Abstract
Background and purpose:
Dopamine inhibits renal cell Na+,K+-ATPase activity and cell sodium transport by promoting the internalization of active molecules from the plasma membrane, whereas angiotensin II (ATII) stimulates its activity by recruiting new molecules to the plasma membrane. They achieve such effects by activating multiple and distinct signalling molecules in a hierarchical manner. The purpose of this study was to investigate whether dopamine and ATII utilize scaffold organizer proteins as components of their signalling networks, in order to avoid deleterious cross talk.
Experimental approach:
Attention was focused on a multiple PDZ domain protein, Pals-associated tight junction protein (PATJ). Ectopic expression of PATJ in renal epithelial cells in culture was used to study its interaction with components of the dopamine signalling cascade. Similarly, expression of PATJ deletion mutants was employed to analyse its functional relevance during dopamine-, ATII- and insulin-dependent regulation of Na+,K+-ATPase.
Key results:
Dopamine receptors and components of its signalling cascade mediating inhibition of Na+,K+-ATPase interact with PATJ. Inhibition of Na+,K+-ATPase by dopamine was prevented by expression of mutants of PATJ lacking PDZ domains 2, 4 or 5; whereas the stimulatory effect of ATII and insulin on Na+,K+-ATPase was blocked by expression of PATJ lacking PDZ domains 1, 4 or 5.
Conclusions and implications:
A multiple PDZ domain protein may add functionality to G protein-coupled and tyrosine kinase receptors signalling during regulation of Na+,K+-ATPase. Signalling molecules and effectors can be integrated into a functional network by the scaffold organizer protein PATJ via its multiple PDZ domains.
Keywords: Na+,K+-ATPase; insulin receptors; PDZ domains; endocytosis; renal epithelial cells
Introduction
Physiological as well as pharmacological modulation of the renal actions of dopamine influences sodium homeostasis (Aperia, 2000). Dopamine exerts its effects on sodium excretion in part by inhibiting the Na+,K+-ATPase/Na+-H+-exchanger activity and thereby reducing trans-epithelial sodium transport across renal tubules (Pedemonte et al., 2005). Dopamine decreases Na+,K+-ATPase activity by promoting the internalization of active Na+,K+-ATPase molecules (Chibalin et al., 1997). Clathrin-mediated endocytosis of Na+,K+-ATPase molecules in response to dopamine is initiated after protein kinase C (PKC)-ζ-dependent phosphorylation of the Ser-18 residue within the α-subunit (Chibalin et al., 1999; Efendiev et al., 1999). The subsequent association of the 14-3-3 protein (regulatory molecule capable of binding phosphorylated Ser/Thr motifs) with the phosphorylated site leads to the binding and activation of phosphatidylinositol 3-kinase (PI 3-kinase) (Yudowski et al., 2000; Efendiev et al., 2005). PI 3-kinase favours the binding of AP-2 to the Tyr-537 residue within the α-subunit and clathrin recruitment (Ogimoto et al., 2000; Done et al., 2002) to the Na+,K+-ATPase complex at the plasma membrane interface. On the contrary, angiotensin II (ATII) signals alter renal Na+,K+-ATPase activity in the opposite direction by increasing its activity (Garvin, 1991) through the incorporation of new/active units into the plasma membrane (Efendiev et al., 2003). Similarly to that of dopamine, this effect requires a complex interplay of distinct signalling networks which begins from the phosphorylation of Ser-11 and Ser-18 within the α-subunit by PKC-β (Efendiev et al., 2003) to the assembly of AP1-mediated clathrin-coated vesicle formation (Efendiev et al., 2008). Altogether, these observations highlight the necessity of protein–protein interaction modules that can organize the signalling networks triggered by dopamine and ATII in time and space in order to provide a hierarchical organization needed to avoid deleterious cross-talk.
PDZ domains (postsynaptic protein PSD-95/SAP90, Drosophila septate junction protein Discs-large, tight junction protein Zo-1) within proteins can provide such organized structures. All PDZ domains have a conserved structure with a hydrophobic binding pocket that interacts in a sequence-specific manner with short peptide motifs located within or at the C-terminus of the target protein (Sheng and Sala, 2001). Their major function is to act as scaffolds for the assembly of large protein complexes at specific subcellular locations (Sheng and Sala, 2001) because they can act in combination with other modular protein interaction domains to generate more complex structures, or be easily linked together to form multi-PDZ proteins.
INAD (inactivation no-after potential) is a multi-PDZ domain protein that was originally discovered by its effects in organizing the phototransduction cascade in Drosophila (Tsunoda et al., 1997). The PDZ-mediated interactions with the involved signalling molecules are mediated by classical C-terminal as well as non-classical internal peptide interactions (Harris et al., 2001). Human INAD-like protein (hINADl), cloned as a homologue of INAD, has 10 PDZ domains among which seven have been well characterized (Philipp and Flockerzi, 1997; Vaccaro et al., 2001; Shin et al., 2005). Later, it was recognized to be more related to the Drosophila Discs lost and it was found to associate with tight junctions in mammalian cells (Lemmers et al., 2002). hINADl also contains one L27 domain in the N-terminus through which it can recruit the Maguk protein Pals1 to the tight junction, and was therefore renamed ‘Pals-associated tight junction protein’ (PATJ) (Roh et al., 2002).
In this study we focused our attention on PATJ as a potential scaffold protein organizing the responses to receptor signals because of its presence in renal (Shin et al., 2005) and intestinal (Lemmers et al., 2002) ion-transporting epithelial cells where it may be involved in the regulation of cell polarity by association with components of the tight junctions. In particular, we examined whether dopamine-, ATII- and insulin-dependent modulation of active sodium transport requires PATJ by measuring the changes in Na+,K+-ATPase activity in opossum kidney (OK) cells transiently expressing PATJ carrying selective deletions in its PDZ domains.
Methods
Preparation of the plasmids
The cDNA for human hINADl/PATJ (pcDNA2.hINAD) was kindly provided by Dr V. Flockerzi (Homburg, Germany) (Philipp and Flockerzi, 1997). To obtain an expression construct for C-terminally green fluorescent protein (GFP)-tagged hINADl/PATJ, we first changed nucleotides encoding Val1549 from GTT to GAT, thus creating a ClaI site, which allowed in-frame fusion with the cDNA of enhanced GFP and generation of pcDNA2.hINADl/PATJ-GFP. Next we subcloned the human CMV promoter in front of the hINADl/PATJ-GFP fusion gene, thus generating pcDNA2.CMV.hINADl/PATJ-GFP. Deletions of individual PDZ domains 1, 2, 4, 5 or 6, respectively, were performed on pcDNA2.CMV.hINAD-GFP by introducing a set of unique restriction sites into the cDNA 5′ and 3′ of the sequences encoding the respective domain. The unique set of restriction sites for each mutant was generated by exchanging nucleotides as follows: EheI for PDZ 1 (Δ130-217a.a, GGCCGG versus GGCGCC, GTGGCC versus GGCGCC), BbrPI for PDZ 2 (Δ248-323a.a, CATGTT versus CACGTG, CTCGTT versus CACGTG), HpaI for PDZ 4 (Δ685-766a.a, GTCAAG versus GTTAAC, GTACAC versus GTTAAC), BlnI for PDZ 5 (Δ1068-1152a.a, CCGAGA versus CCTAGG, CCTGTG versus CCTAGG), SalI for PDZ 6 (Δ1234-1315a.a, GGAGAA versus GTCGAC, GTCAAG versus GTCGAC). All resulting mutant plasmids were then digested with the corresponding enzyme to delete the respective PDZ domain and ligated overnight using a T4 ligase (Roche, IN, USA) and amplified by transformation in competent Escherichia coli. Positive clones were selected by restriction enzyme analysis and deletions were confirmed by DNA sequencing. All mutations were performed by employing the QuikChangeXL site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) and respective oligonucleotides purchased from Proligo (Paris, France). All vector constructions were verified by DNA sequence analysis.
Cell culture and transfection
The OK epithelial cell line has been used as a physiological model system to study renal proximal tubule response to hormones (Efendiev et al., 2002; Khundmiri et al., 2004). OK cells were maintained at 37°C (10% CO2) in Dulbecco's modified Eagle's medium (DMEM) with 10% bovine serum (GIBCOL, Invitrogen, Carlsbad, CA, USA) and antibiotics. Plasmids were introduced into cells using liposomes (LipofectAMINE 2000, Invitrogen). PATJ expression was monitored by confocal microscopy of the GFP tag used as a reporter and by Western blot using a GFP and PATJ antibody. The lower temperature (25°C for at least 72 h) of the culture conditions optimizes PATJ expression level and increases cell viability.
Determination of Na+,K+-ATPase activity
Na+,K+-ATPase activity was determined from the ouabain-inhibitable transport of 86Rb (Efendiev et al., 2000). Briefly, after 2-h incubation (at room temperature) with 10 mM EGTA the cells were transferred to serum-free DMEM containing 50 mM HEPES, pH 7.4 with or without 5 mM ouabain. Thereafter, a trace amount (1 µCi per well) of 86Rb (Perkin-Elmer, Waltham, MA, USA) was added to the cell medium. After 20-min incubation at room temperature, the cells were washed with ice-cold saline and dissolved with 3% SDS, and the accumulated radioactivity was determined. Na+,K+-ATPase-mediated Rb+ transport was calculated from the difference in tracer uptake between samples incubated with and without 5 mM ouabain. Na+,K+-ATPase activity was expressed as nmol of Rb+ mg protein−1 min−1 from triplicate determinations. To assess the effect of dopamine, cells were pre-incubated at room temperature with 5 µM monensin for 30 min (Efendiev et al., 2000), and then with 1 µM dopamine (5 min), or only (without monensin) with ATII (1 pM) for 10 min before the assays. OK cells were incubated with insulin (50 nM) for 10 min at room temperature.
Confocal microscopy
Expression of GFP-tagged PATJ was evaluated by confocal microscopy exactly as described before (Efendiev et al., 2004). PATJ was detected by the intrinsic fluorescence of GFP using a confocal laser-scanning microscope (Leica TCS SP2, Leica Lasertechnik GmbH, Heidelberg, Germany). The confocal microscope was equipped with an Ar/Kr laser and a double dichroic mirror and a 63× lens (Leica HCX PL APO 63x/1.20-0.17, UV, Heidelberg, Germany).
Immunoprecipitation
Opossum kidney cells were washed with PBS and homogenized in 50 mM mannitol, 5 mM HEPES-Tris buffer (pH 7.6) by freezing/thawing followed by vortexing, and by passing through a 27.5-G needle and motor pestle. The cell lysates were centrifuged to remove the nuclear fraction. The supernatants were further centrifuged at 25 000× g for 20 min to isolate the crude membrane fraction. Cell lysates or membranes were solubilized in immunoprecipitation buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 20 mM NaF, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10 µg mL−1 aprotinin, 1 mM PMSF, 5 µg mL−1 pepstatin A, 5 µg mL−1 leupeptin, 5 µg mL−1 antipain). Aliquots of the supernatant (∼400 µg) and/or membranes (∼80 µg) were pre-cleared and transferred to pre-coated (with desired antibody) ExactaCruz beads and incubated overnight. The beads were washed with 150 mM NaCl, and samples analysed by SDS-PAGE using the Laemmli buffer system (Laemmli, 1970), and Western blot was performed using specific antibodies. Proteins were detected by chemiluminescence (GE Healthcare, Uppsala) and quantification performed as described previously (Chibalin et al., 1997).
Statistical analysis
Analysis of the data was performed with the unpaired Student's t-test. P values less than 0.05 were considered significant.
Materials
Antibodies used were as follows – against PATJ, used for Western blots, was a gift of Dr A. Le Bivic (Marseille, France) (Lemmers et al., 2002), against GFP (JL-8) was from Clontech, CA, against the Na+,K+-ATPase α-subunit (6H, 1:500) (Pietrini et al., 1992) that was utilized in Western blots was a gift of Dr M.J. Caplan (Yale University, New Haven, CT, USA) and for immunoprecipitation (5α) was obtained from the Developmental Studies Hybridoma Bank (University of Iowa), against the dopamine D1 receptor (AB1765P) was from Chemicon, CA, against ATII AT1 receptor (sc-1173), against the insulin receptor β subunit (sc-711), against phospholipase C (PLC)-γ1 (sc-426), PKC-ζ (sc-216) and PI 3-kinase (sc-423) were from Santa Cruz Biotech, CA. Monensin, dopamine, ATII and insulin were purchased from Sigma. All reagents were of analytical grade. Receptor nomenclature follows Alexander et al. (2008).
Results
A human PATJ antibody does not recognize the endogenous PATJ in non-transfected OK cells (Figure 1A). We used ectopic expression of PATJ to test the hypothesis that it may serve as a scaffold protein organizing dopamine signals modulating Na+,K+-ATPase activity in OK cells. PATJ-GFP expression levels reached a plateau at 72 h post transfection (Figure 1A), therefore all further studies were performed at this time. Three proteins were detected: a 230 kDa protein (259 kDa including the GFP tag) represents the full-length PATJ whereas the 200 kDa (229 kDa including the GFP tag) and 135 kDa protein (164 kDa including the GFP tag) are possibly due to alternative translation initiation sites. For simplicity, they will subsequently be named in text and figures after their original Mr. Similar expression patterns have been demonstrated previously in HeLa and Caco-2 cells (Lemmers et al., 2002). The majority of all isoforms were similarly distributed between the cytosol and membrane compartments (Figure 1B). The fact that PATJ is also seen in the cytosol may represent proteins that have not yet reached the plasma membrane due to artificially increased synthesis and therefore does not necessarily represent its natural location within the cell, or that our cells in culture dishes are not fully polarized and thereby PATJ resides in intracellular vesicle stores as previously described (Roh et al., 2002).
Figure 1.
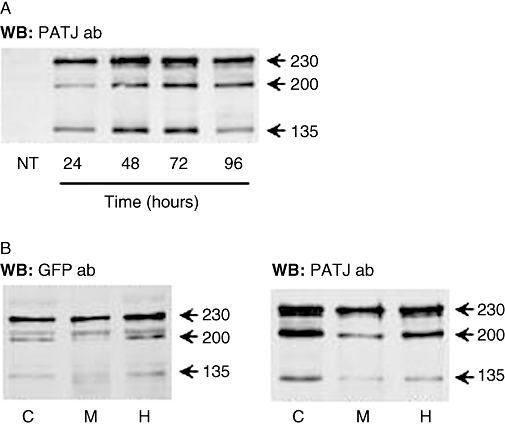
Expression and localization of Pals-associated tight junction protein (PATJ) in opossum kidney (OK) cells. (A) Time-dependent expression of PATJ tagged with green fluorescent protein (GFP) in OK cells (representative Western blot using the PATJ antibody). NT, non-transfected cells. (B) PATJ expression in homogenate (H), membrane (M) and cytosolic (C) fractions (120 µg protein each) from OK cells transfected with PATJ-GFP. Representative Western blot using antibodies against GFP (left panel) and PATJ (right panel).
The interaction of PATJ with potential network partners of the dopamine signalling cascade was further analysed in OK cells expressing PATJ-GFP. PATJ co-immunoprecipitated with the Na+,K+-ATPase α-subunit (Figure 2A) under basal (non-stimulating) conditions, whereas it only co-immunoprecipitated with the dopamine D1 receptor 1 (49 kDa) upon treatment with dopamine (Figure 2B). Additionally, PATJ constitutively associated with the p85α subunit (85 kDa) of PI 3-kinase (Figure 2C), the PKC-ζ (80 kDa) (Figure 2D) and the PLC-γ1 (155 kDa) isoforms (Figure 2E).
Figure 2.
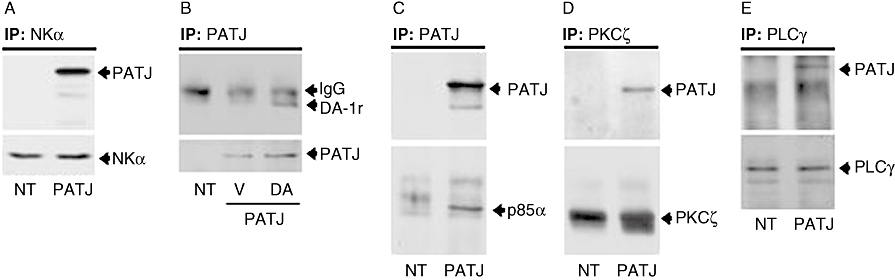
Interaction of Pals-associated tight junction protein (PATJ) with different components of the dopamine/Na+,K+-ATPase signalling network in cells transiently expressing PATJ-GFP (PATJ) and in non-transfected cells (NT). (A) Association of PATJ with the Na+,K+-ATPase α-subunit (Mr = 95 kDa) immunoprecipitated from plasma membranes derived from opossum kidney (OK) cells. (B) Association of PATJ immunoprecipitated with a GFP antibody from plasma membranes from OK cells treated with (DA) or without (V) dopamine (1 µM at 23°C during 5 min; dopamine D1 receptor, DA1-r). (C) Association of PATJ immunoprecipitated with a GFP antibody from OK cells lysates with the PI 3-kinase p85α subunit. (D) Association of immunoprecipitated PKC-ζ from OK cells membranes with PATJ. (E) Association of immunoprecipitated PLCγ from OK cells lysates with PATJ. All co-immunoprecipitation experiments were repeated on three separate occasions and a representative Western blot is shown.
To study the functional relevance of PATJ during dopamine-induced changes in Na+,K+-ATPase activity, we generated PATJ mutants in which individual PDZ domains were deleted. Binding specificity of PDZ domains has been well studied (Table 1), PDZ domain 1, 2, 3 and 4 recognize class II ligands, PDZ domain 5, 6 and 7 recognize class I ligands, while PDZ domain 3 also recognize novel class IV ligands (Vaccaro et al., 2001). We have chosen domain 1, 2, 4, 5 and 6 as examples for each class (Figure 3A). Confocal microscopy (Figure 3B) and Western blot (Figure 3C) using antibodies against GFP and PATJ (epitope conserved in all deletion mutants, Figure 3A) confirmed the successful expression of all three PATJ proteins. Western blot analysis revealed that wild type PATJ-GFP was expressed as three distinct proteins of 230, 200 and 135 kDa. The 230 kDa protein was present in all constructs where obvious mobility shifts were observed between the PATJ wild type (WT) and the deletion mutants, due to the removal of individual PDZ domains. The 200 kDa protein was expressed in all constructs except Del. 2. This could be due to an alternative translation start site within PDZ2 (possibly be Met304) that was removed in Del. 2, the similar expression pattern of 200 kDa in WT and Del. 1 also confirmed this assumption. The 135 kDa protein was also present in all constructs and the shift in Del. 4 is probably due to the alternative translation initiation site which might be Met853 before PDZ 4.
Table 1.
Schematic representation of known PDZ ligand binding preferences
| Distal C-terminus motif | −2 | −1 | 0 |
|---|---|---|---|
| Class I | Ser/Thr | X | Φ |
| Class II | Φ/Ψ | X | Φ |
| Class III | Asp/Glu | X | Φ |
| Class IV | X | Ψ | Asp/Glu |
X: any amino acid
Φ: hydrophobic amino acid
Ψ: aromatic amino acid
Figure 3.
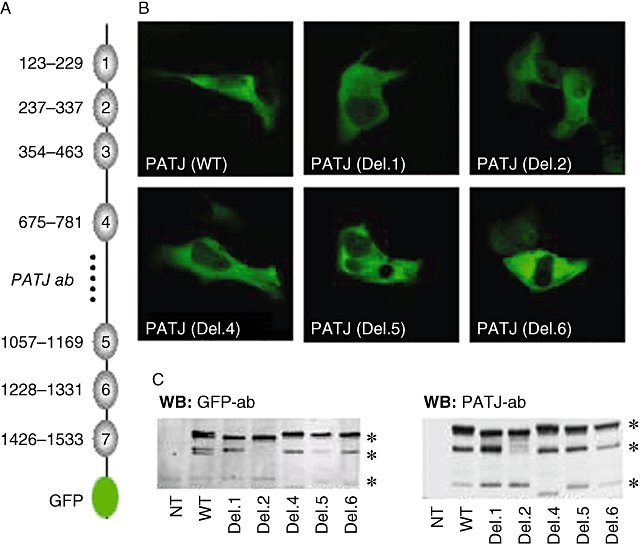
Expression and localization of Pals-associated tight junction protein (PATJ) deletion mutants in opossum kidney (OK) cells. (A) Schematic view of PATJ containing the seven PDZ domains (with corresponding amino acids) and the GFP tag at the C-terminus; the dotted line depicts the area (amino acids: 800–1000) within PATJ recognized by the antibody used. (B) Images of cells expressing the PATJ wild type (WT) and with individual PDZ domain deletions (Del. 1; 2; 4; 5; 6) are shown, as indicated. (C) PATJ wild type and deletion mutants were expressed in OK cells and their presence in membranes examined by Western blot with a GFP antibody (1:1000, left panel) and PATJ antibody (1:200, right panel). Equal amounts of protein were loaded for each condition (150 µg). *Different PTAJ Mr protein.
The presence of various PATJ mutants did not affect the total expression of Na+,K+-ATPase levels in OK cells (Figure 4A). Additionally, Na+,K+-ATPase activity (reflecting plasma membrane abundance) was not significantly affected by the presence of PATJ mutants although there was a trend indicating a slight reduction in cells expressing Del. 2 (Figure 4B). Increases in intracellular sodium concentration induced by monensin resulted in similar increases in Na+,K+-ATPase activity among all groups, although a higher trend was observed in cells expressing Del. 5 (Figure 4C).
Figure 4.
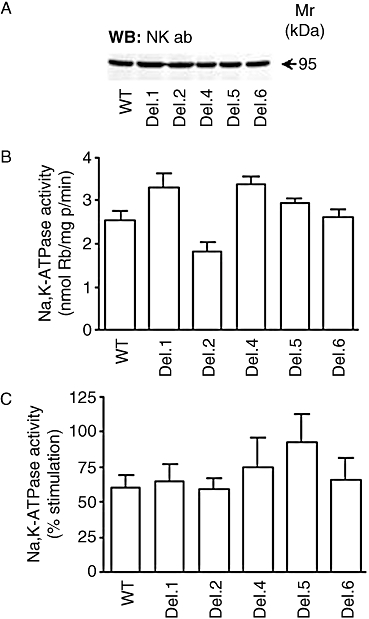
Expression of Na+,K+-ATPase α-subunit and activity in cells transiently transfected with different Pals-associated tight junction protein (PATJ) cDNA mutants. (A) Abundance of Na+,K+-ATPase (Na+,K+-ATPase α-subunit antibody) was examined in cell lysates obtained from opossum kidney (OK) cells expressing different PATJ deletion mutants by Western blot using antibodies against Na+,K+-ATPase (1:500). Equal amount of protein (50 µg) were loaded for each sample. (B) Functional relevance of PATJ during basal and (C) sodium-stimulated Na+,K+-ATPase activity (5 µM monensin for 30 min at room temperature). The studies were performed in OK cells expressing PATJ wild type (WT) and several deletion mutants. Each bar represents the mean + SEM of 8–11 independent experiments performed in triplicate determinations.
We next studied whether PATJ mediated the changes in Na+,K+-ATPase activity in response to dopamine signals. The presence of various PATJ mutants did not affect the total expression of dopamine D1 receptors in OK cells (Figure 5A, upper panel). Dopamine decreases proportionally (∼30% inhibition) the Na+,K+-ATPase activity in cells transfected with the PATJ WT, Del. 1 and 6 mutants (Figure 5A, lower panel), whereas this effect was lost in cells expressing the Del. 2, Del. 4 and Del. 5 mutants. These results demonstrate that PATJ is of functional relevance during dopamine-induced changes in Na+,K+-ATPase activity. It is, however, difficult at this stage to predict the specific role of individual PDZ domains in this regulation, as their deletions may or may not affect the overall structure of the protein. Noteworthy, the presence of wild type PATJ does not affect the basal Na+,K+-ATPase activity, nor that induced by dopamine or ATII, compared with mock-transfected cells (not shown).
Figure 5.
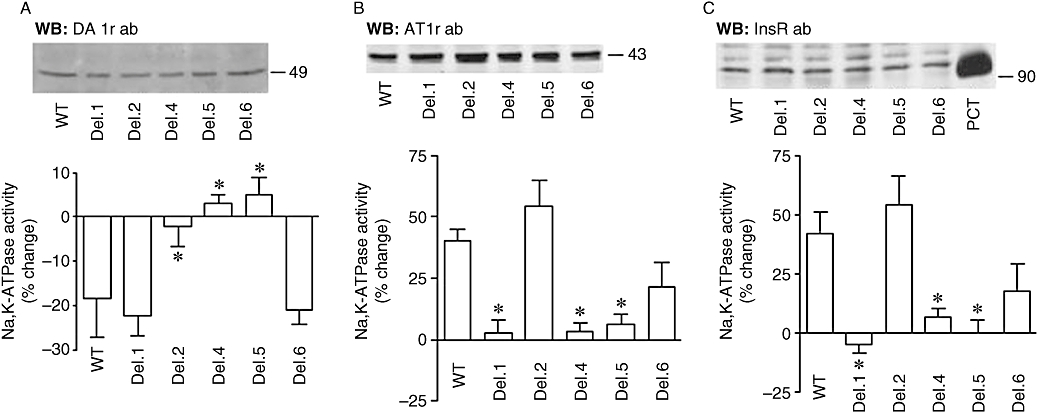
Effect of dopamine, angiotensin II (ATII) and insulin on Na+,K+-ATPase in opossum kidney (OK) cells transiently expressing different Pals-associated tight junction protein (PATJ) mutants. Na+,K+-ATPase activity was examined in OK cells expressing PATJ wild type (WT) or bearing individual PDZ domain deletions. (A) Abundance of dopamine receptors (upper panel) was examined in cell lysates obtained from OK cells expressing different PATJ deletion mutants by Western blot using antibodies against dopamine D1 receptors (DA1-r; 1:500). Equal amount of protein (50 µg) were loaded for each sample. Functional relevance of PATJ during dopamine-dependent inhibition of Na+,K+-ATPase activity in OK cells expressing PATJ wild type (WT) and deletion mutants (lower panel). OK cells were incubated with 5 µM monensin for 30 min and then 1 µM dopamine for 5 min before the assay. Na+,K+-ATPase activity was expressed as % change (in the absence of dopamine). Each bar represents the mean + SEM of five to nine independent experiments performed in triplicate determinations. *P < 0.01 versus WT. (B) Abundance of ATII receptors (AT1r) (upper panel) was examined in cell lysates obtained from OK cells expressing different PATJ deletion mutants by Western blot using a AT1 receptor antibody (1:500). Na+,K+-ATPase activity (lower panel) was determined in OK cells incubated with ATII (1 pM, for 10 min at 23°C). Bars represent the mean + SEM of six experiments performed independently and in triplicate determinations. *P < 0.01 versus WT. (C) Abundance of insulin receptor (InsRβ) (upper panel) was examined in cell lysates obtained from OK cells expressing different PATJ deletion mutants by Western blot using antibodies against the InsR receptor (1:500). Rat proximal convoluted tubules (PCT) lysates were used as positive control. Na+,K+-ATPase activity (lower panel) was determined in OK cells incubated with Ins (50 nM, for 10 min at 23°C). Bars represent the mean + SEM of six experiments performed independently and in triplicate determinations. *P < 0.01 versus WT.
Dopamine decreases Na+,K+-ATPase activity, whereas ATII increases its activity (Efendiev et al., 2003). Because of this, we next examined whether PATJ was also involved in the stimulatory responses to ATII and whether the effect of ATII on Na+,K+-ATPase activity was affected by PATJ mutants in a similar fashion to dopamine. Expression of PATJ WT and deletion mutants did not affect ATII receptor (43 kDa) expression (Figure 5B, upper panel). Transfected OK cells were incubated with ATII 1 pM for 10 min at 23°C. This concentration of ATII (and within this time frame) results in activation of Na+,K+-ATPase activity and incorporation of new molecules in the plasma membrane (Efendiev et al., 2003). In OK cells expressing the wild type form of PATJ, ATII induced a significant increase in Na+,K+-ATPase activity (Figure 5B, lower panel). The magnitude of stimulation was similar to previously described in non-transfected cells (Efendiev et al., 2003). However, the response to ATII in OK cells expressing the different PATJ deletion mutants was varied with the mutant form. The presence of PATJ carrying a deletion in its PDZ domain 2 did not affect the stimulatory effect of ATII on Na+,K+-ATPase activity, whereas expression of PATJ lacking either PDZ1, PDZ4 or PDZ5 prevented the stimulatory effect of ATII on Na+,K+-ATPase activity and the deletion of PDZ6 reduced the ATII stimulatory effect. These data suggest that PATJ was also functionally relevant during ATII-dependent stimulation of Na+,K+-ATPase-mediated sodium transport in epithelial cells. In parallel, we tested the role of PATJ during a non-GPCR-dependent stimulation of Na+,K+-ATPase activity. Insulin through its receptor (a tyrosine kinase receptor) activates Na+,K+-ATPase activity in renal cells derived from proximal tubules (Feraille et al., 1999). Expression of PATJ WT or mutants did not affect expression of the insulin receptor in OK cells (Figure 5C, upper panel). Interestingly, the Na+,K+-ATPase activity in response to insulin was similar to that induced by ATII in terms of magnitude (% change) as well as PDZ domain dependency (Figure 5C, lower panel). However, the effects of both ATII and insulin were different from those obtained with dopamine, regarding the PDZ domains involved.
Discussion and conclusions
This study demonstrates for the first time the ability of a multi-PDZ domain-containing protein of mammalian origin (PATJ) to interact with the dopamine, ATII and insulin receptor signalling network, and to participate functionally during their regulation of Na+,K+-ATPase activity. The predicted importance of these findings is directly related to the contribution of the Na+,K+-ATPase activity in ion-transporting epithelial cells (sodium reabsorption) and possibly to membrane potential (electrical activity and neurotransmitter release). Transport of sodium across the kidney tubules is accomplished by the concerted activity of Na+,K+-ATPase molecules present at the basolateral membrane and specific sodium transporters present at the apical domain of the renal tubule cells. Therefore, activation/inhibition by hormones of basolateral Na+,K+-ATPase would have a significant impact in net sodium excretion and contribute to total body sodium homeostasis.
It is known that each PDZ domain binds to a single peptide ligand and the PDZ peptide-binding groove prefers (although not exclusively) the C-terminus because of the free carboxylate group at the end of the PDZ ligand. The carboxylate-binding loop extends from a highly conserved arginine or lysine residue to the Gly-Leu-Gly-Phe motif (Sheng and Sala, 2001). Binding of the carboxylate-binding loop to the C-terminal residue (position 0) of PDZ ligand projects its side chain into the hydrophobic pocket of the PDZ domain, and the −2 residue of the ligand is an important determinant for PDZ binding and forms the basis for classification of PDZ specificity (Vaccaro and Dente, 2002). As shown in Figure 3, Class I ligands prefer either serine or threonine, Class II ligands prefer a hydrophobic or aromatic residue while Class III ligands select for negatively charged amino acids. The Class IV ligand is characterized by the presence of an acidic residue at the 0 position. Internal motifs (of those mentioned before) can also form binding sites for PDZ domains and are usually found in the β-hairpin structure formed by two anti-parallel β-strands (Brenman et al., 1996; Chevesich et al., 1997). Analysis of the amino acid sequences (rat species) for each binding protein revealed the presence of potential PDZ ligands. PKC-ζ possesses a class III PDZ ligand (-E−2-S−1-V0) at the C-terminus while the dopamine D1 receptor has a potential class III PDZ ligand (-E−13-K−12-I−11-) buried in the distal C-terminal region with a sequence similar to the internal PDZ ligand model proposed for GPCR (Paasche et al., 2005). Furthermore, by analysing the structure of Na+,K+-ATPase α-subunit it also revealed a potential class IV PDZ ligand (-A−498-S−499-E−500-) at the tip of the β-turn within ATP-binding domain. Unfortunately, due to the lack of structural data it was not possible to predict the possible internal PDZ ligands for PLC-γ and the PI 3-kinase p85α subunit.
Dopamine inhibited Na+,K+-ATPase activity in the presence of PATJ whereas removing individual PDZ domains 2, 4 or 5 within PATJ impaired such inhibitory effects on Na+,K+-ATPase activity. This indicates that PATJ through its PDZ domains may potentially organize the dopamine signalling pathway in order to mediate an inhibition of Na+,K+-ATPase activity. All known binding partners of PATJ including the Na+,K+-ATPase α-subunit, the dopamine D1 receptor, PLC-γ, PKC-ζ and PI 3-kinase p85α subunit could participate in forming the signalling complex through PDZ interactions. Similarly, both ATII and insulin stimulated Na+,K+-ATPase activity in the presence of PATJ but not in cells transfected with PATJ lacking either PDZ domain 1, 4 or 5. ATII and insulin stimulate Na+,K+-ATPase activity through distinct initial molecular mechanisms. ATII through its receptor (GPCR) activates PKC-β leading to phosphorylation of Ser-11 and Ser-18 residues within the Na+,K+-ATPase α-subunit (Efendiev et al., 2003) while insulin activates tyrosine kinase to phosphorylate Tyr-5 within Na+,K+-ATPase (Feraille et al., 1999). Interestingly, in the presence of PATJ deletion mutants both ATII and insulin increased Na+,K+-ATPase activity in a remarkably similar fashion which can be hypothetically interpreted as two independent signalling pathways that at one stage converge and share a common molecular mechanism during stimulation of Na+,K+-ATPase activity. Overexpression of PATJ in OK cells could result in other cellular effects because its PDZ domains may lead to the formation of other protein complexes. However, this possibility should not affect the interpretation of these results because the effects of dopamine, ATII, or insulin on Na+,K+-ATPase activity remained in cells expressing the wild type PATJ. Ideally, the information obtained from these studies could be complemented by depleting endogenous PATJ from the OK cells; however, a gene-silencing approach has not been used in this study due to the lack of PATJ sequence and identity in this cell line.
The role of PDZ domains within PATJ and other cellular processes such as distribution (polarity) of proteins in different subcellular domains has been highlighted (Biber et al., 2005). It would, therefore, seem likely that the constitutive association of PATJ with the Na+,K+-ATPase could also represent a relevant interaction governing the polarized distribution of this enzyme in transporting epithelia. Additionally, because in the presence of different PATJ mutants neither the basal nor the sodium-stimulated Na+,K+-ATPase activity was significantly affected, suggesting that its role may be selective to hormonal regulation of this enzyme, rather than being responsible for changing the housekeeping functions of the enzyme. A precedent for the involvement of PDZ domain protein (NHERF-1) during regulation of Na+,K+-ATPase activity has been reported in response to parathyroid hormone in renal epithelial cells (Khundmiri et al., 2005).
In summary, PATJ could serve as a scaffolding mechanism necessary not only for the integration of the dopamine receptor network, to add functionality in time and space and to avoid deleterious crosstalk, but also to organize, similarly, the stimulatory effect of insulin and ATII receptor signalling on Na+,K+-ATPase activity and cell sodium transport.
Acknowledgments
We are grateful to those researchers that provided us with valuable reagents: Dr A. Le Bivic (PATJ antibody, PATJ cDNA) and Dr V. Flockerzi (hINADl/PATJ cDNA). The constructive criticisms of Dr A. Le Bivic and the invaluable support of Professor A. Hamsten are greatly appreciated. This study was supported in part by funds from the Swedish Research Council (32X-10860 and 32P-14879 to AMB), the Swedish Heart and Lung Foundation, the Swedish Kidney Research Fund.
Glossary
Abbreviations:
- ATII
angiotensin II
- dINAD
drosophila INAD
- GFP
green fluorescent protein
- hINADl
human INAD-like
- INAD
inactivation no-after potential
- mINADl
mouse INAD-like
- OK
opossum kidney
- PATJ
Pals-associated tight junction protein
- PDZ
postsynaptic protein PSD-95/SAP90, Drosophila septate junction protein discs-large, tight junction protein Zo-1
- PI 3-K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PLC
phospholipase C
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–647. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]
- Biber J, Gisler SM, Hernando N, Murer H. Protein/protein interactions (PDZ) in proximal tubules. J Membr Biol. 2005;203:111–118. doi: 10.1007/s00232-005-0738-7. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Katz AI, Berggren PO, Bertorello AM. Receptor-mediated inhibition of renal Na(+)-K(+)-ATPase is associated with endocytosis of its alpha- and beta-subunits. Am J Physiol. 1997;273:C1458–C1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Feraille E, et al. Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and Is responsible for the decreased activity in epithelial cells. J Biol Chem. 1999;274:1920–1927. doi: 10.1074/jbc.274.4.1920. [DOI] [PubMed] [Google Scholar]
- Done SC, Leibiger IB, Efendiev R, Katz AI, Leibiger B, Berggren PO, et al. Tyrosine 537 within the Na+,K+-ATPase alpha-subunit is essential for AP-2 binding and clathrin-dependent endocytosis. J Biol Chem. 2002;277:17108–17111. doi: 10.1074/jbc.M201326200. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Bertorello AM, Pedemonte CH. PKC-beta and PKC-zeta mediate opposing effects on proximal tubule Na+,K+-ATPase activity. FEBS Lett. 1999;456:45–48. doi: 10.1016/s0014-5793(99)00925-4. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Bertorello AM, Pressley TA, Rousselot M, Feraille E, Pedemonte CH. Simultaneous phosphorylation of Ser11 and Ser18 in the alpha-subunit promotes the recruitment of Na(+),K(+)-ATPase molecules to the plasma membrane. Biochemistry. 2000;39:9884–9892. doi: 10.1021/bi0007831. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Yudowski GA, Zwiller J, Leibiger B, Katz AI, Berggren PO, et al. Relevance of dopamine signals anchoring dynamin-2 to the plasma membrane during Na+,K+-ATPase endocytosis. J Biol Chem. 2002;277:44108–44114. doi: 10.1074/jbc.M205173200. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem. 2003;278:28719–28726. doi: 10.1074/jbc.M303741200. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Krmar RT, Ogimoto G, Zwiller J, Tripodi G, Katz AI, et al. Hypertension-linked mutation in the adducin alpha-subunit leads to higher AP2-mu2 phosphorylation and impaired Na+,K+-ATPase trafficking in response to GPCR signals and intracellular sodium. Circ Res. 2004;95:1100–1108. doi: 10.1161/01.RES.0000149570.20845.89. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Chen Z, Krmar RT, Uhles S, Katz AI, Pedemonte CH, et al. The 14-3-3 protein translates the NA+,K+-ATPase {alpha}1-subunit phosphorylation signal into binding and activation of phosphoinositide 3-kinase during endocytosis. J Biol Chem. 2005;280:16272–16277. doi: 10.1074/jbc.M500486200. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Budu CE, Bertorello AM, Pedemonte CH. GPCR-mediated traffic of Na,K-ATPase to the plasma membrane requires the binding of adaptor protein1 to a Tyr-255 based sequence in the alpha -subunit. J Biol Chem. 2008;283:17561–17567. doi: 10.1074/jbc.M709260200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraille E, Carranza ML, Gonin S, Beguin P, Pedemonte C, Rousselot M, et al. Insulin-induced stimulation of Na+,K(+)-ATPase activity in kidney proximal tubule cells depends on phosphorylation of the alpha-subunit at Tyr-10. Mol Biol Cell. 1999;10:2847–2859. doi: 10.1091/mbc.10.9.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin JL. Angiotensin stimulates bicarbonate transport and Na+/K+ ATPase in rat proximal straight tubules. J Am Soc Nephrol. 1991;1:1146–1152. doi: 10.1681/ASN.V1101146. [DOI] [PubMed] [Google Scholar]
- Harris BZ, Hillier BJ, Lim WA. Energetic determinants of internal motif recognition by PDZ domains. Biochemistry. 2001;40:5921–5930. doi: 10.1021/bi010142l. [DOI] [PubMed] [Google Scholar]
- Khundmiri SJ, Bertorello AM, Delamere NA, Lederer ED. Clathrin-mediated endocytosis of Na+,K+-ATPase in response to parathyroid hormone requires ERK-dependent phosphorylation of Ser-11 within the alpha1-subunit. J Biol Chem. 2004;279:17418–17427. doi: 10.1074/jbc.M311715200. [DOI] [PubMed] [Google Scholar]
- Khundmiri SJ, Weinman EJ, Steplock D, Cole J, Ahmad A, Baumann PD, et al. Parathyroid hormone regulation of NA+,K+-ATPase requires the PDZ 1 domain of sodium hydrogen exchanger regulatory factor-1 in opossum kidney cells. J Am Soc Nephrol. 2005;16:2598–2607. doi: 10.1681/ASN.2004121049. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemmers C, Medina E, Delgrossi MH, Michel D, Arsanto JP, Le Bivic A. hINADl/PATJ, a homolog of discs lost, interacts with crumbs and localizes to tight junctions in human epithelial cells. J Biol Chem. 2002;277:25408–25415. doi: 10.1074/jbc.M202196200. [DOI] [PubMed] [Google Scholar]
- Ogimoto G, Yudowski GA, Barker CJ, Kohler M, Katz AI, Feraille E, et al. G protein-coupled receptors regulate Na+,K+-ATPase activity and endocytosis by modulating the recruitment of adaptor protein 2 and clathrin. Proc Natl Acad Sci USA. 2000;97:3242–3247. doi: 10.1073/pnas.060025597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasche JD, Attramadal T, Kristiansen K, Oksvold MP, Johansen HK, Huitfeldt HS, et al. Subtype-specific sorting of the ETA endothelin receptor by a novel endocytic recycling signal for G protein-coupled receptors. Mol Pharmacol. 2005;67:1581–1590. doi: 10.1124/mol.104.007013. [DOI] [PubMed] [Google Scholar]
- Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na,K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol. 2005;25:322–327. doi: 10.1016/j.semnephrol.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Philipp S, Flockerzi V. Molecular characterization of a novel human PDZ domain protein with homology to INAD from Drosophila melanogaster. FEBS Lett. 1997;413:243–248. doi: 10.1016/s0014-5793(97)00877-6. [DOI] [PubMed] [Google Scholar]
- Pietrini G, Matteoli M, Banker G, Caplan MJ. Isoforms of the Na,K-ATPase are present in both axons and dendrites of hippocampal neurons in culture. Proc Natl Acad Sci USA. 1992;89:8414–8418. doi: 10.1073/pnas.89.18.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh MH, Makarova O, Liu CJ, Shin K, Lee S, Laurinec S, et al. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J Cell Biol. 2002;157:161–172. doi: 10.1083/jcb.200109010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shin K, Straight S, Margolis B. PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J Cell Biol. 2005;168:705–711. doi: 10.1083/jcb.200408064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, et al. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- Vaccaro P, Dente L. PDZ domains: troubles in classification. FEBS Lett. 2002;512:345–349. doi: 10.1016/s0014-5793(02)02220-2. [DOI] [PubMed] [Google Scholar]
- Vaccaro P, Brannetti B, Montecchi-Palazzi L, Philipp S, Helmer Citterich M, Cesareni G, et al. Distinct binding specificity of the multiple PDZ domains of INADL, a human protein with homology to INAD from Drosophila melanogaster. J Biol Chem. 2001;276:42122–42130. doi: 10.1074/jbc.M104208200. [DOI] [PubMed] [Google Scholar]
- Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, Bertorello AM. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. Proc Natl Acad Sci USA. 2000;97:6556–6561. doi: 10.1073/pnas.100128297. [DOI] [PMC free article] [PubMed] [Google Scholar]


