Abstract
Obesity and type 2 diabetes are related metabolic disorders of high prevalence. The constitutive androstane receptor (CAR) was initially characterized as a xenobiotic receptor regulating the responses of mammals to xenotoxicants. In this study, we have uncovered an unexpected role of CAR in preventing obesity and alleviating type 2 diabetes. Using a high fat diet (HFD)-induced obesity model, we showed that treatment of wild type mice with the CAR agonist 1,4-bis[2-(3,5 dichloropyridyloxy)] benzene (TCPOBOP) efficiently prevented obesity from happening or reversed preinduced obesity. Treatment with TCPOBOP improved insulin sensitivity in both the HFD-induced type 2 diabetic model and the ob/ob mice. In contrast, CAR null mice maintained on a chow diet showed spontaneous insulin insensitivity, which cannot be relieved by TOPOBOP treatment. The hepatic steatosis in HFD-treated mice and ob/ob mice was markedly reduced by the TCPOBOP treatment. The metabolic benefits of CAR activation may have resulted from the combined effect of inhibition of lipogenesis, very low density lipoprotein secretion and export of triglycerides, and gluconeogenesis as well as increases in brown adipose tissue energy expenditure and peripheral fat mobilization. Moreover, the skeletal muscle of CAR-activated mice showed a decreased incomplete oxidation, despite having a lower expression level of peroxisome proliferator-activated receptor α and its target genes involved in fatty acid oxidation. In summary, our results have revealed an important metabolic function of CAR and may establish this “xenobiotic receptor” as a novel therapeutic target for the prevention and treatment of obesity and type 2 diabetes.
Obesity is a metabolic disorder of high prevalence. In the United States, over two-thirds of adults and one-third of children are overweight (1). Obesity is strongly associated with several pathological conditions, including type 2 diabetes, hypertension, and dyslipidemia, which are major causes of morbidity and mortality throughout the world (2). It is conceivable that understanding the molecular basis of obesity and insulin resistance will facilitate the development of therapeutics for the treatment and prevention of these metabolic disorders.
The constitutive androstane receptor (CAR)3 was initially characterized as a xenosensor regulating the responses of mammals to xenotoxicants (3, 4). Recently published results have also implicated this receptor in the control of energy metabolism. It has been proposed that CAR is responsive to nutrient stress, which was evidenced by the induction of CAR and its target gene expression during long term fasting (5, 6). CAR might also regulate energy metabolism by interacting with the peroxisome proliferator-activated receptor α (PPARα) and Pgc-1α (PPARγ coactivator-1α), both of which are key regulators of adaptive responses to starvation (7, 8).
Recent studies have also linked CAR to the lipid metabolism and glucose metabolism. Activation of CAR suppressed lipid metabolism and lowered serum triglyceride by reducing protein levels of the active form of the lipogenic transcription factor SREBP-1 (sterol regulatory element-binding protein 1) (9). The inhibitory effect of CAR on lipid metabolism may also be attributed to induction of Insig-1, a protein with antilipogenic properties (10). Hepatic gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase were reported to be repressed in phenobarbital-treated mice, and this suppression was CAR-dependent (11). This is reminiscent of the suppression of the same gluconeogenic enzymes in pregnane X receptor-activated mice (12, 13). HNF-4α is a positive regulator of gluconeogenesis. It was suggested that CAR inhibits gluconeogenic enzyme gene expression through HNF-4α inhibition, achieved by the competition of CAR with HNF-4α for binding to the DR1 motif in the promoter region of gluconeogenic enzyme genes (14). FoxO1 is a key positive regulator of gluconeogenesis. It has been reported that CAR can physically bind to and suppress the activity of FoxO1 by preventing FoxO1 from binding to the insulin response sequence in gluconeogenic enzyme gene promoters (15). Despite the known function of CAR in energy metabolism, it remains to be determined whether and how CAR signaling might impact obesity and diabetes.
In this study, we have uncovered a metabolic function of CAR in preventing obesity and relieving type 2 diabetes. Our results may establish this “xenobiotic receptor” as a novel therapeutic target for the prevention and treatment of obesity and type 2 diabetes.
EXPERIMENTAL PROCEDURES
Animals, Drug Treatment, Body Composition Analysis, and Histological Evaluation
Mice were housed under a 12-h light-dark cycle and given regular RMH3000 diet from PMI Nutrition International (St. Louis, MO) or high fat diet (catalog number S3282) from Bio-serv (Frenchtown, NJ). Wild type C57BL/6J mice and ob/ob mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The creation of CAR−/− mice has been described previously (4). CAR−/− mice were maintained in the C57BL/6J-SvJ129 mixed background. When necessary, mice received once per week intraperitoneal injections of TCPOBOP (0.5 mg/kg) or vehicle (DMSO). Mice were sacrificed 24 h after the last dose of drug. Body composition was analyzed in live animals using EchoMRI-100TM from Echo Medical Systems (Houston, TX). For histological analysis, tissues were fixed in 4% formaldehyde, embedded in paraffin, sectioned at 5 μm, and stained with hemotoxylin and eosin. Frozen sections (10 μm) were used for Oil-red O staining. The use of mice in this study has complied with relevant federal guidelines and institutional policies.
Measurements of Serum and Fecal Chemistry
Blood samples were collected from fed or fasted mice. Serum levels of bile acids (Bio-Quant, San Diego, CA), alanine aminotransferase (Calchem, Huntington, NY), total triglycerides and cholesterol (Stanbio Laboratory, Boerne, TX), free fatty acids (Biovision, Mountain View, CA), and insulin and leptin (Crystal Chem, Downers Grove, IL) were measured by using commercial assay kits according to the manufacturer's instructions. Feces were collected by using mouse metabolic cages and subjected to the measurement of triglycerides.
Gene Expression Analysis
Total RNA was isolated using the TRIZOL reagent from Invitrogen. For real time PCR analysis, reverse transcription was performed with random hexamer primers and Superscript RT III enzyme from Invitrogen. SYBR Green-based real time PCR was performed with the ABI 7300 real time PCR system, as we have described previously (16). Data were normalized against control cyclophillin. Sequences of the real time PCR probes are listed in supplemental Table S1.
Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
For GTT, mice were fasted for 16 or 4 h before receiving an intraperitoneal injection of d-glucose at 2 g/kg body weight. Blood samples were taken at different time points, and the concentrations of glucose were measured with a glucometer. For ITT, mice were fasted for 4 h before receiving an intraperitoneal injection of human insulin (Novolin) from Novo Nordisk (Princeton, NJ) at 0.5 or 1.5 units/kg body weight. Blood samples were taken, and blood glucose levels were measured.
VLDL Secretion Assay
VLDL secretion rate in vivo was measured as described previously (17). In brief, mice were fasted for 4 h before receiving a tail vein injection of tyloxapol from Sigma at 500 mg/kg body weight. Plasma samples were collected at 0, 1, 2, and 4 h after tyloxapol injection, and triglyceride levels were then measured as described above.
Analysis of Oxygen Consumption and Measurement of Skeletal Muscle Mitochondrial Fatty Acid Oxidation
Oxygen consumption in live animals was measured as described previously (18). Skeletal muscle mitochondrial fatty acid oxidation was measured as described previously (19). In brief, fresh gastrocnemius muscles were excised and placed in ice-cold modified Chappell-Perry buffer. Muscle homogenates were prepared, and mitochondria were isolated using differential centrifugation. Oxidation assays were performed using 0.2 mm [14C]oleate in the absence or presence of 2 mm sodium pyruvate. Reactions were terminated by adding 100 μl of 70% perchloric acid, and [14C]CO2 was trapped in 200 μl of 1 n NaOH. [14C]CO2 and 14C-labeled acid-soluble metabolites were assessed by liquid scintillation counting.
Statistical Analysis
Results are expressed as means ± S.D. Statistical analysis was performed using the unpaired Student's t test for comparison between two groups.
RESULTS
Activation of CAR Prevented and Reversed Obesity
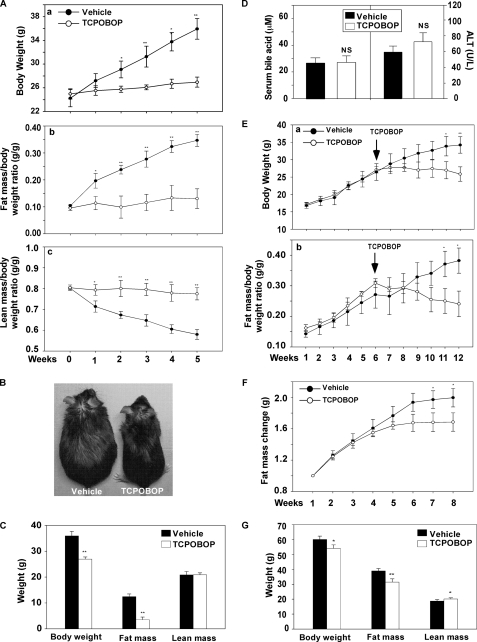
To determine the effect of CAR on obesity, 8-week-old male C57BL/6J mice were fed with a high fat diet (HFD) for 8 weeks and simultaneously treated with the CAR agonist TCPOBOP (0.5 mg/kg, once a week) or vehicle (DMSO). As shown in Fig. 1A, TCPOBOP significantly inhibited the gain of body weight after 2 weeks of drug treatment (Fig. 1A, a). MRI analysis showed that TCPOBOP inhibited the gain of fat mass (Fig. 1A, b), whereas the lean masses between the TCPOBOP and vehicle groups were not significantly different (data not shown). As such, the lean mass/body weight ratio decreased in vehicle-treated mice, whereas the ratio remained steady in the TCPOBOP group (Fig. 1A, c). Fig. 1B shows a representative pair of mice HFD-fed and drug-treated for 5 weeks, at which the body weight difference between TCPOBOP group and vehicle group was completely accountable by the gain of fat mass in the vehicle group (Fig. 1C). A similar pattern of TCPOBOP effect was observed in female mice (supplemental Fig. S1), suggesting that the phenotype was not gender-specific. The TCPOBOP effect was unlikely to be due to toxicity of the drug, because the differences in the serum levels of bile acids (Fig. 1D, left) and alanine aminotransferase (ALT) activity (Fig. 1D, right) between the vehicle and TCPOBOP groups were not statistically significant. As expected, the hepatic mRNA expression of CYP2B10, a prototypical CAR target gene (3, 4), was induced by TCPOBOP (supplemental Fig. S2).
FIGURE 1.
Treatment of mice with a CAR agonist inhibited obesity. A, growth curve (A, a), fat mass/body weight ratio (A, b), and lean mass/body weight ratio (A, c) in male C57BL/6J mice fed with HFD for 5 weeks, in the absence or presence of TCPOBOP treatment (0.5 mg/kg, intraperitoneal, once per week). Fat and lean masses were determined by MRI. n = 5 for each group. B, a representative pair of vehicle- and TCPOBOP-treated mice in A. C, body weight and body composition of mice at the end of a 5-week diet and drug treatment. D, serum levels of bile acids (left) and ALT activity (right) at the end of an 8-week diet and drug treatment. E, growth curve (E, a) and fat mass/body weight ratio (E, b) in female C57BL/6J mice prefed with HFD for 6 weeks followed by 6 weeks of TCPOBOP treatment during which the mice remained on HFD. n = 5 for each group. F, gain of fat mass in female ob/ob mice maintained on a chow diet treated with vehicle or TCPOBOP for 8 weeks (0.5 mg/kg, once per week). n = 5 for each group. G, body weight and body composition of ob/ob mice at the end of the 8-week drug treatment. Results are expressed as means ± S.D. *, p < 0.05; **, p < 0.01, compared with the vehicle group.
To determine whether CAR activation was effective in mice with preexisting obesity, female C57BL/6J mice were fed with HFD for 6 weeks, followed by 6 weeks of TCPOBOP treatment while remaining on HFD. As shown in Fig. 1E, treatment with TOPOBOP stabilized the body weight, whereas the vehicle group continued to gain body weight (Fig. 1E, a). Again, the TCPOBOP effect was largely attributed to the inhibition of gain of fat mass (Fig. 1E, b). TCPOBOP was also effective in inhibiting obesity in the leptin deficient ob/ob mice. In this experiment, 7-week-old female ob/ob mice were maintained on a chow diet and treated with TCPOBOP or vehicle for 8 weeks. The TCPOBOP group had less gain of fat mass (Fig. 1F). By the end of the 8-week treatment, the TCPOBOP group had significantly lower body weight compared with the vehicle group, accompanied by a decrease in fat mass (Fig. 1G). Interestingly, the TCPOBOP-treated ob/ob group had a modest but statistically significant gain of lean mass (Fig. 1G). Taken together, these results suggest that CAR has an anti-obesity effect.
Activation of CAR Improved Insulin Sensitivity
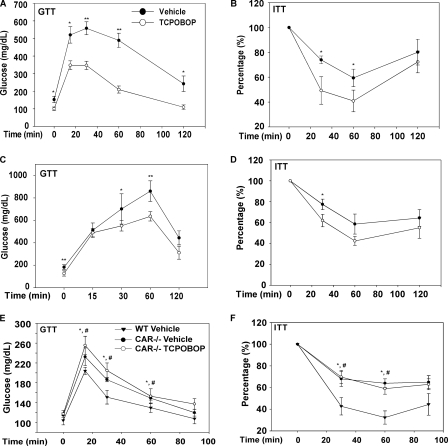
Because obesity is strongly associated with insulin resistance, a typical characteristic of type 2 diabetes, we further examined the effect of CAR on insulin sensitivity. Treatment of HFD-fed male C57BL/6J mice with TCPOBOP for 8 weeks significantly improved insulin sensitivity, as confirmed by both GTT (Fig. 2A) and ITT (Fig. 2B). The insulin-sensitizing effect of TCPOBOP was also evident in ob/ob mice (Fig. 2, C and D), although the changes were consistently smaller than those observed in the C57BL/6J mice. When the serum chemistry was analyzed, we found that treatment with TCPOBOP in HFD-fed C57BL/6J mice decreased the levels of fasting glucose, insulin, and triglycerides (Table 1). In ob/ob mice, TCPOBOP treatment decreased the levels of fasting glucose, serum triglyceride, and cholesterol, but the changes in insulin and free fatty acid were not statistically significant (Table 1). Of note, compared with the HFD-fed C57BL/6J mice, ob/ob mice had a much higher basal level of insulin. The TCPOBOP-induced reductions of insulin and triglycerides were smaller in ob/ob mice (14 versus 52% and 23 versus 42%, respectively). In the loss-of-function CAR−/− mice, we found that the chow-fed CAR−/− mice in C57BL/6J-SvJ129 mixed background had spontaneously impaired insulin sensitivity when compared with their wild type counterparts, and the insulin insensitivity in CAR−/− mice was not responsive to TOPOBOP (Fig. 2, E and F).
FIGURE 2.
Treatment with a CAR agonist ameliorated diabetes, whereas CAR−/− mice exhibited spontaneous insulin insensitivity. A and B, treatment with TCPOBOP ameliorated insulin resistance induced by HFD in male C57BL/6J mice, as determined by GTT (A) and ITT (B). Mice were fed with HFD for 8 weeks in the presence or absence of TCPOBOP (0.5 mg/kg, once per week). C and D, ob/ob mice maintained on chow diet were treated with TCPOBOP or vehicle for 8 weeks before subjecting to GTT (C) and ITT (D) tests. *, p < 0.05; **, p < 0.01, compared with the vehicle group. E and F, GTT (E) and ITT (F) tests on CAR−/− mice maintained on chow diet in the presence or absence of 5-week TCPOBOP treatment. *, p < 0.05, CAR−/−-vehicle versus wild type (WT); #, p < 0.05, CAR−/−-TCPOBOP versus wild type.
TABLE 1.
Serum chemistry in HFD-fed C57BL/6J mice and ob/ob mice treated with vehicle or TCPOBOP
The durations of TCPOBOP treatment are 8 weeks for the C57BL/6J mice and ob/ob mice, respectively. Mice were fasted overnight before blood sampling.
| HFD-fed C57BL/6J |
Chow-fed ob/ob |
|||
|---|---|---|---|---|
| Vehicle | TCPOBOP (percentage decrease) | Vehicle | TCPOBOP (percentage decrease) | |
| Fasting glucose (mg/dl) | 153 ± 18.3 | 102.8 ± 17.7a (33%) | 183.9 ± 21.3 | 128.7 ± 23.4a (31%) |
| Insulin (ng/ml) | 3.26 ± 0.68 | 1.57 ± 0.37b (52%) | 17.89 ± 0.35 | 15.39 ± 1.85 (14%) |
| Total triglyceride (mg/dl) | 230.2 ± 21.3 | 132.8 ± 17.7a (42%) | 162.5 ± 14.1 | 126.3 ± 13.7b (23%) |
| Total cholesterol (mg/dl) | 100.8 ± 9.6 | 89.7 ± 5.3 (11%) | 118.4 ± 5.9 | 90.2 ± 9.9b (24%) |
| Free fatty acid (mm) | 0.84 ± 0.11 | 0.68 ± 0.06 (19%) | 2.59 ± 0.21 | 2.14 ± 0.15 (18%) |
a p < 0.01.
b p < 0.05.
Activation of CAR Suppressed the Expression of Gluconeogenic and Lipogenic Enzyme Genes, Inhibited Hepatic Steatosis, and Inhibited Adiposity
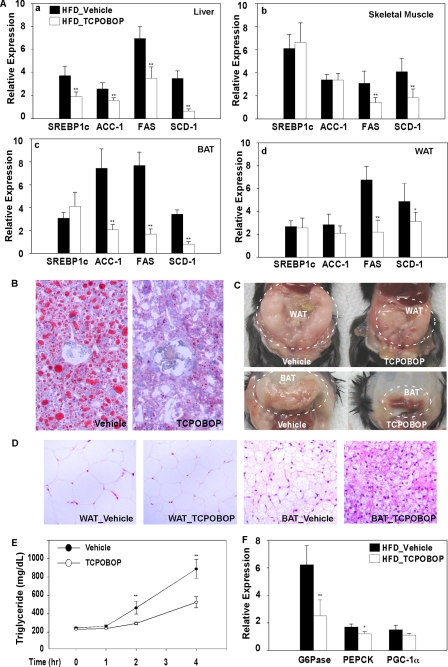
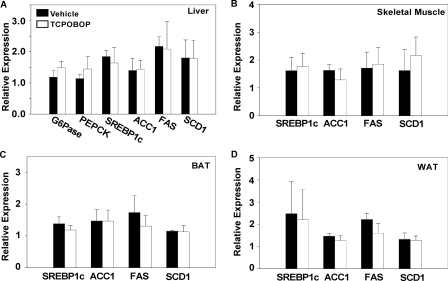
To understand the mechanism by which CAR inhibits obesity and improves insulin sensitivity, we profiled the expression of metabolic genes in HFD-fed C57BL/6J mice treated with TOPOBOP for 1 week. In the liver, TCPOBOP lowered the expression of lipogenic genes, including Srebp-1c, Acc-1, Fas, and Scd-1 (Fig. 3A, a). Significant inhibition of Fas and Scd-1 gene expression was also observed in the skeletal muscle (Fig. 3A, b), brown adipose tissue (BAT) (Fig. 3A, c), and white adipose tissue (WAT) (Fig. 3A, d) of TCPOBOP-treated mice. The expression of Acc-1 was also significantly inhibited by TCPOBOP in BAT (Fig. 3A, c). A similar pattern of gene expression was observed in mice fed with HFD and treated with TCPOBOP for 8 weeks (data not shown). The inhibition of lipogenesis was supported by the ameliorated hepatic steatosis (Fig. 3B), decreased sizes of abdominal WAT and BAT (Fig. 3C), and adipocyte hypotrophy (Fig. 3D) in HFD-fed mice treated with TCPOBOP for 8 weeks. Treatment with TCPOBOP also inhibited VLDL-triglyceride secretion (Fig. 3E), consistent with the reduced serum triglyceride level in these mice (Table 1). In TCPOBOP-treated mice, the hepatic expression of two important gluconeogenic enzymes, glucose-6-phosphatase and phosphoenolpyruvate carboxykinase, was also significantly inhibited (Fig. 3F), consistent with the decreased fasting glucose in these animals (Table 1). The effect of TCPOBOP on lipogenic and gluconeogenic gene expression was CAR-dependent, because this regulation was abolished in CAR−/− mice (Fig. 4), consistent with a previous report (11).
FIGURE 3.
Treatment with a CAR agonist inhibited lipogenesis, VLDL-triglyceride secretion, and gluconeogenesis. A, liver, skeletal muscle, BAT, and WAT were measured for the mRNA expression of genes involved in lipogenesis by real time PCR analysis. Male C57BL/6J mice were HFD-fed and drug-treated for 1 week. n = 4 for each group. B, Oil-red O staining of liver sections from male C57BL/6J mice fed with HFD and treated with TCPOBOP for 8 weeks. C and D, gross appearance (C) and hematoxylin/eosin staining of abdominal WAT and BAT of mice described in B. E, VLDL-triglyceride secretion rate in male C57BL/6J mice treated with vehicle or TCPOBOP for 8 weeks. Mice were fasted for 4 h before receiving an intravenous injection of tyloxapol (500 mg/kg body weight). Blood was sampled at 1, 2, and 4 h and measured for serum concentrations of triglycerides. n = 4 for each group. F, liver mRNA expression of genes involved in gluconeogenesis was measured by real time PCR analysis. Mice were the same as those described in A. *, p < 0.05; **, p < 0.01, compared with the vehicle group.
FIGURE 4.
The suppression of lipogenic and gluconeogenic gene expression by TCPOBOP was abolished in CAR−/− mice. Male CAR−/− mice were HFD-fed and TOPOBOP-treated for 1 week before tissue harvesting. A–D, liver (A), skeletal muscle (B), BAT (C), and WAT (D) were measured for the mRNA expression of lipogenic and gluconeogenic genes by real time PCR analysis. n = 5 for each group. G6Pase, glucose-6-phosphatase; PEPCK, phosphoenolpyruvate carboxykinase.
Activation of CAR Had Little Effect on Lipid Absorption in the Small Intestine
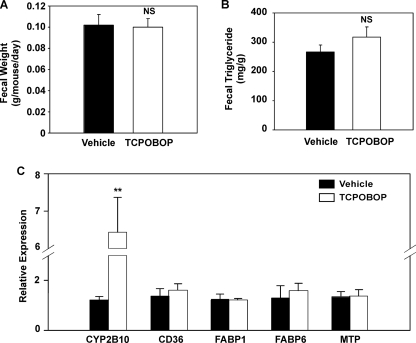
Since CAR is highly expressed in the small intestine, we went on to determine whether activation of CAR affected lipid digestion and absorption in this tissue. Feces were collected by using metabolic cages over a 24-h period in parallel with food intake measurement. As shown in Fig. 5, neither fecal weight (Fig. 5A) nor fecal triglyceride content (Fig. 5B) was affected by TCPOBOP treatment. Moreover, the intestinal expression of genes involved in lipid absorption, synthesis, assembly, and secretion was not significantly affected by TCPOBOP (Fig. 5C). Genes evaluated included the fatty acid translocase Cd36, fatty acid-binding proteins Fabp1 and Fabp6, and the microsomal triglyceride transport protein. The intestinal expression of Cyp2b10 was induced by TCPOBOP, as expected (Fig. 5C). These results suggest that activation of CAR may not have a major effect on fat absorption and metabolism in the intestine.
FIGURE 5.
Activation of CAR had little effect on lipid absorption in the small intestine. Male C57BL/6J mice were HFD-fed and TOPOBOP-treated for 8 weeks. n = 5 for each group. A and B, fecal output (A) and fecal concentration of triglyceride (B). C, hepatic gene expression as measured by real time PCR. FABP, fatty acid binding protein; MTP, microsomal triglyceride transport protein. **, p < 0.01; NS, statistically not significant (p > 0.05) compared with the vehicle group.
Activation of CAR Prevented Fatty Acid Overloading and Incomplete β-Oxidation in Skeletal Muscle
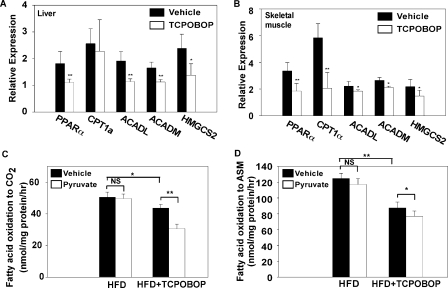
Obesity-related insulin resistance in skeletal muscle is characterized by excessive fatty acid β-oxidation and impaired switch to carbohydrate substrate during the fasted-to-fed transition (19). When the expression of genes involved in fatty acid oxidation was analyzed, we found that an 8-week treatment of TCPOBOP significantly suppressed the expression of PPARα and its target genes involved in β-oxidation and ketogenesis in both the liver (Fig. 6A) and skeletal muscle (Fig. 6B). These include the long-chain acyl-CoA dehydrogenase, medium-chain acyl CoA dehydrogenase, and HMG-CoA synthase (HMGCS2). Interestingly, the expression of β-oxidation genes was largely unaffected in mice treated with TCPOBOP for only 1 week, although the expression of PPARα in the liver was significantly suppressed (supplemental Fig. 3). When the mitochondrial β-oxidation rate was measured in the gastrocnemius muscle of HFD-fed mice, we found that mice treated with TCPOBOP had a modest but significant decrease in the rate of [14C]oleate oxidation to CO2, an indicator of complete β-oxidation (Fig. 6C). In contrast, TCPOBOP treatment resulted in a more dramatic reduction in the accumulation of radiolabeled intermediates in the acid-soluble metabolite (ASM), an indicator of incomplete β-oxidation (Fig. 6D), suggesting that TCPOBOP-treated mice had reduced incomplete β-oxidation. When pyruvate was added to the incubation buffer as a competing carbon source to induce a substrate switch from fatty acid to carbohydrate (20), vehicle-treated mice failed to exhibit a significant substrate switch, which was suggestive of insulin resistance. In contrast, TCPOBOP-treated mice showed a significant switch by having decreased CO2 formation (Fig. 6C). The TCPOBOP group also showed a modest but statistically significant decrease in incomplete β-oxidation (Fig. 6D), which is suggestive of improved insulin sensitivity.
FIGURE 6.
Activation of CAR prevented fatty acid overloading and incomplete β-oxidation in the skeletal muscle. A and B, liver (A) and skeletal muscle (B) mRNA expression of genes involved in β-oxidation. Male C57BL/6J mice were HFD-fed and TOPOBOP-treated for 8 weeks. n = 4 for each group. C and D, complete oxidation of [14C]oleate to CO2 (C) or incomplete oxidation of [14C]oleate to acid-soluble metabolite (ASM) (D) in mitochondria isolated from the gastrocnemius muscle, in the absence or presence of pyruvate. Mice are the same as those described in A and B. *, p < 0.05; **, p < 0.01; NS, statistically not significant (p > 0.05) compared with vehicle group, or the comparisons are labeled.
Activation of CAR Increased BAT Energy Expenditure and Promoted Peripheral Fat Mobilization
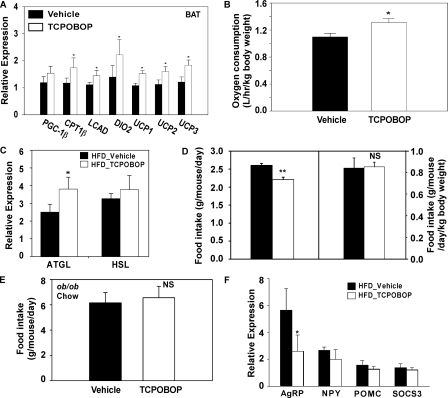
BAT plays an important role in thermogenesis. As shown in Fig. 7A, TCPOBOP treatment significantly increased BAT expression of Pgc-1α, muscle-type carnitine palmitoyltransferase I, long-chain acyl coenzyme A dehydrogenase, thyroid hormone-activating type 2 iodothyronine deiodinase, and uncoupling protein-1, -2, and -3. Mice treated with TCPOBOP also had increased oxygen consumption (Fig. 7B), supporting the notion of increased energy expenditure adapted to a high fat diet. Interestingly, the rectal temperatures between the vehicle and TCPOBOP groups were not significantly different (data not shown).
FIGURE 7.
Activation of CAR increased BAT energy expenditure and promoted peripheral fat mobilization. A, BAT mRNA expression of genes involved in energy expenditure and thermogenesis. Male C57BL/6J mice were HFD-fed and TOPOBOP-treated for 1 week. n = 4 for each group. B, oxygen consumption in male C57BL/6J mice HFD-fed and TOPOBOP-treated for 5 weeks. n = 4 for each group. C, WAT mRNA expression of lipase genes. Mice are the same as those described in A. D, food intake without (left) or with (right) normalization against the body weight. Mice are the same as those described in B. E, food intake of ob/ob mice maintained on a chow diet and treated with vehicle or TCPOBOP for 8 weeks. n = 5 for each group. F, hypothalamic mRNA expression of neuropeptides. Mice are the same as those described in B. *, p < 0.05; **, p < 0.01; NS, statistically not significant (p > 0.05) compared with the vehicle group.
When the WAT was analyzed, we found that the expression of adipose triglyceride lipase but not the hormone-sensitive lipase, was induced in WAT of TCPOBOP-treated mice (Fig. 7C), suggesting that an increased peripheral fat mobilization may have also contributed to the decreased fat mass and adipocyte hypotrophy.
Among other metabolic changes, 8-week treatment of TCPOBOP under HFD conditions modestly but significantly suppressed the food intake (Fig. 7D, left). However, the food intake was unchanged when normalized against the body weight (Fig. 7D, right). TCPOBOP treatment also had little effect on the food intake in chow-fed ob/ob mice (Fig. 7E). Analysis of the expression of food intake-related neuropeptides in the hypothalamus showed a decreased expression of agouti-related peptide (Fig. 7F), a neuropeptide that increases appetite and decreases metabolism and energy expenditure (21). The expression of neuropeptide Y, pro-opiomelanocortin, and SOCS3 (suppressor of cytokine signaling 3), three hypothalamic genes also known to influence appetite and/or metabolism (22–24), was unchanged. It is worthy of notice that the control group has a higher hypothalamic agouti-related peptide expression, even in the condition of high serum leptin level, which indicates that the mice subjected to HFD gradually developed leptin resistance and hyperphagia, whereas TCPOBOP treatment significantly preserved the leptin sensitivity (25).
Activation of CAR Had Little Effect on Endoplasmic Reticulum (ER) Stress in the Liver
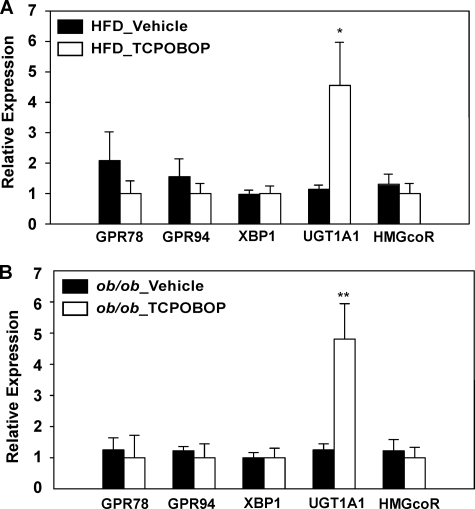
ER stress has been shown to contribute to the pathogenesis of metabolic dysfunction and hepatic steatosis (26). In an effort to determine whether activation of CAR impacts ER stress, we showed that treatment of HFD-fed wild type mice (Fig. 8A) or chow-fed ob/ob mice (Fig. 8B) with TCPOBOP had no significant effect on the expression of several ER stress marker genes, such as the G protein-coupled receptors Grp78 and Grp94, and Xbp1 (X-box-binding protein 1) (27). Among other detoxifying genes, the expression of Ugt1a1, a CAR target gene (28), was induced as expected, whereas the expression of HMG-CoA reductase was not significantly affected. These results suggest that the suppression of ER stress may not be a major mechanism by which CAR inhibits obesity and improves insulin sensitivity.
FIGURE 8.
Activation of CAR had little effect on ER stress in the liver. Shown is liver mRNA expression of genes involved in ER stress as determined by real time PCR analysis. A, male C57BL/6J mice were HFD-fed and TOPOBOP-treated for 8 weeks. n = 4 for each group. B, ob/ob mice maintained on a chow diet and treated with vehicle or TCPOBOP for 8 weeks. n = 5 for each group. HMGcoR, HMG-CoA reductase; GPR, G protein-coupled receptor; XBP1, X-box binding protein 1.
DISCUSSION
Patients of obesity and diabetes often suffer from both hyperglycemia and hyperlipidemia. Although the inhibition of gluconeogenesis and lipogenesis has been suggested largely through the gene expression analysis (9–14), whether the activation of CAR is beneficial in relieving obesity and diabetes has not been reported. In the current study, we showed that CAR was effective in preventing obesity and alleviating type 2 diabetes. The metabolic benefits of CAR may have resulted from the combined effect of inhibition of lipogenesis, VLDL secretion and export of triglycerides, and gluconeogenesis as well as increases in BAT energy expenditure and peripheral fat mobilization.
It is interesting to note that treatment with the CAR agonist inhibited lipogenic gene expression not only in the liver, but also in the WAT, BAT, and skeletal muscle. Among lipogenic enzymes, it has been reported that mice deficient in Acc-2 or Scd-1 were protected from obesity and hepatic steatosis induced by HFD (29, 30). Inhibition of Fas was also shown to inhibit food intake and prevent obesity (31). Thus, we reason that the inhibition of lipogenesis may have played an important role in the anti-obesity effect of CAR.
The pleiotropic effect of CAR on energy metabolism revealed in our study is rather intriguing. Our preliminary studies showed that the skeletal muscle, hypothalamus, and pituitary gland have a low but appreciable expression of CAR, whereas the adipose tissues do not express CAR (data not shown). It remains to be determined whether the phenotypes in gene regulation and fatty acid oxidation in the skeletal muscle were mediated by the local CAR effect. Whether and how the CAR activation in the hypothalamus and pituitary gland affects food intake also remain to be determined. The future creation of tissue-specific CAR knock-out or CAR transgenic mice will help to shed light on the extrahepatic function of CAR in energy metabolism. We also cannot exclude the possibility that the hypothalamic and pituitary function of CAR may have contributed to the hepatic phenotype. Such brain-liver communications have been reported (32). The lack of CAR expression in the adipose tissues suggests that the inhibition of lipogenesis, increased BAT energy expenditure, and activation of adipose triglyceride lipase gene expression in WAT were probably secondary to CAR activation in tissues outside of the adipose tissues.
Recent studies have linked muscle insulin resistance with fatty acid overload and the consequent incomplete fatty acid β-oxidation. In the presence of HFD, skeletal muscles undergo transcriptional remodeling adapting to an increased supply of lipid substrates. However, in the absence of exercise, the tricarboxylic acid cycle remains inactivated at the transcriptional level. As a result, although the complete fat oxidation is decreased or remains unchanged, the rate of incomplete oxidation rises significantly, leading to “mitochondrial stress” and insulin resistance (33). Accordingly, inhibition of β-oxidation and fatty acid influx by using CPT1 inhibitors has been shown to improve insulin sensitivity (19). In the current study, we showed that activation of CAR inhibited the expression of PPARα and its target genes involved in β-oxidation and fatty acid influx, thus reducing incomplete fatty acid β-oxidation of HFD-fed mice. The mechanism by which CAR suppresses the expression of PPARα and its target β-oxidation genes remains to be defined. We showed that a short term (1-week) treatment of TCPOBOP did not affect the expression of many of the β-oxidation genes (supplemental Fig. S3). It is possible that the inhibition of PPARα and its target gene expression is secondary to the reduced plasma triglyceride and free fatty acid levels in TCPOBOP-treated mice. It has been reported that PPARα knock-out mice were resistant to HFD-induced diabetes, and dexamethasone induction of hypertension and diabetes was PPARα-dependent (32). Therefore, the suppression of PPARα gene expression by CAR may have contributed to the metabolic benefit of CAR.
There was an ∼15% decrease in food intake in mice HFD-fed and TCPOBOP-treated for 8 weeks, which correlated with a similar body weight and fat mass decrease. However, the food intake was unchanged when normalized against the body weight. TCPOBOP also had little effect on the food intake in chow-fed ob/ob mice. Moreover, the inhibition of gluconeogenic and lipogenic gene expression by TCPOBOP was also observed in chow-fed mice (data not shown), whose body weights were not affected by this drug. Taken together, although we cannot completely rule out the possibility that the improved insulin sensitivity and steatosis may be secondary to body weight and fat mass loss in TCPOBOP-treated mice, our results suggest that the inhibition of food intake may not be the primary mechanism by which CAR improves insulin sensitivity. It is more likely that the CAR-mediated suppression of gluconeogenesis and lipogenesis preceded and was responsible for the anti-diabetic effect of TCPOBOP.
Dong et al. (35) recently reported that CAR mediates the induction of drug metabolism in a streptozotocin-induced mouse model of type 1 diabetes, which was associated with the induction of Pgc-1α in the liver. In the current study, we showed that hepatic Pgc-1α was induced by HFD feeding (data not shown), consistent with the notion that Pgc-1α promotes gluconeogenesis and insulin resistance in the liver (7). Together, these results suggest that both type 1 and type 2 diabetes are associated with the induction of Pgc-1α. We showed that compared with the vehicle group, treatment with TCPOBOP in HFD-fed mice had the tendency to suppress the expression of Pgc-1α (Fig. 3F), although the suppression did not reach a statistical significance. The lack of Pgc-1α induction and effective suppression of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase gene expression in TCPOBOP-treated mice were consistent with the metabolic benefit of CAR.
In summary, our results have uncovered an important metabolic function for CAR in preventing and relieving obesity and type 2 diabetes. It is interesting to note that the anti-diabetic and anti-obesity effect appeared to be unique for CAR, because the same effect was not observed for pregnane X receptor (data not shown), a sister xenobiotic receptor of CAR that has also been shown to inhibit lipogenesis and gluconeogenesis (9, 12, 13). It is tempting for us to propose that CAR may represent a novel therapeutic target to manage obesity and type 2 diabetes. Indeed, the CAR agonist phenobarbital has been shown to enhance insulin sensitivity and improve glucose and lipid metabolism in diabetic rats (36) and in diabetic patients (37, 38). Obesity is a medical problem of high prevalence. It is encouraging to note that CAR-activating activities have been found not only in clinical drugs but also in neutraceuticals, such as herbal medicines (34), raising the hope that CAR could be a target for neutraceutical prevention and relief of metabolic syndrome.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants ES012479 and CA107011 (to W. X.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S3.
- CAR
- constitutive androstane receptor
- PPARα
- peroxisome proliferator-activated receptor α
- GTT
- glucose tolerance test
- ITT
- insulin tolerance test
- ALT
- alanine aminotransferase
- BAT
- brown adipose tissue
- WAT
- white adipose tissue
- VLDL
- very low density lipoprotein
- HMG
- hydroxymethylglutaryl
- ASM
- acid-soluble metabolite
- ER
- endoplasmic reticulum.
REFERENCES
- 1.Flier J. S. (2004) Cell 116, 337–350 [DOI] [PubMed] [Google Scholar]
- 2.Kahn S. E., Hull R. L., Utzschneider K. M. (2006) Nature 444, 840–846 [DOI] [PubMed] [Google Scholar]
- 3.Honkakoski P., Zelko I., Sueyoshi T., Negishi M. (1998) Mol. Cell. Biol. 18, 5652–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei P., Zhang J., Egan-Hafley M., Liang S., Moore D. D. (2000) Nature 407, 920–923 [DOI] [PubMed] [Google Scholar]
- 5.Maglich J. M., Lobe D. C., Moore J. T. (2009) J. Lipid Res. 50, 439–445 [DOI] [PubMed] [Google Scholar]
- 6.Ding X., Lichti K., Kim I., Gonzalez F. J., Staudinger J. L. (2006) J. Biol. Chem. 281, 26540–26551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koo S. H., Satoh H., Herzig S., Lee C. H., Hedrick S., Kulkarni R., Evans R. M., Olefsky J., Montminy M. (2004) Nat. Med. 10, 530–534 [DOI] [PubMed] [Google Scholar]
- 8.Wieneke N., Hirsch-Ernst K. I., Kuna M., Kersten S., Püschel G. P. (2007) FEBS Lett. 581, 5617–5626 [DOI] [PubMed] [Google Scholar]
- 9.Roth A., Looser R., Kaufmann M., Meyer U. A. (2008) Pharmacogenet. Genomics 18, 325–337 [DOI] [PubMed] [Google Scholar]
- 10.Roth A., Looser R., Kaufmann M., Blättler S. M., Rencurel F., Huang W., Moore D. D., Meyer U. A. (2008) Mol. Pharmacol. 73, 1282–1289 [DOI] [PubMed] [Google Scholar]
- 11.Ueda A., Hamadeh H. K., Webb H. K., Yamamoto Y., Sueyoshi T., Afshari C. A., Lehmann J. M., Negishi M. (2002) Mol. Pharmacol. 61, 1–6 [DOI] [PubMed] [Google Scholar]
- 12.Zhou J., Zhai Y., Mu Y., Gong H., Uppal H., Toma D., Ren S., Evans R. M., Xie W. (2006) J. Biol. Chem. 281, 15013–15020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama S., Moore R., Yamamoto Y., Negishi M. (2007) Biochem. J. 407, 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao J., Fang S., Bae Y., Kemper J. K. (2006) J. Biol. Chem. 281, 14537–14546 [DOI] [PubMed] [Google Scholar]
- 15.Kodama S., Koike C., Negishi M., Yamamoto Y. (2004) Mol. Cell. Biol. 24, 7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J. H., Gong H., Khadem S., Lu Y., Gao X., Li S., Zhang J., Xie W. (2008) Endocrinology 149, 3778–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dresner A., Laurent D., Marcucci M., Griffin M. E., Dufour S., Cline G. W., Slezak L. A., Andersen D. K., Hundal R. S., Rothman D. L., Petersen K. F., Shulman G. I. (1999) J. Clin. Invest. 103, 253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoover-Plow J., Nelson B. (1985) J. Nutr. 115, 303–310 [DOI] [PubMed] [Google Scholar]
- 19.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., Lopaschuk G. D., Muoio D. M. (2008) Cell Metab. 7, 45–56 [DOI] [PubMed] [Google Scholar]
- 20.Randle P. J., Garland P. B., Hales C. N., Newsholme E. A. (1963) Lancet 1, 785–789 [DOI] [PubMed] [Google Scholar]
- 21.Ollmann M. M., Wilson B. D., Yang Y. K., Kerns J. A., Chen Y., Gantz I., Barsh G. S. (1997) Science 278, 135–138 [DOI] [PubMed] [Google Scholar]
- 22.Hanson E. S., Dallman M. F. (1995) J. Neuroendocrinol. 7, 273–279 [DOI] [PubMed] [Google Scholar]
- 23.Fan W., Boston B. A., Kesterson R. A., Hruby V. J., Cone R. D. (1997) Nature 385, 165–168 [DOI] [PubMed] [Google Scholar]
- 24.Bjørbaek C., Elmquist J. K., Frantz J. D., Shoelson S. E., Flier J. S. (1998) Mol. Cell 1, 619–625 [DOI] [PubMed] [Google Scholar]
- 25.Korner J., Savontaus E., Chua S. C., Jr., Leibel R. L., Wardlaw S. L. (2001) J. Neuroendocrinol. 13, 959–966 [DOI] [PubMed] [Google Scholar]
- 26.Rutkowski D. T., Wu J., Back S. H., Callaghan M. U., Ferris S. P., Iqbal J., Clark R., Miao H., Hassler J. R., Fornek J., Katze M. G., Hussain M. M., Song B., Swathirajan J., Wang J., Yau G. D., Kaufman R. J. (2008) Dev. Cell 15, 829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Görgün C., Glimcher L. H., Hotamisligil G. S. (2004) Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 28.Sugatani J., Kojima H., Ueda A., Kakizaki S., Yoshinari K., Gong Q. H., Owens I. S., Negishi M., Sueyoshi T. (2001) Hepatology 33, 1232–1238 [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki M., Flowers M. T., Sampath H., Chu K., Otzelberger C., Liu X., Ntambi J. M. (2007) Cell Metab. 6, 484–496 [DOI] [PubMed] [Google Scholar]
- 30.Oh W., Abu-Elheiga L., Kordari P., Gu Z., Shaikenov T., Chirala S. S., Wakil S. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loftus T. M., Jaworsky D. E., Frehywot G. L., Townsend C. A., Ronnett G. V., Lane M. D., Kuhajda F. P. (2000) Science 288, 2379–2381 [DOI] [PubMed] [Google Scholar]
- 32.Bernal-Mizrachi C., Weng S., Feng C., Finck B. N., Knutsen R. H., Leone T. C., Coleman T., Mecham R. P., Kelly D. P., Semenkovich C. F. (2003) Nat. Med. 9, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 33.Kraegen E. W., Cooney G. J. (2008) Curr. Opin. Lipidol. 19, 235–241 [DOI] [PubMed] [Google Scholar]
- 34.Huang W., Zhang J., Moore D. D. (2004) J. Clin. Invest. 113, 137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong B., Qatanani M., Moore D. D. (2009) Hepatology 50, 622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkatesan N., Davidson M. B., Simsolo R. B., Kern P. A. (1994) Metabolism 43, 348–356 [DOI] [PubMed] [Google Scholar]
- 37.Lahtela J. T., Särkkä P., Sotaniemi E. A. (1984) Res. Commun. Chem. Pathol. Pharmacol. 44, 215–226 [PubMed] [Google Scholar]
- 38.Lahtela J. T., Arranto A. J., Sotaniemi E. A. (1985) Diabetes 34, 911–916 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.