Abstract
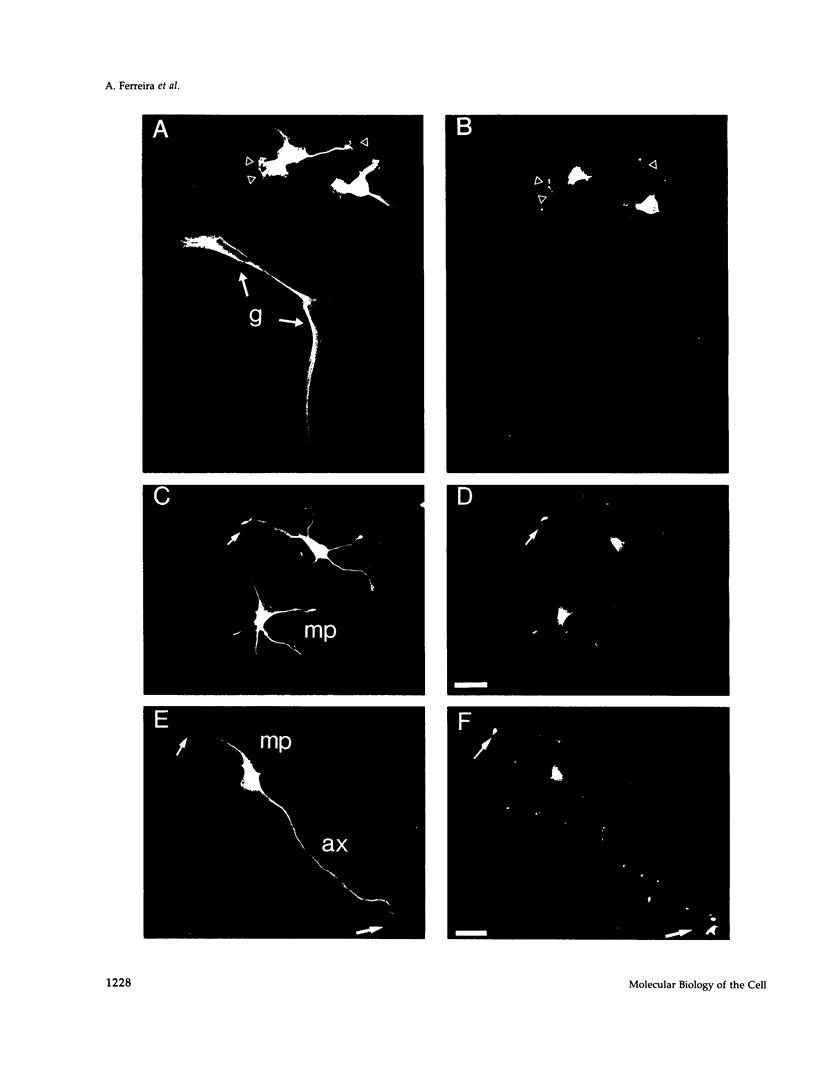
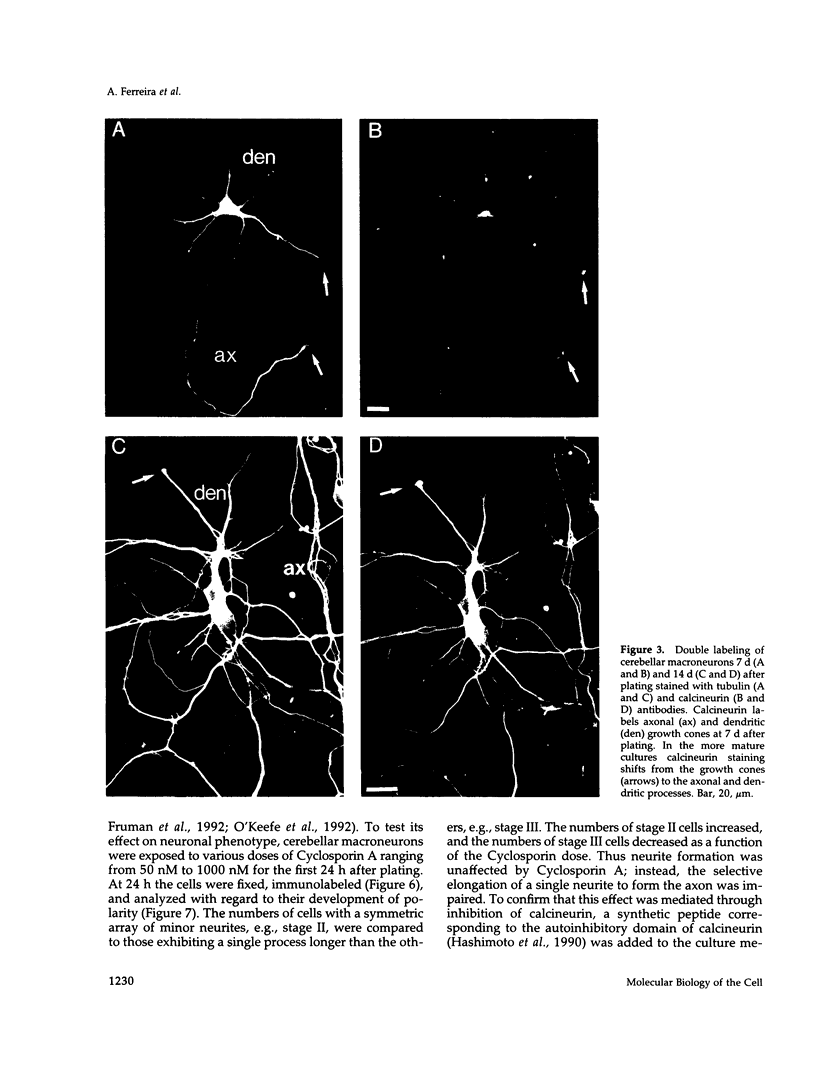
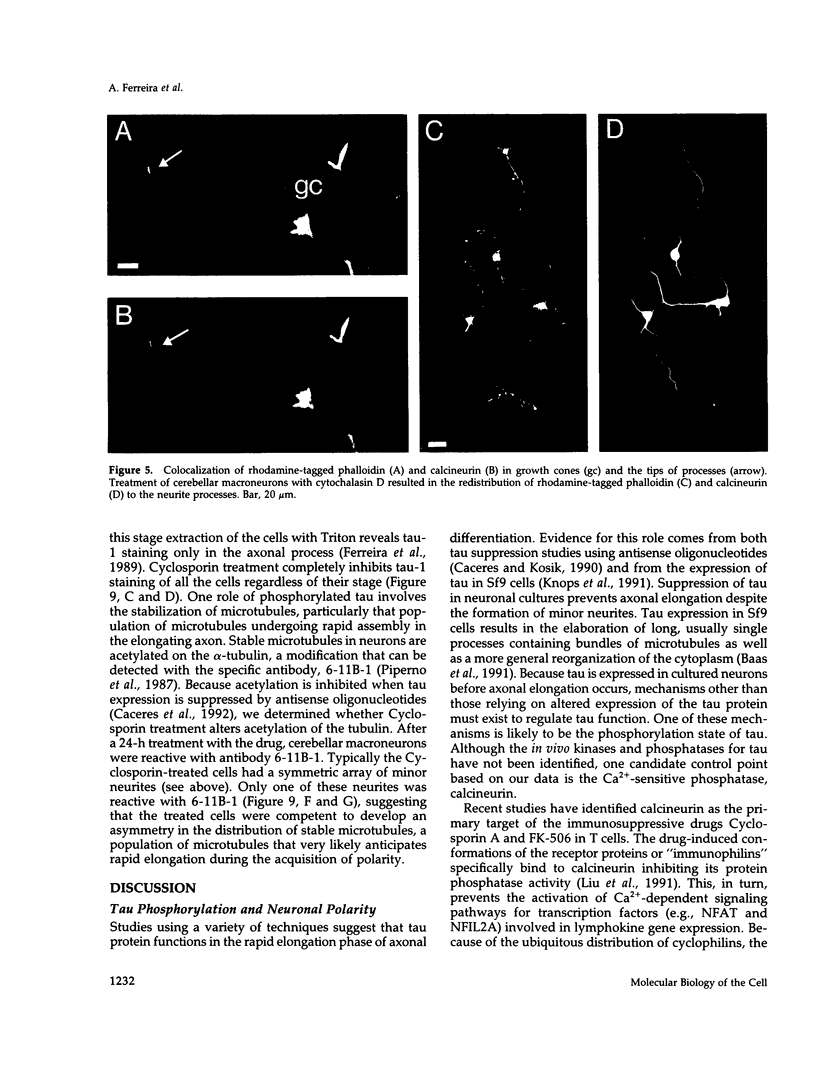
Calcineurin is a calmodulin-dependent serine-threonine phosphatase found in many cell types but most abundant in neurons. To determine its localization in developing neurons, dissociated cultures from embryonic day 15 rat cerebellum were analyzed immunocytochemically after treatment with cytoskeletal-disrupting drugs. During the initial outgrowth of neurites, calcineurin is enriched in growth cones where its localization depends upon the integrity of both microtubules and actin filaments. Treatment with cytochalasin shifts calcineurin from the growth cone to the neurite shaft, and with nocadozole calcineurin translocates to the cell body. Therefore calcineurin is well positioned to mediate interactions between cytoskeletal systems during neurite elongation. By 14 d in culture, when the neurons have developed extensive neuronal contacts and synapses are present, calcineurin is predominantly in the neurite shaft. Incubation of cultured cells with Cyclosporin A or a specific peptide, both of which selectively inhibit calcineurin's phosphatase activity, prevented axonal elongation. Because the microtubule-associated protein tau appears to play a key role in asymmetric neurite elongation, we examined modifications in its phosphorylation state resulting from calcineurin inhibition. In contrast to the normal development of cerebellar macroneurons in which reactivity with the phosphorylation-dependent antibody, tau-1, progressively increases, there was a persistent inhibition of tau-1 reactivity in cells exposed to Cyclosporin A. These findings suggest a role for calcineurin in regulating tau phosphorylation and possibly modulating other steps required for the determination of polarity.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander K. A., Cimler B. M., Meier K. E., Storm D. R. Regulation of calmodulin binding to P-57. A neurospecific calmodulin binding protein. J Biol Chem. 1987 May 5;262(13):6108–6113. [PubMed] [Google Scholar]
- Baas P. W., Pienkowski T. P., Kosik K. S. Processes induced by tau expression in Sf9 cells have an axon-like microtubule organization. J Cell Biol. 1991 Dec;115(5):1333–1344. doi: 10.1083/jcb.115.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg J. R., Bray D., Chapman K. Assembly of microtubules at the tip of growing axons. Nature. 1986 Jun 19;321(6072):788–790. doi: 10.1038/321788a0. [DOI] [PubMed] [Google Scholar]
- Bearer E. L. An actin-associated protein present in the microtubule organizing center and the growth cones of PC-12 cells. J Neurosci. 1992 Mar;12(3):750–761. doi: 10.1523/JNEUROSCI.12-03-00750.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Biernat J., Mandelkow E. M., Schröter C., Lichtenberg-Kraag B., Steiner B., Berling B., Meyer H., Mercken M., Vandermeeren A., Goedert M. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 1992 Apr;11(4):1593–1597. doi: 10.1002/j.1460-2075.1992.tb05204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., Thomas C., Shaw G. Growth cone formation in cultures of sensory neurons. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5226–5229. doi: 10.1073/pnas.75.10.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugg B., Matus A. Phosphorylation determines the binding of microtubule-associated protein 2 (MAP2) to microtubules in living cells. J Cell Biol. 1991 Aug;114(4):735–743. doi: 10.1083/jcb.114.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A., Kosik K. S. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990 Feb 1;343(6257):461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- Caceres A., Mautino J., Kosik K. S. Suppression of MAP2 in cultured cerebellar macroneurons inhibits minor neurite formation. Neuron. 1992 Oct;9(4):607–618. doi: 10.1016/0896-6273(92)90025-9. [DOI] [PubMed] [Google Scholar]
- Carlin R. K., Bartelt D. C., Siekevitz P. Identification of fodrin as a major calmodulin-binding protein in postsynaptic density preparations. J Cell Biol. 1983 Feb;96(2):443–448. doi: 10.1083/jcb.96.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. P., Reese T. S. Polarized compartmentalization of organelles in growth cones from developing optic tectum. J Cell Biol. 1985 Oct;101(4):1473–1480. doi: 10.1083/jcb.101.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988 Apr;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel D. N., Hyman A. A., Cobb M. H., Kirschner M. W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992 Oct;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Busciglio J., Cáceres A. Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and Tau. Brain Res Dev Brain Res. 1989 Oct 1;49(2):215–228. doi: 10.1016/0165-3806(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Cáceres A. The expression of acetylated microtubules during axonal and dendritic growth in cerebellar macroneurons which develop in vitro. Brain Res Dev Brain Res. 1989 Oct 1;49(2):205–213. doi: 10.1016/0165-3806(89)90022-9. [DOI] [PubMed] [Google Scholar]
- Forscher P., Smith S. J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988 Oct;107(4):1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman D. A., Klee C. B., Bierer B. E., Burakoff S. J. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3686–3690. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Cohen E. S., Jakes R., Cohen P. p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A1. Implications for Alzheimer's disease [corrected]. FEBS Lett. 1992 Nov 2;312(1):95–99. doi: 10.1016/0014-5793(92)81418-l. [DOI] [PubMed] [Google Scholar]
- Goldberg D. J. Local role of Ca2+ in formation of veils in growth cones. J Neurosci. 1988 Jul;8(7):2596–2605. doi: 10.1523/JNEUROSCI.08-07-02596.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K., Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989 Apr;108(4):1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K., Birgbauer E., Banker G., Solomon F. The role of cytoskeleton in organizing growth cones: a microfilament-associated growth cone component depends upon microtubules for its localization. J Cell Biol. 1989 Oct;109(4 Pt 1):1621–1631. doi: 10.1083/jcb.109.4.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S., Matsukado Y., Mihara Y., Inoue N., Miyamoto E. Calcineurin in human brain and its relation to extrapyramidal system. Immunohistochemical study on postmortem human brains. Acta Neuropathol. 1986;72(2):150–156. doi: 10.1007/BF00685977. [DOI] [PubMed] [Google Scholar]
- Goto S., Yamamoto H., Fukunaga K., Iwasa T., Matsukado Y., Miyamoto E. Dephosphorylation of microtubule-associated protein 2, tau factor, and tubulin by calcineurin. J Neurochem. 1985 Jul;45(1):276–283. doi: 10.1111/j.1471-4159.1985.tb05504.x. [DOI] [PubMed] [Google Scholar]
- Griffith L. M., Pollard T. D. Evidence for actin filament-microtubule interaction mediated by microtubule-associated proteins. J Cell Biol. 1978 Sep;78(3):958–965. doi: 10.1083/jcb.78.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L. M., Pollard T. D. The interaction of actin filaments with microtubules and microtubule-associated proteins. J Biol Chem. 1982 Aug 10;257(15):9143–9151. [PubMed] [Google Scholar]
- Guerini D., Klee C. B. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9183–9187. doi: 10.1073/pnas.86.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Perrino B. A., Soderling T. R. Identification of an autoinhibitory domain in calcineurin. J Biol Chem. 1990 Feb 5;265(4):1924–1927. [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982 Jul;94(1):129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi H. C., Chu D., Buxbaum R. E., Heidemann S. R. Tension and compression in the cytoskeleton of PC 12 neurites. J Cell Biol. 1985 Sep;101(3):697–705. doi: 10.1083/jcb.101.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Balaban C. D., Billingsley M. L. Differential localization of calmodulin-dependent enzymes in rat brain: evidence for selective expression of cyclic nucleotide phosphodiesterase in specific neurons. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1118–1122. doi: 10.1073/pnas.84.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Giri P. R., Higuchi S., Tamura J., Dixon S. C., Marietta C. A., Amorese D. A., Martin B. M. Cloning and characterization of molecular isoforms of the catalytic subunit of calcineurin using nonisotopic methods. J Biol Chem. 1990 Jul 5;265(19):11312–11319. [PubMed] [Google Scholar]
- Kincaid R. L., Manganiello V. C., Odya C. E., Osborne J. C., Jr, Stith-Coleman I. E., Danello M. A., Vaughan M. Purification and properties of calmodulin-stimulated phosphodiesterase from mammalian brain. J Biol Chem. 1984 Apr 25;259(8):5158–5166. [PubMed] [Google Scholar]
- Kincaid R. L. Preparation, characterization, and properties of affinity-purified antibodies to calmodulin-dependent cyclic nucleotide phosphodiesterase and the protein phosphatase calcineurin. Methods Enzymol. 1988;159:627–652. doi: 10.1016/0076-6879(88)59059-6. [DOI] [PubMed] [Google Scholar]
- King M. M., Huang C. Y., Chock P. B., Nairn A. C., Hemmings H. C., Jr, Chan K. F., Greengard P. Mammalian brain phosphoproteins as substrates for calcineurin. J Biol Chem. 1984 Jul 10;259(13):8080–8083. [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Draetta G. F., Hubbard M. J. Calcineurin. Adv Enzymol Relat Areas Mol Biol. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- Knops J., Kosik K. S., Lee G., Pardee J. D., Cohen-Gould L., McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991 Aug;114(4):725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Finch E. A. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J Neurosci. 1987 Oct;7(10):3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Kumagai H., Nishida E., Sakai H. The interaction between calmodulin and microtubule proteins. IV. Quantitative analysis of the binding between calmodulin and tubulin dimer. J Biochem. 1982 Apr;91(4):1329–1336. doi: 10.1093/oxfordjournals.jbchem.a133819. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Differences in the organization of actin in the growth cones compared with the neurites of cultured neurons from chick embryos. J Cell Biol. 1983 Oct;97(4):963–973. doi: 10.1083/jcb.97.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984 Apr 25;259(8):5301–5305. [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu Y. C., Storm D. R. Dephosphorylation of neuromodulin by calcineurin. J Biol Chem. 1989 Aug 5;264(22):12800–12804. [PubMed] [Google Scholar]
- Meiri K. F., Pfenninger K. H., Willard M. B. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci U S A. 1986 May;83(10):3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T., Giri P. R., Higuchi S., Kincaid R. L. Molecular cloning of a calmodulin-dependent phosphatase from murine testis: identification of a developmentally expressed nonneural isoenzyme. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):529–533. doi: 10.1073/pnas.89.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida E., Kuwaki T., Sakai H. Phosphorylation of microtubule-associated proteins (MAPs) and pH of the medium control interaction between MAPs and actin filaments. J Biochem. 1981 Aug;90(2):575–578. doi: 10.1093/oxfordjournals.jbchem.a133510. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Owada M. K., Hakura A., Iida K., Yahara I., Sobue K., Kakiuchi S. Occurrence of caldesmon (a calmodulin-binding protein) in cultured cells: comparison of normal and transformed cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3133–3137. doi: 10.1073/pnas.81.10.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., LeDizet M., Chang X. J. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987 Feb;104(2):289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli J. W., Billingsley M. L., Kincaid R. L. Expression of the calmodulin-dependent protein phosphatase, calcineurin, in rat brain: developmental patterns and the role of nigrostriatal innervation. Brain Res Dev Brain Res. 1991 Nov 19;63(1-2):105–119. doi: 10.1016/0165-3806(91)90071-p. [DOI] [PubMed] [Google Scholar]
- Sabry J. H., O'Connor T. P., Evans L., Toroian-Raymond A., Kirschner M., Bentley D. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991 Oct;115(2):381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattilaro R. F., Dentler W. L., LeCluyse E. L. Microtubule-associated proteins (MAPs) and the organization of actin filaments in vitro. J Cell Biol. 1981 Aug;90(2):467–473. doi: 10.1083/jcb.90.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Baumrind N. L., Wayne D. B., Pearlman A. L. Concentration of membrane antigens by forward transport and trapping in neuronal growth cones. Cell. 1990 Apr 20;61(2):231–241. doi: 10.1016/0092-8674(90)90804-n. [DOI] [PubMed] [Google Scholar]
- Sobue K., Kanda K., Adachi J., Kakiuchi S. Calmodulin-binding proteins that interact with actin filaments in a Ca2+-dependent flip-flop manner: survey in brain and secretory tissues. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6868–6871. doi: 10.1073/pnas.80.22.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Tanaka T., Ashino N., Kakiuchi S. Ca2+ and calmodulin regulate microtubule-associated protein-actin filament interaction in a flip-flop switch. Biochim Biophys Acta. 1985 Jun 30;845(3):366–372. doi: 10.1016/0167-4889(85)90200-9. [DOI] [PubMed] [Google Scholar]
- Solomon F., Magendantz M. Cytochalasin separates microtubule disassembly from loss of asymmetric morphology. J Cell Biol. 1981 Apr;89(1):157–161. doi: 10.1083/jcb.89.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson S. K., Born T., Zydowsky L. D., Cho H., Chang H. Y., Walsh C. T., Rusnak F. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallant E. A., Cheung W. Y. Calmodulin-dependent protein phosphatase: a developmental study. Biochemistry. 1983 Jul 19;22(15):3630–3635. doi: 10.1021/bi00284a014. [DOI] [PubMed] [Google Scholar]
- Tanaka E. M., Kirschner M. W. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991 Oct;115(2):345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler C. D., Haimo L. T. Regulation of organelle transport in melanophores by calcineurin. J Cell Biol. 1990 Nov;111(5 Pt 1):1939–1948. doi: 10.1083/jcb.111.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K., Muramatsu T., Kincaid R. L. Structure and expression of two isoforms of the murine calmodulin-dependent protein phosphatase regulatory subunit (calcineurin B). Biochem Biophys Res Commun. 1992 Aug 31;187(1):537–543. doi: 10.1016/s0006-291x(05)81527-x. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971 Jun;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Fukunaga K., Goto S., Tanaka E., Miyamoto E. Ca2+, calmodulin-dependent regulation of microtubule formation via phosphorylation of microtubule-associated protein 2, tau factor, and tubulin, and comparison with the cyclic AMP-dependent phosphorylation. J Neurochem. 1985 Mar;44(3):759–768. doi: 10.1111/j.1471-4159.1985.tb12880.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Saitoh Y., Yasugawa S., Miyamoto E. Dephosphorylation of tau factor by protein phosphatase 2A in synaptosomal cytosol fractions, and inhibition by aluminum. J Neurochem. 1990 Aug;55(2):683–690. doi: 10.1111/j.1471-4159.1990.tb04187.x. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., Higbee R., Riederer B. M., Goodman S. R. Spectrin subtypes in mammalian brain: an immunoelectron microscopic study. J Neurosci. 1986 Oct;6(10):2977–2986. doi: 10.1523/JNEUROSCI.06-10-02977.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]