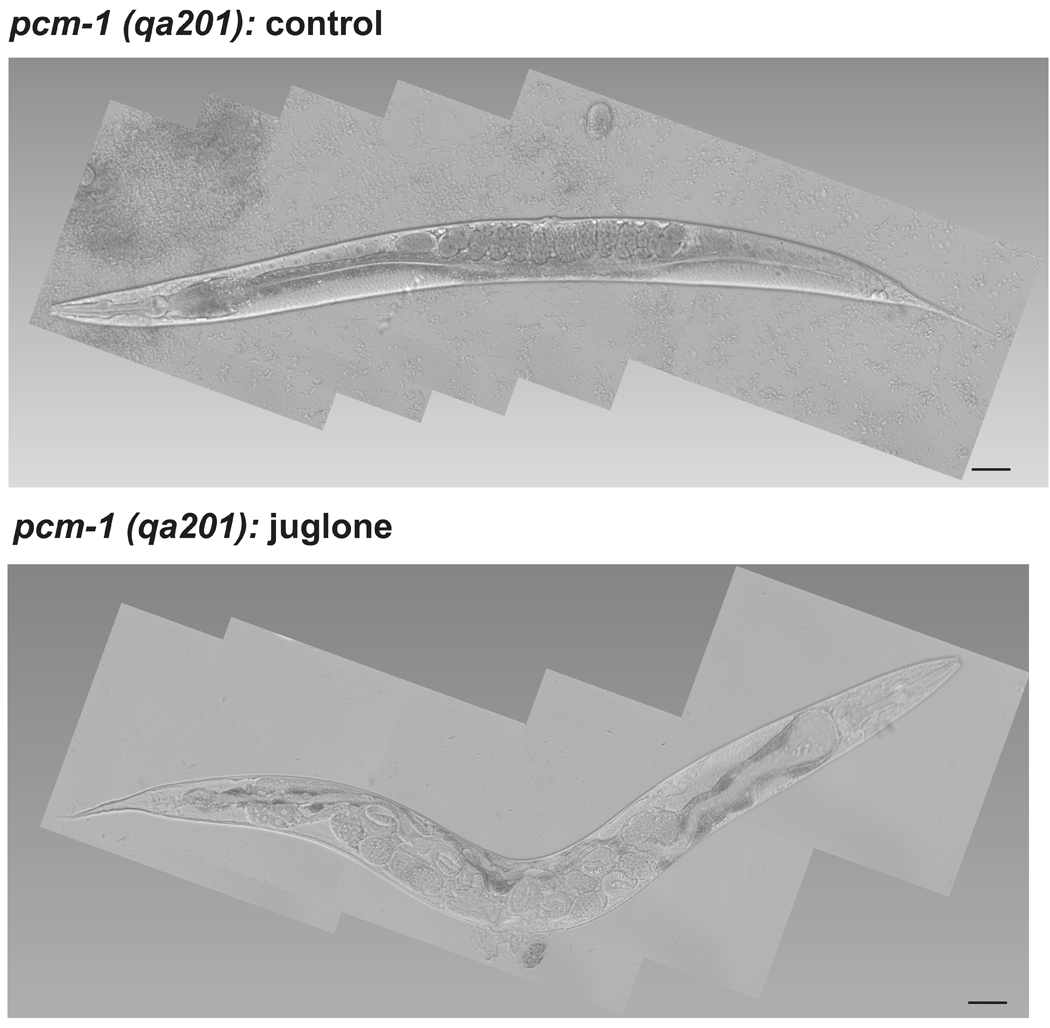
Juglone-treated pcm-1(qa201) mutant dauer larvae develop a defect in egg-laying as gravid adults. Pcm-1(qa201) mutant dauer larvae (total of 300 animals) were incubated in liquid droplets containing either S-media (control) or S-media with 236 µM juglone for 90 min and were transferred NGM + OP50 plates. The animals were monitored for development and viability every 24 hr for 3 days. Most of the 150 control qa201 mutant dauers developed into healthy gravid adults as shown by Nomarski differential interference contrast microscopy at 200-fold magnification (upper panel), with some animals only progressing to L3 or L4 larvae. On the other hand, about one fifth of the 150 juglone-treated mutant dauer larvae developed into adults with a defect in egg-laying shown in the lower panel, with the remainder progressing apparently normally to L3, L4, and gravid adults. Scale bar = 50 µm.
Table 1.
Juglone-treated L-isoaspartyl protein methyltransferase-deficient dauer larvae develop a defect in egg laying (Egl) upon refeeding which is blocked by the addition of vitamin C. Dauer larvae were incubated in the presence or absence of 236 µM juglone with or without 1 mM vitamin C and analyzed for the Egl phenotype as described in “Experimental Procedures”. Three replicate experiments were completed with the total number of animals given (n). The p-value for the difference between the juglone-treated and untreated larvae is given from the two-tailed Student’s t-test of unequal variance.
| Strain | % Egl phenotype |
p-value | |||
|---|---|---|---|---|---|
| n | Juglone | Vitamin C | |||
| N2 | 89 | − | − | 0 | |
| 169 | − | + | 0 | ||
| 140 | + | − | 0 | ||
| 150 | + | + | 0 | ||
| pcm-1(qa201) | 71 | − | − | 0 | |
| 150 | − | + | 0 | ||
| 142 | + | − | 28 ± 3.0 | 2.1 × 10−5 | |
| 146 | + | + | 0 | ||
| pcm-1(tm363) | 75 | − | − | 0 | |
| 90 | − | + | 0 | ||
| 84 | + | − | 24 ± 4.0 | 1.6 × 10−4 | |
| 100 | + | + | 0 | ||
| C10F3.4(tm2679) | 60 | − | − | 0 | |
| 45 | − | + | 0 | ||
| 52 | + | − | 5 ± 2.3 | 0.74 | |
| 68 | + | + | 0 | ||
To confirm that juglone-mediated oxidative stress was the cause for the Egl phenotype in the pcm-1 mutants, dauer larvae were incubated in droplets containing both juglone and the antioxidant vitamin C. At 1 mM vitamin C, the Egl defect was prevented in both pcm-1 mutant strains (Table 1). Hence, juglone-induced oxidative stress results in defects in egg laying in animals lacking PCM-1 activity.
To demonstrate that the Egl defect in the pcm-1 mutant strains was not due to the loss of the C10F3.4 product, we repeated these experiments with the tm2679 C10F3.4 deletion strain. Only a small, statistically insignificant, number of Egl nematodes resulted from treatment of C10F3.4 mutant dauer larvae with juglone (Table 1), suggesting that it is the loss of the methyltransferase that is responsible for the Egl defect. We also measured the adult survival and egg viability of the tm2679 strain treated with juglone but observed no differences when compared to the N2 wild-type strain (Table 1).
To determine if the sensitivity of pcm-1 dauer larvae to juglone was mediated by changes in the DAF-2 signaling pathway, we then asked whether an additional mutation in the insulin-like receptor daf-2 gene suppresses the defects displayed by the pcm-1 mutant dauer larvae under oxidative stress conditions. Dauer larvae from the daf-2(m41), daf-2(e1370);pcm-1(qa201), and daf-2(m596);pcm-1(qa201) strains were treated with juglone as previously described and their development was monitored for 48 hours. Unlike the pcm-1 mutant dauer larvae, a majority of the daf-2(e1370);pcm-1(qa201) and daf-2(m596);pcm-1(qa201) mutant dauers did not display the egg-laying defect and instead developed into gravid, egg-laying adults (Table 2). The progeny were healthy and did not display any defects in motility and development. Thus, the additional mutation in the daf-2 receptor suppressed the defects in egg laying displayed by the pcm-1 mutant dauer larvae exposed to oxidative stress. This result is consistent with PCM-1 exerting its effect by acting upstream of DAF-2 or in a parallel pathway, perhaps in the same way previously shown for dauer formation and longevity (Banfield et al., 2008). This result also provides additional support towards the hypothesis that PCM-1 may be working alongside the insulin-like signaling pathway to regulate cellular responses to stress in C. elegans.
Table 2.
An additional mutation in the daf-2 gene of the insulin-like signaling pathway suppresses the Egl phenotype displayed by pcm-1 mutant dauer larvae following exposure to juglone. Dauer larvae were treated with juglone at 236 µM and/or vitamin C at 1 mM as described in Table 1 and the percentage of animals displaying a defect in egg laying (Egl) is shown at 24 h, along with the percent animals that are dauer and L3 larvae at 48 h. The results are shown from three to six replicate experiments ± standard deviation, with the number of animals scored (n). Each experiment utilized 40–50 dauer larvae from each strain.
| Strain | % Egl phenotype |
% dauer | % L3 | |||
|---|---|---|---|---|---|---|
| n | Juglone | Vitamin C | ||||
| N2 | 140 | − | − | 0 | 0 | 0 |
| 133 | − | + | 0 | 0 | 0 | |
| 145 | + | − | 0 | 0 | 0 | |
| 130 | + | + | 0 | 0 | 0 | |
| daf-2(m41) | 270 | − | − | 0 | 0 | 0 |
| 304 | − | + | 0 | 0 | 0 | |
| 321 | + | − | 0 | 0 | 0 | |
| 282 | + | + | 0 | 0 | 0 | |
| pcm-1(qa201) | 150 | − | − | 0 | 0 | 2.0 ± 2.0 |
| 150 | + | − | 21.3 ± 2.3 | 0 | 0 | |
| daf-2(e1370);pcm-1(qa201) | 438 | − | − | 0 | 0 | 3.0 ± 4.1 |
| 323 | − | + | 0 | 0 | 0 | |
| 472 | + | − | 0 | 0 | 1.8 ± 3.4 | |
| 323 | + | + | 0 | 0 | 0 | |
| daf-2(m596);pcm-1(qa201) | 299 | − | − | 0 | 0 | 0 |
| 299 | − | + | 0 | 0 | 0 | |
| 316 | + | − | 0 | 1.5 ± 2.5 | 0 | |
| 324 | + | + | 0 | 0 | 1.5 ± 2.1 |
3.2. The oxidizing agent paraquat induces a delay in development that is more severe in pcm-1 mutants
We next examined the effects of paraquat to compare the oxidative stress phenotypes induced by a distinct oxidizing agent. Paraquat is a redox-cycling agent that also generates reactive oxygen species. Following entry into the cell, paraquat accepts electrons from iron-sulfur proteins and flavins associated with the electron transport chain and is reduced to the bipyridyl radical. The bipyridyl radical may then reduce molecular oxygen to generate superoxide, which can be converted to hydrogen peroxide by superoxide dismutase and peroxiredoxins (Halliwell and Gutteridge, 2007). Unlike the situation described above with juglone where adults died within 2–5 h, treatment with paraquat induces a delay in development and reduced fecundity in C. elegans (Ishii et al., 1993; Hartman et al., 1995; Fujii et al., 2005). We thus wanted to compare the effects of paraquat with juglone on protein repair-deficient nematodes. We first treated adult wild-type and pcm-1 mutant nematodes (both qa201 and tm363) with 0.1 mM and 0.2 mM paraquat. We found no significant effect on survival rate of wild-type animals, confirming the results of previous studies (Ishii et al., 1993; Hartman et al., 1995; Fujii et al., 2005). Additionally, we found no effect of the pcm-1 mutation upon survival after paraquat treatment (data not shown).
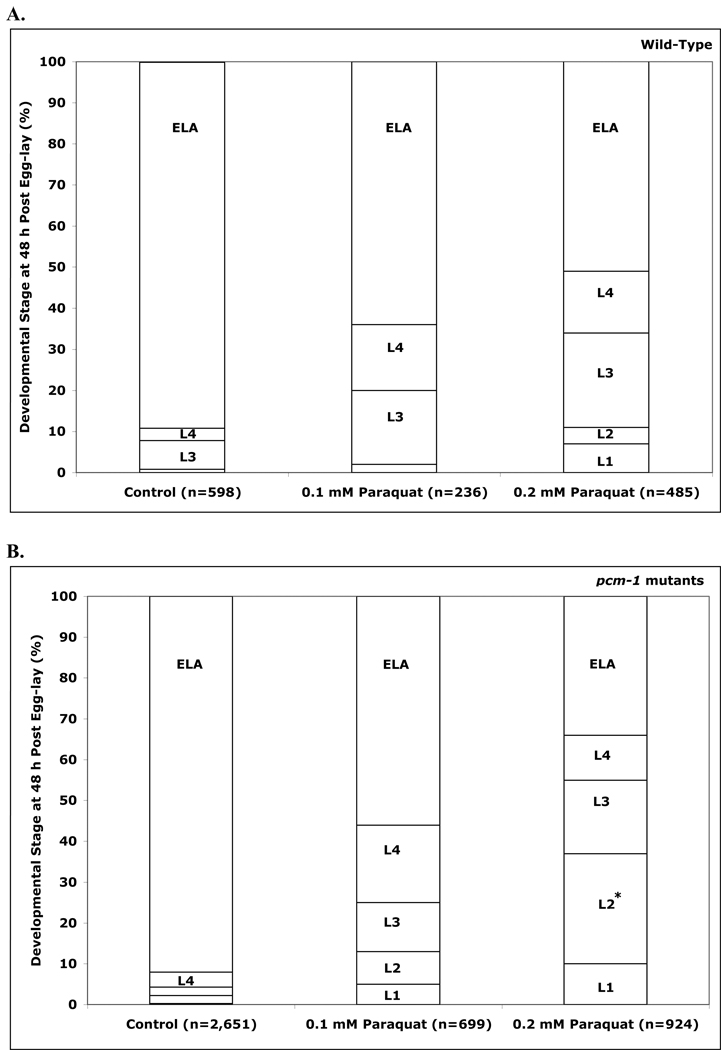
However, we observed a significant delay in development in all strains treated with these concentrations of paraquat and a greater delay in pcm-1 mutant animals, resulting in larger populations of the first and second larval stages (Fig. 3; panels A and B). Upon treatment with 0.1 mM paraquat, 3.5% and 6.3% of the pcm-1 mutant animals delayed at the L1 and L2 stages, respectively, in comparison to the 0% and 2% of the wild-type animals. In 0.2 mM paraquat, 7.5% and 40.5% of the pcm-1 mutants were arrested at the L1 and L2 stages, compared to 5.2% and 3.6% of the wild-type animals. The remaining animals from both wild-type and pcm-1 mutant strains predominantly advanced to the adult stage. Statistical analysis demonstrated significance for a difference of the wild-type and pcm-1 mutants treated with 0.2 mM paraquat at the L2 larval stage (Supplemental Table S3). No significant statistical difference was observed between the two pcm-1 mutants, nor was there a significant statistical difference between each pcm-1 mutant and the wild-type animals following treatment with paraquat. We also subjected the tm2679 C10F4.3 mutant to paraquat stress. We found no differences between this mutant and the wild type nematodes (Supplemental Figure S1; Table S4).
Fig. 3.
(A) Paraquat delays development in wild-type (N2) C. elegans. Eggs (about 100) were transferred to each of two NGM+OP50 plates containing 0, 0.1 mM and 0.2 mM paraquat at 25 °C. The developmental stage of each worm was determined after 48 h as L1, L2, L3, and L4 larvae and as young adult/egg-laying adult (YA/ELA) nematodes. Data from four replicate experiments (2 plates each) are averaged. Asterisks indicate a statistically significant difference in developmental delay between control and treated animals (two-tailed Student’s t-test of unequal variance: *p < 0.05, **p <0.005). (B) Paraquat treatment results in more severe developmental delays in pcm-1 mutant nematodes. Eggs from qa201 (pcm-1 mutant) and tm363 (pcm-1 mutant) were incubated on paraquat-containing plates and the developmental stage was identified as above. Data from each mutant set were combined. (C) Vitamin C reverses the delay in development induced by paraquat in C. elegans. Eggs from three C. elegans strains (N2 (wild-type), qa201 (pcm-1 mutant), and tm363 (pcm-1 mutant)) were transferred to NGM+OP50 plates containing 0, 0.1 mM and 0.2 mM paraquat in the presence and absence of 1 mM vitamin C and were then scored for larval development after 48 h as above. Data from four replicate experiments are shown; the data from the two mutant strains were pooled. Asterisks indicate a statistically significant difference in developmental delay between wild-type and pcm-1 mutant animals treated under the paraquat versus paraquat plus vitamin C concentrations (two-tailed Student’s t-test of unequal variance: *p < 0.05, **p <0.005). The numbers of animals scored in each of the groups in this figure are given in Supplemental Table S7 along with the standard deviation values for the percentage of each larval stage under each condition.
Since PCM-1 overexpression in the PL51 strain enhances adult nematode lifespan at 25 °C (Banfield et al., 2008), we then asked whether there would be enhanced oxidative stress resistance in these animals. We thus repeated the experiments described above with the PL51 strain overexpressing PCM-1, and the mutated PL54 strain, overexpressing a catalytic mutant form of PCM-1. Although we observed a similar developmental delay in the PL54 strain and the pcm-1 mutant animals, we found no difference between the PCM-1-overexpressing PL51 strain and the wild-type N2 strain. However, we noted fewer lower stage larvae (L1 and L2) and young and gravid adults in the PCM-1 overexpressing rescue strain than in the wild-type strain following treatment with 0.2 mM paraquat (Supplemental Figure S2; Table S5).
To determine if the developmental delay was due to oxidative stress, wild-type and pcm-1 mutant worm strains were incubated on plates containing both paraquat and vitamin C. Upon addition of either 0.5 or 1 mM vitamin C, the delay in development caused by paraquat was prevented in both wild-type and pcm-1 mutant nematodes, and development into young and egg-laying adults occurred normally (Fig. 3, panel C).
3.3. Homocysteine and homocysteine thiolactone induce a delay in development that is more severe in pcm-1 mutants
Homocysteine is a metabolite that has been linked to degenerative cardiovascular and neuronal pathologies (Jakubowski, 2007). The detrimental physiological effects associated with homocysteine may be mediated by reactive oxygen species that are produced following its auto-oxidation (Starkebaum 1986; Perna et al., 2003). Additionally, homocysteine can affect methylation reactions by reacting with adenosine to form S-adenosylhomocysteine, a potent inhibitor of many methyltransferases, including the L-isoaspartyl protein repair enzyme (Clarke and Banfield, 2001; Perna et al., 2003). A cyclized derivative of homocysteine, homocysteine thiolactone, is formed following intramolecular nucleophilic attack of its thiol group on its carboxylate group and can be hydrolyzed to give homocysteine. In the thiolactone form, homocysteine can covalently modify lysine residues within proteins and subsequently alter tertiary structure and induce protein aggregation (Jakubowski, 2007). Since homocysteine and homocysteine thiolactone can both induce oxidative stress and potentially inhibit protein repair reactions, we were interested in comparing the effects of these two compounds to those induced by the oxidizing agents juglone and paraquat on wild-type and pcm-1 mutant nematodes. As a control, we utilized the inactive disulfide homocystine.
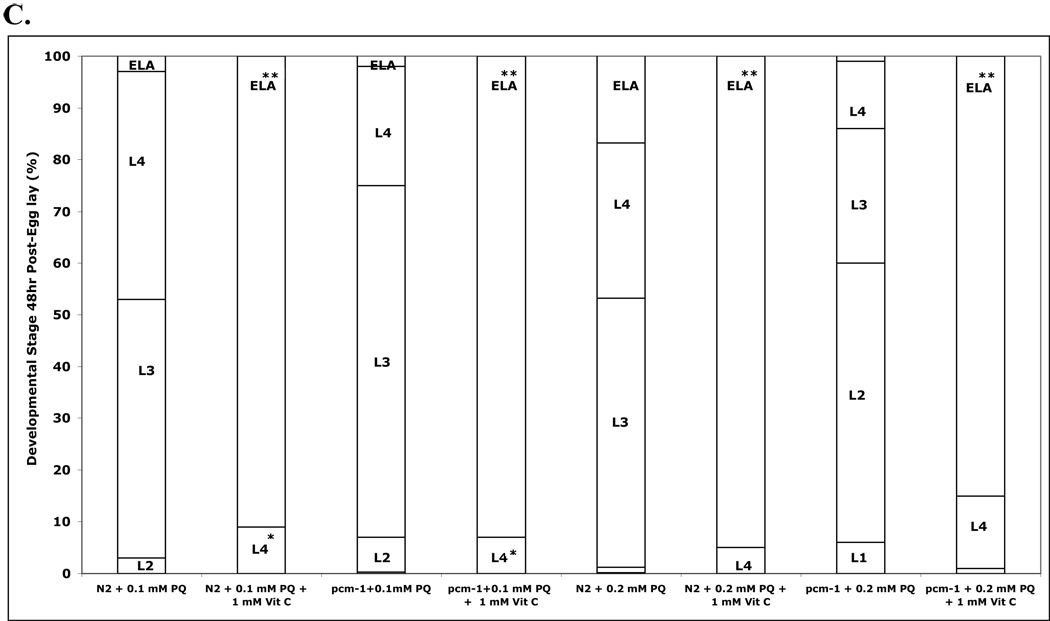
Wild-type (N2) and pcm-1 mutant (qa201 and tm363) strains were exposed to homocysteine, homocysteine thiolactone and homocystine. In the lifespan analysis, adult nematodes (both wild-type and pcm-1 mutant animals) treated with 10 mM or 20 mM of each of these compounds had very similar survival rates when compared to nematodes in untreated conditions (data not shown). However, in terms of development, homocysteine and homocysteine thiolactone caused delays in wild-type and pcm-1 mutant nematodes that were more severe in the mutants. The delay in development in wild-type nematodes treated with homocysteine and homocysteine thiolactone was similar to the paraquat-induced developmental delay at the lower dosage (10 mM) but that were more severe at the higher dosage (20 mM) of homocysteine derivatives used (Fig. 4, panel A). Under the latter conditions, L2 larvae were the predominant stage present. In contrast, nematodes exposed to homocystine did not undergo a delay in development.
Fig. 4.
(A) Homocysteine and homocysteine thiolactone delay development in wild-type (N2) C. elegans. Eggs (about 100) were transferred to NGM+OP50 plates containing 0, 10 mM and 20 mM homocysteine (Hcy), homocysteine thiolactone (HCTL), and homocystine (Hcy-Hcy) and the developmental stage of each worm was determined after 48 h at 25 °C as L1, L2, L3, and L4 larvae and as young adult/egg-laying adult (YA/ELA) nematodes. Data from a total of five experiments each with duplicate plates are shown. Asterisks indicate a statistically significant difference in developmental delay between control and treated animals (two-tailed Student’s t-test of unequal variance: *p < 0.05, **p <0.005). (B) Homocysteine treatment results in more severe developmental delays in pcm-1 mutant nematodes. Eggs from qa201 and tm363 pcm-1 mutants were exposed to homocysteine, homocysteine thiolactone, and homocystine and development was scored as above, again with five duplicate experiments for each mutant. Asterisks indicate a statistically significant difference in developmental delay between wild-type and pcm-1 mutant animals treated under the specified homocysteine compound concentrations (two-tailed Student’s t-test of unequal variance: *p < 0.05, **p <0.005). The numbers of animals scored in each of the groups in this figure are given in Supplemental Table S7 along with the standard deviation values for the percentage of each larval stage under each condition.
Pcm-1 mutant C. elegans (both qa201 and tm363 strains) underwent a more pronounced delay in development at the first larval stage (L1) in comparison to wild-type C. elegans following treatment with homocysteine and homocysteine thiolactone (Fig. 4, panel B; Supplemental Table S6). The percentage of wild-type C. elegans that delay at the L1 stage in 10 mM and 20 mM homocysteine is 1.6% and 6.2%, respectively, whereas the pcm-1 mutants delay in L1 at 5% and 41%, respectively, under these conditions. The difference in the animals treated with 20 mM homocysteine is statistically significant (Fig. 4, panel B; Supplemental Table S6). Similar effects were seen following treatment with homocysteine thiolactone (Fig. 4, panel B).
Whereas a majority of the pcm-1 mutant animals delay at the L1 stage following treatment with 20 mM homocysteine or homocysteine thiolactone (41% and 46%, respectively), approximately 50% of the wild-type animals delay at the second larval stage (L2) following treatment with these compounds (Fig. 4, panels A and B). We also showed that 20 mM homocysteine causes a statistically significant increase in the number of pcm-1 mutant nematodes delayed in L2 as well, though relatively fewer than are seen for the wild-type nematodes (Fig. 4, panels A and B; Supplemental Table S6).
Statistical analysis was performed to further distinguish the effects of homocysteine on wild-type and pcm-1 mutant development. We combined the data from a “control” group consisting of untreated animals and animals incubated with 10 mM and 20 mM of the inactive homocystine derivative and an “experimental” group consisting of animals treated with 20 mM homocysteine and 10 mM and 20 mM homocysteine thiolactone. We found that the delay in development at the first and second larval stages in the experimental data set for pcm-1 mutant animals was statistically significant (Supplemental Table S6).
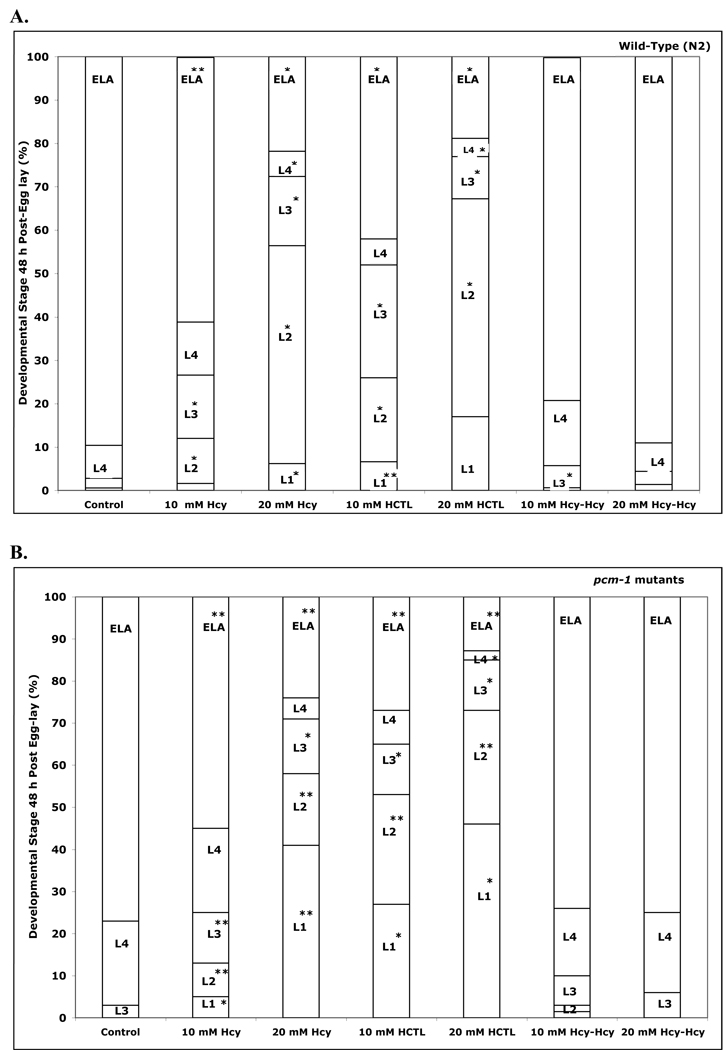
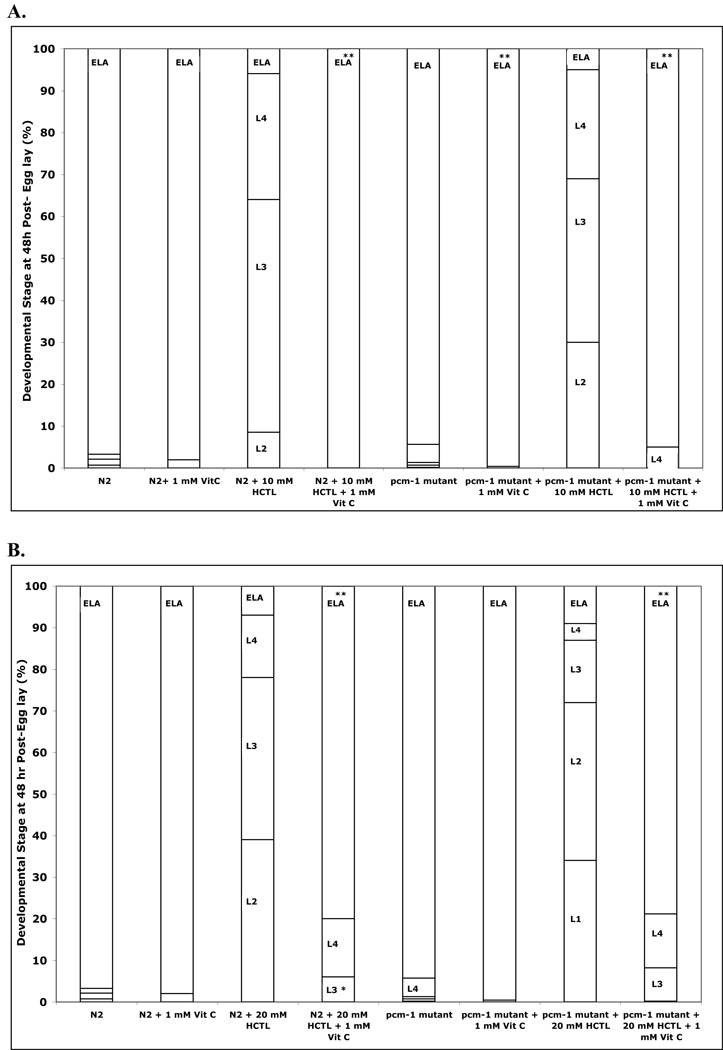
As the detrimental effects of homocysteine can be mediated by oxidative stress, we then tested whether vitamin C could prevent the delay in development. The effects of 1 mM vitamin C on the homocysteine thiolactone-induced delay in development are shown in Fig. 5. In all cases, the developmental delay was prevented in the presence of vitamin C in both wild-type and pcm-1 mutant animals. For animals treated with 10 mM homocysteine thiolactone and vitamin C, development proceeded normally to the adult stage for both the wild-type and pcm-1 mutants. For animals treated with 20 mM homocysteine thiolactone and vitamin C, there were a small number of wild-type L4 larvae (about 10%) and pcm-1 mutant L3 (12%) and L4 larvae (20%) remaining, indicating that the reversal was not complete.
Fig. 5.
Vitamin C reverses the delay in development induced by homocysteine thiolactone (HCTL) in C. elegans. Eggs (about 100) from strains N2 (wild-type), qa201 (pcm-1 mutant), and tm363 (pcm-1 mutant) were transferred to duplicate NGM+OP50 plates containing 0 and 10 mM homocysteine thiolactone (panel A) or 0 and 20 mM homocysteine thiolactone (panel B) in the presence and absence of 1 mM vitamin C at 25 °C and scored for larval development at 48 h as in Fig. 4. Data are shown for four replicate experiments. Data for the two pcm-1 mutants are combined. Asterisks indicate a statistically significant difference in developmental delay between wild-type and pcm-1 mutant animals treated under the specified homocysteine compound versus homocysteine plus vitamin C concentrations (two-tailed Student’s t-test of unequal variance: *p < 0.05, **p <0.005). The numbers of animals scored in each of the groups in this figure are given in Supplemental Table S7 along with the standard deviation values for the percentage of each larval stage under each condition.
Finally, we found no difference in development between wild-type and tm2679 C10F4.3 mutant nematodes treated with homocysteine. When we tested the PCM-1 overexpression and mutant strains, PL51 and PL54, we found similar results as with wild-type and pcm-1 mutant animals, respectively, confirming the rescue of the phenotype by active PCM-1 enzyme (Supplemental Figure S2; Table S5).
3.4. Homocysteine treatment results in defects in egg laying (Egl phenotype)
Interestingly, not only did homocysteine induce a delay in development in pcm-1 mutant animals, it also had detrimental effects on their ability to lay eggs as adults. This egg-laying defect was only present in the pcm-1 mutant strain (Table 3). Following treatment with 20 mM homocysteine, approximately 75% and 42% of pcm-1 mutant qa201 and tm363 animals displayed egg-laying defects, respectively. The addition of vitamin C (both 0.5 mM and 1 mM vitamin C) prevented the formation of the Egl defect in pcm-1 mutant nematodes in a similar fashion to the juglone-treated Egl animals. Significantly, we did not observe the Egl phenotype in homocysteine thiolactone-treated nematodes.
Table 3.
Homocysteine-treated L-isoaspartyl protein methyltransferase-deficient eggs develop into gravid adults exhibiting a defect in egg laying (Egl phenotype) which is prevented in the presence of vitamin C. Eggs (about 100) were transferred to NGM+OP50 plates containing 0, 10 mM and 20 mM homocysteine (Hcy) in the absence or presence of 1 mM vitamin C. The developmental stage of each worm was determined after 48 h at 25 °C as L1, L2, L3, and L4 larvae and as young adult/egg-laying adult (YA/ELA) nematodes. The percentage of ELAs displaying a defect in egg laying (Egl phenotype) was determined. Data from a total of five experiments each with duplicate plates are shown, with the number of animals given (n). Statistical analyses show that the differences in Egl phenotype between the wild-type and pcm-1 mutant C. elegans worm strains were significant following exposure to 20 mM homocysteine.
| Strain | % Egl phenotype |
p-value | |||
|---|---|---|---|---|---|
| n | 20 mM Homocysteine |
1 mM Vitamin C |
|||
| N2 | 307 | − | − | 0 | |
| 435 | − | + | 0 | ||
| 73 | + | − | 0 | ||
| 435 | + | + | 0 | ||
| pcm-1(qa201) | 129 | − | − | 0 | |
| 400 | − | + | 0 | ||
| 34 | + | − | 75 ± 25 | 1.1 × 10−5 | |
| 550 | + | + | 0 | ||
| pcm-1(tm363) | 100 | − | − | 0 | |
| 439 | − | + | 0 | ||
| 41 | + | − | 42 ± 16 | 1.6 × 10−5 | |
| 528 | + | + | 0 | ||
| C10F3.4(tm2679) | 410 | − | − | 0 | |
| 392 | − | + | 0 | ||
| 40 | + | − | 0 | ||
| 82 | + | + | 0 |
4. Discussion
In mammals, oxidative stress can increase the cellular content of proteins containing age-damaged aspartyl residues that can be recognized for repair by the protein L-isoaspartyl methyltransferase (O’Connor et al., 1988; Ingrosso et al., 2000; Ingrosso et al., 2002; D’Angelo et al., 2005; Zhu et al., 2006). However, the connection between oxidative and isomerization damage is not clear. This enzyme is widely conserved in nature, and we were thus interested in studying how C. elegans, a model organism for aging studies, responds to environmental oxidative stress conditions in the absence of the protein repair methyltransferase.
Upon exposure to the oxidizing agent juglone, we found that pcm-1 mutant dauer larvae develop a defect in egg laying upon exiting dauer diapause. Previous research has shown juglone to be a potent oxidizing agent that is taken up directly by C. elegans (Blum and Fridovich, 1983; De Castro et al., 2004). Wild-type dauer larvae were able to combat the oxidative stress to exit dauer diapause, but pcm-1 mutant dauer larvae were not, suggesting a defect in the neurosensory machinery necessary for normal vulval development and for the timely release of eggs. PCM-1 is expressed in the neural cords and neuronal cells, body wall, and reproductive tissues including the vulva (Gomez et al., 2008). Thus, the defect in egg laying observed in the juglone-treated pcm-1 mutants may be due to damage in the vulva and/or neurons involved in signaling needed for egg-laying. Additional work is needed to determine what role PCM-1 plays in preventing defects in egg-laying imposed by stress, whether it be one involving protein repair or signaling.
The redox-cycling agent paraquat induced delays in development in wild-type and pcm-1 mutant nematodes, but in the mutants the delay in development was more pronounced at the second larval stage. In comparison to juglone, paraquat exposure was much less toxic to wild-type and pcm-1 mutant nematode larvae. It is unclear whether this distinction results from differences in the uptake or differences in the metabolism of both compounds by the nematodes. For example, paraquat is less permeable to C. elegans than juglone (Blum and Fridovich, 1983); it is possible that it is largely taken up by ingesting the paraquat-incubated E. coli food source. However, with both juglone and paraquat, defects were seen in the pcm-1 mutant nematodes that were antagonized by vitamin C treatment, suggesting that elimination of reactive oxygen species prevents the effect of both of these drugs.
Significantly, pcm-1 mutant nematodes treated with homocysteine displayed both Egl and developmental delay phenotypes, whereas the homocysteine thiolactone-treated pcm-1 mutant nematodes experienced a delay in development only; both phenotypes induced by the homocysteine derivatives were prevented upon exposure to vitamin C. There is no information to our knowledge of how homocysteine and its derivatives are absorbed by C. elegans. Taken together, however, the results from the treatment of nematodes with juglone, paraquat, homocysteine and homocysteine thiolactone indicate that the loss of the PCM-1 repair methyltransferase significantly reduces the resistance of C. elegans to oxidative stress.
The role of vitamin C itself in the C. elegans oxidative stress response has not been extensively characterized. Initial examination of the effects of vitamin C has shown that, unlike vitamin E, it does not extend lifespan in C. elegans (Harrington and Harley, 1988). In fact, it is unclear whether vitamin C is synthesized in C. elegans or not. In our experiments, vitamin C treatment alone did not alter morphology or have any detrimental effects on development. However, our results clearly show that exogenous vitamin C can prevent the oxidative stress phenotypes in nematodes induced by juglone, paraquat, homocysteine, and homocysteine thiolactone; whether there are endogenous compounds that function in similar ways is not known.
As the effects of oxidative stress are either more pronounced (developmental delay) or are only observed (Egl phenotype) in pcm-1 mutants, we hypothesize that PCM-1 may play an important role in the mechanisms by which C. elegans regulates stress response and development. It is possible that this role simply involves the repair of aspartyl isomerization damage in proteins and enzymes that are needed to respond to oxidative stress. However, it is also possible that the methyltransferase functions in a signaling role to up-regulate the expression of proteins that protect against oxidative stress. In mice, the loss of the PCMT1 gene for the repair methyltransferase has been shown to activate the insulin/insulin-like growth factor I pathway (Farrar et al., 2005). The mechanism of this activation is not known; it is possibly a cellular response to the increased concentration of damaged proteins. If a similar activation of the corresponding DAF-2 mediated insulin-like signaling pathway occurs in the C. elegans pcm-1 mutants, the inactivation of the DAF-16 transcription factor would be expected to reduce the levels of superoxide dismutase, catalase, and HSP-70 and HSP-16 (McElwee et al., 2003). This effect may account in itself for the phenotypes observed in this work, or may contribute to it. Importantly, we have shown here that mutations in the daf-2 insulin-like receptor gene effectively suppress the Egl phenotype of pcm-1 mutant animals exposed to juglone. This result suggests that PCM-1 may be functioning as an upstream regulator of the DAF-2 signaling pathway, possibly by a similar mechanism to the one postulated for the control of dauer formation and survival by PCM-1 (Banfield et al., 2008). At this point, however, we cannot rule out that PCM-1 may give resistance to oxidative stress by a pathway parallel to the DAF-2 signaling pathway.
The interaction of PCM-1 and the DAF-2 pathway may also explain the more pronounced delay in development seen in pcm-1 mutants under oxidative stress. The possible up-regulation of DAF-2 signaling leading to DAF-16 deactivation in pcm-1 mutants could account for the inhibition of G2/M progression that would delay normal progression through the L1 to L4 larval stages (Furukawa-Hibi et al., 2005; Wolf et al., 2007). Furthermore, it has been demonstrated that a mutant in the DAF-2 signaling pathway displays similar characteristics to the pcm-1 mutant animals exposed to juglone or homocysteine. Specifically, C. elegans lacking the serum- and glucocorticoid-inducible kinase 1 (SGK-1), a protein that forms a complex with protein kinase B of the DAF-2 pathway, undergoes a delay in development and displays the Egl phenotype (Hertweck et al., 2004). Further work will be needed to determine the mechanism(s) by which PCM-1 plays a beneficial role in oxidative stress resistance.
Supplementary Material
Acknowledgments
We would like to thank Dr. Carole Linster, Dr. Jonathan Lowenson, and Lital Alder for their measurement of PCM-1 methyltransferase activities. We are grateful to Brian Head and Dr. Alex van der Bliek for their guidance in microscopy. Additionally, we would also like to thank these colleagues as well as Fernando Gomez, Cecilia Zurita-Lopez, and Dorothy Trogler for their helpful advice and assistance throughout this study. We also thank Shohei Mitani at Tokyo Women’s Medical University in Tokyo for generously providing strains tm363 and tm2679. This work was supported by USPHS Training Program GM07185, and USPHS grants GM026020 and AG032303.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arkblad EL, Tuck S, Pestov NB, Dmitriev RI, Kostina MB, Stenvall J, Tranberg M, Rydstrom J. A Caenorhabditis elegans mutant lacking nicotinamide nucleotide transhydrogenase displays increased sensitivity to oxidative stress. Free Rad. Biol. Med. 2005;38:1518–1525. doi: 10.1016/j.freeradbiomed.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- Banfield KL, Gomez TA, Lee W, Clarke S, Larsen PL. Protein-repair and hormone-signaling pathways specify dauer and adult longevity and dauer development in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:798–808. doi: 10.1093/gerona/63.8.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani C, Mendel J. Heterotrimeric G proteins in C. elegans (October 13, 2006) In: Wormbook, editor. The C. elegans Research Community. Wormbook; 2006. http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J, Fridovich I. Superoxide, hydrogen peroxide, and oxygen toxicity in two free-living nematode species. Arch. Biochem. Biophys. 1983;222:35–43. doi: 10.1016/0003-9861(83)90499-x. [DOI] [PubMed] [Google Scholar]
- Braekman BP, Houthoofd K, Vanfleteren JR. Insulin-like signaling, metabolism, stress resistance, and aging in Caenorhabditis elegans. Mech. Ageing Dev. 2001;122:673–693. doi: 10.1016/s0047-6374(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Cassada RC, Russell RL. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Chávez V, Mohri-Shiomi A, Maadani A, Vega LA, Garsin DA. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics. 2007;176:1567–1577. doi: 10.1534/genetics.107.072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavous DA, Jackson FR, O’Connor CM. Extension of the Drosophila lifespan by overexpression of a protein repair methyltransferase. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14814–14818. doi: 10.1073/pnas.251446498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A, Capasso R, Muller F, Sambri I, Masella L, Raimo M, Luigia De Bonis H, D’Angelo S, Zappia V, Galletti P, Ingrosso D. Protein isoaspartate methyltransferase prevents apoptosis induced by oxidative stress in endothelial cells: Role of Bcl-XL deamidation and methylation. PLoS ONE. 2008;3:e3258. doi: 10.1371/journal.pone.0003258. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Clarke S, Banfield K. In: S-adenosylmethionine-dependent methyltransferases. In Homocysteine in Health and Disease. Carmel R, Jacobsen DW, editors. Cambridge: Cambridge University Press; 2001. pp. 63–78. [Google Scholar]
- Clarke S. Aging as a war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res. Rev. 2003;2:263–285. doi: 10.1016/s1568-1637(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J. Gerontol. Biol. Sci. 2002;57:B109–B114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- D’Angelo S, Ingrosso D, Migliardi V, Sorrentino A, Donnarumma G, Masella L, Tufano MA, Zappia M, Galletti P. Hydroxytyrosol, a natural antioxidant from olive oil, prevents protein damage induced by long-wave ultraviolet radiation in melanoma cells. Free Rad. Biol. Med. 2005;38:908–919. doi: 10.1016/j.freeradbiomed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- De Castro E, De Castro SH, Johnson TE. Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to juglone. Free Rad. Biol. Med. 2004;37:139–145. doi: 10.1016/j.freeradbiomed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Desai C, Horvitz RH. Caenorhabditis elegans mutants defective in the functioning of the motor neurons responsible for egg-laying. Genetics. 1989;121:703–721. doi: 10.1093/genetics/121.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar CE, Houser CR, Clarke SG. Activation of the PI3K/Akt signal transduction pathway and increased levels of insulin receptor in protein repair-deficient mice. Aging Cell. 2005;4:1–12. doi: 10.1111/j.1474-9728.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- Fujii M, Tanaka N, Miki K, Hossain MN, Endoh M, Ayusawa D. Uncoupling of longevity and paraquat resistance in mutants of the nematode Caenorhabditis elegans. Biosci. Biotechnol. Biochem. 2005;69:2015–2018. doi: 10.1271/bbb.69.2015. [DOI] [PubMed] [Google Scholar]
- Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid. Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- Gami MS, Wolkow CA. Studies in Caenorhabditis elegans DAF-2/Insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell. 2006;5:31–37. doi: 10.1111/j.1474-9726.2006.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. A pheromone-induced developmental switch in Caenorhabditis elegans: Temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc. Natl. Acad. Sci. U. S. A. 1984;81:819–823. doi: 10.1073/pnas.81.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TA, Banfield KL, Trogler DM, Clarke SG. The L-isoaspartyl-O-methyltransferase in Caenorhabditis elegans larval longevity and autophagy. Dev. Biol. 2007;303:493–500. doi: 10.1016/j.ydbio.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TA, Banfield KL, Clarke SG. The protein L-isoaspartyl-O-methyltransferase functions in the Caenorhabditis elegans stress response. Mech. Ageing Develop. 2008;129:752–758. doi: 10.1016/j.mad.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Fourth Edition. New York: Oxford University Press; 2007. [Google Scholar]
- Harrington LA, Harley CB. Effect of vitamin E on lifespan and reproduction in Caenorhabditis elegans. Mech. Ageing Dev. 1988;43:71–78. doi: 10.1016/0047-6374(88)90098-x. [DOI] [PubMed] [Google Scholar]
- Hartman P, Childress E, Beyer T. Nematode development is inhibited by methyl viologen and high oxygen concentrations at a rate inversely proportional to lifespan. J. Geron. A Bio. Sci. Med. Sci. 1995;50:B322–B326. doi: 10.1093/gerona/50a.6.b322. [DOI] [PubMed] [Google Scholar]
- Hertweck M, Gobel C, Baumeister R. Caenorhabditis elegans is the critical component of the Akt/PKB kinase complex to control stress response and lifespan. Dev. Cell. 2004;6:577–588. doi: 10.1016/s1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Ingrosso D, D’Angelo S, di Carlo E, Perna AF, Zappia V, Galletti P. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur. J. Biochem. 2000;267:4397–4405. doi: 10.1046/j.1432-1327.2000.01485.x. [DOI] [PubMed] [Google Scholar]
- Ingrosso D, Cimmino A, D’Angelo S, Alfinito F, Zappia V, Galletti P. Protein methylation as a marker of aspartate damage in glucose-6-phosphate dehydrogenase-deficient erythrocytes; role of oxidative stress. Eur. J. Biochem. 2002;269:2032–2039. doi: 10.1046/j.1432-1033.2002.02838.x. [DOI] [PubMed] [Google Scholar]
- Ishii N, Suzuki N, Hartman PS, Suzuki K. The radiation-sensitive mutant rad-8 of Caenorhabditis elegans is hypersensitive to the effects of oxygen on aging and development. Mech. Ageing Dev. 1993;68:1–10. doi: 10.1016/0047-6374(93)90135-e. [DOI] [PubMed] [Google Scholar]
- Jakubowski H. The molecular basis of homocysteine thiolactone-mediated vascular disease. Clin. Chem. Lab. Med. 2007;45:1704–1716. doi: 10.1515/CCLM.2007.338. [DOI] [PubMed] [Google Scholar]
- Kagan RM, Niewmierzycka A, Clarke S. Targeted gene disruption of the Caenorhabditis elegans L-isoaspartyl protein repair methyltransferase impairs survival of dauer stage nematodes. Arch. Biochem. Biophys. 1997;348:320–328. doi: 10.1006/abbi.1997.0362. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Fleming JT. Basic culture methods. Methods Cell Biol. 1995;48:3–29. [PubMed] [Google Scholar]
- Mahfouz MM, Kummerow FA. Vitamin C or vitamin B6 supplementantion prevent the oxidative stress and decrease of prostacyclin generation in homocysteinemic rats. Int J. Biochem. Cell Biology. 2004;36:1919–1932. doi: 10.1016/j.biocel.2004.01.028. [DOI] [PubMed] [Google Scholar]
- McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann Cl, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Murakami S. Caenorhabditis elegans as a model system to study aging of learning and memory. Mol. Neurobiol. 2007;35:85–94. doi: 10.1007/BF02700625. [DOI] [PubMed] [Google Scholar]
- O’Connor CM, Yutzey KE. Enhanced carboxyl methylation of membrane-associated hemoglobin in human erythrocytes. J. Biol. Chem. 1988;263:1386–1390. [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The forkhead transcription factor DAF-16 transduces insulin-like metabolic longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Perna AF, Ingrosso D, De Santo NG. Homocysteine and oxidative stress. Amino Acids. 2003;25:409–417. doi: 10.1007/s00726-003-0026-8. [DOI] [PubMed] [Google Scholar]
- Starkebaum G, Harlan JM. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J. Clin. Invest. 1986;77:1370–1376. doi: 10.1172/JCI112442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J, Hodgkin J. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Springs Harbor Laboratory Press; 1988. [Google Scholar]
- Trent C, Tsung N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Nunes F, Henkel A, Heinick A, Rudiger JP. The MAP kinase JNK-1 of Caenorhabditis elegans: Location, activation, and influences over temperature-dependent insulin-like signaling, stress responses, and fitness. J. Cell. Physiol. 2007;214:721–729. doi: 10.1002/jcp.21269. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Honda S, Ishii N. Properties of an oxygen-sensitive mutant mev-3 on the nematode Caenorhabditis elegans. Mut. Res. 1996;358:1–6. doi: 10.1016/0027-5107(96)00070-x. [DOI] [PubMed] [Google Scholar]
- Yanase S, Yasuda K, Ishii N. Adaptive responses to oxidative damage in three mutant of Caenorhabditis elegans (age-1, mev-1, and daf-16) that affect lifespan. Mech. Ageing Dev. 2002;123:1579–1587. doi: 10.1016/s0047-6374(02)00093-3. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Doyle HA, Mamula MJ, Aswad DW. Protein repair in the brain, proteomic analysis of endogenous substrates for protein L-isoaspartyl methyltransferase in mouse brain. J. Biol. Chem. 2006;281:33802–33813. doi: 10.1074/jbc.M606958200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.