Abstract
Cellular responsiveness to nitric oxide (NO) is shaped by past history of NO exposure. The mechanisms behind this plasticity were explored using rat platelets in vitro, specifically to determine the relative contributions made by desensitization of NO receptors, which couple to cGMP formation, and by phosphodiesterase-5 (PDE5), which is activated by cGMP and also hydrolyzes it. Repeated delivery of brief NO pulses (50 nm peak) at 1-min intervals resulted in a progressive loss of the associated cGMP responses, which was the combined consequence of receptor desensitization and PDE5 activation, with the former dominating. Delivery of pulses of differing amplitude showed that NO stimulated and desensitized receptors with similar potency (EC50 = 10–20 nm). PDE5 activation was highly sensitive to NO, with a single pulse peaking at 2 nm being sufficient to evoke a 50% loss of response to a subsequent near-maximal NO pulse. However, the activated state of the PDE subsided quickly after removal of NO, the half-time for recovery being 25 s. In contrast, receptor desensitization reverted much more slowly, the half-time being 16 min. Accordingly, with long (20-min) exposures, NO concentrations as low as 600 pm provoked significant desensitization. The results indicate that PDE5 activation and receptor desensitization subserve distinct short term and longer term roles as mediators of plasticity in NO-cGMP signaling. A kinetic model explicitly describing the complex interplay between NO concentration, cGMP synthesis, PDE5 activation, and the resulting cGMP accumulation successfully simulated the present and previous data.
Nitric oxide (NO) is an intercellular messenger molecule in most tissues of the body and exerts physiological effects by binding to receptors possessing intrinsic guanylyl cyclase (GC)4 activity. The receptor proteins are known by various names, including the homogenate-based one, soluble guanylyl cyclase, but here we simply call them NO receptors because this terminology is conceptually more informative in a cellular context. Synthesis of cGMP from GTP that follows receptor activation can engage a number of downstream targets, including cGMP-dependent protein kinase, to bring about alterations in cell function, such as smooth muscle relaxation and neural transmission (1–3).
In common with other hormone or transmitter signaling pathways, the sensitivity of the NO-cGMP pathway is subject to short term and long term regulation. Enduring exposure of cells to NO (hours or more) leads to a loss of NO responsiveness that in the cardiovascular system contributes to the clinical problem of tolerance to nitrovasodilator therapy (4–6). One mechanism here is a gradual loss of the NO receptor mRNA and protein (7, 8). Conversely, a chronic lack of NO leads to supersensitivity that has been attributed to increased NO receptor activity (9, 10).
Short term regulatory mechanisms serve to shape acute cellular cGMP responses to NO and may involve NO receptor desensitization, reducing the rate of cGMP formation (11), activation of phosphodiesterases (PDEs) that consume cGMP (12), or combinations of the two (13, 14). These short term mechanisms may also be quite sustained. For example, relatively brief (5 min) exposures of cells to NO can reduce responsiveness to NO an hour later, a phenomenon that correlated with increased activity of PDE5 (15). This PDE isoform, which is inhibited by drugs like sildenafil (Viagra) used to treat erectile dysfunction, contains a non-catalytic cGMP binding site whose occupation stimulates catalytic activity (16, 17). Reciprocally, shortly after removing the endothelial source of NO, blood vessels develop a supersensitivity to NO-induced relaxation, an effect that was speculated to reflect increased NO receptor responsiveness (18).
Addressing directly the relative contributions of NO receptors and PDEs to the regulation of NO-cGMP signaling is problematic in complex tissues because it is very difficult to measure their activities accurately. Rat platelets maintained in vitro, on the other hand, have merit as an experimental model for these purposes because they are physiological NO targets, homogenous, and are also extremely small (about 1 μm in diameter), minimizing problems of compartmentation of the signaling proteins and of diffusion delays in access of applied agents to the cell interior. In addition, they exist naturally in suspension, which is ideal for kinetic studies, they have an abundance of NO receptors of just one type (α1β1), and the only detectable PDE that hydrolyzes NO-evoked cGMP signals is PDE5 (13). Exposure of these cells to persistent NO generates only very transient cGMP responses, mostly terminating within 10 s of NO application; this profile has been explained by a combination of receptor desensitization and PDE5 enhancement (13). The questions addressed here are whether or not these adaptations persist beyond the period of NO application to influence subsequent cellular responsiveness and, if so, for how long, and what are their relative contributions to the plasticity? Central to the feasibility of obtaining answers was our recent development of a method for delivering repeated NO pulses of known amplitude and duration (14), allowing controlled conditioning NO exposures to be followed by test exposures after selected time intervals in NO-free solution.
EXPERIMENTAL PROCEDURES
Materials
Proline-NONOate, diethylenetriamine-NONOate, spermine NONOate, and 2–4-carboxyphenyl-4,4,5,5,-tetramethylimidazoline-1-oxyl-3-oxide (CPTIO) were obtained from Axxora Ltd. (Nottingham, UK); sildenafil was supplied by the Chemistry Division of the Wolfson Institute for Biomedical Research (London, UK). Other special chemicals were from Sigma-Aldrich. The human embryonic kidney 293 cell line expressing bovine α1β1-GC and human PDE5A1 (HEK-GC/PDE5 cells (19)) and the phospho-PDE5 antibody were kindly supplied by Professor Doris Koesling (Univ. of Bochum, Germany).
Platelets
These were prepared from rat blood using methods described previously (13) and incubated at 37 °C in a solution containing 137 mm NaCl, 2.7 mm KCl, 0.5 mm MgCl2, 0.55 mm NaH2PO4, 25 mm Tris, 5.6 mm d-glucose, 0.3 mm urate, and 0.1 mm l-nitroarginine supplemented with superoxide dismutase (1000 units/ml), pH 7.4. The platelets were maintained at 0.5 mg of protein/ml or, for investigating PDE5 phosphorylation, at twice this concentration.
NO Delivery and cGMP Measurement
Calibrated pulses of NO were administered using a combination of a fast NO releaser, proline-NONOate (half-life = 1.8 s), and a slow NO-consumer, CPTIO (50 μm), as described in detail (14). As before, calibration was carried out by directly measuring the NO concentration attained by standard mixtures. Stock proline-NONOate solutions were then prepared to give the desired final peak NO concentrations (which are proportional to the donor concentration) when diluted 1:100 into the platelet suspension containing CPTIO. The pulses illustrated in the figures are simulations based on the kinetics of the reaction and closely resemble the recorded NO profiles, except that the recorded profiles are artificially slowed by the electrode response time (14). Clamped NO concentrations were achieved with a mixture of CPTIO (50 μm) and one of the slower NO releasers, spermine-NONOate (half-life = 39 min) or diethylenetriamine NONOate (half-life = 20 h), depending on the duration of the experiment (20). Aliquots of platelets were inactivated in boiling buffer, and the amounts of cGMP and protein were measured as before (14). In many of the experiments, rapid (2-s) exposures to NO in the presence or absence of sildenafil were made to measure the initial rate of cGMP accumulation as precisely as possible. Sampling was done by rapidly dispensing an aliquot of platelets (50 μl) preincubated with CPTIO onto a 0.5-μl bead of NO donor (and a separate 0.5-μl bead of sildenafil, when used) deposited immediately beforehand in the bottom of a 1.5-ml Eppendorf tube prewarmed to 37 °C (time 0) and then quickly withdrawing the aliquot back into the pipette before inactivating it (time = 2 s). Based on measurements using 0.5-μl beads of India ink, mixing was complete 72 ± 10 ms (mean ± S.E.; n = 5) after the initial addition of the platelets (see supplemental Fig. S1). At the applied concentration (100 μm), maximally effective concentrations of sildenafil are expected intracellularly almost instantaneously; from the molecular weight (494) and polar surface area (109 Å2), the membrane permeability coefficient of sildenafil is expected to be about 1.3 × 10−6 cm/s (21), which predicts an intracellular concentration of 0.1 μm after 14 ms in a platelet (assumed to be a sphere of radius = 0.5 μm). The effect of 0.1 μm sildenafil on the early rise in cGMP would be indistinguishable from maximum PDE5 inhibition, as determined from the kinetics of inhibition of PDE5 by sildenafil at steady state in rat platelets (13). In each experiment there were 3–4 independent runs of each condition; results are presented as the means ± S.E. Except where impractical, extracellular/bound cGMP sustained through prior NO exposure was measured by inactivating an aliquot of platelets immediately before re-exposure. In agreement with prior detailed studies (13), the values ranged from 5 to 25 pmol/mg protein depending on conditions. Unless indicated otherwise (Fig. 3), results are given after subtraction of this pool of cGMP.
FIGURE 3.
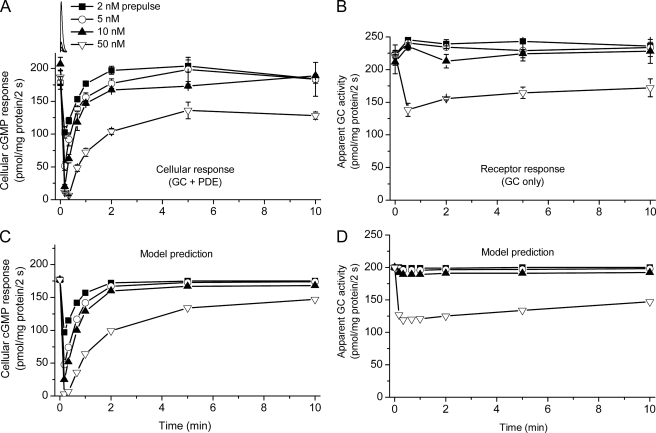
Differential rates of recovery of PDE5 activity and receptor desensitization. Platelets were exposed to single NO pulses of varying amplitude (simulated in the inset at the top), and cGMP responses (2 s) to near-maximal test NO pulse (50 nm) in the absence (A) and presence (B) of sildenafil were then charted over time in comparison with the amplitude of control responses to the same test pulse, shown at time 0. Because of the complexity of this experiment, it was not practicable to measure (and correct for) extracellular/bound cGMP resulting from prior NO exposure. The predictions of the model (Fig. 6 and Table 1) for the experiment are shown in C and D with GCmax = 127 μm/s, Vp2 = 75 μm/s, and k3 = 1 × 107 m−1s−1.
PDE5 Phosphorylation
After incubation and treatment, aliquots of platelets (0.1 ml) were inactivated with 25 μl of a Laemmli-type buffer, giving a final concentration of 62 mm Tris-HCl, pH 6.8, 2% (w/v) SDS, 0.01% (w/v) bromphenol blue, 10% (w/v) glycerol, and 5% (v/v) β-mercaptoethanol. HEK-GC/PDE5 cells were grown in 48-well plates and were then incubated and exposed to S-nitrosoglutathione as described (19). After treatment, the medium was aspirated and replaced with the above Laemmli-type buffer. All samples were kept on ice until the end of the experiment when they were frozen at −80 °C until needed. Electrophoresis was carried out in 4–15% gradient SDS-PAGE gels (Bio-Rad). Gels were equilibrated for 30 min in transfer buffer (50 mm Tris base, 384 mm glycine, 5% methanol) with the addition of 0.05% Triton X-100 and 1/1000 (v/v) antioxidant solution (2 m sodium bisulfite in 10% dimethylformamide). Proteins were transferred to hydrated polyvinylidene difluoride membranes (Immun-Blot, Bio-Rad) in a submerged blotting apparatus (TE22, Hoefer Inc., Holliston, MA) using a current of 0.38 A for 90 min in transfer buffer plus 0.01% SDS, after which the blots were allowed to dry completely at room temperature before being stored at 4 °C until required. Blots were submerged in methanol for ∼10 s, equilibrated in Tris-buffered saline for 5 min, and then blocked for 1 h in 3% skimmed milk diluted in Tris-buffered saline with 0.2% Tween 20. Anti-phospho-PDE5 antibody was diluted 1/1000 into blocking buffer for overnight incubation at 4 °C with agitation. Blots were washed 3 times (5 min each) in Tris-buffered saline containing 0.05% Tween 20. Secondary antibody conjugated to horseradish peroxidase (goat anti-rabbit, Pierce) was applied at 10 ng/ml in blocking buffer for 2 h at room temperature with agitation. After washing (as before), blots were developed in chemiluminescence substrate (Supersignal West Pico, Pierce) and exposed to Amersham Biosciences Hyperfilm ECL (GE Healthcare). Images from autoradiographs were captured in GeneSnap (Syngene, Cambridge, UK), and the relative intensity of bands was quantified with GeneTools (Syngene).
Vasodilator-stimulated Phosphoprotein (VASP) Phosphorylation
Phosphorylated VASP in platelets was detected by immunoblotting as described previously (22).
Modeling
Equations encapsulating the scheme presented under “Results” were solved in Mathcad 14 (Adept Scientific, Letchworth, Herts, UK). The NO pulse input was as described before (14). The results of the calculations were in molar units (concentrations or rates), which were converted into the experimental units (pmol/mg protein) by assuming 1 mg of protein is equivalent to 2.5 μl (23).
RESULTS
Progressive Tachyphylaxis of NO-cGMP Signaling
In the first experiment pulses of NO peaking at a concentration of 50 nm after 2 s and declining to 0 after 15–20 s were delivered at 1-min intervals. This pulse height gives near-maximal NO receptor activation (14). The apparent GC activity was recorded during each pulse by measuring cGMP accumulation over a 2-s period in the presence of sildenafil in high enough concentration (100 μm) to effectively abolish PDE5 activity (13). From a control activity of around 230 pmol/mg of protein/2 s, a single preceding NO pulse caused the activity to fall by 45%. With succeeding pulses, further reductions occurred until a steady 78% desensitization was reached after 5–6 pulses (Fig. 1A).
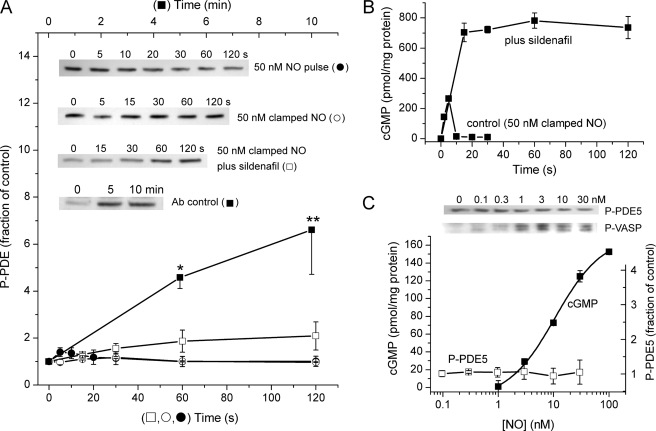
FIGURE 1.
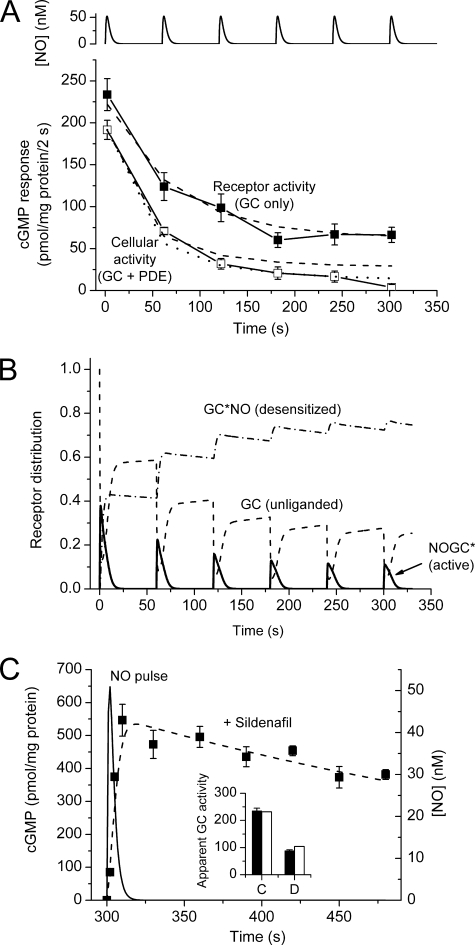
Progressive tachyphylaxis after repeated NO pulses. A, pulses of NO peaking at 50 nm were delivered to platelets at 1-min intervals (simulated at the top), and cGMP accumulation over 2 s was measured each minute in the presence and absence of sildenafil (100 μm). Dashed lines are predictions of the model (Fig. 6 and Table 1) with GCmax = 140 μm/s and Vp2 = 90 μm/s and k3 = 1 × 107 m−1s−1. The dotted line providing a better fit to the cellular activity (open squares) is an extended version of that shown in Fig. 6B, in which the active, cGMP-bound form of PDE5 (cGMP-PDE5*) undergoes a transition to a more stable (putatively phosphorylated) state, with forward and backward rate constants of 2 × 10−3 and 6 × 10−4 s−1, respectively. The latter value was chosen to match the 20-min reported half-life of phosphorylated PDE5 (19) and the former to fit the experimental data. B, model predictions for the different states of the receptor accounting for the output seen in A. C, test for the appearance of new PDE activity associated with tachyphylaxis. Platelets given 6 pulses (as in A) have desensitized NO receptors, as indicated by the reduction in cGMP generation during the first 2 s of the last pulse (filled bars in the inset: control response (C), desensitized response (D)). In the main panel, samples of platelets were subjected to the 6th pulse in the presence of sildenafil (100 μm), and cGMP was measured periodically over the next 3 min to assess PDE activity. The dashed line is a fit to the model (Fig. 6 and Table 1) assuming GCmax = 140 μm/s and k3 = 0.53 × 107 m−1s−1, giving the levels of control and desensitized GC indicated by the open bars in the inset. The time-course of the NO pulse is simulated for comparison (solid line).
To gauge concomitant alterations in PDE5 activity, cGMP accumulation during the first 2 s of each pulse was measured in the same experiments in the absence of sildenafil. To begin with, the control responses were not much smaller than with sildenafil present (180 pmol/mg of protein/2 s), signifying a low initial activity of PDE5, but 1 min after the first NO pulse the response was down to about a third of its initial amplitude. Additional pulses elicited further reductions until, after 4–6 pulses, the response amplitude became relatively constant at about 15 pmol/mg of protein/2 s, which was less than 10% that of the starting value (Fig. 1A).
Conceivably, some of the fade taking place in the presence of sildenafil under these conditions could be caused by the appearance of PDE activity other than that of PDE5. To examine this possibility, PDE activity in the presence of sildenafil was measured after the same sequence of pulses. This was carried out by adding sildenafil just before the final (sixth) pulse and then charting the decline in platelet cGMP levels once the NO pulse had decayed. The outcome (Fig. 1C) showed a very slow rate of loss of cGMP that was quantitatively the same as predicted from the measured kinetics of inhibition by sildenafil under steady-state conditions in rat platelets (broken line, Fig. 1C). This result shows that there was no unsuspected PDE activity and also attests to the expected very rapid penetration of sildenafil into the platelets (see “Experimental Procedures”).
These findings demonstrate that brief periods of NO exposure induce a progressive reduction of subsequent responsiveness (tachyphylaxis) and that the tachyphylaxis is caused by a mixture of receptor desensitization and PDE5 activation, with the former dominating under the conditions of this experiment. Moreover, the alterations in both components must be quite enduring; otherwise, summation with repeated pulses would not have occurred.
Progress and NO Concentration Dependence of Receptor Desensitization
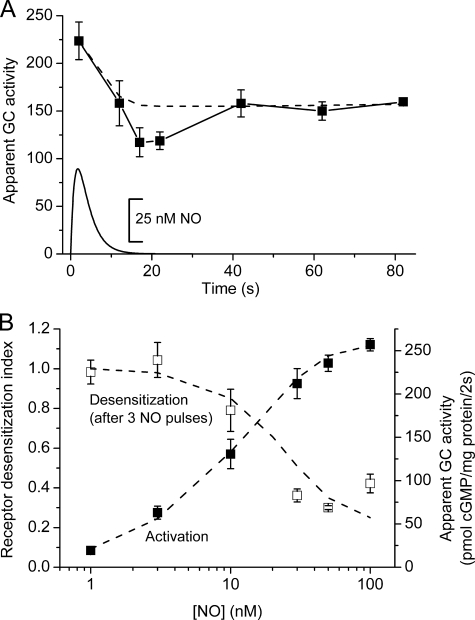
The evolution of the desensitized state was examined by measuring the apparent GC activity (2-s exposure to a 50 nm pulse in the presence of sildenafil) at various times after delivering a single NO pulse (50 nm peak amplitude). There was an initial fall in apparent GC activity, bottoming near the end of the NO pulse (15–20 s), and then a partial recovery to a steady desensitized level (Fig. 2A). A persistence of the desensitized state for more than a minute explains the summation observed in Fig. 1A.
FIGURE 2.
Characteristics of NO receptor desensitization. A, platelets were given a single NO pulse peaking at 50 nm (simulated by a solid line at the bottom), and the apparent GC activity was monitored by measuring cGMP accumulation in response to a second NO pulse (2-s exposure) delivered at various times afterward in the presence of 100 μm sildenafil. The first point is a control 2-s exposure to the pulse in the presence of sildenafil. The broken line is the model prediction (Fig. 6 and Table 1) assuming GCmax = 138 μm/s and k3 = 0.7 × 107 m−1s−1. B, platelets were given three NO pulses peaking at different concentrations (abscissa) and, a minute after the last pulse, were given a test pulse (50 nm amplitude) in the presence of 100 μm sildenafil. The rise in cGMP over 2 s was normalized to the value from control (not NO-pretreated) platelets (open symbols). In the same experiment the receptor activation curve for NO was measured by exposing control platelets to the same range of NO pulses for 2 s in the presence of sildenafil (filled symbols). Broken lines are model predictions (Fig. 6 and Table 1) with GCmax = 138 μm/s and k3 = 0.7 × 107 m−1s−1.
The concentration-response curve for NO-induced desensitization was examined by giving three identical pulses of NO (1 per min) of differing peak amplitude (0.1–100 nm) and, a minute later, measuring the apparent GC activity (2-s exposure) generated in response to a fixed 50 nm pulse. The results showed that 50% desensitization was achieved with a pulse height of about 20 nm NO (Fig. 2B). Measurement of the receptor activation curve in the same experiment (in untreated platelets) showed an EC50 of about 10 nm, in agreement with previous findings with this method (14) and with measurements using clamped NO concentrations (13). Thus, acute receptor activation and desensitization occur over similar NO concentration ranges.
Progress and NO Concentration Dependence of PDE5 Activation in Comparison with Receptor Desensitization
Single NO pulses of different amplitudes were administered, and at varying times afterward, the cGMP accumulation occurring over 2 s in response to a fixed (50 nm) pulse was recorded in the presence and absence of sildenafil. The aim was to differentiate changes in function of the receptor alone (test plus sildenafil) and the receptor plus PDE5 (test minus sildenafil). The net cellular response (comprising changes in receptor plus PDE5) altered radically. After an NO pulse as low as 2 nm (peak), the cellular response was depressed by 50% after 10 s but then recovered, apparently completely, by 2 min (Fig. 3A). NO pulses of increased amplitude (5, 10, and 50 nm) gave greater initial depressions whose amplitudes were graded with the NO pulse height, the largest causing effectively complete inhibition of cGMP accumulation 10–20 s afterward. In all cases, however, the responses recovered at similar rates, reaching an apparent steady-state value after about 2 min with a half-time of around 25 s. After the 50 nm NO pulse, the resulting steady-state amplitude was clearly depressed relative to the control (no pre-exposure to NO). When assayed in the presence of sildenafil, there was little obvious change in the receptor output over the whole time-course after pulses of 2–10 nm NO, but a 50 nm pulse gave ∼30% depression that lasted for the 10-min duration of the experiment (Fig. 3B).
Recovery from Desensitization
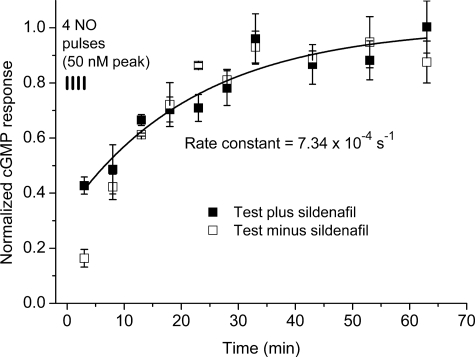
The foregoing experiment indicates that PDE5 activation makes a profound contribution to short term tachyphylaxis, whereas receptor desensitization becomes evident with larger pulses and has a longer-lasting role. To find out how long desensitization persists, platelets were given a sequence of 4 NO pulses (50 nm peak; 1 per min) to provoke tachyphylaxis, and then recovery was monitored over the next hour by periodically testing the response (2 s) to a 50 nm NO pulse in the presence of sildenafil. Controls included performing the test sampling in the absence of sildenafil (to check for possible changes in PDE5 activity) and the running of untreated samples alongside. The results showed that there was a slow but apparently complete recovery of receptor function, the half-time being near 16 min (Fig. 4). With the exception of the first test sample (assayed during the last of the conditioning pulses), there was little effect of omitting sildenafil from the test samples, consistent with PDE5 activity reverting quickly to its very low resting level.
FIGURE 4.
Recovery from receptor desensitization. Platelets were given 4 NO pulses (50 nm peak, 1 per min), and subsequent responsiveness were tested periodically over the next hour by exposing them to a further 50 nm NO pulse for 2 s in the absence (open symbols) or presence (filled symbols) of sildenafil (100 μm). The first points in the time-course represent the values measured during the last of the conditioning NO pulses. Values are normalized to control responses measured over the same time period as the tests; the line is an exponential fit to the data obtained in the presence of sildenafil, giving the indicated rate constant.
PDE5 Phosphorylation
The recovery of the PDE5 activity in these experiments was unexpectedly rapid considering previous evidence from human platelets that activation of PDE5 can last much longer, being still evident 1 h after a 5-min exposure to 1 μm diethylamine NONOate (15). From studies on model cell lines, the enduring activity has been attributed to phosphorylation by cGMP-dependent protein kinase of a serine residue on the PDE5 enzyme, resulting in a slowed dissociation of cGMP from its agonist site (19). The much faster reversal observed in the present experiments, therefore, suggests either that PDE5 does not become phosphorylated with the pulses of NO used here (amplitude up to 50 nm) or that phosphorylation reverses much more rapidly than in human platelets.
On monitoring the level of PDE5 phosphorylation by immunoblotting (Fig. 5A), no significant alteration was detectable during a 2-min period, after giving a 50 nm NO pulse. The same result was obtained when NO was present throughout the 2-min period in a clamped concentration of 50 nm when, as reported before (13), cGMP also rose only transiently (Fig. 5B). In contrast, when faced with a clamped 50 nm NO concentration in the presence of sildenafil (100 μm), cGMP rose to very high levels which were maintained over 2 min (Fig. 5B), in agreement with previous findings (13). Under these conditions, a trend toward increased PDE5 phosphorylation with time was evident in some runs (inset, Fig. 5A), but the mean levels were not significantly different from controls (Fig. 5A). When many more samples were analyzed, however, a 2-min exposure to 50 nm clamped NO in the presence of sildenafil generated a modest but significant increase in PDE5 phosphorylation (145 ± 17% of control; n = 13; p = 0.0027 by paired t test). As a positive control for the phospho-PDE5 antibody, HEK cells expressing NO-activated GC and PDE5 were challenged with 100 μm S-nitrosoglutathione for 5 or 10 min; mean increases of up to 700% of basal levels were observed (Fig. 5A), consistent with a prior publication using the same cells and methodology (19).
FIGURE 5.
PDE5 phosphorylation. A, time-course of the levels of phospho-PDE5 (P-PDE5) assayed by immunoblotting lysates of platelets previously exposed to a 50 nm NO pulse (●) or to a persistent (clamped) NO concentration of 50 nm without (○) or with (□) 100 μm sildenafil. For a positive control, HEK-GC/PDE5 cells were exposed to 100 μm S-nitrosoglutathione in the presence of sildenafil (■) (see Ref. 19). Data are the means ± S.E.; p < 0.05 (*) and p < 0.01 (**) by one-way analysis of variance and Dunnett's post-hoc test (n = 3); all other results (3–4 independent platelet samples) were not significantly different (p > 0.05) from their corresponding controls (untreated platelets). Representative blots are shown in the inset, and sample cGMP responses of platelets from the same experiments are shown in B. Ab, antibody. In C the platelets were exposed to a range of clamped NO concentrations for 2 min, and the level of phosphorylated PDE5 was assayed (□; n = 3); as controls in the same experiments, the phosphorylation of VASP (P-VASP) was checked (example shown in inset together with a sample P-PDE blot), and the rise in cGMP in response to different NO concentrations was measured after 2-s exposure (■). Clamped NO concentrations in all cases were achieved using a mixture of spermine-NONOate and CPTIO (50 μm).
The possibility then arose that the concentration of NO to which the platelets were exposed in the above experiments (50 nm) was too high, particularly in view of evidence that the concentration-response curve for NO-induced, cGMP-dependent phosphorylation of VASP in rat platelets is biphasic, with NO concentrations higher than 3 nm giving progressively less VASP phosphorylation (13). Exposure to a range of clamped NO concentrations (0.1–30 nm) for 2 min, however, did not lead to a significant change in phospho-PDE5 levels (Fig. 5C). As controls in the same experiments, NO produced the usual concentration-dependent increase in cGMP levels (EC50 = 10 nm; Fig. 5C) and increased VASP phosphorylation, with a maximal effect occurring at about 3 nm NO followed by a decline (inset, Fig. 5C). All these results suggest that the behavior of PDE5 in the platelets under the conditions of our experiments is largely uncomplicated by phosphorylation of the enzyme.
A Quantitative Analysis
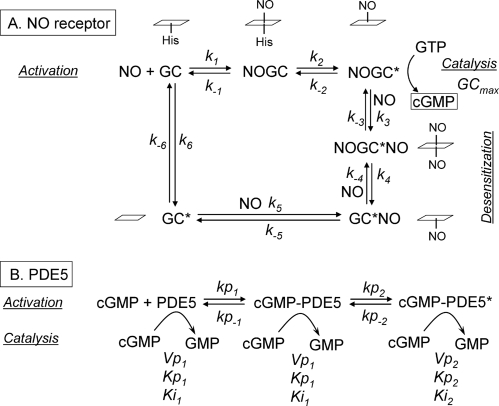
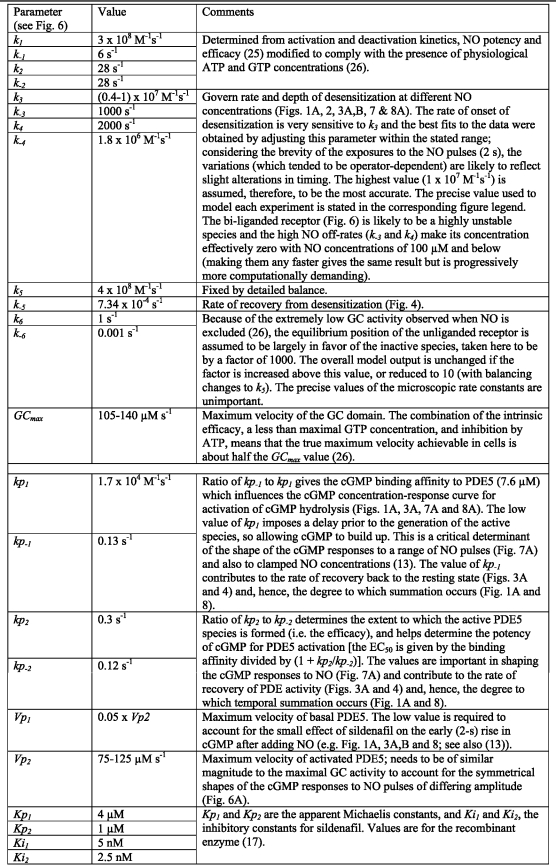
The data obtained in the present study, when combined with the current scheme for NO receptor activation (14, 24–26), allow a kinetic model for NO-cGMP signaling in rat platelets to be formulated (Fig. 6). There are two main components to the model; that is, the NO receptor whose activation generates cGMP, and PDE5, which is activated by the binding of cGMP to an agonist site and which hydrolyzes cGMP at its catalytic site. Both components are represented as two-step reactions; binding of the agonist (NO or cGMP) followed by a conformational change leading to a catalytically active (or more active) species. In the case of the NO receptor, the basic model used is a utilitarian version of a detailed enzyme-linked receptor mechanism that takes into account the likely concentrations of GTP and ATP existing in cells (26). Desensitization was incorporated using a scheme derived from studies of purified NO-activated GC (27). In this scheme receptor activation is associated with NO binding distally to the heme prosthetic group and then the proximal coordinating histidine bond breaks, facilitating a conformational change. Under conditions of low GTP, a second NO can bind to the now-vacant proximal heme site. Dissociation of the distal NO results in an NO-bound inactive state of the receptor from which NO leaves very slowly (see Fig. 6 and “Discussion”). Values for the rate constants governing the transitions to and recovery from the desensitized state were based on the experimental data (see Table 1). For PDE5, it is assumed that all three species (unbound and the two cGMP-bound states) are able to hydrolyze cGMP in ways that can be adequately described using Michaelis-Menten kinetics, as has been found experimentally in rat platelets and other cells (11, 13). The rate constants describing PDE5 activation and deactivation are all constrained by experimental data (Table 1).
FIGURE 6.
Model of cellular NO-cGMP kinetics comprising the receptor kinetics leading to cGMP synthesis and desensitization (A) and the activation of PDE5 by cGMP, leading to cGMP hydrolysis (B). In A the states of the heme NO binding site corresponding to each of the receptor states are indicated in the adjacent schematics, the heme group being represented by a parallelogram. The values of the kinetic parameters in italics are given in Table 1.
TABLE 1.
Model parameters
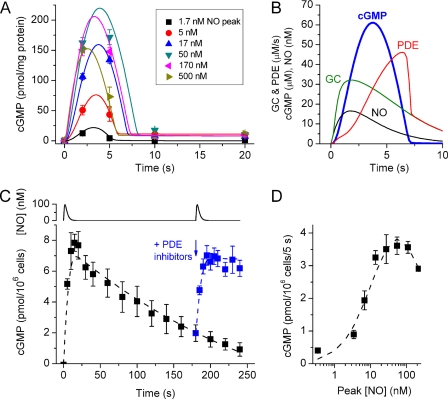
A stringent test of any model of this type is to describe the complete time-course of cGMP changes in platelets in response to a range of NO concentrations because this entails widely differing rates of cGMP formation, levels of cGMP, and PDE activity, all of them changing with time. The model replicates well the transient cGMP responses to NO pulses covering a very wide range of peak amplitudes, namely 1.7–500 nm, in rat platelets (Fig. 7A). In addition to the responses to NO pulses, the cGMP transients observed on exposure to a range of clamped NO concentrations (13) are similarly replicated (not illustrated). Important in reproducing the data faithfully are a low resting PDE5 activity and a relatively slow rate of activation by the binding of cGMP to the agonist site, allowing cGMP to build up for a couple of seconds before hydrolysis becomes overwhelming, as is illustrated in the example of a 17 nm NO pulse (Fig. 7B). The alternative of having fast binding to the PDE and a slow transition to the active state (while maintaining recovery at its observed rate) fails to fit the data in Fig. 7A. Desensitization can be seen most obviously with the higher NO pulses (170 and 500 nm peaks) where cGMP at the 5-s time point becomes progressively lower than after a smaller (50 nm) pulse.
FIGURE 7.
Model simulation of the acute cGMP responses of rat platelets to a range of NO pulse amplitudes (A and B) and of the behavior of rat cerebellar cells (C and D). The data in A are taken from our recent study (14); the model (Fig. 6 and Table 1) had GCmax = 105 μm/s, Vp2 = 90 μm/s, and k3 = 0.4 × 107 m−1s−1. Extracellular/bound cGMP (seen as the plateau at 10–20 s) was assumed to be formed exponentially with a rate constant of 0.5 s−1. The profiles of a sample NO pulse (peaking at 17 nm) and the resulting GC and PDE activities predicted by the model are illustrated together with the consequent cGMP profile, in B. The data in C and D are from the same study as A. C, time-course of cGMP levels in a cerebellar cell suspension after 2 NO pulses (100 nm peak amplitude) given 3 min apart (simulated at the top), the second one in the presence of inhibitors of the operative PDEs (PDE5 and PDE4), namely sildenafil (100 μm) and rolipram (1 μm). Broken lines are model predictions using the parameters: GCmax = 88 μm/s, Vp2 = 0.8 μm/s, and k3 = 0.7 × 107 m−1s−1. The PDE4 parameters additionally incorporated were: Vmax = 9.5 μm/s, Km for cGMP = 3 mm, and KI for rolipram = 30 nm. D, concentration-response curve after 5-s exposures to a range of amplitudes of NO pulses. The broken line is the model simulation assuming GCmax = 77 μm/s and the other parameters as in C. For further details, see Ref. 14.
On applying the model to the present results, it was found to describe accurately the progressive desensitization observed on giving repeated 50 nm NO pulses (Fig. 1A) and reproduces quite well the progressive cellular tachyphylaxis taking place when PDE5 is active (assay minus sildenafil), although the experimental data indicated a slightly greater PDE5 activity toward the end of the train of pulses (Fig. 1A). The changes in the proportions of unliganded, active, and desensitized receptor predicted by the model for this experiment are illustrated in Fig. 1B. The model also provides a reasonable fit to the NO concentration-response curves for receptor activation and desensitization (Fig. 2B) and gives a good depiction of the time-courses of the depression and recovery from single NO pulses of different amplitude assayed both with and without sildenafil (Fig. 3, C and D).
Tachyphylaxis during Long Exposure to NO
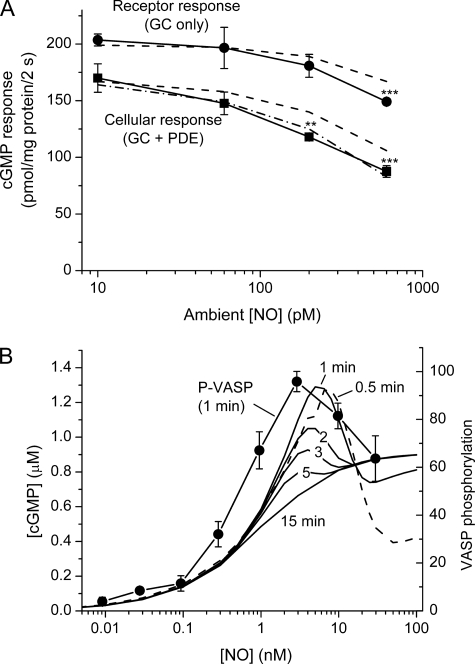
One prediction of the model is that NO concentrations much lower than those evoking acute desensitization (EC50 = 20 nm, Fig. 2B) or PDE5 activation (EC50 = 2 nm, Fig. 3A) would be effective if present tonically, because the two corresponding states would build up over time. To test this prediction, enduring (20 min) exposures to fixed NO concentrations in the subnanomolar range were made using a combination of the slow NO releaser, diethylenetriamine NONOate (half-life = 20 h), and CPTIO (20). As usual, changes in GC and PDE activities were assayed using 2-s exposures to a test NO pulse (50 nm peak) in the presence or absence of sildenafil. With a tonic 10 or 60 pm NO, no significant receptor desensitization or PDE activation was detected, whereas at 200 pm NO there was a significant reduction in the cGMP response in the absence of sildenafil (Fig. 8A), signifying enhanced PDE5 activity. An NO concentration of 600 pm gave an ∼50% loss of cellular responsiveness which was associated also with a significant fall in GC activity. The concentration-response relationships resemble those predicted by the model (dashed lines, Fig. 8A).
FIGURE 8.
Effects of tonic NO exposure in rat platelets. Data in A show cGMP responses after 2-s applications of a 50 nm NO pulse with and without sildenafil (100 μm) measured after 20 min of incubation in a range of clamped NO concentrations in the subnanomolar range. p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) compared with control (untreated) platelets (t test). Broken lines are model predictions with GCmax = 127 μm/s, Vp2 = 90 μm/s, and k3 = 1 × 107 m−1s−1. The dash-dot line assumes an additional more stable active PDE5 state (see the legend to Fig. 1A). The clamped NO concentrations were achieved using a mixture of diethylenetriamine NONOate and CPTIO (50 μm). In B the lines are the cGMP concentrations predicted by the model (Fig. 6 and Table 1) to exist in platelets subjected to various NO concentrations for 0.5–15 min (indicated for each curve; the 0.5 min line is dashed for clarity); data represent the levels of VASP phosphorylation (arbitrary units) measured in rat platelets after 1 min of exposure to different NO concentrations (13).
DISCUSSION
Knowledge of the kinetics of cellular NO-cGMP signaling is needed to understand the behavior of complex tissues in normal and disease states. Despite much evidence that the NO-cGMP pathway is subject to acute, activity-dependent regulation, the underlying mechanisms have not been clear. In general, those authors focusing on NO-activated GC have attributed the tachyphylaxis to that component while ignoring possible changes in PDE activity and vice versa (see the Introduction). Using rat platelets as a preparation that yields the quantitative data needed to address the problem, we find that changes in activity of both PDE5 and the NO receptor contribute, but in distinct ways; acutely, PDE5 activation has the lower threshold (in terms of NO concentration) but only a short term influence once NO is removed, whereas NO receptor desensitization requires higher NO concentrations but is much more enduring.
Considering first the role of PDE5, the results showed that activation of cGMP hydrolysis can have profound consequences in that it can effectively abolish subsequent NO-induced cGMP accumulation, although recovery starts as soon as the NO disappears. In addition, the phenomenon occurs at low NO concentrations, a brief pulse peaking at 2 nm NO being enough to engender a 50% loss of the cGMP response. No data on the NO concentration dependence of PDE5 activation in cells were previously available, although the results are compatible with the low nm potency of sodium nitroprusside for activating PDE5 in gastric smooth muscle cells in vitro during a 1-min exposure, although here the PDE activity was measured after cell lysis (28).
No previous attempt to measure the duration of the enhanced PDE5 activity in cells has been made either, although this has been addressed by measuring PDE activity in lysates of human platelets previously treated in vitro for 5 min with a 1 μm concentration of the NO donor diethylamine NONOate (half-life = 2.1 min; expected [NO], ∼1 μm) and then incubated with the NO scavenger CPTIO (15). It was found that the lysate PDE activity, although maximal immediately after the NO stimulation, remained elevated for more than an hour and that the elevated activity correlated with cGMP-dependent phosphorylation of PDE5, which in cell-free conditions at least, stabilizes the activated state (19). It appears that substantial long term activation requires higher NO concentrations and/or more prolonged exposure than used in the present experiments. We usually restricted the NO pulse height to 50 nm because this is near the top of the NO-cGMP concentration-response curve. In addition, application of higher concentrations creates problems of secondary sources of NO being created in the incubation medium, leading to inadvertent longer term stimulation (14). Even after this near-maximal conditioning pulse, there was no discernible long-term component to PDE5 activation. Accordingly, on examining the amount of phosphorylated PDE5 under several relevant conditions, no change could be detected, although a small increase was observable when the platelets were exposed to 50 nm NO for 2 min in the presence of sildenafil, a condition leading to high, sustained cGMP levels. Hence, the measured kinetics of PDE5 appear largely uncomplicated by alterations in PDE5 phosphorylation and are likely, therefore, to reflect the rates of cGMP binding and unbinding at the agonist site on PDE5.
This does not necessarily imply that a faster rate of deactivation of PDE5 signifies a lack of phosphorylation. An investigation using rat cultured smooth muscle cells in vitro found that atrial natriuretic factor-induced cGMP accumulation resulted in PDE5 phosphorylation within a minute, but then it declined to undetectable levels after 3 min, an interval that also corresponded to a 50% loss of the activated state of PDE5 measured after lysis (29). Obviously, dephosphorylation and the associated deactivation of PDE5 may occur at different rates in different cells, providing a mechanism for variably delaying the return of cellular PDE5 activity to normal beyond the 2-min period operating under the conditions of our experiments. Indeed, it cannot be excluded that there is a small component of more persistent PDE5 activation in the experiments involving repeated application of 50 nm NO pulses (Fig. 1A) or prolonged (20-min) exposure to subnanomolar NO concentrations (Fig. 8A) because a rather better fit to these experimental results was achieved by assuming that the activated PDE5 can undergo a slow transition to a more stable active species (dotted lines in Figs. 1A and 8A; see Fig. 1A legend for rate constants). Only about 10% of the enzyme is required to be in this more stable active state to provide the fits to the data; if caused by phosphorylation, such a change would be very difficult to detect by Western blotting, and so our results with this technique cannot exclude it. The predominantly rapid recovery of PDE5 activity observed in rat platelets may apply more generally; for example, many physiological studies have shown that nitrergic nerve-mediated smooth muscle relaxations, including of human material, can be repeated at regular short intervals (1–5 min) without demonstrable tachyphylaxis (see the references in Ref. 30).
Turning to NO receptor desensitization, the acute potency of NO for inducing desensitization recorded using pulse applications (Fig. 2B) is very similar to that found when rat platelets are exposed to clamped NO concentrations, where 10 nm NO generated progressive desensitization and 3 nm did not, during exposures lasting up to 1.5 min (13). Despite the relatively high threshold for inducing desensitization compared with PDE5 activation during short exposures, the phenomenon contributes to the shaping of the acute cGMP response in rat platelets to NO at the higher concentrations (13, 14), as illustrated in Fig. 7A. Moreover, in cerebellar astrocytes, which have very low PDE activity, desensitization is the primary mechanism governing the shape of the cGMP response over time (11). A feature revealed in the present experiments is the much more enduring influence of desensitization compared with PDE5 activation once NO is removed, permitting summation of the process over long time intervals. Hence, an NO concentration more than 10-fold lower than that found to be effective acutely in this and the previous study (13) could induce significant desensitization with a 20-min exposure. From its properties, desensitization is likely to serve as a gain control, titrating cellular sensitivity to NO according to the past history of NO exposure.
Tonic levels of NO are found in the cardiovascular system and brain as a result of endothelial NO synthase activity (31–34). Moreover, the enhanced relaxant potency of NO on blood vessels in vivo and in vitro shortly after inhibiting NO synthase or removing the endothelium (18) shows that the smooth muscle normally exists in a state of subsensitivity to NO as a result of prior exposure to NO. From our findings, the subsensitivity would be mainly the result of receptor desensitization, as an enhanced PDE5 activity would mostly revert to normal quickly. In agreement, the supersensitivity observed after endogenous NO is eliminated does not immediately subside after re-exposure to NO (18), contrary to expectations were the deactivation of PDE5 chiefly responsible for the supersensitive state. Under more pathological conditions, desensitization may contribute to the early loss of NO-stimulated GC activity observed when the inducible NO synthase, which synthesizes NO continuously, is expressed (35). It may also underlie the reduced NO-evoked GC activity found in blood vessels when endothelial NO synthase is overexpressed (36), and its absence could explain the increased NO-stimulated GC activity observed in mice lacking endothelial NO synthase (9, 10), as in both cases no changes in the level of NO receptor protein were found.
Previous analyses of the cGMP profiles of cerebellar astrocytes, striatal neurons, and rat platelets exposed to NO (11, 13, 37) gave optimism that it would be feasible to understand quantitatively the complex interplay between NO receptor activation, desensitization, and cGMP hydrolysis in cells. The earlier attempts using rat platelets (13, 14) were hampered by a lack of direct information about the kinetics of PDE5 that the data from the present experiments partially rectifies, enabling an explicit mechanism to be proposed (Fig. 6) that provides a good simulation of most of the data from this and previous studies.
For the PDE5 component, an important feature required to match the data was a slow rate of activation by cGMP, allowing cGMP to accumulate before being destroyed. Despite being an empirical value, the low bimolecular rate constant deduced for cGMP binding (1.7 × 104 m−1s−1) transpires to be very similar to the measured association rate constant for cGMP binding to the non-catalytic site on the rod photoreceptor PDE (PDE6), a close relative of PDE5 (38). Another feature of note is the potency of cGMP for PDE5 activation. In the model (Fig. 6 and Table 1), the binding affinity is about 8 μm, and the EC50 is close to 2 μm. Various estimates of the EC50 in cell-free conditions have been made, but the results have been variable, ranging from about 1 μm (39) to about 50 μm (Fig. 7B of Ref. 19) with in both cases a small reduction when the enzyme was phosphorylated by cGMP-dependent protein kinase. The inherent complexity of this approach (cGMP being both an agonist and a substrate) has been circumvented by measuring stimulation of the binding of competitive inhibitors acting at the PDE5 catalytic site, which indicated a maximal effect at 25 μm cGMP (40), and by studying a chimeric protein with an adenylyl cyclase substituted for the catalytic domain (41), where the cGMP EC50 came to 10 μm, reducing to 2 μm when phosphorylation was mimicked by mutation. In all, our empirical estimate of the potency of cGMP in the intact cell is compatible with those measured under cell-free conditions.
To incorporate desensitization into the model, we initially examined classical mechanisms in which the desensitized state is reached reversibly from one or more agonist-bound states (42, 43). Although adequate for describing changes occurring over the first few seconds of NO exposure (14), such mechanisms failed the more stringent tests reported here. In particular, the classical models predict that, with such a slow rate of recovery from desensitization (Fig. 4), the receptor must eventually become almost totally desensitized after repeated or prolonged NO exposure, whereas in the present experiments and those reported previously (13), desensitization stabilized maximally at about 80%. A satisfactory solution was achieved by formalizing a scheme proposed from studies on purified NO-activated GC (27) in which NO exposure in the absence of substrate leads to the formation of a stable inactive receptor with NO bound on the “wrong” side of the heme. The existence of such a species is consistent with crystallographic evidence from a related bacterial cytochrome (44) and with the finding that GC activity could be restored by adding excess NO in the presence of a high substrate concentration (27). In agreement with this mechanism subserving desensitization in cells, the rate of recovery of platelet GC activity from desensitization that we measured (k = 7.34 × 10−4 s−1) is remarkably similar to the rate of dissociation of NO from the NO-bound inactive GC species (produced by exposure to NO in the absence of GTP) for which rate constants of 7 × 10−4 s−1 at 20 °C (45) and 3 × 10−4 s−1 at 10 °C (46) have been reported.
Although direct evidence would be hard to find, it is reasonable to suppose that there is a transient depletion of GTP in the vicinity of active GC, bearing in mind that the total cellular GTP concentration is about 100 μm (47–50) and that, when maximally stimulated by NO, cellular GC in platelets consumes about 70 μm GTP/s. The transient dip in GC activity seen during exposure to a 50 nm NO pulse before the appearance of steady desensitization (Fig. 2A) plausibly reflects a reduction in GTP concentration followed by recovery.
It should be mentioned that the inactive NO-bound species referred to above has been considered by one group to display a low (10–20% of maximum) intrinsic activity (46). That the species in question acts as an NO donor (half-life ∼15 min), which on releasing its NO becomes an active GC again (as in the model, Fig. 6), would explain the observed activity. In addition, the experiments may have been compromised by the formation of secondary NO donors in the buffer that could artifactually add to the activity (14).
Without direct evidence, the proposed desensitization scheme must be regarded as tentative; nevertheless, it has the merits of replicating experimental results on platelets in detail and of making correct predictions regarding the effect of long term NO exposure (Fig. 8A) as well as being concordant with data from purified NO-activated GC. Other proposed mechanisms of desensitization have included oxidation and loss of the NO binding heme, which was found not to contribute measurably to desensitization in rat platelets (22), thiol nitrosation (51), and cGMP-dependent phosphorylation (52, 53). Thiol nitrosation is unlikely to have intruded on our experiments because it has been observed only under extreme conditions, for example after 1 h of exposure to 0.1–1 mm S-nitrosocysteine (53). A study in cerebellar astrocytes found no effect of kinase or phosphatase inhibitors on desensitization occurring during a 2-min exposure to NO (37), implying that changes in phosphorylation were not instrumental. The phosphorylation mechanism appears to require longer exposures to NO, and it also seems unable to give the required degree (80%) of desensitization (51). Its participation in the lesser effect found after long (20-min) exposure to subnanomolar NO concentrations (Fig. 8A), however, is not excluded.
To scrutinize the generalizability of our model, we analyzed results from cerebellar astrocytes (14), a cell type having a very low cGMP-hydrolyzing PDE activity comprising a mixture of PDE5 and PDE4 (54). The effects of twin 100 nm NO pulses given 3 min apart are accurately simulated, including the degree of desensitization (50%) evident on applying the second pulse (Fig. 7C). Also well simulated is the bell-shaped NO concentration-response curve found in astrocytes with 5-s applications of NO pulses of differing amplitude (Fig. 7D), the biphasic shape of the curve being caused by desensitization (55). Hence, the kinetics of desensitization deduced using platelets appears to apply to other cells.
Having a quantitative model helps in interpreting results and in making predictions. To take just one example relevant to the present study, cGMP-dependent phosphorylation of VASP in rat platelets shows a puzzling biphasic relationship to NO concentration (delivered in the clamped mode) in that low NO concentrations (EC50 = 0.5 nm) give more phosphorylation than higher ones (>3 nm) even though the higher ones give more cGMP (Fig. 5C and Ref. 13). The intuitive interpretation is that higher NO concentrations inhibit phosphorylation independently of cGMP (13). However, cGMP was measured during its transient increase (lasting about 10 s), whereas phosphorylation was assessed after an exposure of 1 min (2 min in Fig. 5C). With the model, we can analyze what happens to cGMP levels beyond the transient peak. The result (Fig. 8B) would not have been foreseen. It shows a strongly biphasic relationship between NO concentration and cGMP accumulation after 0.5–1 min of exposure, arising from the non-linearities between GC activity, cGMP concentration, and PDE activity over time. The EC50 for NO now becomes about 1 nm instead of 10 nm initially and, even if too small to measure directly, cGMP still is present at the submicromolar concentrations active on cGMP-dependent protein kinase (56). NO concentrations of 5–25 nm give progressively less cGMP. With exposures longer than 1 min, the curve gradually resolves to a conventional sigmoidal type (EC50 = 0.9 nm after 15 min; Fig. 8B). The shapes of the curves with 0.5–1 min exposures to NO, therefore, provide a good match to the biphasic shape of the concentration-response curve for VASP phosphorylation measured with a 1-min exposure (Fig. 8B). With more sustained NO application, therefore, it is probably the persisting low cGMP level that primarily determines the extent of downstream phosphorylation rather than the size of the initial cGMP surge. This interpretation is consistent with data showing that VASP phosphorylation continues to increase after the surge is over (13).
Supplementary Material
Acknowledgments
We are very grateful to Professor Doris Koesling (Dept. of Pharmacology, University of Bochum, Germany) for kindly giving us both the phospho-PDE5 antibody and the HEK-GC/PDE5 cell line used in the work and to Dr. Andrew Batchelor for performing the mixing experiments shown in supplemental Fig. S1.
This work was supported in part by a Programme grant from The Wellcome Trust.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1.
- GC
- guanylyl cyclase
- PDE
- phosphodiesterase
- CPTIO
- 2–4-carboxyphenyl-4,4,5,5,-tetramethylimidazoline-1-oxyl-3-oxide
- VASP
- vasodilator-stimulated phosphoprotein
- HEK
- human embryonic kidney.
REFERENCES
- 1.Garthwaite J. (2008) Eur. J. Neurosci. 27, 2783–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobbs A. J., Higgs A., Moncada S. (1999) Annu. Rev. Pharmacol. Toxicol. 39, 191–220 [DOI] [PubMed] [Google Scholar]
- 3.Rand M. J., Li C. G. (1995) Annu. Rev. Physiol. 57, 659–682 [DOI] [PubMed] [Google Scholar]
- 4.Axelsson K. L., Andersson R. G. (1983) Eur. J. Pharmacol. 88, 71–79 [DOI] [PubMed] [Google Scholar]
- 5.Ujiie K., Hogarth L., Danziger R., Drewett J. G., Yuen P. S., Pang I. H., Star R. A. (1994) J. Pharmacol. Exp. Ther. 270, 761–767 [PubMed] [Google Scholar]
- 6.Waldman S. A., Rapoport R. M., Ginsburg R., Murad F. (1986) Biochem. Pharmacol. 35, 3525–3531 [DOI] [PubMed] [Google Scholar]
- 7.Scott W. S., Nakayama D. K. (1998) J. Surg. Res. 79, 66–70 [DOI] [PubMed] [Google Scholar]
- 8.Filippov G., Bloch D. B., Bloch K. D. (1997) J. Clin. Invest. 100, 942–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain M. B., Hobbs A. J., MacAllister R. J. (1999) Br. J. Pharmacol. 128, 1082–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandes R. P., Kim D., Schmitz-Winnenthal F. H., Amidi M., Gödecke A., Mülsch A., Busse R. (2000) Hypertension 35, 231–236 [DOI] [PubMed] [Google Scholar]
- 11.Bellamy T. C., Wood J., Goodwin D. A., Garthwaite J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 2928–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullershausen F., Russwurm M., Thompson W. J., Liu L., Koesling D., Friebe A. (2001) J. Cell Biol. 155, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mo E., Amin H., Bianco I. H., Garthwaite J. (2004) J. Biol. Chem. 279, 26149–26158 [DOI] [PubMed] [Google Scholar]
- 14.Roy B., Garthwaite J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12185–12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullershausen F., Friebe A., Feil R., Thompson W. J., Hofmann F., Koesling D. (2003) J. Cell Biol. 160, 719–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada D., Asakawa S. (2002) Biochemistry 41, 9672–9679 [DOI] [PubMed] [Google Scholar]
- 17.Rybalkin S. D., Rybalkina I. G., Shimizu-Albergine M., Tang X. B., Beavo J. A. (2003) EMBO J. 22, 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moncada S., Rees D. D., Schulz R., Palmer R. M. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 2166–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullershausen F., Russwurm M., Koesling D., Friebe A. (2004) Mol. Biol. Cell 15, 4023–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths C., Wykes V., Bellamy T. C., Garthwaite J. (2003) Mol. Pharmacol. 64, 1349–1356 [DOI] [PubMed] [Google Scholar]
- 21.Clark D. E. (1999) J. Pharm. Sci. 88, 807–814 [DOI] [PubMed] [Google Scholar]
- 22.Roy B., Mo E., Vernon J., Garthwaite J. (2008) Br. J. Pharmacol. 153, 1495–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eigenthaler M., Nolte C., Halbrügge M., Walter U. (1992) Eur. J. Biochem. 205, 471–481 [DOI] [PubMed] [Google Scholar]
- 24.Bellamy T. C., Wood J., Garthwaite J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 507–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garthwaite J. (2005) Front. Biosci. 10, 1868–1880 [DOI] [PubMed] [Google Scholar]
- 26.Roy B., Halvey E. J., Garthwaite J. (2008) J. Biol. Chem. 283, 18841–18851 [DOI] [PubMed] [Google Scholar]
- 27.Russwurm M., Koesling D. (2004) EMBO J. 23, 4443–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murthy K. S. (2001) Biochem. J. 360, 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyatt T. A., Naftilan A. J., Francis S. H., Corbin J. D. (1998) Am. J. Physiol. 274, H448–H455 [DOI] [PubMed] [Google Scholar]
- 30.Gibson A. (2001) Eur. J. Pharmacol. 411, 1–10 [DOI] [PubMed] [Google Scholar]
- 31.Garthwaite G., Bartus K., Malcolm D., Goodwin D., Kollb-Sielecka M., Dooldeniya C., Garthwaite J. (2006) J. Neurosci. 26, 7730–7740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold M. E., Wood K. S., Byrns R. E., Fukuto J., Ignarro L. J. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 4430–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopper R. A., Garthwaite J. (2006) J. Neurosci. 26, 11513–11521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin W., Villani G. M., Jothianandan D., Furchgott R. F. (1985) J. Pharmacol. Exp. Ther. 232, 708–716 [PubMed] [Google Scholar]
- 35.Fernandes D., da Silva-Santos J. E., Duma D., Villela C. G., Barja-Fidalgo C., Assreuy J. (2006) Mol. Pharmacol. 69, 983–990 [DOI] [PubMed] [Google Scholar]
- 36.Yamashita T., Kawashima S., Ohashi Y., Ozaki M., Rikitake Y., Inoue N., Hirata K., Akita H., Yokoyama M. (2000) Hypertension 36, 97–102 [DOI] [PubMed] [Google Scholar]
- 37.Wykes V., Bellamy T. C., Garthwaite J. (2002) J. Neurochem. 83, 37–47 [DOI] [PubMed] [Google Scholar]
- 38.Mou H., Grazio H. J., 3rd, Cook T. A., Beavo J. A., Cote R. H. (1999) J. Biol. Chem. 274, 18813–18820 [DOI] [PubMed] [Google Scholar]
- 39.Corbin J. D., Turko I. V., Beasley A., Francis S. H. (2000) Eur. J. Biochem. 267, 2760–2767 [DOI] [PubMed] [Google Scholar]
- 40.Blount M. A., Beasley A., Zoraghi R., Sekhar K. R., Bessay E. P., Francis S. H., Corbin J. D. (2004) Mol. Pharmacol. 66, 144–152 [DOI] [PubMed] [Google Scholar]
- 41.Bruder S., Schultz A., Schultz J. E. (2006) J. Biol. Chem. 281, 19969–19976 [DOI] [PubMed] [Google Scholar]
- 42.Jones M. V., Westbrook G. L. (1996) Trends Neurosci. 19, 96–101 [DOI] [PubMed] [Google Scholar]
- 43.Katz B., Thesleff S. (1957) J. Physiol 138, 63–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawson D. M., Stevenson C. E., Andrew C. R., Eady R. R. (2000) EMBO J. 19, 5661–5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kharitonov V. G., Russwurm M., Magde D., Sharma V. S., Koesling D. (1997) Biochem. Biophys. Res. Commun. 239, 284–286 [DOI] [PubMed] [Google Scholar]
- 46.Cary S. P., Winger J. A., Marletta M. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13064–13069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breitwieser G. E., Szabo G. (1988) J. Gen. Physiol. 91, 469–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatakeyama K., Harada T., Kagamiyama H. (1992) J. Biol. Chem. 267, 20734–20739 [PubMed] [Google Scholar]
- 49.Otero A. D. (1990) Biochem. Pharmacol. 39, 1399–1404 [DOI] [PubMed] [Google Scholar]
- 50.Horie M., Irisawa H. (1989) J. Physiol. 408, 313–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sayed N., Baskaran P., Ma X., van den Akker F., Beuve A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12312–12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murthy K. S. (2004) Neurochem. Int. 45, 845–851 [DOI] [PubMed] [Google Scholar]
- 53.Zhou Z., Sayed N., Pyriochou A., Roussos C., Fulton D., Beuve A., Papapetropoulos A. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1803–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellamy T. C., Garthwaite J. (2001) Mol. Pharmacol. 59, 54–61 [DOI] [PubMed] [Google Scholar]
- 55.Bellamy T. C., Garthwaite J. (2001) J. Biol. Chem. 276, 4287–4292 [DOI] [PubMed] [Google Scholar]
- 56.Francis S. H., Corbin J. D. (1999) Crit. Rev. Clin. Lab. Sci. 36, 275–328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.