Abstract
Inherent cancer phenotypes that are independent of fluctuating cross talk with the surrounding tissue matrix are highly desirable candidates for targeting tumor cells. Our novel study design employs epithelial cell lines derived from low vs. high histologic grade primary breast cancer to effectively diminish the breadth of transient variability generated within the tumor microenvironment of the host, revealing a “paracrine-independent expression of grade-associated” gene signature (PEGA). PEGA members extended beyond ‘proliferation-driven’ signatures commonly associated with aggressive, high grade breast cancer. The calcium-binding protein, S100P, was prominent among PEGA genes overexpressed in high grade tumors. A three-member fingerprint of S100P correlated genes, consisting of GPRC5A, FXYD3, and PYCARD, conferred poor outcome in multiple breast cancer datasets, irrespective of estrogen receptor status but dependent on tumor size (P <0.01). S100P silencing markedly diminished coregulated gene transcripts, and reversed aggressive tumor behavior. Exposure to pathway implicated agents including the calmodulin inhibitor, N- (6-aminohexyl)-5-chloro-1-naphthalenesulphonamide, and the phenothiazine, chlorpromazine, resulted in rapid apoptotic cell death in high grade tumor cells resistant to the chemotherapeutic drug, cisplatin. This is the first comprehensive study describing molecular phenotypes intimately associated with histologic grade whose expression remains relatively fixed despite an unavoidably changing environment to which tumor cells are invariably exposed.
Introduction
It has long been known that the microscopic appearance and arrangement of tumor cells, defined as histologic grade, holds key information regarding malignant behavior and patient outcome. Criteria for the widely used Scarff-Bloom-Richardson (SBR) system of grading and its modification by Elston and Ellis (1) subclassify primary breast cancer into low grade or well differentiated (grade I), intermediate grade or moderately differentiated (grade II), and high grade or poorly differentiated (grade III) tumors. High grade tumors result in early treatment failures whereas low and intermediate grade tumors recur after longer time intervals, independently of clinical stage (2–4) (with the exception of medullary carcinomas, which display high tumor grade and have a relatively favorable prognosis (5)). The 10-year survival is reduced from 76% for patients with grade l, to 39% for those with grade III tumors (6). Based on the microscopic evidence of a striking proliferative differential, conventional systemic chemotherapy exploits the higher mitotic index of high grade tumors. However the effects of such drugs on non-malignant tissues and organ systems are undesirable and often intolerable. Additionally, therapeutic resistance is a major obstacle in achieving complete response. Current research strives to address these deficiencies through the identification of molecular markers at diagnostic presentation, which predict long-term efficacy of systemic therapy in both adjuvant and neoadjuvant settings.
In this regard, gene expression profiles offer the scope and sensitivity to unveil aberrant changes underlying tumor aggressiveness, and therapeutic vulnerability. A critical limitation in the clinical translation of such phenotypes, displayed as a single snapshot in time, includes the incorporation of transient, localized stromal influences within the afflicted tissue. While differentially expressed signatures, such as the ‘grade gene index’ have contributed towards improving grade-based subclassification (7–9), due to inordinate proliferative differences between the sample classes, functional insights required for tumor targeting remain limited. We have implemented an approach, which overrides the proliferative differential between tumor cells of varying histologic grade, thereby revealing additional functional phenotypes that contribute collectively to the biological basis of this key clinical parameter. Technical roadblocks that have previously persisted in the execution of this goal include maintenance of pristine, continuously proliferating epithelial cell populations from the full spectrum of primary breast cancer. Even when isolated from fresh tumor tissue, such malignant populations routinely display a finite in vitro lifespan in contrast to the common belief that cell immortalization is an inevitable consequence of tumorigenesis. On the other hand, rare spontaneously immortalized breast cancer cell lines have originated exclusively from metastatic, or high-grade primary carcinoma (10–16). To provide a representation of the histologic and clinical heterogeneity of human breast disease, we have utilized experimental models of early and late clinical stage, and low, intermediate, and high grade primary breast tumors (17–21), and more recently of non malignant high risk breast tissue (22), developed through incremental improvements in selective cell isolation, short-term propagation, genotypic and phenotypic characterization, and immortalization. This study describes the molecular profiles of clinically based novel cellular models of low and intermediate grade primary breast cancer, as yet unavailable in the field. It employs these towards the identification of a constitutively expressed gene signature to facilitate an understanding of the functional biology of histologic grade for application in drug discovery efforts. A practical application of the tumor biology revealed through this innovative approach is exemplified by the in vitro recapitulation of differential S100P expression associated with tumor grade, its role in clinical stratification with novel coregulated gene fingerprints, and in the functional verification of predictive target response to pathway guided therapy.
Materials and Methods
Tissue Culture, RNA isolation and Microarrays
epithelial cultures were derived from clinical primary breast cancer specimens under IRB approved guidelines as previously described (18, 19). For cell immortalization, sixteen independent cases were transduced with the hTERT gene as described (21). RNA isolated from subconfluent cultures of the immortalized cell lines was subjected to routine quality control measures and hybridized to the HG-U133A chip (Affymetrix, Santa Clara, CA) according to manufacturers protocols. Affymetrix software was used for image analysis and probe quantification to generate CEL files. Data collection and analysis are as described in Chin et al (23). For quantitative real-time reverse transcription-polymerase chain reaction (QPCR) analysis, cDNA was synthesized and analyzed as before (21) by an Applied Biosystems 5700 Sequence Detection System (Foster City, CA). For primer sequences, see Supplementary Table 1.
S100P was silenced by siRNA transfection of the sense strand sequence: 5’ACAAGGAUGCCGUGGAUAA3’ Dharmacon Research (Lafayette, CO, USA) using Lipofectamine 2000 (Invitrogen). siCONTROL non-targeting siRNA#1 served to evaluate off-target effects. S100P expression was normalized to that of the reference gene, ACTB, to control for differences in the amount and/or quality of total RNA isolated from different cell pellets.
Immunofluorescence and FACS analysis
Expression of S100P protein in tumor cell lines was measured by indirect immunofluorescence of a mouse monoclonal primary antibody to S100P (BD Biosciences) and detection with Alexa 488 conjugated secondary antibody (Invitrogen).
To measure growth rate after S100P knockdown, starting on day 4 after transfection with S100P siRNA or siCONTROL, triplicate sets of BrdU incorporated cell suspensions were immunostained with anti BrdU, acquired by FACScan (BD Biosciences), and analyzed using CellQuest Pro software. Ten thousand cells were acquired in each of three independent runs. Apoptotic cell fractions were analyzed 48 hours post transfection using Annexin V-FITC and propidium iodide kit (MBL International) followed by FACScan analysis as described above.
Drug response
Cells were plated at a density of 200,000 cells/well in a 6-well plate and 24 hours later treated with 200µM cisplatin, 10µM chlorpromazine, or N- (6-aminohexyl)-5-chloro-1-naphthalenesulphonamide, also known as W7 (Sigma) for 24 hours. Cells were then stained with Annexin V-FITC and propidium iodide and analyzed by FACScan as above.
Migration assay
Cells were placed in inserts with hanging geometry (Becton Dickinson, Franklin Lakes, NJ) in 6-well plates and allowed 4 days for migration through the 8 micron pores in the polyester membrane bottom of the insert. Inserts were removed, and migrated cells in the plate bottom were propagated as colonies for an additional 4 days, fixed, stained with crystal violet, and counted. Migration potential was determined by the number of colonies at the end of the experiment divided by the number of cells initially seeded in the insert.
Coculture of primary tumor cell lines and fibroblasts were set up in a similar format with the exception that the insert pore size was reduced 0.4 micron. Tumor cell RNA was isolated after 3 days of continuous coculture.
Statistical analysis
Further analyses of annotated and filtered data was based on 6053 probe sets that displayed >=2-fold variation across low vs. high grade primary breast cancer cell lines. Validation of the array data as described below follows published guidelines (24).
PEGA
We used the R program samr (Significance Analysis of Microarrays (25)) to select genes whose expression levels were significantly correlated with the pathologist assigned histologic grade of the tumor tissue from which each of the cell lines was derived. The initial comparison included 3 grade I vs. 4 grade III cell lines. Setting the samr parameter delta equal to 0.35 gave a false discovery rate of 1.19% for 105 induced and 0.79% for 536 repressed probe IDs. The expression pattern associated with high grade cells was designated as the PEGA signature. The validity of the association between cell culture-based PEGA genes and histologic grade of patient tumors was tested on datasets mentioned below. Using a hierarchical tree program in conjunction with the top 10 induced and 10 repressed PEGA probe IDs, false positive and false negative rates were calculated to demonstrate agreement between true grade and the PEGA classifier-based grade assignment in published cancer datasets. Kaplan-Meier survival curves for recurrence free survival were computed from followup data in these datasets.
S100P-COR
Genes specifically associated with S100P in primary tumor cell lines in the 95th percentile (most correlated) or in the 5th percentile (most anti-correlated) were identified. For tumor tissue data, using a criterion of 0.3 or greater correlation with S100P, genes common to two datasets were identified, and used for analysis of enriched Gene Ontology terms. Kaplan-Meier analysis was used to compute recurrence-free survival in these datasets. The statistical significance of the resultant hazard ratios was determined by the P value of the likelihood-ratio test.
Patient Array Datasets
The following publicly available primary breast cancer microarray datasets based on the same array platform as employed by us were used for clinical validation of PEGA-derived genes:
For clinical followup
Dataset 1 (E-TABM -158, Ref. 23; http://www.ebi.ac.uk/arrayexpress) – comprised of 112 cases with 30 recurrences and median followup of 7.9 yrs; Dataset 2 (GSE 6532, Ref. 26; http://www.ncbi.nlm.nih.gov/geo) -comprised of 317 cases with 106 recurrences and median followup of 8.9 yrs.
For grade distribution
Dataset 1 – comprised of 12 GI vs. 55 GIII tumors; Dataset 2 – comprised of 61 GI vs. 52 GIII tumors.
Results
An autonomously regulated profile represented by PEGA (paracrine-independent expression of grade-associated) genes
Fourteen independent primary breast tumor cell cultures of known histologic grade (and 2 additional cell lines from ungraded primary breast tumors) were isolated and immortalized with hTERT transduction in a single step. These are designated as the CCdl series (Table1). Morphologically, epithelial cultures originating from high tumor grade (HTG) were distinct from those of low tumor grade (LTG) even after the emergence of homogeneous, immortalized cell populations (Figure 1A). The new tumor cell lines were rigorously authenticated by direct comparison with the patient’s constitutive genotype obtained from nonmalignant tissue DNA (data not shown).
Table 1.
Clinicopathological characteristics of Novel Primary Breast Cancer Cell Lines
| Sample ID |
Histologic type | Age at diagnosis (yrs) |
TNM stage |
Histologic grade |
Current in vitro passages |
|---|---|---|---|---|---|
| CCdl22 | Invasive ductal carcinoma | NA | 1 | Low | 171 |
| CCdl67 | Invasive ductal carcinoma | 61 | 2 | Low | 55 |
| CCdl68 | Invasive ductal carcinoma | 52 | 2 | Low | 100 |
| CCdl61 | Invasive lobular carcinoma | 62 | 3 | Intermediate | 79 |
| CCdl66 | Invasive ductal carcinoma | 47 | 2 | Intermediate | 57 |
| CCdl78 | Invasive ductal carcinoma | 78 | 1 | Intermediate | 50 |
| CCdl631 | Invasive ductal/lobular carcinoma |
49 | 3 | Intermediate | 105 |
| CCdl1570 | Invasive ductal/lobular carcinoma |
66 | 2 | Intermediate | 70 |
| CCdl1599 | Invasive ductal carcinoma | 64 | 1 | Intermediate | 90 |
| CCdl329 | Invasive lobular carcinoma | 42 | 2B | Intermediate | 37 |
| CCdl54 | Invasive ductal carcinoma | 59 | 4 | High | 99 |
| CCdl257 | Invasive ductal carcinoma | 41 | 2B | High | 94 |
| CCdl672 | Invasive ductal carcinoma | 60 | 2 | High | 80 |
| CCdl675 | Invasive ductal carcinoma | 29 | 2B | High | 79 |
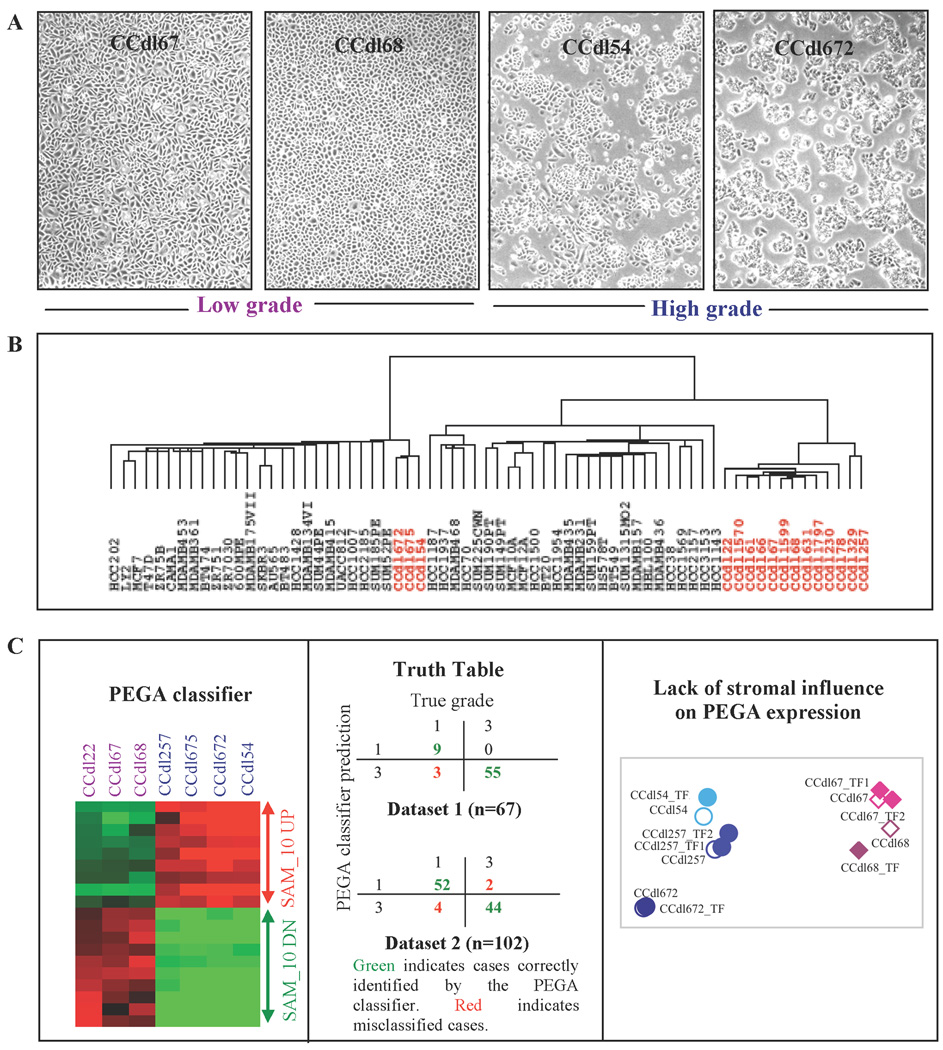
Figure 1. Distinctive profiles of proliferating primary breast tumor cells of varying histologic grade.
A, Microscopic phenotype of histologic grade in four independent breast epithelial cell lines derived from low grade tumors (LTG - 2 left panels) and high grade tumors (HTG - 2 right panels). B, Hierarchical clustering of 67 independent breast epithelial cell lines based on global gene expression profiling. Sixteen novel primary tumor cell lines encompassing low, intermediate, and high histologic grade are displayed in red lettering (CCdl1797 and CCdl230 are ungraded). C - Left panel, SAM- identified, PEGA-based top 10 upregulated (UP), and downregulated (DN) probe IDs distinguish proliferating tumor cells of low grade (purple, n=3) vs. high grade (blue, n=4). Middle panel, Cross-classification analysis of independent clinical breast cancer datasets using the PEGA classifier depicted by the adjoining heat map. Right panel, Plot of the first 2 principal components of the top 5 UP and DN PEGA genes expression levels in HTG and LTG cell lines in the presence or absence of tumor fibroblast (TF) coculture. TF1 and TF2 are independent cases. Open symbols represent control tumor cell lines. Filled symbols denote tumor + fibroblast cocultures. Circles represent HTG cell lines; diamonds indicate LTG cell lines.
Global gene expression data of the CCdl lines was combined with that of 51 previously established breast epithelial cell lines, and examined by unsupervised hierarchical clustering. Three of 4 HTG CCdl cell lines were well integrated with other known HTG derived tumor cell lines of a putative luminal molecular subtype. The remaining 13 cell lines, representing 3 LTG, 7 intermediate tumor grade (ITG), 1 HTG, and 2 ungraded cases, clustered apart in a distinct subgroup suggesting the presence of attributes that distinguish them from the malignant and non malignant breast epithelial cell lines represented in the 2 major clusters (Figure 1B). Significance Analysis of Microarrays (SAM) of HTG (n=4) and LTG (n=3) CCdl tumor cell line data identified 641 differentially expressed (105 induced and 536 repressed in HTG) probe IDs, designated as the PEGA signature.
Towards minimizing the effect of background noise, a SAM-defined classifier based on the top 10 induced and 10 repressed (in high grade cultures) PEGA probe IDs (Figure 1C, left panel) was used to confirm association with pathologist designated histologic grade in clinical breast cancer samples. This PEGA classifier achieved 95% correct classifications for grade I (LTG), and grade III (HTG) in two independent breast cancer datasets (Figure 1C, middle panel). In comparison, an arbitrary classifier based on marginal relative frequencies of the two grades provided only 42% correct classifications. Remarkably, grade-associated expression profiles of both HTG and LTG cell cultures, were maintained despite the induction of cell immortalization.
To ascertain the absence of a significant effect induced by stromal fibroblasts on PEGA profiles, gene expression was measured in both HTG and LTG cell lines cocultured with one or more independent samples of tumor-derived fibroblasts. Principal component analysis (PCA) based on the top 10 PEGA genes (5 UP and 5 DN), namely: ASS1, ALDH2, S100P, CLU, FGF13, FYN, FLRT2, AKT3, F2R, and SNCA, was used to evaluate changes in gene expression levels of cocultured tumor cells. Although HTG and LTG cell lines clustered in different parts of a two-dimensional space, however each of the 5 controls was tightly grouped with its test sample counterpart exposed to paracrine signaling from stromal fibroblasts (Figure 1C, right panel).
Overexpression of a PEGA gene, S100P, stratifies intermediate grade tumors in vivo and in vitro
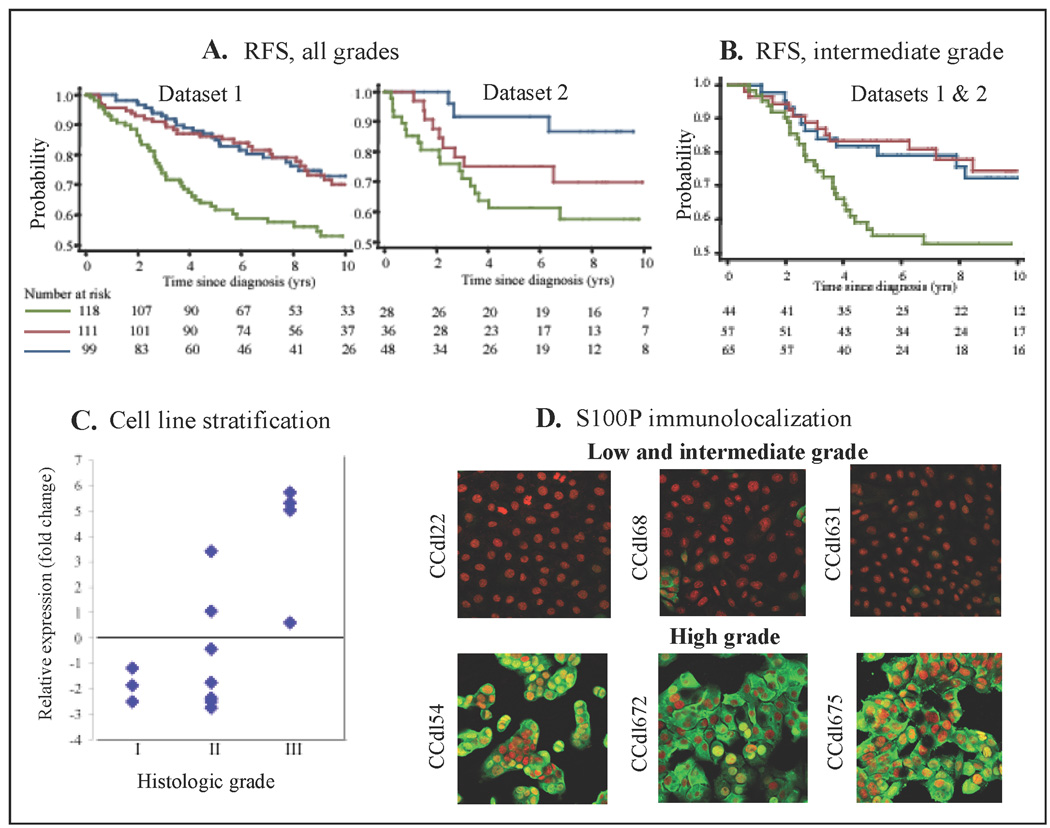
The top 10 overexpressed PEGA genes were further tested in conjunction with two independent clinical datasets for predicting patient outcome. Using backward stepwise selection, among the grade predictors retained for joint significance at p<0.05, only S100P, a calcium ion binding and calcium-dependent protein binding protein, attained significance in predicting recurrence-free survival (RFS) at the single gene level. S100P expression tertiles defined for the combined data from 2 independent datasets were used to separately evaluate RFS in each dataset. Significant associations were observed for both datasets (Dataset 1: hazard ratio=1.8, 95%CI 1.2 to 1.74, p=0.001; Dataset 2: hazard ratio 1.3, 95%CI 1.1 to 1.6, p=0.002 - Figure 2A).
Figure 2. Identification of aggressive primary breast cancer by expression levels of the PEGA gene, S100P.
A, Kaplan-Meier analysis - S100P expression divided into tertiles; blue – low, red – intermediate; green - high; Log normalized data values for each group: low risk − 3.0 to 7.07, intermediate risk − 7.08 to 9.46, high risk − 9.47 to 13.2. Recurrence-free survival (RFS) for all grades, displayed independently for dataset 1, and dataset 2. B, RFS for intermediate grade tumors in datasets 1 and 2 combined. C, Tumor cell lines of varying grade accurately stratified by S100P expression levels relative to ACTB, and grade of original tumor tissue. C, Immunofluorescence localization of anti-S100P (green) in LTG or ITG (top panels) vs. HTG (bottom panels) cell lines. Nuclei counterstained with propidium iodide (red).
We then asked whether expression of S100P could serve to dichotomize intermediate tumor grade (ITG). In tumor tissue, S100P expression was significantly associated with RFS at the cutpoint used above (Dataset 2: Hazard ratio=2.09, 95%CI 1.2 to 3.4, p=0.004 - (Figure 2B). Similarly in vitro, S100P expression in 7 ITG derived cell lines ranged from HTG-like (n=2) to LTG-like (n=5) transcript levels (Figure 2C). In agreement with the expression profiling data, indirect immunofluorescence of S100P protein in HTG cell lines displayed strong nuclear/cytoplasmic staining, whereas in LTG and some ITG cell lines, immunostaining was undetectable to minimal (Figure 2D).
A role for S100P correlated genes in the manifestation of tumor grade
In the search for a broader scope of biological processes, which link high tumor grade and poor clinical outcome to S100P, we identified genes whose expression levels were significantly correlated to S100P in clinical tumor tissue (S100P-CORtt). A criterion of 0.3 or greater correlation with S100P, identified 17 probe IDs (14 genes) common between two independent breast cancer datasets (Supplementary Table 2). A higher correlation threshold produced too few genes, while lower resulted in too many. Known functions for the 14-gene set of S100P-CORtt genes included: calcium ion binding, transport, and regulation (PLA2G10, CAPN9, CALML5, GPRC5A, ST6GALNAC2), glycosylation (PMM2), cell adhesio n (CEACAM6, MUC5B, CAPN9, PLA2G10), oxidoreductase activity (KMO), apoptosis (PYCARD, NTHL1), cell differentiation and TGFβ-signaling (SLC2A10, CEACAM6, CAPN9, FXYD3, CAPN9, CALML5, CTSD), and cell motility and invasion (CTSD, CEACAM6).
In CCdl cell lines, expression of genes at significantly higher correlation (>= 0.9, or −0.9) to S100P, designated as S100P-CORcc, represented association with biological processes similar to those observed for the 14-member S100P-CORtt gene set. For example: FGFR1, MYO1B, GNA15, SFN (calcium flux), JAG1, S100A2, EHD2 (calcium ion binding), BSPRY (ion transport), TMEM8, CD44, AZGP1, FLRT3, COL4A6 (cell adhesion), ALDH5A1 (oxidoreductase activity), SFN, TP73L (apoptosis), GNAI1, RRAS (G-protein signaling), INHBB, JAG1, AGR2, INHBA (cell differentiation and TGFβ-signaling), and MMP14, RRAS2 (cell migration).
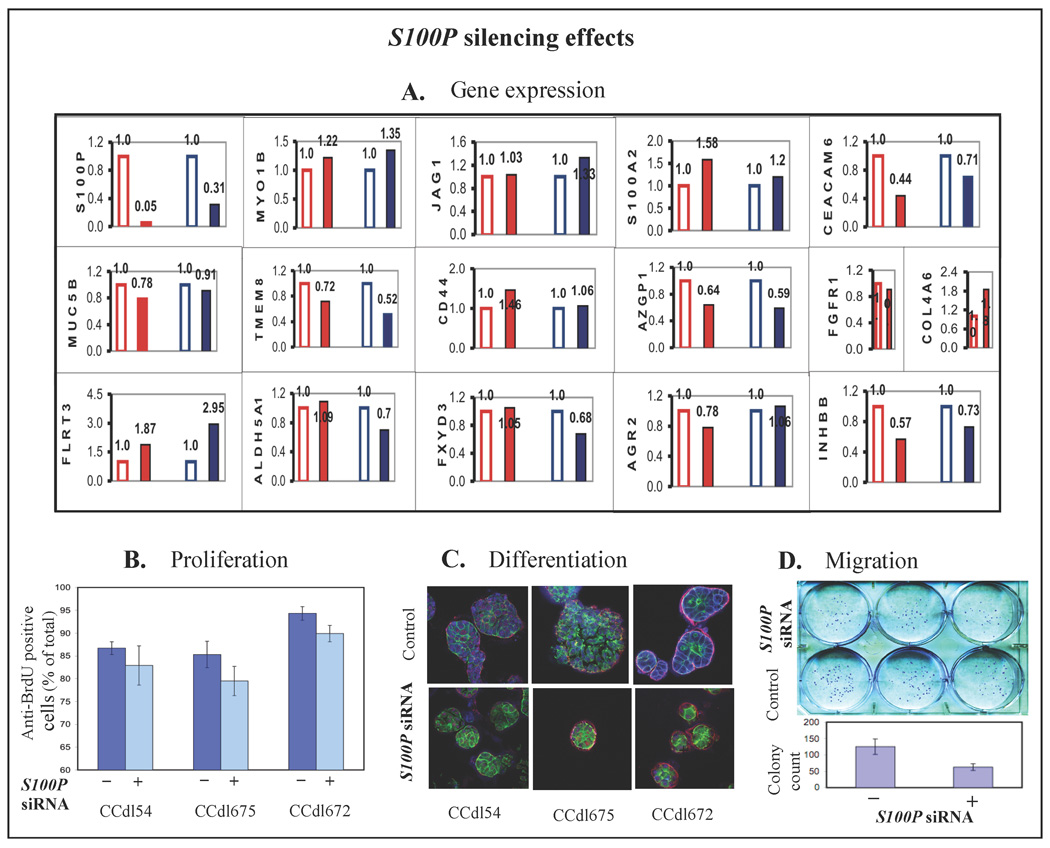
Towards a functional understanding of the role of the PEGA gene, S100P, and genes correlated to it in tumor cells of varying histologic grade, targeted transcript reduction was induced using S100P siRNA. In these cells, a significant reduction in S100P protein levels was confirmed by indirect immunofluorescence of anti S100P (data not shown). Together with a reduction in S100P transcript levels, siRNA transfected HTG cell lines displayed a rapid reversal of several S100P-CORcc and S100P-CORtt genes providing a functional association for the correlative data (Figure 3A). Dramatic cellular effects induced by diminished levels of S100P-CORcc and S100P-CORtt transcripts included: (i) an appreciable loss of the BrdU-incorporating proliferating cell fraction (Figure 3B) (ii) marked growth reduction in the 3-dimensional substrate, Matrigel, resulting in significantly smaller colony size, and rearrangement of cell nuclei from a random, apolar configuration to a relatively organized central alignment (Figure 3C), and (iii) a significant reduction in migration potential (Figure 3D).
Figure 3. Pleiotropic effects of S100P silencing.
A, Expression levels of S100P-COR genes in HTG cell lines altered by S100P knock down. Red bars - CCdl54. Blue bars - CCdl675. Open bars - control siRNA. Solid bars - S100P siRNA. B, Reduction in the number of anti BrdU stained proliferating cells in 3 HTG cell lines transfected with S100P siRNA. Error bars indicate standard deviation between triplicate assays. C, Apolar morphology of HTG colonies propagated in matrigel (top); normalized by S100P silencing to resemble polarized acinar structures (bottom). Blue - immunolocalization of anti S100P, green – actin localization by phalloidin, and red - immunolocalization of anti integrin alpha6. D, Reduction in migration potential induced by S100P siRNA in HTG cell lines. Standard error bars represent triplicate assays.
Targeting high grade primary breast tumors through a PEGA gene pathway
To test the impact of the functional attributes of tumor aggressiveness conferred by S100P-COR genes at a clinical level, we applied the 14-gene S100P-CORtt classifier towards outcome stratification, independently of S100P. First, classical clinical parameters, such as, patient age at diagnosis, clinical stage, histologic grade, tumor size, lymph node status, and estrogen receptor (ER) status were tested for RFS prediction by combining two independent datasets. These provided information for all 6 parameters in 440 tumors with 139 recurrent cases. Among the classical variables, only tumor size and ER status were found to be significantly related to outcome in this diverse group of patients, For further analysis, we divided this data into 2/3-training (n=292) and 1/3-test (n=148) sets using a uniform random number generator.
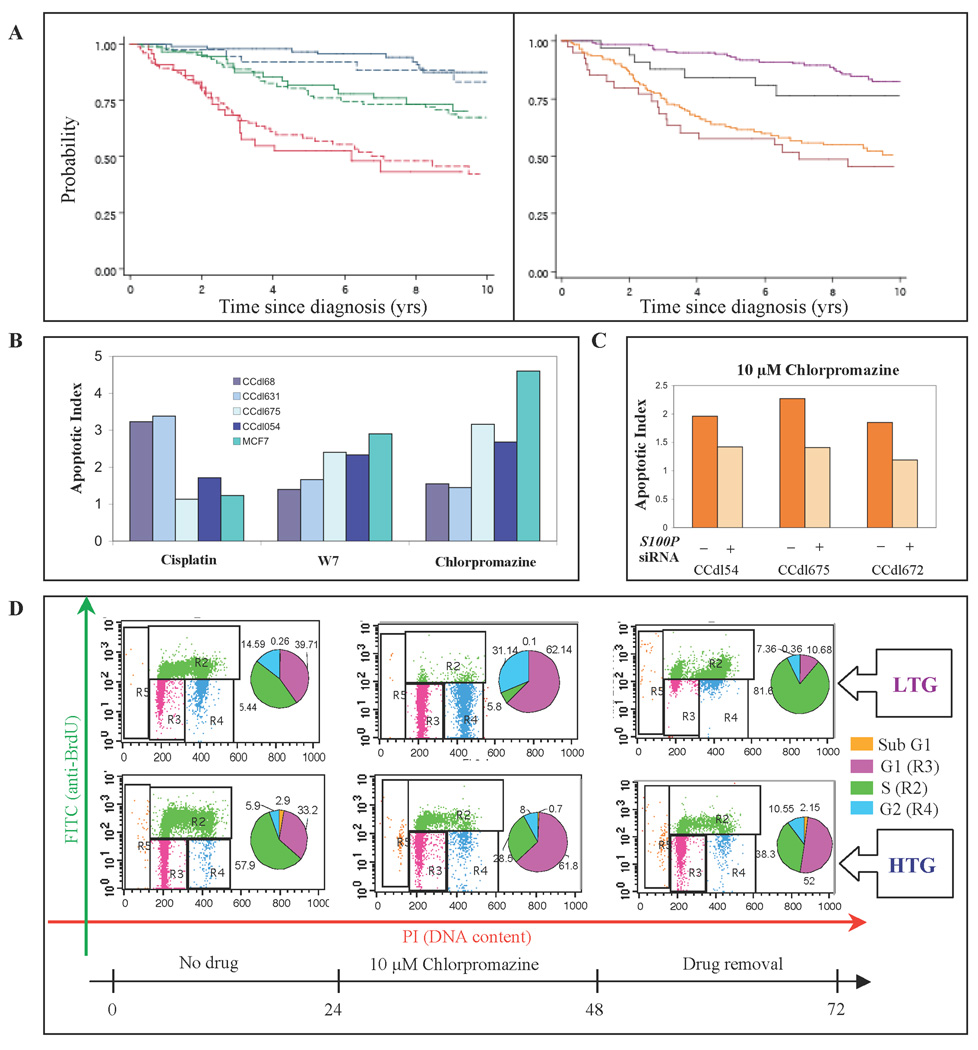
In the next step, the 14-gene classifier was added to ER status and tumor size for backward stepwise selection of significant predictors of RFS in the training set. Tumor size, and 3/14 genes: FXYD3, GPRC5A, and PYCARD, maintained statistical significance (p<0.01) (Supplementary Table 3). The size + 3-gene predictor was combined linearly, and using coefficients from the Cox proportional hazards model applied to the training set, to generate a risk score for each patient. The 33rd and 67th percentiles of the risk scores in the training set subdivided both training and test sets into patient groups at high, medium, and low risk of recurrence. Survival in the three risk subgroups differed significantly in each data set (p < 0.001 by log-rank test in each set) (Figure 4A, left panel). Moreover, the log rank test was significant in each ER category (p=0.0274 for ER-negative, and p<0.001 for ER-positive tumors) (Figure 4A, right panel). Table 2 summarizes the results, and demonstrates that RFS in the test set, using the same classification scheme as the training set, gave closely similar results thereby providing robust data validation.
Figure 4. Stratification of primary breast cancer by tumor size + S100P-COR 3- gene fingerprint, and chemotherapeutic response measurements.
A – Left panel, Comparison of Training vs. Test datasets. Training set – solid lines. Test set - dashed lines. Blue - low risk tertile; green - intermediate risk tertile; red - high risk tertile. Right panel, Comparison of ER-negative vs. ER positive cases in the combined datasets based on a median value of the classifier. Purple – low risk/ER-positive; black – low risk/ER-negative; yellow - high risk/ER-positive; maroon – high risk/ER-negative. B, Apoptosis induction measured as Annexin V positive cells in tumor cell lines of varying grade exposed to 200µM cisplatin, 10µM chlorpromazine or W7. Values represent statistically significant differences between unexposed controls and drug-treated cells (p<0.05). C, S100P siRNA-induced loss of chlorpromazine sensitivity demonstrated as declining number of Annexin V positive apoptotic cells in 3 independent HTG cell lines. D, Cell cycle analysis of LTG (CCdl68) and HTG (CCdl675) cell lines in the presence and absence of chlorpromazine. Representative FACS analysis of cells without drug treatment (left panels); with 24 hr. drug exposure (middle panels); or 24 hr. post recovery from drug exposure (right panels). The numbers for pie chart segments indicate the percentage of cells within each cell cycle phase (sub G1, G1, S, and G2).
Table 2.
Recurrence free survival estimates for Training and Test datasets based on tumor size + 3-gene classifier
| Dataset | Risk Group |
5-year survival probability (%) |
(95% CI) | 10-year survival probability (%) |
(95% CI) |
|---|---|---|---|---|---|
| Training | Low | 97 | 0.90 – 0.99 | 88 | 0.77 – 0.93 |
| Medium | 77 | 0.67 – 0.84 | 67 | 0.56 – 0.76 | |
| High | 58 | 0.47 – 0.68 | 42 | 0.30 – 0.54 | |
| Test | Low | 92 | 0.77 – 0.97 | 83 | 0.63 – 0.93 |
| Medium | 82 | 0.69 – 0.90 | 70 | 0.55 – 0.81 | |
| High | 52 | 0.36 – 0.66 | 43 | 0.26 – 0.59 |
Confidence intervals for 5 and 10-year survival in each subgroup of the training set include the survival estimates of the test subgroup and vice versa (Table 2). A log-rank test for survival differences within each subgroup revealed no differences between survival in the training and test group within each stratum (p=0.98 for difference in the lowest risk; p=0.54 for differences in the middle risk and p=0.84 for differences in the highest risk stratum). Our model was further validated using the PSEP test, defined as the difference between the predicted probability of survival in the best and worst groups at a selected time (27). In our study, at 5 yrs, survival was 0.97 for the low and 0.58 for the high risk group, with a PSEP of 0.39 (0.97-0.58) in the training set. In the test set PSEP was 0.40 (0.92-0.52). Thus PSEP for training and test set survival was almost identical. Another validation test consisted of comparing Harrell's C statistic in the training and test sets. The statistic is equal to the number of times a pair of patients, selected at random, has their survival time correctly predicted by the model. In our training data the model correctly predicted the survival order 70% of the time, while in the test set the correct order was predicted 68% of the time. Both of these percentages are considered to be quite high for breast cancer prediction (28).
Finally, towards the goal of defining candidate drugs, which effectively address molecular heterogeneity in breast cancer for targeting pertinent pathways, tumor cell response was compared between a conventional chemotherapeutic drug, cisplatin, and compounds whose mode of action is known to be calcium dependent, such as the phenothiazine, chlorpromazine, and the calmodulin inhibitor, N- (6-aminohexyl)-5-chloro-1-naphthalenesulphonamide (also known as W7). Almost no apoptosis induction was observed in HTG primary breast cancer cell lines, or in the MCF7 cell line in response to 200µM cisplatin. However, these cell lines displayed striking sensitivity to the calcium-binding drugs (Figure 4B). Reversal of drug response in S100P siRNA transfected HTG cell lines demonstrated a direct role for S100P and coregulated genes in this regard (Figure 4C). In these experiments, the sensitivity of HTG cell lines to both chlorpromazine, and W7 was persistent over time (Figure 4D). Upon removal of drugs after a 24 hr exposure period, HTG cells continued to be eliminated through apoptotic cell death, whereas G1-arrested LTG cells rapidly reentered the cell cycle.
Discussion
Here our approach of evaluating global expression profiles of tumor cell cultures of extreme histologic grades yet proliferating at relatively comparable rates, has significantly amplified the detectability of unsuspected grade associated targets. Based on the widely accepted fact that routine tissue culture conditions are inadequate in representing the tumor microenvironment with regard to oxygen, and glucose concentrations, and a variety of paracrine interactions, our experimental strategy uniquely afforded the level of resolution required to uncover a functional basis for the diagnostic differential in histologic differentiation patterns long used clinically for breast cancer subclassification and patient management. Such a context independent profile of tumor aggressiveness as depicted by PEGA could assist in sifting apart cellular programs of cancer cell autonomy from the broad interactive dimension of an infiltrating malignant population amidst a wide spectrum of surrounding host cells. Clinically, this could enable rapid improvements in disease stratification and tumor targeting.
As demonstrated, top-tier differentially expressed PEGA genes are a robust discriminant of histologic grade in tumor derived cell cultures as well as in clinical tumor tissue. Among these, CEACAM6, MUC5B, and S100P are known indicators of patient outcome in many tumor types including breast cancer (29–31). However, little is known about their functional role or mechanism of action during tumor progression. In previous microarray analyses of archived tumors with respect to grade or outcome, differential expression of PEGA genes was masked and not found to be as distinctive as proliferation-related genes. In fact, as shown in a recent meta-analysis (32), proliferation was the single common driving force for 9 independent prognostic signatures (7, 33–40). In contrast, PEGA genes defined here, are predictive without prior selection based on patient outcome, and reflect the basic infrastructure of cell signaling pathways underlying the prognostic performance of proliferation genes. Uniquely, in the derivation of this context independent signature, issues related to stromal and epithelial heterogeneity within archived tumor tissue, observed to be a limitation in the verification and clinical application of candidate prognostic cancer markers in general, are easily circumvented.
In view of the significance of the calcium ion in the cell differentiation process (41) - the ‘backbone’ of histologic grading schemes, we have highlighted the role of the PEGA member, S100P and coregulated genes in the maintenance of the dedifferentiated, high grade tumor phenotype both in vivo and in vitro. S100 proteins display cell type-specific expression patterns and regulate cytoplasmic Ca2+ levels (42). Consistent with this role, we have demonstrated that transcript levels of S100P and coregulated genes associated with high tumor grade in primary breast carcinoma parallel increased proliferation, decreased apoptosis, and greater migration potential in high grade tumor derived cell cultures. Conversely, experimental silencing of S100P initiates the appearance of epithelial polarity, a phenotype characteristic of well-differentiated human breast epithelial cells (43).
Defining comprehensive phenotypes of histologic grade, which portray molecular and functional traits, is a pivotal objective towards unambiguous, quantitative measures of breast cancer stratification. Based on gene expression cutpoint, a ‘grade gene index’ classification suggests that intermediate grade tumors essentially represent high or low histologic grade, and reflect the outcome predicted for the two extreme grades (8). Such an approach significantly enhances the usefulness of pathological evaluation of malignant tumors. Further mechanistic insights regarding temporal events within the afflicted tissue that lead to a specific tumor grade gene index at clinical presentation could be invaluable. In this regard, induction of high grade phenotypes in non malignant breast epithelial cells of high risk individuals exposed to natural and synthetic estrogens, suggests that specific grade determining events occur early in tumorigenesis (22). This is consistent with the observation of S100P overexpression at early stages of breast cancer development (44).
It could be speculated that early in tumorigenesis, selective expansion of cells harboring PEGA phenotypes, such as altered calcium regulation, cell adhesion, oxidative stress management, apoptosis, cell differentiation, and cell migration facilitate the manifestation of tumor dedifferentiation, recorded as microscopically-determined histologic grade. Notably in our study, a 3-gene fingerprint comprised of GPRC5A, PYCARD, and FXYD3 expression, replaces ER status as a significant predictor of outcome, and enhances the predictive power of tumor size, another long measured clinical parameter. While it can be appreciated that underlying these highly predictive genes are key cellular processes related to calcium regulation, apoptosis, and differentiation, respectively, a biological basis for the contribution of tumor size to this algorithm in particular, is presently unclear. In generating a reliable risk score for breast cancer patients, this 3-gene fingerprint potentially serves as a molecular adjunct to two clinicopathological variables: tumor size and histologic grade. High grade primary breast tumors are generally treated with radiation, chemotherapy and hormonal therapy (if ER positive). Response to these agents is highly variable. Consequently, a sizeable proportion of patients with high grade tumors succumb to their disease. Prior knowledge of such cases could assist in more aggressive and targeted clinical management. By employing cell lines spanning a wide spectrum of risk phenotypes identified by the PEGA profile, our data further demonstrate exquisite sensitivity of high risk tumor cells to calcium regulating drugs, while low risk counterparts display resistance by rapidly reversing to a proliferative state upon drug removal. These data provide robust functional evidence towards a therapeutic approach worthy of further consideration.
Towards fruitful drug discovery efforts, our experimental strategy advocates continued tumor cell characterization through the implementation of new model systems. While routinely used cancer cell lines provide an unlimited supply of high grade, and metastatic cells at late stages of cancer progression, an important application of early stage, and well- to moderately differentiated tumor cell models is to provide functional validation for presumed phenotypes of tumor aggressiveness. Using these model systems, a biologically derived gene signature of tumor cell immortalization, ImmSig, was previously identified, which portrays the importance of oxidative stress-reducing genes in maintaining continuously proliferating tumor populations (21), recently confirmed by others (45). In the search for new effective therapeutic targets against aggressive breast tumor cells, it is important to acknowledge that the successful application of autonomously regulated gene products, such as ERBB2 and EGFR, represents the validity of our described approach. Moreover, since expression patterns of such genes are more accurately replicated in preclinical experimental models, the potential for clinical reagent development is likely to be realized within a shorter time frame. The PEGA signature sets forth a new paradigm, and provides a clear rationale for employing the full range of grade-associated molecular phenotypes, and model systems for refining approaches in tumor targeting to ensure data reliability and clinical benefit.
Supplementary Material
Acknowledgement
This work was supported by the National Institutes of Health under grant CA109325.
References
- 1.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 2.Black MM, Barclay TH, Hankey BF. Prognosis in breast cancer utilizing histologic characteristics of the primary tumor. Cancer. 1975;36:2048–2055. doi: 10.1002/cncr.2820360919. [DOI] [PubMed] [Google Scholar]
- 3.Rosen PP, Saigo PE, Braun DW, Jr, Weathers E, DePalo A. Predictors of recurrence in stage I (T1N0M0) breast carcinoma. Ann Surg. 1981;193:15–25. doi: 10.1097/00000658-198101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopton DS, Thorogood J, Clayden AD, MacKinnon D. Histological grading of breast cancer; significance of grade on recurrence and mortality. Eur J Surg Oncol. 1989;15:25–31. [PubMed] [Google Scholar]
- 5.Ridolfi RL, Rosen PP, Port A, Kinne D, Mike V. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977;40:1365–1385. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Pereira H, Pinder SE, Sibbering DM, et al. Pathological prognostic factors in breast cancer. IV: Should you be a typer or a grader? A comparative study of two histological prognostic features in operable breast carcinoma. Histopathology. 1995;27:219–226. doi: 10.1111/j.1365-2559.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 7.Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 8.Ivshina AV, George J, Senko O, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66:10292–10301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 9.Yu K, Ganesan K, Miller LD, Tan P. A modular analysis of breast cancer reveals a novel low-grade molecular signature in estrogen receptor-positive tumors. Clin Cancer Res. 2006;12:3288–3296. doi: 10.1158/1078-0432.CCR-05-1530. [DOI] [PubMed] [Google Scholar]
- 10.Lasfargues EY, Ozzello L. Cultivation of human breast carcinomas. J Natl Cancer Inst. 1958;21:1131–1147. [PubMed] [Google Scholar]
- 11.Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 12.Cailleau R, Young R, Olivé M, Reeves WJ., Jr Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974;53:661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 14.Gazdar AF, Kurvari V, Virmani A, et al. Characterization of paired tumor and non-tumor cell lines established from patients with breast cancer. Int J Cancer. 1998;78:766–774. doi: 10.1002/(sici)1097-0215(19981209)78:6<766::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Ethier SP. Identifying and validating causal genetic alterations in human breast cancer. Breast Cancer Research and Treatment. 2003;78:285–287. doi: 10.1023/a:1023078722316. [DOI] [PubMed] [Google Scholar]
- 16.Smith HS, Wolman SR, Dairkee SH, et al. Immortalization in culture occurs at a late stage in progression of breast cancer. J Natl Cancer Inst. 1987;78:611–615. [PubMed] [Google Scholar]
- 17.Dairkee SH, Deng G, Stampfer MR, Waldman FM, Smith HS. Selective cell culture of primary breast carcinoma. Cancer Res. 1995;55:2516–2519. [PubMed] [Google Scholar]
- 18.Dairkee SH, Paulo EC, Traquina P, Moore DH, Ljung B-M, Smith HS. Partial Enzymatic Degradation of Stroma Allows Enrichment and Expansion of Primary Breast Tumor Cells. Cancer Res. 1997;57:1590–1596. [PubMed] [Google Scholar]
- 19.Li Z, Bustos V, Miner J, et al. Propagation of Genetically Altered Tumor Cells Derived from Fine Needle Aspirates of Primary Breast Carcinoma. Cancer Res. 1998;58:5271–5274. [PubMed] [Google Scholar]
- 20.Dairkee SH, Ji Y, Ben Y, Moore DH, Meng Z, Jeffrey SS. A molecular ‘fingerprint’ of primary breast cancer cultures; patterns resembling tumor tissue. BMC Genomics. 2004;5:47. doi: 10.1186/1471-2164-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dairkee SH, Nicolau M, Sayeed A, et al. Oxidative stress pathways are highlighted in an immortalization signature in breast cancer. Oncogene. 2007;26:6269–6279. doi: 10.1038/sj.onc.1210452. [DOI] [PubMed] [Google Scholar]
- 22.Dairkee SH, Seok J, Champion S, et al. Bisphenol A induces a profile of tumor aggressiveness in high-risk cells of breast cancer patients. Cancer Res. 2008;68:2076–2080. doi: 10.1158/0008-5472.CAN-07-6526. [DOI] [PubMed] [Google Scholar]
- 23.Chin K, DeVries S, Fridlyand J, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Dupuy A, Simon RM. Critical review of published microarray studies for cancer outcome and guidelines on statistical analysis and reporting. J Natl Cancer Inst. 2007;99:147–157. doi: 10.1093/jnci/djk018. [DOI] [PubMed] [Google Scholar]
- 25.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loi S, Haibe-Kains B, Desmedt C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 27.Altman DG, Royston P. What do we mean by validating a prognostic model? Statist Med. 2000;19:453–473. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Chow E, Abdolell M, Panzarella T, et al. Predictive model for survival in patients with advanced cancer. J Clin Oncol. 2008;26:5863–5869. doi: 10.1200/JCO.2008.17.1363. [DOI] [PubMed] [Google Scholar]
- 29.Maraqa L, Cummings M, Peter MB, et al. Carcinoembryonic antigen cell adhesion molecule 6 predicts breast cancer recurrence following adjuvant tamoxifen. Clin Cancer Res. 2008;14:405–411. doi: 10.1158/1078-0432.CCR-07-1363. [DOI] [PubMed] [Google Scholar]
- 30.Yu CJ, Yang PC, Shun CT, Lee YC, Kuo SH, Luh KT. Overexpression of MUC5 genes is associated with early post-operative metastasis in non-small-cell lung cancer. Int J Cancer. 1996;69:457S–465S. doi: 10.1002/(SICI)1097-0215(19961220)69:6<457::AID-IJC7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Platt-Higgins A, Carroll J, et al. Induction of metastasis by S100P in a rat mammary model and its association with poor survival of breast cancer patients. Cancer Res. 2006;66:1199–1207. doi: 10.1158/0008-5472.CAN-05-2605. [DOI] [PubMed] [Google Scholar]
- 32.Wirapati P, Sotiriou C, Kunkel S, et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008;10:R65. doi: 10.1186/bcr2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 34.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 35.Miller LD, Smeds J, George J, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102:13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield ML, George LK, Grant GD, Perou CM. Common markers of proliferation. Nat Rev Cancer. 2006;6:99–106. doi: 10.1038/nrc1802. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 38.Naderi A, Teschendorff AE, Barbosa-Morais NL, et al. A gene-expression signature to predict survival in breast cancer across independent data sets. Oncogene. 2007;26:1507–1516. doi: 10.1038/sj.onc.1209920. [DOI] [PubMed] [Google Scholar]
- 39.Teschendorff AE, Naderi A, Barbosa-Morais NL, et al. A consensus prognostic gene expression classifier for ER positive breast cancer. Genome Biol. 2006;7:R101. doi: 10.1186/gb-2006-7-10-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang HY, Sneddon JB, Alizadeh AA, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bikle DD, Oda Y, Xie Z. Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. Review. J Steroid Biochem Mol Biol. 2004;89–90:355–360. doi: 10.1016/j.jsbmb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Becker T, Gerke V, Kube E, Weber K. S100P, a novel Ca(2+)-binding protein from human placenta: cDNA cloning, recombinant protein expression and Ca(2+) binding properties. Europ J Biochem. 1992;207:541–547. doi: 10.1111/j.1432-1033.1992.tb17080.x. [DOI] [PubMed] [Google Scholar]
- 43.Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guerreiro Da Silva ID, Hu YF, Russo IH, et al. S100P calcium-binding protein overexpression is associated with immortalization of human breast epithelial cells in vitro and early stages of breast cancer development in vivo. Int J Oncol. 2000;16:231–240. [PubMed] [Google Scholar]
- 45.Rai P, Onder TT, Young JJ, et al. Continuous elimination of oxidized nucleotides is necessary to prevent rapid onset of cellular senescence. Proc Natl Acad Sci U S A. 2009;106:169–174. doi: 10.1073/pnas.0809834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.