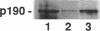Abstract
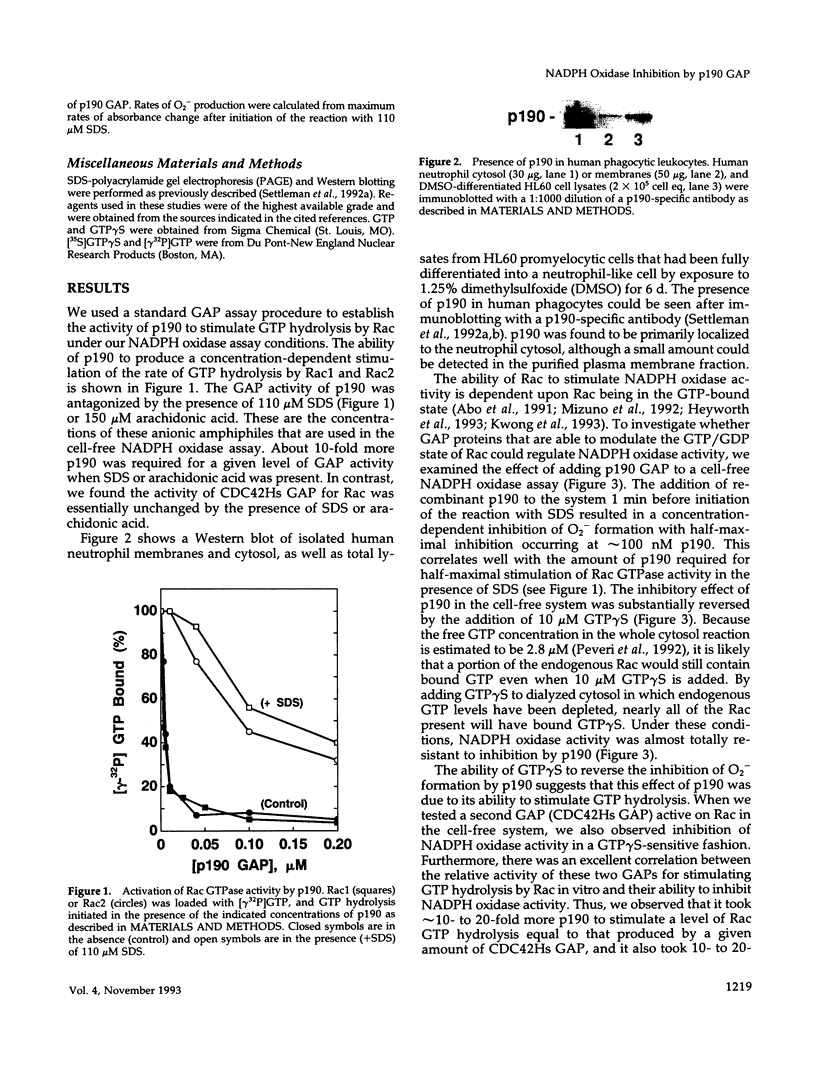
Activation of the NADPH oxidase of phagocytic cells requires the action of Rac2 or Rac1, members of the Ras superfamily of GTP-binding proteins. Rac proteins are active when in the GTP-bound form and can be regulated by a variety of proteins that modulate the exchange of GDP for GTP and/or GTP hydrolysis. The p190 Rac GTPase Activating Protein (GAP) inhibits human neutrophil NADPH oxidase activity in a cell-free assay system with a K1 of approximately 100 nM. Inhibition by p190 was prevented by GTP gamma S, a nonhydrolyzable analogue of GTP. Similar inhibition was seen with a second protein exhibiting Rac GAP activity, CDC42Hs GAP. The effect of p190 on superoxide (O2-) formation was reversed by the addition of a constitutively GTP-bound Rac2 mutant or Rac1-GTP gamma S but not by RhoA-GTP gamma S. Addition of p190 to an activated oxidase produced no inhibitory effect, suggesting either that p190 no longer has access to Rac in the assembled oxidase or that Rac-GTP is not required for activity once O2- generation has been initiated. These data confirm the role of Rac in NADPH oxidase regulation and support the view that it is the GTP form of Rac that is necessary for oxidase activation. Finally, they raise the possibility that NADPH oxidase may be regulated by the action of GAPs for Rac proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Boyhan A., West I., Thrasher A. J., Segal A. W. Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1, and cytochrome b-245. J Biol Chem. 1992 Aug 25;267(24):16767–16770. [PubMed] [Google Scholar]
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991 Oct 17;353(6345):668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Der C. J. Emerging concepts in the Ras superfamily of GTP-binding proteins. FASEB J. 1993 Jun;7(9):750–759. doi: 10.1096/fasebj.7.9.8330683. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Prossnitz V. Isoprenoid metabolism is required for stimulation of the respiratory burst oxidase of HL-60 cells. J Clin Invest. 1992 Feb;89(2):402–408. doi: 10.1172/JCI115599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg Y., Pick E. Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cell Immunol. 1984 Oct 1;88(1):213–221. doi: 10.1016/0008-8749(84)90066-2. [DOI] [PubMed] [Google Scholar]
- Chuang T. H., Xu X., Knaus U. G., Hart M. J., Bokoch G. M. GDP dissociation inhibitor prevents intrinsic and GTPase activating protein-stimulated GTP hydrolysis by the Rac GTP-binding protein. J Biol Chem. 1993 Jan 15;268(2):775–778. [PubMed] [Google Scholar]
- Clark R. A. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990 Jun;161(6):1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Volpp B. D., Leidal K. G., Nauseef W. M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J Clin Invest. 1990 Mar;85(3):714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986 Jul 1;237(1):111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J Clin Invest. 1985 May;75(5):1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Scott P. J., Mayo L. A. Cytosolic components of the respiratory burst oxidase: resolution of four components, two of which are missing in complementing types of chronic granulomatous disease. Proc Natl Acad Sci U S A. 1989 Feb;86(3):825–829. doi: 10.1073/pnas.86.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann D., Brill S., Garrett M. D., Totty N., Hsuan J., Monfries C., Hall C., Lim L., Hall A. Bcr encodes a GTPase-activating protein for p21rac. Nature. 1991 May 30;351(6325):400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- Downward J. Regulatory mechanisms for ras proteins. Bioessays. 1992 Mar;14(3):177–184. doi: 10.1002/bies.950140308. [DOI] [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Garrett M. D., Self A. J., van Oers C., Hall A. Identification of distinct cytoplasmic targets for ras/R-ras and rho regulatory proteins. J Biol Chem. 1989 Jan 5;264(1):10–13. [PubMed] [Google Scholar]
- Grand R. J., Owen D. The biochemistry of ras p21. Biochem J. 1991 Nov 1;279(Pt 3):609–631. doi: 10.1042/bj2790609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Hall A. Signal transduction through small GTPases--a tale of two GAPs. Cell. 1992 May 1;69(3):389–391. doi: 10.1016/0092-8674(92)90441-e. [DOI] [PubMed] [Google Scholar]
- Hart M. J., Shinjo K., Hall A., Evans T., Cerione R. A. Identification of the human platelet GTPase activating protein for the CDC42Hs protein. J Biol Chem. 1991 Nov 5;266(31):20840–20848. [PubMed] [Google Scholar]
- Heyneman R. A., Vercauteren R. E. Activation of a NADPH oxidase from horse polymorphonuclear leukocytes in a cell-free system. J Leukoc Biol. 1984 Dec;36(6):751–759. doi: 10.1002/jlb.36.6.751. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Curnutte J. T., Nauseef W. M., Volpp B. D., Pearson D. W., Rosen H., Clark R. A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J Clin Invest. 1991 Jan;87(1):352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Knaus U. G., Xu X., Uhlinger D. J., Conroy L., Bokoch G. M., Curnutte J. T. Requirement for posttranslational processing of Rac GTP-binding proteins for activation of human neutrophil NADPH oxidase. Mol Biol Cell. 1993 Mar;4(3):261–269. doi: 10.1091/mbc.4.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg M. E., Malech H. L., Rotrosen D. The phagocyte 47-kilodalton cytosolic oxidase protein is an early reactant in activation of the respiratory burst. J Biol Chem. 1990 Sep 15;265(26):15577–15583. [PubMed] [Google Scholar]
- Knaus U. G., Heyworth P. G., Evans T., Curnutte J. T., Bokoch G. M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991 Dec 6;254(5037):1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- Knaus U. G., Heyworth P. G., Kinsella B. T., Curnutte J. T., Bokoch G. M. Purification and characterization of Rac 2. A cytosolic GTP-binding protein that regulates human neutrophil NADPH oxidase. J Biol Chem. 1992 Nov 25;267(33):23575–23582. [PubMed] [Google Scholar]
- Kwong C. H., Malech H. L., Rotrosen D., Leto T. L. Regulation of the human neutrophil NADPH oxidase by rho-related G-proteins. Biochemistry. 1993 Jun 1;32(21):5711–5717. doi: 10.1021/bi00072a029. [DOI] [PubMed] [Google Scholar]
- Li B. Q., Kaplan D., Kung H. F., Kamata T. Nerve growth factor stimulation of the Ras-guanine nucleotide exchange factor and GAP activities. Science. 1992 Jun 5;256(5062):1456–1459. doi: 10.1126/science.1604323. [DOI] [PubMed] [Google Scholar]
- Marti K. B., Lapetina E. G. Epinephrine suppresses rap1B.GAP-activated GTPase activity in human platelets. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2784–2788. doi: 10.1073/pnas.89.7.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., Shirley P. S., Clayton C. C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J Clin Invest. 1985 May;75(5):1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Peveri P., Heyworth P. G., Curnutte J. T. Absolute requirement for GTP in activation of human neutrophil NADPH oxidase in a cell-free system: role of ATP in regenerating GTP. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2494–2498. doi: 10.1073/pnas.89.6.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips M. R., Pillinger M. H., Staud R., Volker C., Rosenfeld M. G., Weissmann G., Stock J. B. Carboxyl methylation of Ras-related proteins during signal transduction in neutrophils. Science. 1993 Feb 12;259(5097):977–980. doi: 10.1126/science.8438158. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Evans T., Loetterle L. R., Jesaitis A. J., Bokoch G. M. Translocation of Rac correlates with NADPH oxidase activation. Evidence for equimolar translocation of oxidase components. J Biol Chem. 1993 Oct 5;268(28):20983–20987. [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Yeung C. L., Leto T. L., Malech H. L., Kwong C. H. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992 Jun 5;256(5062):1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- Settleman J., Albright C. F., Foster L. C., Weinberg R. A. Association between GTPase activators for Rho and Ras families. Nature. 1992 Sep 10;359(6391):153–154. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- Settleman J., Narasimhan V., Foster L. C., Weinberg R. A. Molecular cloning of cDNAs encoding the GAP-associated protein p190: implications for a signaling pathway from ras to the nucleus. Cell. 1992 May 1;69(3):539–549. doi: 10.1016/0092-8674(92)90454-k. [DOI] [PubMed] [Google Scholar]
- Ueda T., Kikuchi A., Ohga N., Yamamoto J., Takai Y. Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. J Biol Chem. 1990 Jun 5;265(16):9373–9380. [PubMed] [Google Scholar]
- Uhlinger D. J., Burnham D. N., Lambeth J. D. Nucleoside triphosphate requirements for superoxide generation and phosphorylation in a cell-free system from human neutrophils. Sodium dodecyl sulfate and diacylglycerol activate independently of protein kinase C. J Biol Chem. 1991 Nov 5;266(31):20990–20997. [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]