Abstract
The infectious agent of transmissible spongiform encephalopathies is believed to consist of an oligomeric isoform, PrPSc, of the monomeric cellular prion protein, PrPC. The conversion of PrPC to PrPSc is characterized by a decrease in α-helical structure, an increase in β-sheet content, and the formation of PrPSc amyloid. Whereas the N-terminal part of PrPC comprising residues 23–120 is flexibly disordered, its C-terminal part, PrP(121–231), forms a globular domain with three α-helices and a small β-sheet. Because the segment of residues 90–231 is protease-resistant in PrPSc, it is most likely structured in the PrPSc form. The conformational change of the segment containing residues 90–120 thus constitutes the minimal structural difference between PrPC and a PrPSc monomer. To test whether PrP(121–231) is also capable to undergo conformational transitions, we analyzed its urea-dependent unfolding transitions at neutral and acidic pH. We identified an equilibrium unfolding intermediate of PrP(121–231) that is exclusively populated at acidic pH and shows spectral characteristics of a β-sheet protein. The intermediate is in rapid equilibrium with native PrP(121–231), significantly populated in the absence of urea at pH 4.0, and may have important implications for the presumed formation of PrPSc during endocytosis.
Keywords: transmissible spongiform encephalopathies, protein folding and stability, endocytosis, prion replication
Prions constitute the infectious agents of fatal neurodegenerative illnesses such as the Creutzfeldt–Jakob disease, the Gerstmann–Sträussler–Scheincker syndrome, fatal familial insomnia, and kuru in humans, bovine spongiform encephalopathy in cattle, and scrapie in sheep (1–3). According to the “protein-only” hypothesis (4–6), the prion is believed to consist mostly, if not entirely, of the oligomeric scrapie isoform prion protein PrPSc, of the host-encoded monomeric cellular prion protein PrPC. PrPSc is most likely identical in its covalent structure with PrPC (7). Both forms of the prion protein thus appear to differ only in their three-dimensional structure, as Fourier-transform infrared spectra and CD spectra indicate that the β-sheet content of PrPSc is significantly increased compared with PrPC (8, 9).
Two different kinetic models for the self-replication of PrPSc have been proposed. In the first model, the rate-limiting step would be the irreversible autocatalytic conversion of monomeric PrPC to a monomeric PrPSc subunit, followed by fast oligomerization of the subunits (10). This model has, however, been questioned recently by simulations of the kinetics of prion formation (11). In the second model, a fast equilibrium between monomeric PrPC and a monomeric PrPSc precursor is proposed and the rate-limiting reaction would be the formation of a PrPSc oligomer, which acts as nucleus for the growth of larger oligomers of the infectious agent (12).
PrPC is a secretory cell surface glycoprotein. Its mature form made up of the residues 23–231 [amino acid numbering according to human PrP (13)] is anchored to the cell membrane via a glycosyl phosphatidylinositol anchor at its C terminus (7). It has a single disulfide bridge and two N-glycosylation sites (7). The three-dimensional NMR structure of the recombinant murine prion protein, PrP(23–231), has revealed that the whole N-terminal segment 23–120 is flexibly disordered and that only the segment containing the C-terminal 111 residues, PrP(121–231), possesses a defined three-dimensional structure (14–17). Murine PrP(121–231) is a self-folding domain consisting of three α-helices and a two-stranded antiparallel β-sheet (14, 15). The structure of the isolated domain PrP(121–231) is the same as that in the context of the murine full-length protein (15, 17). Very similar results were obtained for the structure of the full-length prion protein, PrP(29–231), from hamster (18).
Although mature PrPC is soluble and protease-sensitive, the PrPSc oligomer is insoluble and partially protease-resistant. Digestion of PrPSc yields a PrP species termed PrP27–30 that retains infectivity. The frayed N termini of the PrP27–30 subunits are found within residues 73–90 (1). Within the framework of the “protein-only” hypothesis, the segment containing residues 90–231 of the prion protein thus appears to be the minimal infectious unit. This is in accordance with the finding that transgenic mice that exclusively express an N-terminally truncated prion protein lacking residues 23–80 are still susceptible to prion infection and able to propagate the infectious agent (19). Because segment 90–120 is protected from protease digestion in PrPSc, it is very likely that residues 90–120 adopt a defined conformation in the PrPSc subunits. This was confirmed by the identification of PrPC- and PrPSc-specific epitopes in segment 90–120 with recombinant antibody Fab fragments (20). A structural change in segment 90–120 thus appears to be the minimal requirement for the transition from PrPC to PrPSc.
The aim of this study was to test whether segment 90–120 is the only region of prion protein that can undergo a structural change during formation of PrPSc or whether the C-terminal domain PrP(121–231) is also able to adopt alternative conformations that may be present in PrPSc. For this purpose, we analyzed the pH dependence of the urea-induced unfolding transition of murine PrP(121–231) that folds reversibly in a one-step transition at pH 7.0 (14). Recent experiments on the folding of the recombinant human and hamster prion protein fragments 90–231 showed that PrP(90–231) forms an unfolding intermediate at acidic pH with β-sheet character (21, 22). Herein we show that formation of this intermediate is independent of segment 90–120, fully reversible, and an intrinsic property of the C-terminal domain PrP(121–231). We present quantitative data on the population of the intermediate at acidic pH and discuss its possible implications for the present models of prion propagation and the presumed formation of PrPSc during endocytosis.
MATERIALS AND METHODS
Expression and Purification of PrP(121–231).
Murine PrP(121–231) was expressed in the periplasm of Escherichia coli BL21(DE3) as described (14), except that the bacteria were grown at 37°C prior to induction and that the expression vector pPrP-CRR was used. This plasmid differs from the described plasmid pPrP-C (14) at the codons for Arg-228 and Arg-229 that were replaced by the most frequent Arg codons in strongly expressed E. coli genes. The modified gene encoding PrP(121–231) was amplified from pPrP-C by the PCR with the following oligonucleotide primers: N-terminal primer, 5′-GGCTGCGGCAGTCGCGAGTGTAGTGGGGGGCCTTGGTGGC-3′; C-terminal primer, 5′-AGGAGGGGAGGGGATCCAAGCTTACTAGCTGGAACGACGCCCGTCGTAATAGGCCTGGGACTCC-3′.
The amplified gene was cut with NruI and BamHI and cloned into the plasmid pRBI-PDI-T7 (23) that had been digested with StuI and BamHI. pPrP-CRR provided about 2-fold higher yields of PrP(121–231) compared with pPrP-C. In addition, a modified form of PrP(121–231) with an apparent molecular mass that was about 1 kDa higher on SDS/polyacrylamide gels and that accumulated to about 10% compared with PrP(121–231) was no longer observed when pPrP-CRR was used. N-terminal sequencing and mass spectrometry indicated that this modified form had the correct N terminus but an enlongation at the C-terminus, obviously caused by the natural codons of Arg-228 and Arg-229 in the mouse PrP gene, which are the rarest Arg codons in E. coli. Recombinant PrP(121–231) was purified as described (14), dialyzed against distilled water, and stored at −20°C. The concentration of PrP(121–231) was measured by its absorbance at 280 nm (A280 nm, 1 mg/ml, 1 cm = 1.55).
CD Spectroscopy.
Far-UV CD spectra were measured on a Jasco J710 CD spectropolarimeter at protein concentrations of 0.3–0.4 mg/ml in 0.02-cm cuvettes. Buffers were the same as those used for measuring the unfolding/refolding equilibria (see below). Spectra were corrected for the buffers.
Unfolding and Refolding of PrP(121–231).
Reversible unfolding and refolding of PrP(121–231) was measured between pH 4.0 and pH 7.0 at 22°C and a constant ionic strength of 88 mM in the following buffers that contained different concentrations of urea: pH 7.0, 50 mM sodium phosphate; pH 6.0, 10 mM Mes⋅NaOH/84 mM NaCl; pH 5.0, 50 mM acetic acid⋅NaOH/56 mM NaCl; pH 4.5, 50 mM acetic acid⋅NaOH/70 mM NaCl; pH 4.0, 50 mM formic acid⋅NaOH/56 mM NaCl. Native or denatured PrP(121–231) (in 8 M urea) was diluted 1:11 with buffer [final concentration of PrP(121–231), 22–40 μM] and incubated for 36 h. The mean residue ellipticity at 222 nm was then recorded for 2 min in 0.1-cm cuvettes and averaged. The original CD data obtained at pH 7.0, 6.0, and 5.0 were analyzed according to the two-state model of folding, by using a six-parameter fit as described (24). Eqs. 1–3 (25) were used for the three-state analysis of the data measured at pH 4.5 and 4.0, where θN, θI, and θU are the mean residue ellipticities at 222 nm of the native protein, the intermediate, and the unfolded protein at 0 M urea; sN and sI correspond to the slopes of the urea dependence of θN and θU; ΔGNI and ΔGIU are free energy differences between the native protein and the intermediate and between the intermediate and the unfolded protein, respectively; and mNI and mIU are the cooperativities of the transitions.
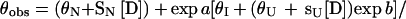 |
1 |
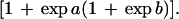 |
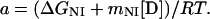 |
2 |
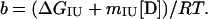 |
3 |
The fractions of the native protein, the intermediate, and the unfolded protein at different urea concentrations were calculated from the urea dependence of the equilibrium constant of each transition (KNI and KIU) according to Eqs. 4 and 5.
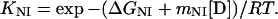 |
4 |
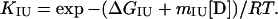 |
5 |
RESULTS
Urea-Induced Unfolding Transitions of PrP(121–231).
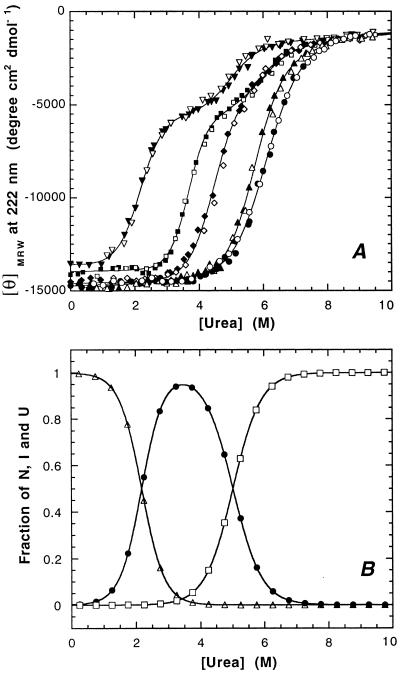
We investigated the pH dependence of the urea-induced unfolding/refolding transition of murine PrP(121–231) at 22°C and a constant ionic strength of 88 mM. The transitions were monitored by the far-UV CD signal of PrP(121–231) at 222 nm. Fig. 1A shows the urea-induced unfolding and refolding of PrP(121–231) between pH 4.0 and 7.0. All transitions were completely reversible, and no protein aggregation was observed under the conditions used. The transitions at pH 6.0 and 7.0 are apparent two-state transitions with similar cooperativities, transition midpoints, and free energies of folding of −28 and −31 kJ mol−1, respectively (Table 1). In contrast, the unfolding transitions at pH 4.0 and 4.5 are shifted to lower urea concentrations and are characterized by the presence of an equilibrium unfolding intermediate seen as a plateau in the transition profiles (Fig. 1A). Three-state analysis of the data revealed that the unfolding intermediate is maximally populated to 95% at pH 4.0 and 3.5 M urea (Fig. 1B) and to about 85% at pH 4.5 and 4.5 M urea. A mixed situation is observed at pH 5.0, where PrP(121–231) is significantly less stable compared with pH 6.0 and 7.0, but the plateau is only marginally developed (Fig. 1A). Table 1 shows the relative stabilities of the intermediate, native, and unfolded PrP(121–231) in the absence of denaturant. The intermediate is closer in its free energy to native PrP(121–231) at pH 4.0 and closer to unfolded PrP(121–231) at pH 4.5 (Table 1), indicating that the intermediate is absent at higher pH values. The higher stability of the intermediate at pH 4.0 is reflected by its fraction in the absence of denaturant, which is 0.2% at pH 4.0 and 0.0002% at pH 4.5.
Figure 1.
(A) Reversible unfolding transitions of PrP(121–231) at 22°C in the presence of urea at pH 4.0 (▾), 4.5 (▪), 5.0 (⧫), 6.0 (▴), and 7.0 (•). (A) Mean residue ellipticities at 222 nm. The solid lines result from fitting the data according to a two-state model for pH 5.0, pH 6.0, and pH 7.0 and according to a three-state model for pH 4.0 and pH 4.5. The corresponding refolding experiments are represented by open symbols. (B) Three-state analysis of the fractions of the native (▵), intermediate (•), and unfolded state (□) of PrP(121–231) at pH 4.0.
Table 1.
Thermodynamic parameters of urea-induced folding of PrP(121–231) between pH 4.0 and pH 7.0 at 22°C
| Parameter | pH 7.0 | pH 6.0 | pH 4.5 | pH 4.0 |
|---|---|---|---|---|
| ΔGNU, kJ mol−1 | −28.6 ± 0.95 | −32.1 ± 1.30 | ||
| mNU, kJ mol−1 M−1 | 4.73 ± 0.16 | 5.61 ± 0.16 | ||
| Urea1/2, M | 6.05 | 5.72 | ||
| ΔGNI, kJ mol−1 | −31.1 ± 1.3 | −15.5 ± 1.2 | ||
| mNI, kJ mol−1 M−1 | 8.53 ± 0.41 | 7.12 ± 0.48 | ||
| Urea1/2, M | 3.65 | 2.18 | ||
| ΔGIU, kJ mol−1 | −22.4 ± 5.4 | −27.4 ± 3.2 | ||
| mIU, kJ mol−1 M−1 | 3.82 ± 0.89 | 5.54 ± 0.64 | ||
| Urea1/2, M | 5.86 | 4.94 |
Acid-Induced Unfolding of PrP(121–231) at Constant Denaturant Concentration.
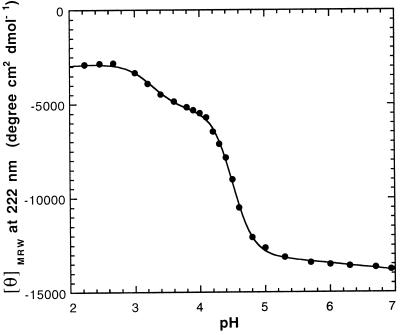
The pH dependence of the formation of the intermediate was followed at a constant urea concentration of 3.5 M where all molecules are native at pH 7.0 and the intermediate is maximally populated at pH 4.0 (see. Fig. 1A). Two pH-dependent processes were observed (Fig. 2). The first transition with an apparent pKa of 4.5 corresponds to the transition from native PrP(121–231) to the unfolding intermediate. The second process with an apparent pKa of 3.3 was assigned to the transition from the intermediate to an unfolded state at acidic pH. The apparent pKa of 4.5 of the first transition suggests that acidic residues in PrP(121–231) are titrated upon formation of the intermediate. The transitions are significantly steeper than expected from two single acid/base equilibria, demonstrating that several acidic side chains are protonated in each of the transitions (25). The intermediate is not measurably populated in 3.5 M urea above pH 5.5 but is still present during urea-induced unfolding of PrP(121–231) at pH 5.0.
Figure 2.
Acid-induced unfolding of PrP(121–231) in the presence of 3.5 M urea monitored by the mean residue ellipticity at 222 nm.
Spectroscopic Characterization of the Intermediate.
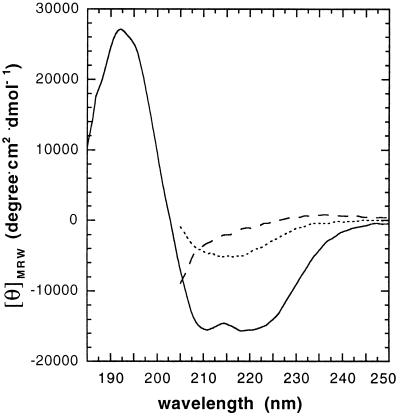
To compare the structural features of the intermediate with native and unfolded PrP(121–231), the far-UV CD spectra of all three states were recorded at pH 4.0 (Fig. 3). The shape of the spectrum of native PrP(121–231) is identical with the spectrum at pH 7.0 with minima at 208 and 222 nm (mean residue ellipticity at 222 nm, −13,600 degrees⋅cm2/degrees⋅ cm2⋅dmol−1) that are diagnostic for the high α-helix content in PrP(121–231). In contrast, the spectrum of the intermediate has a minimum at 215 nm with a mean residue ellipticity of −7,000 deg⋅cm2/dmol that indicates a high content of β-sheet secondary structure and a strong decline of α-helix content in the intermediate (26).
Figure 3.
Far-UV CD spectra of native PrP(121–231) (solid line), the equilibrium unfolding intermediate (dotted line), and acid denatured PrP(121–231) (dashed line) at 22°C. The spectra of native PrP(121–231) and the intermediate were recorded at pH 4.0 and zero M and 3.5 M urea, respectively. The spectrum of acid denatured PrP(121–231) was measured at pH 2.0 in 3.5 M urea (see Fig. 2). Spectra recorded in the presence of urea could not be recorded below 210 nm due to the high absorbance of the denaturant.
Kinetics of the Formation of the Intermediate.
The kinetics of formation of the equilibrium unfolding intermediate were analyzed at pH 4.0. The native protein and the unfolded protein (in 8 M urea) were diluted with buffer to a final urea concentration of 3.5 M where the intermediate is maximally populated. In both cases, the CD signal of the intermediate was formed within the dead time of manual mixing (about 15 s; data not shown), demonstrating that the equilibria between the native or unfolded state and the intermediate of PrP(121–231) are very rapid.
DISCUSSION
In this study we have demonstrated that the C-terminal domain of the prion protein (i.e., the only segment in PrPC with defined tertiary structure) can adopt an alternative conformation at acidic pH with spectral characterisitics of a β-sheet protein. Therefore, structural differences between PrPC and PrPSc subunits may not be restricted to the prion protein segment 90–120, and the C-terminal domain PrP(121–231) may also undergo a conformational change during the conversion of PrPC into PrPSc. This is strongly supported by the fact that the epitope recognized by the only PrPSc-specific antibody that is presently available is composed of three segments from PrP(121–231) (27). An acid-induced unfolding intermediate with β-sheet properties has recently also been observed during guanidinium chloride-induced unfolding transitions of the recombinant fragment 90–231 from human PrP (21). We have now shown that the formation of the acid-induced intermediate is an intrinsic property of the C-terminal domain PrP(121–231) and independent of segment 90–120.
Monomeric folding intermediates of amyloidogenic proteins have previously been proposed as precursors of amyloid for a variety of amyloidogenic diseases (28, 29). We have demonstrated that the equilibrium unfolding intermediate of PrP(121–231) is in a rapid equilibrium with the native and unfolded state. This is consistent with the nucleation/condensation model for the formation of the infectious PrPSc oligomer (12), which postulates a fast equilibrium between monomers of PrPC and PrPSc and a rate-limiting association of PrPSc monomers to an oligomer of critical size that forms a nucleus for further irreversible incorporation of PrPSc monomers into a growing PrPSc oligomer. Within the framework of this model, any change in solvent conditions that shifts the PrPC/PrPSc equilibrium toward PrPSc would accelerate the formation of oligomeric PrPSc. A change in solvent conditions, namely a shift from physiological to acidic pH, may indeed occur during the conversion of PrPC into PrPSc in vivo: The cell surface protein PrPC is normally exposed to physiological pH. PrPC appears to be clustered in cholesterol-rich invaginations of the plasma membrane termed caveolae (30), which may bud from the membrane and fuse with endosomes in a clathrin-independent endocytosis pathway. Intriguingly, insoluble PrPSc amyloid accumulates in the endosomal lumen of scrapie-infected cells (31, 32) where the pH varies between 4.0 and 6.0 (33), indicating that the propagation of the infectious scrapie agent may occur during endocytosis at acidic pH. This agrees with our experimental data that show that the unfolding intermediate of PrP(121–231) with β-sheet characteristics is only populated below pH 5.0 (Fig. 2). A quantitative analysis demonstrates that the intermediate is also significantly present in the absence of urea at pH 4.0. The following scheme presents a deduced model for the propagation of PrPSc in endosomes, where the unfolding intermediate (I) should be populated at equilibrium in addition to the native (N) and unfolded (U) state and could be removed from the equilibrium by irreversible incorporation into the PrPSc oligomer.
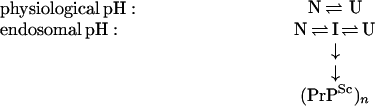 |
In this context, it is important to note that equilibrium unfolding intermediates are rather frequently observed during protein folding studies at acidic pH and have been extensively analyzed in the case of apomyoglobin (25) and staphylococcal nuclease (34). However, these intermediates generally contain native-like secondary structures, whereas the far-UV CD spectrum of the unfolding intermediate of PrP(121–231) lacks the native α-helical features. The apparent pKa of 4.5 measured for the transition from native PrP(121–231) to the intermediate (Fig. 2) is reminiscent of the conformational change in influenza hemagglutinin that is also induced by mildly acidic conditions (pH 5.0) and required for fusion of the viral membrane with the endosomal membrane of infected animal cells (35). The conformational transition in hemagglutinin is, however, irreversible and kinetically controlled (35). For the domain PrP(121–231), the conformational change is reversible but may be followed by irreversible oligomerization of PrP. Importantly, acid pH has also been shown to promote the formation of amyloid fibrils of another amyloidogenic protein, transthyretin, which represents the main β-sheet amyloid deposit of familial amyloidotic polyneuropathy (36, 37).
The model of an acid-induced unfolding intermediate of PrPC that acts as possible monomeric precursor of the scrapie agent is also supported by the known amino acid exchanges in human PrP that have been associated with inherited human prion diseases (2): Like the protonation of acidic residues at low pH, the disease-related point mutations in human PrP that involve acidic residues (Asp178Asn and Glu200Lys) remove negative charges. Asp-178 and Glu-200 are located at the N terminus of the second and third helix of PrP(121–231), respectively (15). The mutations may thus eliminate favorable interactions of these residues with the helix dipoles and facilitate the formation of the intermediate. The fact that the intermediate can be almost quantitatively populated at equilibrium opens the possibility to determine its structure in solution by NMR spectroscopy. Acidic pH and endocytosis may also represent important experimental parameters for successful propagation of the infectious scrapie agent in vitro.
Acknowledgments
We gratefully acknowledge discussions with A. Aguzzi, M. Billeter, M. Huber-Wunderlich, S. Liemann, R. Riek, S. Tan, C. Weissmann, G. Wider, and K. Wüthrich. This work was supported by the Eidgenössische Technische Hochschule Zürich, the Schweizerische Nationalfonds [Project 438+.050285 (R.G.)], and the Boehringer-Ingelheim-Fonds (grant to S.H.).
ABBREVIATIONS
- PrP
prion protein
- PrPC
cellular PrP
- PrPSc
insoluble oligomeric “scrapie” isoform of the PrP
- PrP(121–231)
C-terminal domain of PrPC comprising residues 121–231
References
- 1.Weissmann C, Fischer M, Raeber A, Büeler H, Sailer A, Shmerling D, Rülicke T, Brandner S, Aguzzi A. Cold Spring Harbor Symp Quant Biol. 1996;61:511–522. [PubMed] [Google Scholar]
- 2.Prusiner S B. Science. 1997;278:245–250. doi: 10.1126/science.278.5336.245. [DOI] [PubMed] [Google Scholar]
- 3.Horwich A L, Weissman J S. Cell. 1997;89:499–510. doi: 10.1016/s0092-8674(00)80232-9. [DOI] [PubMed] [Google Scholar]
- 4.Alper T, Cramp W A, Haig D A, Clarke M C. Nature (London) 1967;214:764–766. doi: 10.1038/214764a0. [DOI] [PubMed] [Google Scholar]
- 5.Griffith J S. Nature (London) 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- 6.Prusiner S B. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 7.Stahl N, Prusiner S B. FASEB J. 1991;5:2799–2807. doi: 10.1096/fasebj.5.13.1916104. [DOI] [PubMed] [Google Scholar]
- 8.Caughey B W, Dong A, Bhat K S, Ernst D, Hayes S F, Caughey W S. Biochemistry. 1991;30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- 9.Pan K-M, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick R J, Cohen F E, Prusiner S B. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prusiner S B. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 11.Eigen M. Biophys J. 1996;63:A1–A18. doi: 10.1016/s0301-4622(96)02250-8. [DOI] [PubMed] [Google Scholar]
- 12.Jarrett J E, Lansbury P T., Jr Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 13.Schätzl H M, Da Costa M, Taylor L, Cohen F E, Prusiner S B. J Mol Biol. 1995;245:362–374. doi: 10.1006/jmbi.1994.0030. [DOI] [PubMed] [Google Scholar]
- 14.Hornemann S, Glockshuber R. J Mol Biol. 1996;261:614–618. doi: 10.1006/jmbi.1996.0487. [DOI] [PubMed] [Google Scholar]
- 15.Riek R, Hornemann S, Wider R, Billeter M, Glockshuber R, Wüthrich K. Nature (London) 1996;382:180–382. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
- 16.Hornemann S, Korth C, Oesch B, Riek R, Wider R, Glockshuber R, Wüthrich K. FEBS Lett. 1997;413:277–281. doi: 10.1016/s0014-5793(97)00921-6. [DOI] [PubMed] [Google Scholar]
- 17.Riek R, Hornemann S, Wider R, Glockshuber R, Wüthrich K. FEBS Lett. 1997;413:282–288. doi: 10.1016/s0014-5793(97)00920-4. [DOI] [PubMed] [Google Scholar]
- 18.Donne D G, Viles J H, Groth D, Mehlhorn I, James T L, Cohen F E, Prusiner S B, Wright P E, Dyson H J. Proc Natl Acad Sci USA. 1997;94:13452–13457. doi: 10.1073/pnas.94.25.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 20.Peretz D, Williamson R A, Matsunaga Y, Serban H, Pinilla C, Bastidas R B, Rozenshteyn R, James T L, Houghten R A, Cohen F E, et al. J Mol Biol. 1997;273:614–622. doi: 10.1006/jmbi.1997.1328. [DOI] [PubMed] [Google Scholar]
- 21.Swietnicki W, Peterson R, Gambetti P, Surewicz W K. J Biol Chem. 1997;272:27517–27520. doi: 10.1074/jbc.272.44.27517. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Stöckel J, Mehlhorn I, Groth D, Baldwin M, Prusiner S B, James T L, Cohen F E. Biochemistry. 1997;36:3543–3553. doi: 10.1021/bi961965r. [DOI] [PubMed] [Google Scholar]
- 23.Strobl S, Mühlhahn P, Bernstein R, Wiltscheck R, Maskos K, Wunderlich M, Huber R, Glockshuber R, Holak T. Biochemistry. 1995;34:8281–8293. doi: 10.1021/bi00026a009. [DOI] [PubMed] [Google Scholar]
- 24.Santoro M M, Bolen D W. Biochemistry. 1988;27:8063–8068. doi: 10.1021/bi00421a014. [DOI] [PubMed] [Google Scholar]
- 25.Barrick D, Baldwin R L. Biochemsitry. 1993;32:3790–3796. doi: 10.1021/bi00065a035. [DOI] [PubMed] [Google Scholar]
- 26.Fasman G D. Circular Dichroism and the Conformational Analysis of Biomolecules. New York: Plenum; 1996. [Google Scholar]
- 27.Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, et al. Nature (London) 1997;390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- 28.Kelly J W. Curr Opin Struct Biol. 1996;6:11–17. doi: 10.1016/s0959-440x(96)80089-3. [DOI] [PubMed] [Google Scholar]
- 29.Booth D R, Sunde M, Bellotti V, Robinson C V, Hutchinson W L, Fraser P E, Hawkins P N, Dobson C M, Radford S E, Blake C C F, Pepys M B. Nature (London) 1997;385:787–793. doi: 10.1038/385787a0. [DOI] [PubMed] [Google Scholar]
- 30.Vey M, Pilkuhn S, Wille H, Nixon R, DeArmond S J, Smart E J, Anderson R G W, Taraboulos A, Prusiner S B. Proc Natl Acad Sci USA. 1996;93:14945–14949. doi: 10.1073/pnas.93.25.14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold J E, Tipler C, Laszlo L, Hope J, Landon M, Mayer R J. J Pathol. 1995;176:403–411. doi: 10.1002/path.1711760412. [DOI] [PubMed] [Google Scholar]
- 32.Borchelt D R, Taraboulos A, Prusiner S B. J Biol Chem. 1992;267:16188–16199. [PubMed] [Google Scholar]
- 33.Lee R J, Wang S, Low P S. Biochim Biophys Acta. 1996;1312:237–242. doi: 10.1016/0167-4889(96)00041-9. [DOI] [PubMed] [Google Scholar]
- 34.Ionescu R M, Eftink M R. Biochemistry. 1997;36:1129–1140. doi: 10.1021/bi9609681. [DOI] [PubMed] [Google Scholar]
- 35.Carr M C, Kim P S. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- 36.Lai Z, Colon W, Kelly J W. Biochemistry. 1996;35:6470–6482. doi: 10.1021/bi952501g. [DOI] [PubMed] [Google Scholar]
- 37.Blake C, Serpell L. Structure. 1996;4:989–998. doi: 10.1016/s0969-2126(96)00104-9. [DOI] [PubMed] [Google Scholar]