Abstract
Background & Aims
Retinoic acid plays a positive role in induction of FoxP3+ regulatory T cells. Because retinoic acid is produced as a metabolite of vitamin A in the intestine and FoxP3+ T cells regulate intestinal inflammation, we investigated the impact of vitamin A status on the regulatory T cells and inflammation in the intestine.
Methods
The SAMP1/YP model is a mouse model of Crohn’s disease. We made vitamin A deficient, excessive and normal SAMP1/YP mice and assessed the intestinal inflammation. We investigated also the phenotype and function of FoxP3+ T cells induced in different vitamin A availability in regulation of intestinal inflammation in a T cell-induced inflammation model in SCID mice.
Results
The limited and excessive vitamin A conditions induced distinct FoxP3+ T cell subsets in vivo and both ameliorated the intestinal inflammation in SAMP1/YP mice. The limited vitamin A condition greatly induced unusual CD103+CCR7+ FoxP3+ cells, while the high vitamin A condition induced CCR9+α4β7+ FoxP3+ T cells in the intestine. Both FoxP3+ T cell populations, when transferred into mice with ongoing intestinal inflammation, were highly effective in reversing the inflammation. Blockade or lack of occupancy of RARα is a mechanism to induce the highly suppressive CD103+CCR7+ FoxP3+ cells in both thymus and periphery in the limited vitamin A availability.
Conclusions
Our results identify novel pathways of inducing highly suppressive FoxP3+ regulatory T cells that can effectively control intestinal inflammation. The results have significant ramifications in treating inflammatory bowel diseases.
Introduction
FoxP3+ T cells represent a major subset of regulatory T cells.1-3 FoxP3+ T cells are made in the thymus as natural FoxP3+ T cells and periphery as induced FoxP3+ T cells.4-6 Both natural and induced FoxP3+ regulatory T cells are highly effective in suppression of intestinal inflammation.7, 8 Consistently, one of the most notable clinical symptoms of immune dysregulation, polyadenopathy, enteropathy, and X-linked inheritance (IPEX) patients with mutations in the FOXP3 gene is severe enteritis.9, 10 A growing body of evidence suggests that defects in FoxP3+ T cells may underlie inflammatory bowel diseases in humans.11 FoxP3+ T cells, induced in gut-associated lymphoid tissues, preferentially migrate to the intestine, while FoxP3+ T cells induced in periphery acquire the migration capacity to other tissue sites.5, 12
Vitamin A and retinoic acids are required for development of proper immunity to pathogens by promoting IgA response and phagocytic functions.13-15 Moreover, induction of gut homing receptors in T cells and B cells depends on retinoic acid.15, 16 On the other hand, retinoic acid can promote immune tolerance through induction of gut homing FoxP3+ T cells.17-21 Gut dendritic cells can produce retinoic acid and turn naïve T cells into induced FoxP3+ T cells in a retinoic acid-dependent manner.19-21 Both direct and indirect roles of retinoic acid and RARα have been suggested.22, 23 Another function of retinoic acid in vitro is to suppress the differentiation of naïve T cells into Th17 cells.17, 21, 22 The role of vitamin A in regulation of regulatory T cells in physiological settings remain unknown and, the functional consequence of this pathway on regulation of intestinal inflammation has yet to be determined.
We hypothesized that increased vitamin A intake (Hi-A) would alleviate tissue inflammation by increasing the numbers of FoxP3+ T cells in the intestine, while limited vitamin A intake (Low-A) would exacerbate the inflammation by decreasing the numbers of FoxP3+ T cells. We found that increasing the vitamin A intake can increase the frequency of CCR9+ FoxP3+ T cells and ameliorate the intestinal inflammation as expected. Strikingly, we found also that limiting vitamin A intake induces specialized regulatory FoxP3+ T cells that are equally efficient in suppressing intestinal inflammation. The FoxP3+ T cells induced in limited vitamin A availability have a homing behavior distinct from the retinoid-induced FoxP3+ T cells. These results provide new insights into the roles of the vitamin A-dependent and independent immune regulatory mechanisms in control of intestinal inflammation.
Methods
Generation of Hi-A, Mid-A and Low-A mice
AKR/J mice and SCID (C3Hsmn.C-Prkdcscid/J) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). BALB/c mice and DO11.10 (-/-) mice were purchased from Harlan (Indianapolis, IN). SAMP1/YP mice have been described before.24 All the experiments with animals in this study were approved by the Purdue animal care and use committee (PACUC). BALB/c, AKR/J and SAMP1/YP mice were kept on custom research diets based on AIN-93G and containing high (25,000 IU/kg, Hi-A; 10-fold higher than the normal dietary range), normal (2,500 IU/kg, Mid-A, a normal dietary range) or low (0 IU/kg, Low-A, no dietary consumption of vitamin A causing vitamin A deficiency) levels of retinyl acetate (Harlan Teklad TD-06528, 00158, and 07267) immediately following birth for 13-20 weeks. The pups were weaned at 4 weeks old and maintained on the same diets for 13-20 weeks. Serum retinol concentrations were examined to verify vitamin A deficiency in early studies but it was later replaced with flow cytometric determination of CCR9 expression by small intestinal T cells as a surrogate indicator for vitamin A deficiency as demonstrated by Iwata et al.15
Cell isolation and culture
CD4+ T cells were isolated from mesenteric lymph nodes (MLN) or spleen with the CD4+ T cell isolation kit (Miltenyi Biotec, Auburn, CA). Total Tregs were isolated by positively selecting CD25+ cells from the CD4+ T cells, and CD103- Tregs were isolated by depleting CD103+ Tregs with anti-CD103 antibody and then positively selecting CD25+ cells (∼95% pure). To prepare antigen presenting cells (APC), thymus or spleen of Mid-A or Low-A mice were digested with 1.5 mg/mL of collagenase (type 3; Worthington, Lakewood, NJ) for 45 min at 37 °C. Irradiated total splenocytes were used as spleen APC. For thymic APC, total thymocytes were incubated with biotinylated anti-mouse CD3 (145-2C11), CD4 (RM4-5) and CD2 (RM2-5) antibodies and then the T cells were depleted with streptavidin-beads (Miltenyi Biotec). The spleen and thymic APC were irradiated before culture. For isolation of dendritic cells, spleen and MLN were digested with collagenase for 45 min, and CD11c+ cells (∼80% pure based on CD11c expression) were positively selected with anti-CD11c-PE (N418, Biolegend) and anti-PE-beads. 1×105 naïve CD4+ cells were co-cultured with the APC isolated from spleen (5×105), thymus (2×104), or CD11c+ cells (2×104) of normal mice in the presence of staphylococcal enterotoxin B (500 ng/ml) or anti-CD3 (1 μg/ml) with or without Ro41-5253 (200 nM) for 5∼6 days in U-bottomed 96 well plates.
Flow cytometry
Intracellular cytokine staining was performed as described previously.25 Detection of surface antigens and FoxP3 was performed as described previously.24 Stained cells were analyzed using a BD Canto II (BD Bioscience).
Generation of antigen-specific FoxP3+ cells in vivo in response to exogenous retinoic acid
5×106 of DO11.10 (-/-) splenocytes were transferred i.v. into Hi-A or Low-A BALB/c mice. The mice were immunized i.p with ovalbumin (100 μg/mouse) in incomplete Freund’s adjuvant. Some Low-A BALB/c mice immunized with ovalbumin were injected i.p. with all-trans-retinoic acid (ATRA; 1 mg/Kg) every 2 days. 7 days later, the mice were sacrificed, and indicated organs such as peripheral lymph node (PLN), bone marrow (BM) and 8 others tissues were examined for expression of DO11.10 TCR (KJ-1.26), CCR9 (clone 242504) and FoxP3 (FJK-16s).
Ex vivo and in vivo suppression assays
CD4+CD25+ cells were isolated from MLN of Hi-A, Mid-A or Low-A mice. CD4+CD25- cells were isolated from the spleen of Mid-A mice as target T cells. The cells were co-cultured at indicated ratio for 72 h in U-bottomed 96 well plates in the presence of soluble anti-CD3 (5 μg/ml of 145-2C11) and 1×105 of irradiated splenocytes as antigen presenting cells (APC). Cells were further incubated with 1 μCi/ml of 3H-thymidine for 8 h before measurement of cell proliferation.
For the in vivo assay, 0.2×106 of CD4+CD25- cells were prepared from MLN of SAMP1/YP mice (>20 weeks old) and injected i.p. into 6 week-old C3Hsmn.C-Prkdcscid/J SCID mice to induce intestinal inflammation. Sometime between day 15-21 when mice started to lose weight, 0.5×106 of CD4+CD25+ cells, isolated from MLN of Mid-A, Hi-A or Low-A SAMP1/YP mice, were injected i.p. into the SCID mice. The weight change was monitored every 2 days. All mice were sacrificed 6 weeks after cell transfer and the intestine tissues were evaluated histologically as described below. The frequencies of Th1, Th17 and FoxP3+ cells in indicated organs were determined as previously described.25
Homing experiment
Total unprocessed lymphocytes (2×107 cells) from MLN and Peyer’s patches (PP) of Low-A and Mid-A AKR/J mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Low-A) or tetramethylrhodamine isothiocyanate (TRITC; Mid-A) and co-injected into AKR/J mice via a tail vein. ∼20 hrs later, the mice were sacrificed and single cell suspensions were prepared from selected organs. The numbers of the injected CFSE+ or TRITC+ CD4+FoxP3+/- cells in each organ was determined with flow cytometry. The relative homing index for Low-A versus Mid-A T cells was determined. Relative homing index = (# of CFSE+ cells in organ A)/(# of TRITC+ cells in organ A) ÷ (# of CFSE+ cells in input)/(# of TRITC+ cells in input).
Assessment of intestinal inflammation
The intestinal inflammation in SAMP1/YP mice and SCID mice was scored as previously described.24
Statistical analyses
Student’s paired 2-tailed t test (most data), Mann-Whitney test (Figure 4C) or Wilcoxon matched pairs test (Figure 8) was used. p values < or = 0.05 were considered significant.
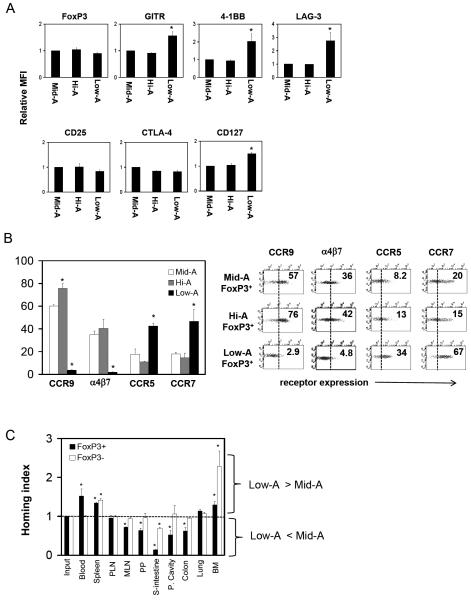
Figure 4. Surface and homing phenotypes of Low-A and Hi-A FoxP3+ T cells.
(A) Expression levels of Treg-associated surface antigens in relative mean fluorescence intensity (MFI). CD4+ CD103+FoxP3+ T cells are gated. (B) Expression of trafficking receptors. (C) Relative homing capacity of Low-A FoxP3+ T cells in vivo. A 20 h homing study was performed in AKR/J mice. A set of combined data of three (A) and four (B and C) experiments or a set of representative data out of four independent experiments (B; dot plots) are shown. *Significant differences from the Mid-A group (A and B) or from the frequencies in the input (C).
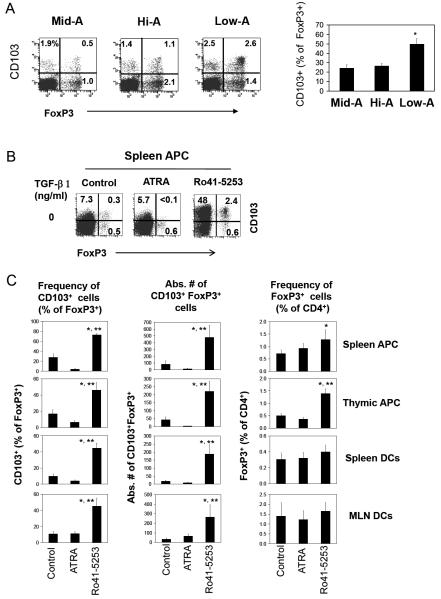
Figure 8. Increased production of CD103+FoxP3+ T cells in the thymus and by antigen presenting cells following RARα blockade.
(A) The frequencies of CD103+FoxP3+ T cells in the thymi of Mid-A, Hi-A or Low-A mice. (B) Induction of CD103+ FoxP3+ T cells by irradiated APC (prepared from the indicated organs of normal mice) in the presence of RO41-5253 or ATRA. Naïve CD4+CD25- T cells were co-cultured with irradiated APC or CD11c+ DCs for 5-6 days. One representative set of data out of at least 3 independent experiments are shown. The graphs in panel A (n=3) and C (n=4) show combined data with SEM. Significant differences from the control (*) or ATRA group (**).
Results
Population of antigen-specific CCR9+ FoxP3+ T cells in the intestine is dependent on vitamin A and retinoic acid
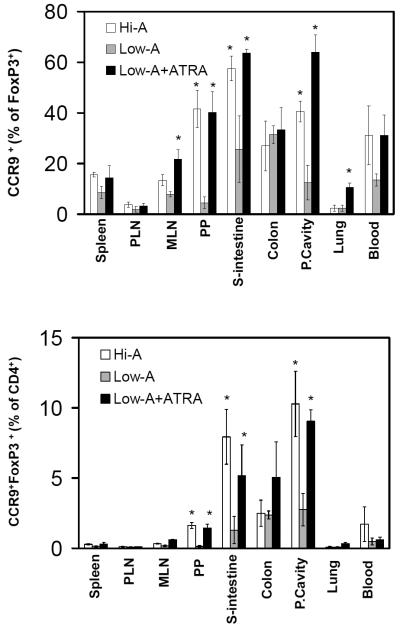
To determine if retinoid-dependent FoxP3+ T cells are induced in a physiological setting, we prepared Low-A mice and Hi-A mice fed with special diets as described in the methods section. We adoptively transferred ovalbumin-specific FoxP3- T cells from DO11.10 Rag2 (-/-) mice5 into Hi-A and Low-A mice and immunized them with ovalbumin. We examined the emergence of ovalbumin-specific CCR9+FoxP3+ T cells in secondary lymphoid tissues and other tissues 7 days after the immunization (Figure 1A and B). While many CCR9+ FoxP3+ T cells were found in the intestine of Hi-A mice, fewer CCR9+ FoxP3+ T cells were found in the small intestine of Low-A mice (Figure 1 and supplementary Figure 1A). Decreased frequencies of ovalbumin-specific CCR9+ FoxP3+ T cells were observed in other gut-associated tissue sites (MLN, peritoneal cavity, and Peyer’s patches) of Low-A versus Hi-A mice (Figure 1A and B). Repetitive injection of ATRA increased the frequency of ovalbumin-specific CCR9+ FoxP3+ T cells in Low-A mice (Figure 1). The frequency of total ovalbumin-specific FoxP3+ T cells was decreased in the small intestine of Low-A mice but increased following the ATRA administration (supplementary Figure 1B). These results suggest that the vitamin A or retinoic acid signal is required for optimal population of induced CCR9+FoxP3+ T cells in the gut.
Figure 1. Short-term peripheral induction of FoxP3+ T cells in Low-A and Hi-A conditions in an antigen-specific manner.
Splenocytes of DO11.10 Rag2 (-/-) mice were injected into Low-A and Hi-A BALB/c mice. The mice were immunized with ovalbumin and sacrificed at day 7. Some Low-A mice were injected with retinoic acid following the immunization. Frequencies of KJ1.26+CCR9+FoxP3+ T cells as % of FoxP3+ T cells and % of CD4+ T cells are shown. Combined data of three independent experiments (total n=3) are shown. *Significant differences between Low-A and Hi-A or Low-A and Low-A mice injected with ATRA (p<0.05).
Suppressed intestinal inflammation in both excessive and limited vitamin A availability
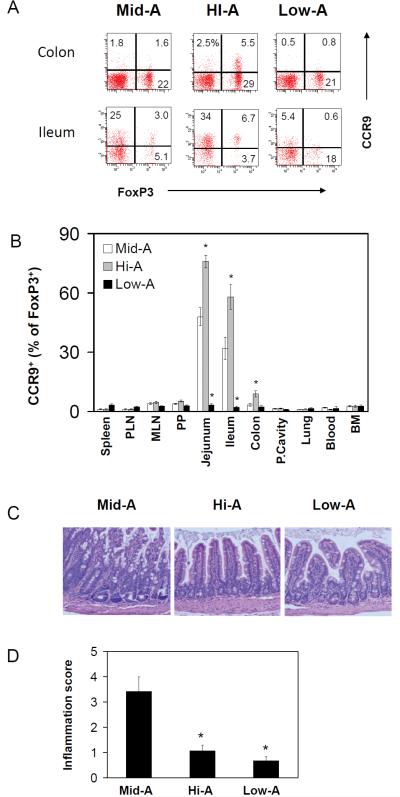
The results shown in Figure 1 imply that it is possible to control the numbers of certain FoxP3+ T cells and therefore to control inflammation in the small intestine by modulating the vitamin A status. We, next, investigated the impact of vitamin A status on long-term induction/accumulation of FoxP3+ T cells and on the intestinal inflammation. As an animal model of intestinal inflammation, we used SAMP1/YP mice which develop spontaneous inflammation reminiscent of human Crohn’s disease.24 We prepared Low-A, Mid-A (a normal dietary range of vitamin A) and Hi-A SAMP1/YP mice by feeding the mice with special diets. We found that the high vitamin A therapy increased and low vitamin A therapy decreased the long-term generation and accumulation of CCR9+ FoxP3+ T cells in jejunum, ileum and colon as expected (Figure 2A and Figure 2B). Importantly, the high vitamin A therapy suppressed inflammation in the terminal ileum as evidenced by decreased mucosa hyperplasia and leukocyte infiltration compared to Mid-A mice (Figure 2C and D). While we expected exacerbated inflammation in Low-A mice, the inflammation in these mice was surprisingly ameliorated compared to the control Mid-A mice (Figure 2C and D).
Figure 2. Effects of Low-A and Hi-A statuses on ileitis of SAMP1/YP mice.
Frequencies of CCR9+FoxP3+ T cells in the intestine (A) and eleven tissue sites (B) of Mid-A, Hi-A and Low-A SAMP1/YP mice are shown. The cells shown in panel A is gated on CD4+ T cells. Hematoxylin and eosin staining (C) and inflammation scores for the ileum (D) are shown. SAMP1/YP mice were fed with Low-A, Mid-A or Hi-A diet for 20 weeks for this study. Averages of five mice with SEM are shown. *Significant differences from Mid-A (p<0.05).
Induction of a different subset of FoxP3+ T cells in limited vitamin A availability
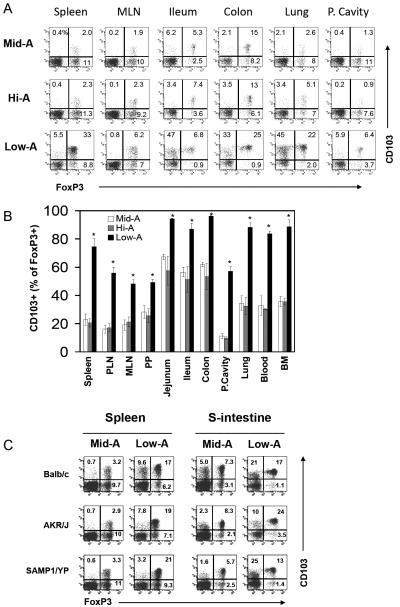
Interestingly, the frequencies of total FoxP3+ T cells in Low-A SAMP1/YP mice were similar or actually increased over those of Mid-A mice in some organs including small intestine, lung, spleen and blood (supplementary Figure 2A). This implies that other FoxP3+ T cells may be induced in vitamin A deficiency in the absence of CCR9+ FoxP3+ T cells. When CD103+FoxP3+ T cells were examined, we found that this population was greatly increased in Low-A SAMP1/YP mice (Figure 3A and B; supplementary Figure 2B). It is notable that these FoxP3+ T cells were increased not only in gut tissues (MLN, PP, jejunum, ileum, and colon) but also in other tissue sites (spleen, PLN, lung and marrow). In most tissues including the intestine, the absolute numbers of CD103+ FoxP3+ T cells were increased 3-5 times in Low-A mice compared to Mid-A mice (not shown). The induction of the CD103+ FoxP3+ T cells in Low-A mice occurred in all of the three mouse strains that we examined in this study suggesting that it is not a strain-specific biological phenomenon (Figure 3C).
Figure 3. Low-A therapy induces a novel subset of FoxP3+ T cells enriched with CD103+ cells.
Frequencies of CD103+ FoxP3+ T cells in Mid-A, Hi-A or Low-A SAMP1/YP mice were examined. Data are shown in dot plot (A) and graph (B) forms. (C) Also shown is the induction of CD103+ FoxP3+ T cells in three mouse strains (BALB/c, AKR/J, and SAMP1/YP). Averages of four mice with SEM are shown in panel B. *Significant differences from the Mid-A group.
Surface phenotype and trafficking behavior of the FoxP3+ T cells induced in limited vitamin A availability
An interesting feature of the Low-A CD103+FoxP3+ T cells is that they expressed Treg-associated molecules such as GITR,26 4-1BB,27 and LAG-328 at levels higher than the control (Hi-A or Mid-A) Tregs (Figure 4A and supplementary Figure 3). The expression of CD127, which is decreased in conventional Tregs compared to non-Tregs, was increased on these Tregs. They do not express the gut homing receptors, α4β7 and CCR9, but express CCR7 and/or CCR5 at high levels (Figure 4B). Thus, the Low-A FoxP3+ T cells are best represented by CCR7+CD103+CCR5+/- Tregs in the intestine. The Low-A FoxP3+ T cells have a lower capacity in short-term (∼20 h) homing to the small intestine and peritoneal cavity reflecting the low CCR9 and α4β7 expression (Figure 4C). In contrast, the difference between Low-A and Mid-A CD4+FoxP3- T cells in migration to the intestine was relatively small.
Normal vitamin A status is required for optimal population of Th17, Th1 and Th2 cells in the intestine
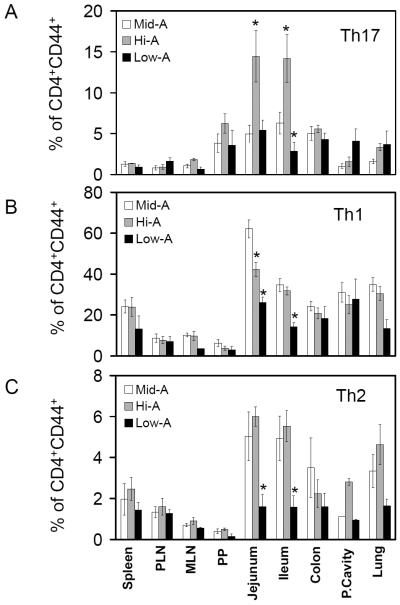
Retinoids can decrease the generation of Th17 cells in vitro.17, 21, 22 Therefore, we additionally examined the frequencies of Th17 cells in Hi-A and Low-A SAMP1/YP mice. Unexpectedly, Th17 cells were increased in the gut tissues (jejunum and ileum) of Hi-A mice and decreased in the ileum of Low-A mice (Figure 5A and supplementary Figure 4). This suggests that the suppression of Th17 cells by retinoids would not happen readily in vivo. In fact, it provides evidence that the vitamin A signal increases the frequency of Th17 cells in the small intestine.
Figure 5. Impacts of the Low-A and Hi-A statuses on the balance of inflammatory effector T cells in the intestine of SAMP1/YP mice.
The frequencies of Th17 (A), Th1 (B) and Th2 cells (C) among CD4+CD44+ T cells were examined in Low-A, Mid-A or Hi-A SAMP1/YP mice. Averages of four mice with SEM are shown. *Significant differences from the Mid-A group.
It has been reported that retinoic acid promotes Th2 cell polarization, while increased Th1 cells were observed in vitamin A deficiency in certain animal models.29-31 Considering that the generation and expansion of effector T cells can be suppressed by FoxP3+ T cells,32, 33 it is possible that the numbers of Th1 and Th2 cells in vitamin A deficiency may be linked to the number of Tregs induced in this condition. Our results showed that the frequency of Th1 cells was actually decreased in several tissue sites including jejunum and ileum of Low-A mice and in the jejunum of Hi-A SAMP1/YP mice (Figure 5B). Th2 cells were greatly reduced in many tissue sites of Low-A mice but not in Hi-A SAMP1/YP mice (Figure 5C). Overall, the decreased Th1 and Th2 cells closely correlate with the decreased inflammation in Low-A SAMP1/YP mice.
The FoxP3+ T cells induced in both limited and excessive vitamin A availability are highly effective in control of ongoing intestinal inflammation
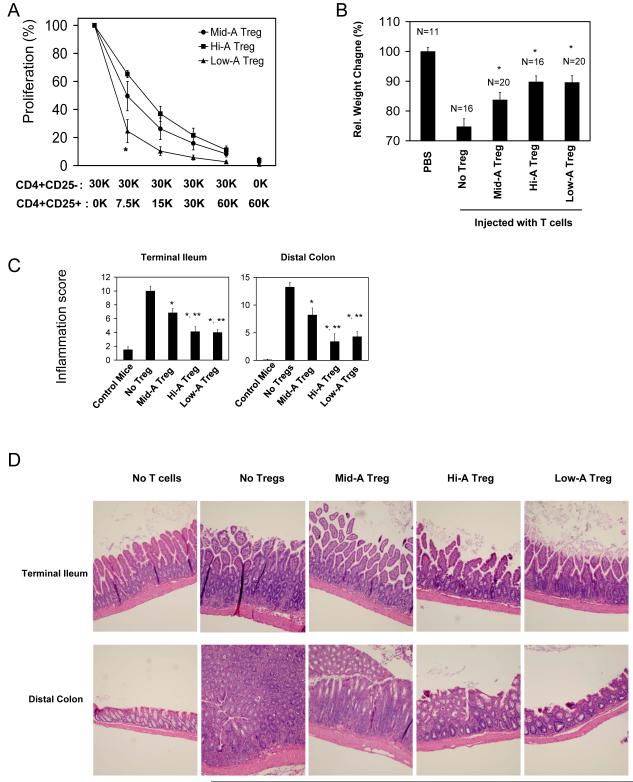
The greatly increased induction of the CD103+ FoxP3+ T cells in Low-A mice is intriguing. We isolated and compared the in vitro suppressive activity of the CD4+CD25+ Tregs isolated from Low-A, Hi-A, and control Mid-A mice (Figure 6). While all of the Tregs were suppressive, it was the Low-A Tregs that were most suppressive in vitro (Figure 6A). CD103- Low-A Tregs were as efficient as total Low-A Tregs (supplementary Figure 5), suggesting that the superior suppressive ability of Low-A Tregs is not limited to the CD103+ cells at least in vitro.
Figure 6. Low-A and Hi-A FoxP3+ T cells are highly efficient in suppression of intestinal inflammation.
(A) In vitro suppressive activity of Tregs of Mid-A, Hi-A or Low-A mice. [3H]-thymidine incorporation by CD4+CD25- target T cells cultured in the presence of indicated numbers of Tregs was measured. (B) In vivo suppressive activity of Tregs of Mid-A, Hi-A or Low-A mice. (C) Inflammation scores for terminal ileum and distal colon. (D) Intestinal histology images with H&E staining. For the in vivo study, SCID mice were injected with MLN CD4+CD25- cells of SAMP1/YP mice at day 0 and with MLN CD4+CD25+ cells of Mid-A, Low-A mice or Hi-A mice sometime between 15 and 21 days when weight loss started to occur. Significant differences from the No-Treg group* or Mid-A Treg group**.
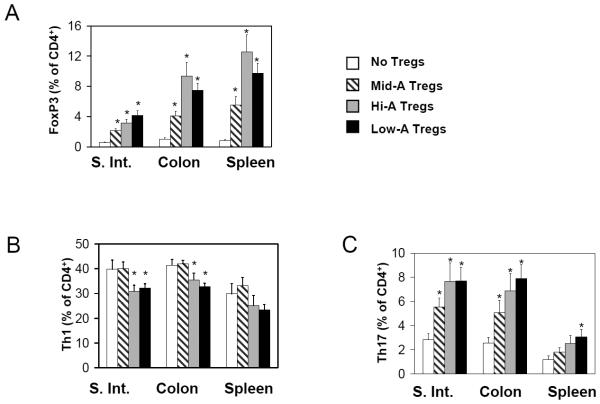
We compared the in vivo suppressive activity of the Mid-A, Low-A and Hi-A Tregs on the intestinal inflammation in SCID mice induced by SAMP1/YP T cells (Figure 6B). For this experiment, SCID mice were injected first with Treg-depleted SAMP1/YP CD4+ T cells at day 0 and then injected with the Tregs isolated from the Mid-A, Low-A or Hi-A mice when the mice began to lose weight. The Low-A and Hi-A Tregs were more potent than Mid-A Tregs in suppression of weight loss and tissue inflammation (Figure 6B-D). The potent suppressive activity of the transferred Low-A or Hi-A Tregs is consistent with the high frequency of persisting FoxP3+ T cells and the low frequency of Th1 cells in the small and large intestine of the mice (Figure 7A and B). Along with Tregs, Th17 cells were also increased in the Hi-A and Low-A mice (Figure 7C).
Figure 7. Both the Low-A and Hi-A FoxP3+ T cells can decrease Th1 cells but increase Th17 cells in inflamed intestine.
SCID mice were injected with MLN CD4+CD25- cells of SAMP1/YP mice at day 0, and MLN CD4+CD25+ cells of Mid-A, Hi-A or Low-A mice when mice started to lose weight. The mice were sacrificed between day 30 and 40. The frequencies of FoxP3+ T cells (A), Th1 cells (B), and Th17 cells (C) are shown (n=11-20). *Significant differences from the no Treg group.
Blockade of RARα induces the highly suppressive CD103+ FoxP3+ cells
FoxP3+ T cells are made in the thymus as natural naïve FoxP3+ T cells and in the periphery by antigen presenting cells as induced FoxP3+ T cells. It is possible that one or both of the FoxP3+ T cell populations are increased in the limited vitamin A availability. To determine this, we first examined the frequencies of CD103+ FoxP3+ T cells in the thymus (Figure 8A). The frequency of CD103+ FoxP3+ T cells was greatly increased in the thymus of Low-A mice, suggesting that the thymic output of natural CD103+ FoxP3+ T cells is increased.
It is also possible that the increase of CD103+FoxP3+ T cells in limited vitamin A availability may be due to increased generation of induced FoxP3+ T cells due to vitamin A deficiency. We co-cultured CD4+CD25- naïve T cells with antigen presenting cells isolated from various organs. The cells were stimulated with APC (isolated from spleen, thymus or MLN) plus SEB (Figure 8B) or anti-CD3 (supplementary Figure 6) as TCR activators in the presence of a RARα antagonist (Ro41-5253) or ATRA to mimic antigen priming of naïve T cells in vitamin A deficiency or excessiveness. We found that the RARα blockade greatly increased the conversion of naïve T cells into CD103+FoxP3+ T cells (Figure 8 B) with 5-11 fold increases in absolute numbers of CD103+FoxP3+ T cells (Figure 8C). Addition of TGF-β1 greatly increased the number of CD103+FoxP3+ T cells in all groups (supplementary Figure 6B).
Discussion
We investigated the impact of vitamin A status on the phenotype and function of FoxP3+ T cells in regulation of intestinal inflammation. We found that both high and low vitamin A therapies induce distinct subsets of FoxP3+ T cells with potent regulatory activities. A population of FoxP3+ T cells termed “Hi-A FoxP3+ T cells” was greatly increased by retinoic acid under excessive vitamin A availability. On the other hand, a population of FoxP3+ T cells termed “Low-A FoxP3+ T cells” was greatly increased under limited vitamin A availability in the absence RARα occupancy. Interestingly, both the Hi-A and Low-A FoxP3+ T cells were significantly more efficient in suppression of intestinal inflammation than control FoxP3+ T cells in vivo.
It has been reported that retinoic acid can enhance TGF-β1-mediated induction of FoxP3 and CD103 in vitro.17-22 Indeed, we found that ∼70% of the FoxP3+ T cells induced in the intestine but not other organs of Mid-A or HI-A mice had this phenotype (Figure 3A). This organ specificity is thought to be due to the fact that dendritic cells and epithelial cells of the intestine have the ability to produce retinoic acid.21 Moreover, CCR9+FoxP3+ T cells were increased in HI-A mice but decreased in the intestine of Low-A mice. Importantly, Hi-A FoxP3+ T cells were highly effective in reversing intestinal inflammation in SCID mice. Thus, the pathway of induction of FoxP3+ T cells by retinoic acid operates in vivo in a physiological setting. We, unexpectedly, found in this study that a novel subset of FoxP3+ T cells enriched with CD103+ cells is highly induced in limited vitamin A availability in most tissues including the intestine.
It has been reported that CD103+CD25+ Tregs isolated from lymphoid tissues were highly suppressive in regulation of inflammation.34 While they share the CD103 expression, the relationship between these CD103+CD25+ Tregs of normal mice and the Low-A FoxP3+ T cells is unclear at the moment. CD103+CD25+ Tregs highly express the Treg-associated molecules such as GITR, 4-1BB and LAG3 compared to control Tregs. Perhaps the most intriguing phenotype of the Low-A FoxP3+ T cells is their homing receptor expression pattern. The Low-A Tregs preferentially express CD103, CCR7 and CCR5 but not the gut homing receptors CCR9 and α4β7, and they have better circulating ability in blood, spleen and marrow compared to control Tregs. A rather surprising fact is that, despite the deficiency of CCR9 and α4β7, Low-A FoxP3+ T cells are found at high frequencies in the small and large intestine. Based on the fact that the Low-A FoxP3+ T cells do not migrate well to the small intestine (Figure 4B), it is likely that these cells are the result of induction or expansion rather than migration to the intestine.
We propose that unavailability of RARα ligands in the Low-A condition, which makes the RAR-RXR heterodimer receptor inactive (“apo state”),35 is responsible for the increased induction of the specialized FoxP3+ T cells. This is supported by the increased conversion of naïve CD4+ T cells into CD103+FoxP3+ T cells by antigen presenting cells of both thymus and peripheral lymphoid tissues in the presence of a RARα antagonist. In this regard, the frequency of CD103+FoxP3+ T cells was significantly increased in the thymus and most peripheral tissues of Low-A mice (Figure 8A), suggesting that both the thymic output and peripheral induction of the specialized FoxP3+ T cells are greatly increased in the Low-A mice.
In this study, we found that Th17 cells were highly enriched in the gut of Hi-A mice. This was surprising given the fact that retinoic acid can suppress the induction of Th17 cells in vitro.17, 21, 22 This may be due to the fact that retinoic acid production in vivo is tightly regulated in space and time that the retinoid-induced suppression of Th17 cells does not happen widely in the body within the physiological concentration range of retinoic acid (∼10 nM). Another possibility is that even the migration and retention of Th17 cells in the gut would require the retinoic acid signal in a manner similar to other effector T cells. Indeed, vitamin A is required also for the optimal population of Th1 and Th2 in the small intestine. Th17 cells were also increased in SCID mice after transfer of Low-A or HI-A FoxP3+ T cells, and this is consistent with others’ finding that FoxP3+ T cells could promote the generation of Th17 cells.36 We observed a negative correlation between frequencies of Th17 cells and intestinal inflammation and a positive correlation between Th1 and intestinal inflammation. The negative correlation between Th17 cells and intestinal inflammation is in line with the protective role of IL-17 and Th17 cells in this model.37 In Low-A SAMP/YP mice, however, there were decreases in both Th17 cells and intestinal inflammation. In this case, CD103+FoxP3+ cells were increased and Th1 cells were decreased. Thus, it appears that the intestinal pathology in the Low-A and Hi-A SAMP1/YP mice and in the SCID mice would be regulated by the balance between protective (Tregs and perhaps Th17) and inflammatory (Th1) cells.
It has long been observed in animals and humans that, vitamin A deficiency leads to defective immunity and increased susceptibility to infection.38 There are several explanations available for this phenomenon such as defective maturation of neutrophils and IgA deficiency. The results of this study provide another potential explanation. The greatly increased frequencies of highly suppressive Low-A FoxP3+ regulatory T cells and concomitantly decreased effector T cells could induce the typical immunodeficiency associated with vitamin A deficiency. Taken together, our results provide novel therapeutic strategies to suppress inflammation in the intestine and potentially other tissue sites by regulating vitamin A availability. It is expected also that the Hi-A and Low-A FoxP3+ T cells would have the potential to be used as effective cellular therapeutics in suppression of inflammatory diseases.
Supplementary Material
Acknowledgments
This study was supported, in part, from grants from NIH (1R01AI074745 and 1R01DK076616) and Crohn’s and Colitis Foundation of America to CHK.
Abbreviations
- Low-A
low vitamin A or vitamin A deficient
- Hi-A
high vitamin A
- Mid-A
medium or a normal range of vitamin A
- ATRA
all-trans retinoic acid
- FoxP3
Forkhead box P3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist
References
- 1.Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, Bacchetta R, Levings MK. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 2.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–10. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–6. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JH, Kang SG, Kim CH. FoxP3+ T Cells Undergo Conventional First Switch to Lymphoid Tissue Homing Receptors in Thymus but Accelerated Second Switch to Nonlymphoid Tissue Homing Receptors in Secondary Lymphoid Tissues. J Immunol. 2007;178:301–11. doi: 10.4049/jimmunol.178.1.301. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J Immunol. 2003;171:5012–7. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 8.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–21. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 10.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 11.Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125:145–53. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–47. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokuyama Y, Tokuyama H. Retinoids as Ig isotype-switch modulators. The role of retinoids in directing isotype switching to IgA and IgG1 (IgE) in association with IL-4 and IL-5. Cell Immunol. 1996;170:230–4. doi: 10.1006/cimm.1996.0156. [DOI] [PubMed] [Google Scholar]
- 14.Wiedermann U, Tarkowski A, Bremell T, Hanson LA, Kahu H, Dahlgren UI. Vitamin A deficiency predisposes to Staphylococcus aureus infection. Infect Immun. 1996;64:209–14. doi: 10.1128/iai.64.1.209-214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 17.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 18.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A Metabolites Induce Gut-Homing FoxP3+ Regulatory T Cells. J Immunol. 2007;179:3724–33. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 22.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–9. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 23.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–70. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang SG, Piniecki RJ, Hogenesch H, Lim HW, Wiebke E, Braun SE, Matsumoto S, Kim CH. Identification of a chemokine network that recruits FoxP3(+) regulatory T cells into chronically inflamed intestine. Gastroenterology. 2007;132:966–81. doi: 10.1053/j.gastro.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–83. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 27.Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, Kwon BS. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75:785–91. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 28.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–22. [PubMed] [Google Scholar]
- 30.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15:1017–25. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 31.Stephensen CB, Rasooly R, Jiang X, Ceddia MA, Weaver CT, Chandraratna RA, Bucy RP. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol. 2002;168:4495–503. doi: 10.4049/jimmunol.168.9.4495. [DOI] [PubMed] [Google Scholar]
- 32.Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394–9. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 33.Bellinghausen I, Klostermann B, Knop J, Saloga J. Human CD4+CD25+ T cells derived from the majority of atopic donors are able to suppress TH1 and TH2 cytokine production. J Allergy Clin Immunol. 2003;111:862–8. doi: 10.1067/mai.2003.1412. [DOI] [PubMed] [Google Scholar]
- 34.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25-regulatory T cells. Proc Natl Acad Sci U S A. 2002;99:13031–6. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–9. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor W, Jr., Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–9. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semba RD. Vitamin A, immunity, and infection. Clin Infect Dis. 1994;19:489–99. doi: 10.1093/clinids/19.3.489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.