Abstract
Objective
Neurovascular niches have been proposed as critical components of the neural stem cell (NSC) response to acute central nervous system (CNS) injury, however, it is unclear whether these potential reparative niches remain functional during chronic injury. Here we asked how CNS inflammatory injury regulates the intrinsic properties of NSCs and their niches.
Methods
We investigated the sonic hedgehog (Shh)-Gli1 pathway, an important signaling pathway for NSCs, in experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis (MS) and its regulation of by inflammatory cytokines.
Results
We show that Shh is markedly up-regulated by reactive and perivascular astroglia in areas of injury in MS lesions and during EAE. Astroglia outside the subventricular zone (SVZ) niche can support NSC differentiation towards neurons and oligodendrocytes and Shh is a critical mediator of this effect. Shh induces differential upregulation of the transcription factor Gli1, which mediates Shh-induced NSC differentiation. However, despite the increase in Shh and the fact that Gli1 was initially increased during early inflammation of EAE and active lesions of MS, Gli1 was significantly decreased in spinal cord oligodendrocyte precursor cells (OPCs) after onset of EAE and in chronic active and inactive lesions from MS brain. The Th1 cytokine IFN-γ was unique in inducing Shh expression in astroglia and NSCs, while paradoxically suppressing Gli1 expression in NSCs and inhibiting Shh-mediated NSC differentiation.
Interpretation
Our data suggest that endogenous repair potential during chronic injury appears to be limited by inflammation-induced alterations in intrinsic NSC molecular pathways such as Gli1.
INTRODUCTION
MS and its animal model EAE are inflammatory and demyelinating diseases of the CNS characterized by robust perivascular immune cell infiltration, demyelination, astroglia activation and axonal injury followed by astroglial plaques1. Although spontaneous remyelination occurs in MS, it is greatly limited2. Premyelinating oligodendrocytes have been described in MS and EAE lesions1,3. However, OPC differentiation and functional remyelination is lackingin MS or EAE lesions, unlike what happens in neurotoxin induced demyelination4. In addition, the activation of the SVZ niche in EAE and in MS is limited5,6, compared to stroke or other models of focal neurodegeneration, where the SVZ stem cells migrate to areas of injury and differentiate to replace the missing cells7. These results suggest that the microenvironment may be permissive for the neural stem/precursor cells in some diseases and not permissive in others8.
Astroglia are the most abundant cells in the CNS and are part of the typical neural stem cell niches in adult forebrain, namely the SVZ and the dentate gyrus. Astroglia act as multipotent stem cells as well as niche cells, supporting the NSCs by cell-cell contact and by producing niche molecules9,10. It is unclear whether astroglia outside the typical niche can function as niche cells under certain conditions. It is hypothesized that the injury itself could reactivate programs in astroglia and endothelial cells. We have shown that the SVZ niche responds to the inflammatory microenvironment11 in EAE and those areas of injury create atypical injury-induced niches formed by perivascular as well as parenchymal astroglia and endothelial cells. One of the mediators is SDF-1α that acts through CXCR4 receptor on NSCs facilitating the migration and differentiation of exogenous stem cells8,12. Thus, the area of injury can re-create a transient permissive microenvironment or ectopic niches to facilitate stem cell migration, differentiation and integration, raising the possibility that other stem cell regulators may be re-expressed in these areas but confirmation of their role is still lacking13.
Sonic hedgehog is one member of the hedgehog family morphogens that play important roles in development of many tissues and organs. Shh is crucial in regulating stem cell niches and NSC proliferation in postnatal telecephalon14, adult hippocampus15, and SVZ16. Shh is also required for oligodendrogenesis14,17 and plays a role in OPC migration18. Shh signaling is tightly regulated; when Shh binds to its receptor Patched1, it releases the inhibition of the associated signaling receptor Smoothened (Smo), resulting in upregulation and nuclear translocation of Gli transcription factors, Gli1, Gli2 and Gli319. Increased Shh reactivity has been reported after neural injury20,21, and Shh may play a role in neuronal repair and oligodendrocyte maturation22,23. However, it is unclear how Shh is regulated during inflammation outside the classical NSC niche in adult CNS.
Here, we demonstrate that inflammation in EAE and MS induces reactivation of the developmental molecule Shh, but paradoxically inhibits its downstream transcription factor Gli1. We show that non-niche astroglia regulate the NSCs differentiation by inducing the expression of Shh. Inflammatory cytokines, specifically IFN-γ can increase Shh in astroglia but paradoxically down-regulate Gli1 expression in NSCs, thus disrupting the program of Shh induced NSCs differentiation. These data suggest a novel mechanism underlying the negative effects of inflammation on repair during chronic injury.
METHODS
Animals
C57BL/6 and C57BL/6-Tg (UBC-GFP) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). MOG-TCR transgenic 2D2 mice were provided by Dr. Vijay K. Kuchroo in Harvard Medical School. All mice were housed according to NIH guidelines and the Animal Care Committee of Harvard University approved all experiments.
Human brain specimen
Human brain samples were obtained from Rocky Mountain MS Center Tissue Bank (Englewood, Colorado, USA) and Human Brain and Spinal Fluid Resource Center, University of California (Los Angeles, CA, USA). MS samples from 25 cases are listed in Supplemental Table 1 (See supplemental method for detail).
Primary neural stem cell and astroglia isolation and culture
E14.5 or E12.5 NSCs were isolated from cerebral cortices of timed C57BL/6 or UBC-GFP mouse embryos. Adult NSCs and astroglia were isolated from the SVZ or the spinal cords of adult C57BL/6 mice, respectively11. For the cytokine treatment experiment, astroglia were isolated from the neonatal mice hemispheres on postnatal day 0 (See supplemental methods for detail).
NSC differentiation
Neurospheres initially cultured in FGF/EGF containing media were plated on poly-D-lysine (PDL)-coated coverslips or on top of adult astroglia monolayer without FGF/EGF for 5 days in the present with 5 μg/ml Shh neutralizing antibodies (Sigma-Aldrich or 5E1 from Developmental Studies Hybridoma Bank, Iowa City, IA, USA) or the same concentration of control IgG.
Gli1 gene silencing
NSCs were treated with recombinant mouse Shh, amino-terminal peptide (1μg/ml) for 24 hours then transfected with 2 μM SMARTpool Gli1 siRNA (Dharmacon Inc. Lafayette, Colorado, USA) with mouse NSC nucleofector device (Amaxa, Gaithersburg, Maryland, USA). Suppression of RNA expression was evaluated after 24 hours and 4 days.
Statistical Analysis
The comparisons for percentage of cells in this paper were presented as mean ± SD. We used unpaired t-test with Welch’s correction for statistical analysis of percentage data and expression profiles and Mann-Whitney test for fold difference data using Prism 4.0.
RESULTS
The stem cell regulator Shh is upregulated by astroglia in MS and EAE
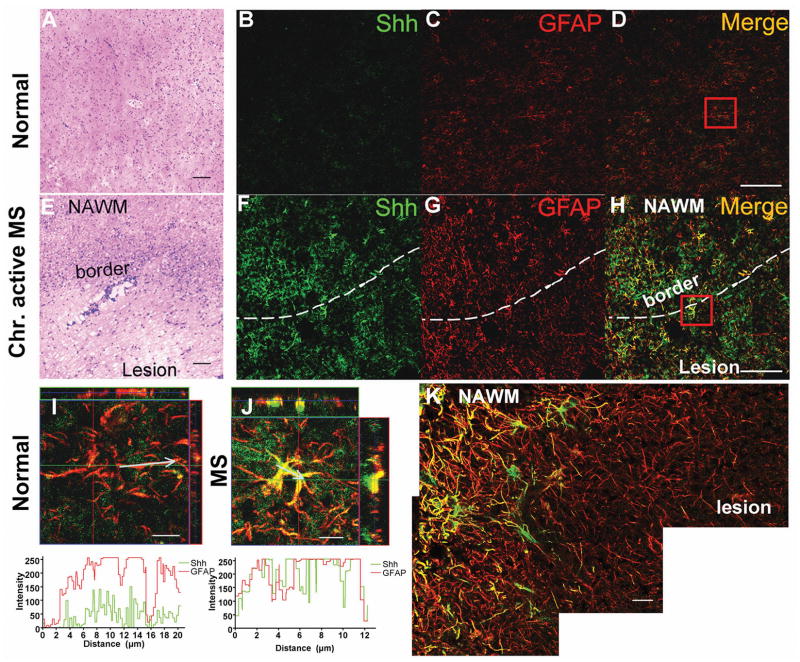
We found that Shh is expressed in the SVZ (Supplemental Figure 1A), and constitutively expressed at low levels in the spinal cord of adult naive mice, where it co-localizes with glial fibrillary acidic protein (GFAP) (Supplemental Figure 1B). We then examined Shh expression in normal human brain and chronic active MS (Figure 1A, E). We found significant upregulation of Shh in hypertrophic astroglia (Figure 1F) associated with enhanced GFAP expression (Figure 1G, H) in the MS lesions compared to normal brain (Figure 1B, C and D). Shh expression was most intense at the border of the lesions and the adjacent normal appearing white matter (NAWM), and less so at the center of the lesions (Figure 1K). Shh expression profile of astroglia from MS was much higher than that was in normal brain (Figure 1I, J).
Figure 1. Shh expression in human MS lesions.
(A, E) H&E staining of frozen sections from normal and MS brain. The MS brain shows increased cellularity and perivascular infiltration at the lesion border. Scale bar, 100 μm. Immunofluorescence staining for Shh (green) and GFAP (red) from normal human brain (B, C, D) and chronic active MS lesions (F, G, H) shows upregulation of Shh in hypertrophic astroglia around the MS lesion border and NAWM, less so inside the lesion. Scale bar, 50 μm. (I, J) 3-D view and profile analysis of Shh (green) and GFAP (red) intensity on representative astroglia from normal (D) and MS brain (H) by LSM510 software. The chart indicates the intensity of Shh and GFAP alone the arrow line represented areas in the images. Scale bar, 10 μm. The images were acquired using the same parameters. (K) Stitched image of Shh and GFAP co-expression in chronic active MS lesions, showing Shh is up-regulated around the lesion border and NAWM, but less so inside the lesion. Scale bar, 20 μm. NAWM, normal appearing white matter.
We next examined the effect of the CNS inflammation on Shh expression during EAE. Shh expression was profoundly increased in hypertrophic astroglia in the spinal cord during the course of EAE, associated with the increased expression of GFAP. The upregulation of Shh was observed as early as day 10 post-immunization (dpi), before the onset of clinical signs, peaking between days 20 and 30 (Supplemental Figure 1B, B′). Furthermore, Shh was also up-regulated in perivascular astroglia (Supplemental Figure 1C) that have been proposed as atypical niches in stroke and EAE8,12,13. Shh was not up-regulated in the SVZ niche but was significantly increased in the corpus callosum astroglia of diseased animals (Supplemental Figure 2A–D). The upregulation of Shh was confirmed by western blot of EAE spinal cord from day 20 post-immunization (Supplemental Figure 1D) and its co-localization with GFAP confirmed by FACS analysis (Supplemental Figure 1E). Slight upregulation of Shh was also detected in neurons in the EAE spinal cord. No Shh expression was detected on oligodendrocyte lineage cells (data not shown). No change in the stem cell regulators BMP2 and 4 were observed in EAE (Supplemental Figure 2E).
Next, we investigated the specific inflammatory mediators that may up-regulate Shh in astroglia. Astroglia from postnatal day 0 were cultured for 7 days with a panel of cytokines including IFN-γ, TGF-β, IL-4, IL-10, IL-17 or co-cultured with splenocytes from MOG-transgenic mice that had been activated with MOG in vitro. Western blot analysis showed that most of the cytokines tested were able to up-regulate Shh on astroytes, but IFN-γ was the most potent inducer of Shh compared to untreated cells (p< 0.05) (Supplemental Figure 3A). The astroglial upregulation of Shh by IFN-γ was confirmed by immunofluorescence staining (Supplemental Figure 3B).
Adult astroglia regulate NSCs neuronal and glial differentiation by Shh
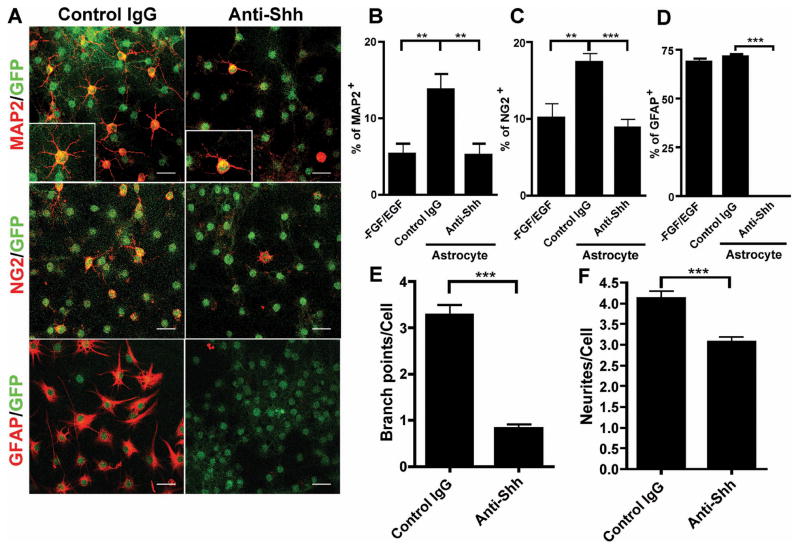
We hypothesized that adult spinal cord astroglia may play a role in maintaining endogenous progenitor population by providing a favorable microenvironment similar to a typical stem cell niche. To test this hypothesis, we cultured GFP-tagged E12.5 NSCs on a monolayer of adult spinal cord astroglia in serum free and FGF/EGF free conditions for 5 days. Differentiation of neural stem cells in medium alone was very low (Supplemental Figure 4C), but when co-cultured with astroglia, there was an increase in the number of differentiated progeny. Immunofluorescence staining for neurons (MAP2), oligodendrocyte precursors (NG2), and astroglia (GFAP) among the GFP+ cell population (Figure 2A) showed that co-culture with astroglia induced differentiation of a substantial number of neurons (13.7±7.1%, Figure 2B), as well as oligodendrocyte precursors (17.4±3.7%, Figure 2C) and astroglia (71.8±5.0%, this number may not represent total differentiated astroglia, as the GFAP can be expressed in a portion of undifferentiated NSCs as well, Figure 2D). Addition of anti-Shh neutralizing antibody to the co-culture inhibited differentiation compared to rat IgG control (Figure 2A). Blocking Shh reduced neuronal differentiation by 70% (5.2±4.9%, p= 0.0070), and oligodendrocyte precursors by 50% (8.8±3.7%, p<0.0001), which is comparable to FGF/EGF withdrawal induced differentiation. Interestingly, the GFAP+ astroglia differentiation was totally abrogated by anti-Shh antibody (p< 0.0001). We also found that the maturation of the differentiated neurons was greatly delayed by blocking the Shh pathway (Inserts in Figure 2A), as shown by a significant reduction of neurites and branch-point complexity (Figure 2E, F) (p< 0.0001). These data suggest that adult spinal cord astroglia can induce NSCs neuronal, oligodendroglial, and astroglia differentiation and that this process is at least partially mediated by Shh.
Figure 2. Adult astroglia-induced NSC differentiation is mediated by Shh.
E12.5 GFP+ NSCs were cultured on a monolayer of adult spinal cord astroglia under FGF/EGF withdrawal condition for 5 days with anti-Shh neutralizing antibody or the same concentration of control IgG treatment. (A) Immunofluorescence staining for MAP2, NG2 and GFAP on NSCs differentiated in co-culture with adult astroglia monolayer. Scale bar, 20 μm. The inserts show a higher magnification of neurons to demonstrate their complexity. Quantification of NSCs differentiation by co-culture with adult astroglia: (B) the percentage of MAP2+ neurons to total GFP+ cells is increased compared to FGF/EGF withdrawal (p=0.0068) and this is abrogated by anti-Shh (p=0.0070), (C) the percentage of NG2+ OPCs is increased in the co-culture (p=0.0062) and this is abrogated by addition of anti-Shh (p<0.0001), (D) the percentage of GFAP+ cells is unchanged in the co-culture but anti-Shh totally abrogates GFAP+ astroglial differentiation (p<0.0001). The percentage (out of the total cells) of the MAP2, NG2, and GFAP positive cells were calculated from ten 200x fields, ** p< 0.01, ***p< 0.001. Neutralizing Shh by anti-Shh antibody significantly delayed adult astroglia induced neuronal differentiation with decreased complexity of neuronal dendrites, as shown by the number of branch points (E) and neurites (F). The figure shows the average of 100 cells. ***p<0.0001. The statistics was done by unpaired t-test with Welch’s correction. The experiment shown represents three independent repeats.
Unique role of Gli1 during Shh-induced NSC differentiation
To confirm the role of Shh induced-differentiation and investigate the downstream mediators, we pretreated NSCs with Shh, followed by FGF/EGF withdrawal induced differentiation in the presence of Shh. Under conditions of FGF/EGF withdrawal, NSCs stop proliferating and start to down-regulate the expression of the immature marker nestin. Notably on day 4 after FGF/EGF withdrawal, 77.4% of the NSCs remained nestin positive, while only 26% of the cells pre-treated with Shh were nestin positive (Supplemental Figure 4A). Furthermore, Shh-treated NSCs generated significantly more MAP2+ neurons (22.6± 6.0%) than those under FGF/EGF withdrawal (17.0± 2.3%, p= 0.0019) (Supplemental Figure 4D). Similarly, Shh promoted more NG2+ OPCs (p= 0.0003) (Supplemental Figure 4F) but significantly less GFAP+ astroglia (p<0.0001) (Supplemental Figure 4E) than control. Interestingly, while control cultures showed no O4+ cells on day 5, we observed a substantial number of O4+ cells in the Shh-treated culture (Supplemental Figure 4C). Differentiation markers MAP2 and O4 positive cells started to emerge as early as day 3 in Shh-treated conditions (Supplemental Figure 4B), suggesting that Shh accelerates oligodendrocyte differentiation. These data confirm the critical role of Shh on oligodendrocyte differentiation.
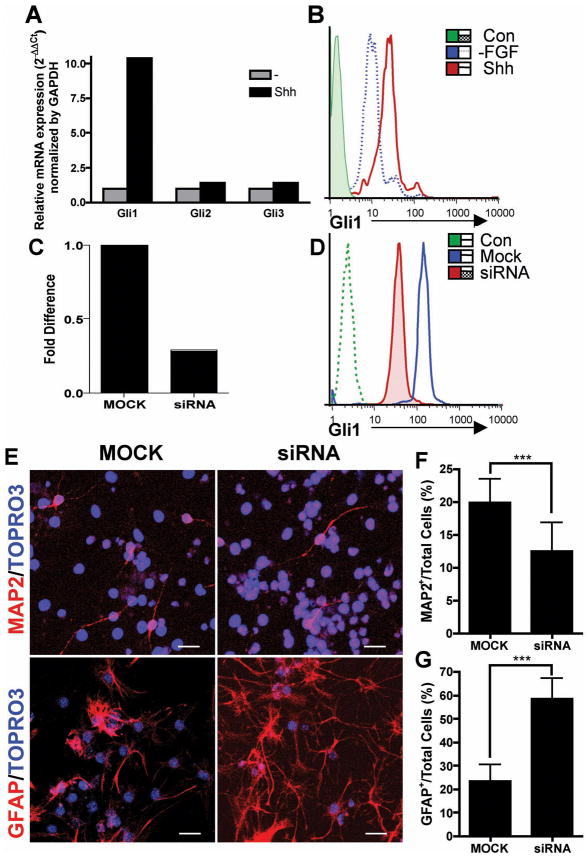
Shh signaling is mediated by the transcription factors Gli1, Gli2 and Gli3. Gli1 was found to be important in the Shh-responding neural stem cells24, but less is known about whether it plays the major role in neural stem cell differentiation during development and in disease. To identify the Shh downstream signals in NSCs differentiation, we examined Gli1, Gli2 and Gli3 mRNA expression in Shh-treated E14.5 NSCs. We found 10-fold induction of Gli1 by Shh, but no significant induction of Gli2 and Gli3, suggesting that Gli1 is the predominant signaling pathway of Shh in NSCs (Figure 3A). We confirmed the induction of Gli1 at the protein level by intracellular staining of Gli1 in Shh stimulated NSCs. Shh-treated cells showed about 3-fold Gli1 induction (MFI=35.1), compared to Shh-untreated cells (MF1=12.9) and secondary antibody control (MFI=1.73) (Figure 3B).
Figure 3. Role of Gli1 in neuronal and astroglial fate commitment of NSCs.
(A) NSCs were treated with Shh for 24 hours followed by real-time PCR analysis for mRNA expression of transcription factors Gli1, Gli2 and Gli3. (B) NSCs were treated with Shh in FGF/EGF containing media for 48 hours followed by FGF/EGF withdrawal of NSCs plated on PDL-coated coverslips. Gli1 protein expression was examined by intracellular staining and FACS analysis at 4 days after FGF/EGF withdrawal. Protein levels of Gli1 after Shh treatment (solid red line) were compared to FGF/EGF withdrawal only (dashed blue line) and secondary antibody control (shaded green area) by mean fluorescence intensity (MFI). (C) NSCs were treated with Shh for 48 hours, then transfected with Gli1 siRNA or transfection reagent alone as mock control. Gli1 knock-down was verified by real-time RT-PCR for Gli1 mRNA level 24 hours after the siRNA transfection. Comparison of the mRNA levels of siRNA transfected and the mock transfected NSCs was normalized to GAPDH and shown as fold difference indicating 2−ΔΔCt value. (D) Verification of Gli1 protein knockdown by FACS analysis 96 hours after the transfection. The blue line represents mock transfection, the shaded red area represents Gli1 siRNA transfection and the green line represents secondary antibody alone as control. (E) Gli1 siRNA or MOCK transfected NSCs were differentiated in presence of Shh. Immunofluorescence staining reveals that the siRNA transfected NSCs significantly decreased neuronal (MAP2) differentiation (F, average of twenty 200x fields, p=0.0009), but increased astroglial (GFAP) differentiation (G, p< 0.0001) The statistics was done by unpaired t-test with Welch’s correction. Scale bar, 20 μm. Each result represents three similar experiments.
We confirmed the role of Gli1 in NSCs differentiation by performing transient knockdown of Gli1. We evaluated the effects of Gli1 small interfering RNA (siRNA) on the differentiation of Shh-treated E14.5 NSCs. Gli1 mRNA level was knocked down by 71.3%, 24 hours after siRNA transfection compared to the cells with mock transfection (Figure 3C). Reduction of Gli1 protein persisted for at least 4 days after the transfection as detected by FACS analysis, as shown by MFI reduction from 147 to 38.2 (secondary control 2.44) (Figure 3D). We observed a significant reduction of MAP2+ neuronal differentiation in siRNA-transfected cells (12.5±4.5%), compared to 19.8±3.7% in mock transfected cells (p= 0.0009,), and an increase in GFAP+ astroglial differentiation in siRNA-transfected cells (58.4±8.8%), compared to 23.6±6.9% in control (p<0.0001) (Figure 3E–G). The effect of siRNA transfection on oligodendrocyte differentiation could not be evaluated because of failure of OPC differentiation even after mock transfection, since OPCs are more sensitive to environmental damage25. These data confirm the critical role of Gli1 in mediating Shh-induced NSC differentiation.
Interestingly, we found that neurons preserve Gli1 immunoreactivity and aggregation in the nuclei of MAP2+ cells (Supplemental Figure 5A, B), but GFAP+ astroglia had low expression without nuclear localization and aggregation (Supplemental Figure 5C, D). We confirmed these findings by FACS analysis showing that MAP2+ neurons express higher levels of Gli1 (MFI=149), compared to GFAP+ astroglia (MFI=75) (Supplemental Figure 5E), suggesting a role of Gli1 in mature neuron function.
In vivo inflammation suppresses Gli1 expression in EAE and MS lesions
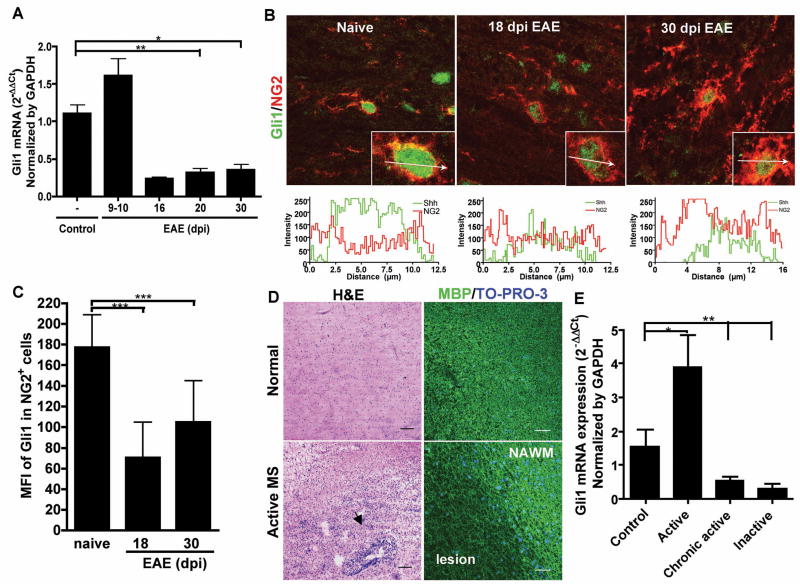
Since we found increased Shh expression in the CNS after injury, we examined the expression of Shh receptor and downstream signaling molecules in EAE and MS. The Shh receptor smoothened (Smo) expression was up-regulated in the spinal cord in EAE with kinetics similar to the upregulation of Shh (Supplemental Figure 6A). Interestingly, despite a slight increase in Gli1 mRNA before the onset of EAE (day 10), we found Gli1 expression markedly decreased after the onset of the EAE (day16) and throughout the course of the disease (Figure 4A). By immunofluorescence staining, we found significant amount of Gli1 immunoreactivity localized in the nuclei of OPCs and neurons as expected (Figure 4B, Supplemental Figure 6B, C) whereas astroglia show very low Gli1 immunoreactivity in both EAE and naive CNS (Supplemental Figure 6D). However, we observed a reduction of Gli1 expression in the spinal cord OPCs during the peak and chronic phases of EAE (Figure 4B). The MFI of Gli1 expression in the naïve OPCs was 177.01±31.94, incompared to 70.55±34.4 and 104.95±40.17 on days 18 and 30 dpi respectively in EAE mice (Figure 4C). Compared to the naive mice, the OPCs in EAE mice exhibited significant downregulation of Gli1 (p< 0.001). Although Gli1 was expressed by neurons, there is no significant difference in Gli1 expression between naive and xEAE animals (Supplemental Figure 6C). Thus, confirming that the reduction of Gli1 expression in EAE spinal cords was due to the downregulation of Gli1 in the OPCs.
Figure 4. Shh downstream transcription factor Gli1 is down-regulated in EAE and MS.
(A) Real-time PCR analysis for Gli1 mRNA expression of naive (n=6, number of mice) and EAE lumbosacral section of the spinal cords on days 9–10 (before onset, n=5), 16 (n=2) and 20 (n=4) (acute phase) and 30 (chronic phase, n=3) after immunization. (B) Immunofluorescence staining for Gli1 (green) in spinal cord white matter OPCs (NG2+, red) from naive and EAE on days 18 (peak) and 30 (chronic phase) after immunization. (C) Mean fluorescence intensity (MFI) of Gli1 expression profile analysis on NG2+ OPCs from naive (n=41, number of cells) and EAE on days 18 (n=40) and 30 (n=40) after immunization. ***p<0.0001 by unpaired t-test with Welch’s correction. (D) H&E staining of an active lesion from MS patient and normal brain tissues. The area of perivascular infiltration is indicated by the arrowhead. Immunofluorescence staining shows reduction of MBP (green) expression in the demyelinated lesion. Nuclei were counterstained by TO-PRO-3 (blue). Scale bar, 100 μm. (E) Real-time PCR analysis on the active (n=3), chronic active (n=9) and inactive (n=5) MS lesions and normal brain tissues reveals that Gli1 is up-regulated in the active lesions, but down-regulated in the chronic active and inactive MS lesions. * p<0.05, ** p< 0.01, by Mann Whitney test.
Olig1 and Olig2 genes are important for remyelination26, and Olig genes were shown to be regulated by Shh27. We observed a similar regulation in EAE; Olig2-expressing cells are increased in all stages of EAE (supplementary figure 7A), but we did not detect significant changes in Olig1 and Olig2 expression levels during EAE progression (supplementary figure 7B).
We further examined Gli1 expression in MS lesions of different stages and in normal controls (Figure 4D, E). Consistent with our findings in EAE, Gli1 mRNA was up-regulated in active lesions, but was significantly decreased in chronic active lesions (p=0.0025) and inactive lesions (p=0.0031) from MS brain compared to normal brain, suggesting that long-term inflammation impairs Gli1 signal in chronic MS lesions. Thus, despite the notable increase of Shh in the lesions, Gli1 the critical downstream mediator for NSCs differentiation is decreased in MS and EAE. Taken together, our data suggest that downregulation of Gli1 signal in the OPCs may contribute to the impaired oligodendrocyte maturation in EAE and MS.
IFN-γ suppresses Gli1 expression and perturbs the Shh-induced NSC differentiation
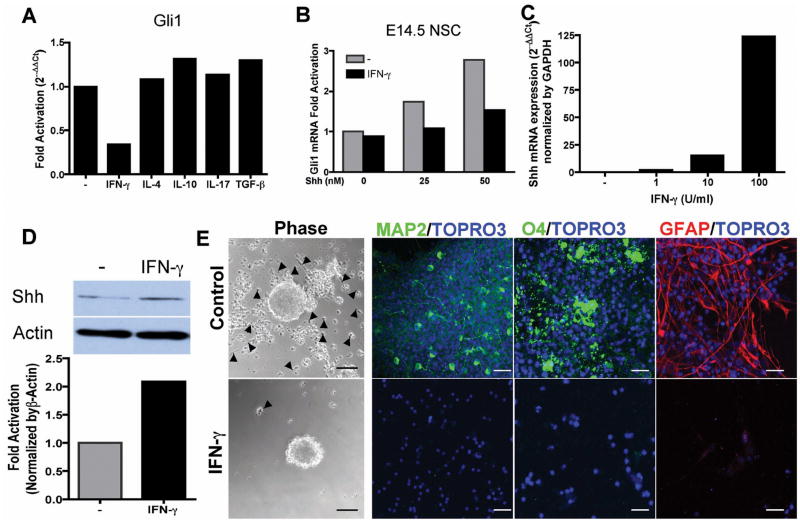
In order to identify the mechanism of Gli1 downregulation by inflammation, we analyzed Gli gene expression in E14.5 NSCs in the presence of inflammatory cytokines. As shown in Figure 5A, Gli1 expression was reduced 3-fold by IFN-γ. We focused on Gli1 since it is the major transcription factor of Shh in NSCs. There were less than 2-fold change in Gli2 and Gli3 regulation by the cytokines, which is considered not significant for real-time quantitative RNA assay (Supplemental Figure 8A–B). IFN-γ suppressed Shh-induced upregulation of Gli1 in embryonic NSCs (Figure 5B) as well as adult SVZ NSCs (Supplemental Figure 8C) in a dose dependent manner, while it paradoxically increased Shh mRNA expression by more than 100 fold (Figure 5C) and Shh protein levels by more than 2 fold (Figure 5D). Thus, IFN-γ inhibits Shh-Gli1 signaling in the Shh responder cells despite upregulating an autocrine increase in Shh in NSCs.
Figure 5. Inflammatory cytokines regulate Shh-Gli1 signaling in NSCs in vitro.
(A) Downregulation of Gli1 expression by IFN-γ. NSCs were treated with Shh together with IFN-γ, TGF-β, IL-4, IL-10 or IL-17 for 48 hours then processed for real-time PCR. (B) Dose response of IFN-γ inhibition of Shh-Gli1 signaling in E14.5 NSCs. (C) Dose response of Shh mRNA expression in E14.5 NSCs treated with IFN-γ for 24 hours by real-time PCR. (D) Western blot analysis of Shh protein expression in E14.5 NSCs-treated with IFN-γ for 48 hours. Densitometry of Shh fold increase normalized to β-actin. (E) IFN-γ abrogates Shh mediated NSC differentiation. E14.5 NSCs were pretreated with Shh for 48 hours and IFN-γ was added for 24 hours followed by FGF/EGF withdrawal for 5 days on PDL-coated coverslips. Phase-contrast images showed that IFN-γ-untreated NSCs attached to PDL surface and migrated out of the neurospheres (arrow head) and immunofluorescent staining revealed NSCs differentiated to neurons (MAP2), oligodendrocytes (O4) and astroglia (GFAP). Whereas, IFN-γ treated cells remained as unattached neurospheres with few cells attached and migrating and no differentiation was observed. Scale bars, 50 μm, phase-contrast, 20 μm immunofluorescence.
We then examined the consequences of IFN-γ mediated Shh-Gli1 dysregulation on NSCs differentiation. IFN-γ was added to Shh-induced NSC differentiation before FGF/EGF withdrawal, and after Gli1 had already been induced by Shh. Shh-treated neurospheres attached to the poly-D-lysine-coated surface, and NSCs migrated out of the neurospheres and differentiated into neurons, oligodendrocytes and astroglia. In contrast, the IFN-γ-treated neurospheres remained unattached with very few cells migrating out and remained in an undifferentiated state (Figure 5E). This was not due to IFN-γ toxicity on NSCs, as we found no difference in the percentage of apoptotic or necrotic cells by Annexin V and 7-AAD staining (Supplemental Figure 8D). Taken together, our data suggest that inflammatory cytokines such as IFN-γ paradoxically inhibit Gli1 that mediates NSC differentiation even in the presence of exogenous Shh or autocrine increase in Shh by the NSCs.
DISCUSSION
Adult stem cells are viewed as repositories for repair potential. But it is becoming clear that repair potential requires both stem cell competence and a permissive microenvironment. The mobilization of endogenous precursor cells has been proposed as a strategy to increase the regeneration in neurological diseases. In MS, progenitor cells are seen around the lesions but there is a lack of mature oligodendrocytes1,3. The lack of repair in MS has been attributed to increased Jagged 1 or hyaluronan in lesions that impairs the maturation of OPCs26,28. Here we show that astroglia contribute to the creation of ectopic niches during EAE and produce Shh that should lead to differentiation of NSCs. However, the inflammatory microenvironment, specifically IFN-γ, perturb the Shh-induced differentiation by down-regulating Gli1 in vivo and in vitro. Our data suggest that inflammation may initiate the establishment of ectopic niches in the CNS, but chronic inflammation impairs effective repair.
Glial cells have well-established roles that include maintaining the ionic milieu of neurons, modulating synaptic action by controlling the uptake of neurotransmitters, and providing a scaffold during neural development29. Furthermore, astroglia in the hippocampus and SVZ are multipotent stem cells, and also function as niche cells supporting stem cell proliferation and maturation9,30. A large number of neural progenitor cells, such as OPCs and doublecortin (Dcx)+ migrating neuronal progenitors are maintained in the adult CNS and mobilized during CNS injury31,32. The local microenvironment, especially adult astroglia may contribute to the progenitor response, raising the question of whether astroglia outside the typical niche areas could become niche-like cells under circumstances such as injury or inflammation. Our workdata show that Shh is expressed in adult spinal cord astroglia at a low level under normal conditions and that astroglia support neurogenesis and oligodendrogenesis. Thus, we demonstrate that astroglia outside the niche areas can sustain the differentiation of NSCs. We also report that this effect is mediated in part by Shh, since Shh blockade inhibits neuronal and oligodendrocyte differentiation of NSCs co-cultured with astroglia.
We further observed that Shh itself is a potent mediator of neuronal and oligodendroglial differentiation, and it signals through the transcription factor Gli1. Interestingly, astroglia and maturing oligodendrocytes did not have significant expression of Gli1, while post-mitotic neurons preserve high levels of Gli1 in the nuclei, suggesting that Gli1 can control neuronal function, since motor neurons can up-regulate Shh and its receptor Smo after injury in adult rats20 and in mouse EAE (our observation).
Hedgehog signaling has been postulated to play a pivotal role in healing and repair processes33 and inappropriate activation of this pathway has been implicated in several types of cancer34. In vertebrates, the Gli genes, Gli1, Gli2, and Gli3, mediate the Shh morphogenetic signal by regulating expression of Shh target genes. Although the three Gli molecules function combinatorially in a context-dependent manner, the response to Shh signaling largely relies on regulation of Gli1 transcripts35. Our observations highlight the fact that Shh-Gli1 signal is critical for NSC differentiation which mediates the astroglia-induced neuronal and oligodendroglial differentiation.
Olig1 and Olig2 genes are important in remyelination in models of toxin-induced demyelination and are also expressed in MS lesions36. Shh is believed to be critical for Olig gene expression during development as demonstrated by gain or loss of function studies27. We have observed dysregulation of Shh-Gli1 signaling with up-regulated Shh but down-regulated Gli1, despite an increase in Olig1+ and Olig2+ cells in chronic EAE. These apparently contradictory findings suggest the complexity of Shh-Olig pathway. For instance, little Shh is expressed in adult CNS even when Olig2 is increased in toxin-induced demyelination model, which also suggests independence of Olig gene activation from Shh37. Furthermore, there is no evidence of direct correlation between Olig gene expression and the Shh downstream signaling molecule Gli1. Our data suggest that Olig1 and Olig2 genes may be upstream of Gli1, or more likely use different signal transductiontransductionpathways than Gli1 and have different responses to inflammatory signals. On the other hand, our observations show that Shh is involved in differentiation of neural progenitors through Gli1, while previous reports have associated Shh and Gli1 with proliferation of neural stem cells and progenitors38. These findings suggest a dual function for Gli1 that is context dependent: during conditions of self-renewal Gli1 helps maintain the NSC proliferation and during differentiation it contributes to oligodendrogenesis. This is in keeping with other oligodendrocyte genes like Olig1/2 genes that have dual roles in neural stem cell proliferation and differentiation39.
During neuronal injury or inflammation, activated astroglia can up-regulate a series of molecules including GFAP, MHC class II but also neural regulatory factors26. However, the local inflammatory response involves multiple cytokines and the outcome for the exposed activated astroglia may be supportive or inhibitory for neural precursor cell proliferation and differentiation. Although Shh is a developmental molecule, upregulation of Shh is observed with neuronal injury20. Shh can be regulated by IFN-γ in cerebellar neurons40, and we found that activated astroglia in the spinal cord of EAE and in MS lesions strongly up-regulate the niche molecule Shh. We further demonstrate in vitro that Shh can be regulated by multiple cytokines, and most potently by IFN-γ. Conversely, it has been reported that Shh is produced by CD4+ T cells and promotes proliferation and survival of activated T cells41. We did not detect significant expression of Shh in infiltrating cells in EAE, however, the Shh secreted by reactive astroglia may play a role in local T cell survival and proliferation, which may further contribute to astroglia activation in a positive feed-back loop. Our data suggest that inflammation can redirect mature astroglia from outside the typical adult stem cell niches to form a niche-like microenvironment that can support precursor cell proliferation and differentiation.
In contrast to the favorable changes to the stem cell microenvironment, we demonstrate that the stem cell response to Shh is attenuated by inflammation both in vivo and in vitro. The same pro-inflammatory cytokine, namely IFN-γ also down-regulates Gli1 signal in the Shh responder cells, thus directly abrogating the neural stem/progenitor cell differentiation induced by Shh. This paradoxical effect of IFN-γ on Shh producing astroglia and Shh responder NSCs may explain the fact that neural progenitor cells or OPCs proliferate in MS and EAE, but do not undergo full maturation because of impaired Shh-Gli1 signal. Our findings confirm the hypothesis that inflammation initiates the neural repair process, but chronic inflammation impairs repair. This may also suggest that any potential therapy to up-regulate Shh signal has to be combined with modulation of inflammation.
Supplementary Material
Acknowledgments
We thank Ken Dole of Rockey Mountain Multiple Sclerosis Center Tissue Bank, Englewood, CO and Human Brain and Spinal Fluid Resource Center, University of California, Los Angeles, CA for providing the human brain samples. We thank Dr. Adi Vaknin-Dembinsky for help in siRNA transfection. This work was supported by grants AI043496 and AI071448 from NIH and RG3945 from NMSS, to SJK
Footnotes
The authors have no conflict of interest.
References
- 1.Scolding N, Franklin R, Stevens S, et al. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121 (Pt 12):2221–2228. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- 2.Imitola J, Snyder EY, Khoury SJ. Genetic programs and responses of neural stem/progenitor cells during demyelination: potential insights into repair mechanisms in multiple sclerosis. Physiol Genomics. 2003;14:171–197. doi: 10.1152/physiolgenomics.00021.2002. [DOI] [PubMed] [Google Scholar]
- 3.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 4.Blakemore WF. Remyelination of the superior cerebellar peduncle in the mouse following demyelination induced by feeding cuprizone. J Neurol Sci. 1973;20:73–83. doi: 10.1016/0022-510x(73)90119-6. [DOI] [PubMed] [Google Scholar]
- 5.Nait-Oumesmar B, Picard-Riera N, Kerninon C, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. 2007;104:4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picard-Riera N, Decker L, Delarasse C, et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 8.Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia AD, Doan NB, Imura T, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 11.Imitola J, Comabella M, Chandraker AK, et al. Neural stem/progenitor cells express costimulatory molecules that are differentially regulated by inflammatory and apoptotic stimuli. Am J Pathol. 2004;164:1615–1625. doi: 10.1016/S0002-9440(10)63720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pluchino S, Zanotti L, Rossi B, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 14.Tekki-Kessaris N, Woodruff R, Hall AC, et al. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development (Cambridge, England) 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- 15.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 16.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Miller RH. Specification of optic nerve oligodendrocyte precursors by retinal ganglion cell axons. J Neurosci. 2006;26:7619–7628. doi: 10.1523/JNEUROSCI.0855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merchan P, Bribian A, Sanchez-Camacho C, et al. Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. Molecular and cellular neurosciences. 2007;36:355–368. doi: 10.1016/j.mcn.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Jacob J, Briscoe J. Gli proteins and the control of spinal-cord patterning. EMBO Rep. 2003;4:761–765. doi: 10.1038/sj.embor.embor896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akazawa C, Tsuzuki H, Nakamura Y, et al. The upregulated expression of sonic hedgehog in motor neurons after rat facial nerve axotomy. J Neurosci. 2004;24:7923–7930. doi: 10.1523/JNEUROSCI.1784-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seifert T, Bauer J, Weissert R, et al. Differential expression of sonic hedgehog immunoreactivity during lesion evolution in autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2005;64:404–411. doi: 10.1093/jnen/64.5.404. [DOI] [PubMed] [Google Scholar]
- 22.Mastronardi FG, Min W, Wang H, et al. Attenuation of experimental autoimmune encephalomyelitis and nonimmune demyelination by IFN-beta plus vitamin B12: treatment to modify notch-1/sonic hedgehog balance. J Immunol. 2004;172:6418–6426. doi: 10.4049/jimmunol.172.10.6418. [DOI] [PubMed] [Google Scholar]
- 23.Bambakidis NC, Wang RZ, Franic L, Miller RH. Sonic hedgehog-induced neural precursor proliferation after adult rodent spinal cord injury. J Neurosurg. 2003;99:70–75. doi: 10.3171/spi.2003.99.1.0070. [DOI] [PubMed] [Google Scholar]
- 24.Palma V, Lim DA, Dahmane N, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development (Cambridge, England) 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirianov G, Jesurasa A, Mehmet H. Developmentally regulated changes in c-Jun N-terminal kinase signalling determine the apoptotic response of oligodendrocyte lineage cells. Cell death and differentiation. 2006;13:531–533. doi: 10.1038/sj.cdd.4401805. [DOI] [PubMed] [Google Scholar]
- 26.John GR, Shankar SL, Shafit-Zagardo B, et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- 27.Lu QR, Yuk D, Alberta JA, et al. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 28.Back SA, Tuohy TM, Chen H, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 29.Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- 30.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 31.Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita T, Ninomiya M, Hernandez Acosta P, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusano KF, Pola R, Murayama T, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 34.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science (New York, NY) 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 35.Dahmane N, Lee J, Robins P, et al. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 36.Arnett HA, Fancy SP, Alberta JA, et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science (New York, NY) 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- 37.Fancy SP, Zhao C, Franklin RJ. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Molecular and cellular neurosciences. 2004;27:247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Dahmane N, Sanchez P, Gitton Y, et al. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development (Cambridge, England) 2001;128:5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- 39.Ligon KL, Huillard E, Mehta S, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–517. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Pham-Mitchell N, Schindler C, Campbell IL. Dysregulated Sonic hedgehog signaling and medulloblastoma consequent to IFN-alpha-stimulated STAT2-independent production of IFN-gamma in the brain. J Clin Invest. 2003;112:535–543. doi: 10.1172/JCI18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowrey JA, Stewart GA, Lindey S, et al. Sonic hedgehog promotes cell cycle progression in activated peripheral CD4(+) T lymphocytes. J Immunol. 2002;169:1869–1875. doi: 10.4049/jimmunol.169.4.1869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.