Abstract
The ClC protein family includes voltage-gated chloride channels and chloride/proton exchangers. In eukaryotes, ClC proteins regulate membrane potential of excitable cells, contribute to epithelial transport, and aid in lysosomal acidification. Although structure/function studies of ClC proteins have been aided greatly by the available crystal structures of a bacterial ClC chloride/proton exchanger, the availability of useful pharmacological tools, such as peptide toxin inhibitors, has lagged far behind that of their cation channel counterparts. Here we report the isolation, from Leiurus quinquestriatus hebraeus venom, of a peptide toxin inhibitor of the ClC-2 chloride channel. This toxin, GaTx2, inhibits ClC-2 channels with a voltage-dependent apparent KD of ∼20 pm, making it the highest affinity inhibitor of any chloride channel. GaTx2 slows ClC-2 activation by increasing the latency to first opening by nearly 8-fold but is unable to inhibit open channels, suggesting that this toxin inhibits channel activation gating. Finally, GaTx2 specifically inhibits ClC-2 channels, showing no inhibitory effect on a battery of other major classes of chloride channels and voltage-gated potassium channels. GaTx2 is the first peptide toxin inhibitor of any ClC protein. The high affinity and specificity displayed by this toxin will make it a very powerful pharmacological tool to probe ClC-2 structure/function.
ClC proteins form a family of voltage-gated Cl− channels and Cl−/H+ exchangers that are found in animals, plants, and bacteria (1). These proteins are expressed on the plasma membrane and some intracellular membranes in both excitable and nonexcitable cells (1, 2). There are nine mammalian members of the ClC family that perform functions as varied as maintenance of membrane potential in neuronal cells (ClC-2) (3), Cl− transport across plasma membranes of epithelial and skeletal muscle cells (ClC-1, ClC-2, and ClC-Ka/b) (1, 4), and participation in lysosomal acidification (ClC-5 and ClC-6) (2). Defects in the genes encoding ClC proteins are linked to a number of diseases including myotonia, epilepsy, Dent's disease, and Bartter's syndrome (1–3). It has been suggested recently that ClC-2 may play a role in constipation-associated irritable bowel disease as well as in atherosclerosis (5, 6). Most ClC channels show localized tissue expression; ClC-1, for example, is expressed solely in skeletal muscle, whereas ClC-Ka/b is localized to the kidney. ClC-2, on the other hand, is expressed nearly ubiquitously, suggesting that this channel plays an important, yet largely undefined, physiological role (1, 2).
ClC proteins are structurally unrelated to cation channels, with the functional unit being a homodimer (1). ClC channels display two equidistant conductance levels for a single channel opening. In 2002, the crystal structure of a bacterial ClC protein from Salmonella typhimurium was solved, revealing a very complicated membrane topology consisting of 18 α-helical units/subunit in the homodimer, only some of which fully traverse the membrane (7). Examination of the crystal structure revealed no obvious pore, such as is evident in K+ channel structures, even though bound Cl− ions were present near the proposed selectivity filter (7, 8). Shortly after the crystal structure was solved, it was shown that the bacterial ClC protein was actually a Cl−/H+ exchanger and not a channel (9). Comparison of the amino acid sequence of the bacterial ClC protein with that of the eukaryotic ClC channels ClC-0, -1, and -2 revealed only 22, 16, and 19% overall identity, respectively (data not shown). The divergence is largely in the cytoplasmic domains, which are absent in bacterial ClC proteins; sequence identity is much higher in the transmembrane domains.
Single-channel gating in ClC proteins is complicated, involving both fast and slow gating processes, which are thought to involve separate regions of the protein (1). Fast gating controls the opening and closing of both protopores independently, operating on the millisecond time scale or faster. Through examination of the crystal structure and subsequent electrophysiological analysis, the fast gating process was revealed to involve a conserved glutamate residue deep within each pore (10). This acidic residue lies near a Cl−-binding site and moves slightly to open the pathway in response to changes in membrane voltage and subsequent changes in occupancy of that site, thus providing the link between permeation and gating observed in ClC channels (4). In contrast, slow gating controls both pores simultaneously, operating on the hundreds of milliseconds to seconds time scale. Unlike with fast gating, the regions of the ClC protein involved in slow gating are still unknown, despite the availability of the bacterial ClC crystal structure. It is believed that the dimer interface contributes to slow gating, as well as the long cytoplasmic C-terminal domain, an isolated version of which was recently crystallized (11–13). However, the conformational changes involved in the fast and slow gating processes are still largely unknown. Also, in both ClC-1 and -2, fast and slow gating are linked through an undetermined mechanism (14, 15).
Despite the availability of the bacterial ClC protein crystal structure, our understanding of gating mechanisms and structural rearrangements of ClC proteins has lagged behind that of their cation channel counterparts. This is due in large part to a lack of useful pharmacological agents, such as peptide toxins, that may be used as tools. Toxins from venomous animals such as scorpions, snakes, and cone snails have been used for a number of years to define the permeation pathways and gating processes of cation channels (16). However, no peptide toxins have been isolated that inhibit a ClC channel, and only one toxin has been isolated that inhibits any Cl− channel of known molecular identity (17). We recently showed that venom from the scorpion Leiurus quinquestriatus hebraeus contains a peptide component that inhibits the ClC-2 chloride channel (18). Here, we report the isolation of this peptide toxin, its proteomic properties, and primary characteristics of the biophysical mechanism of inhibition.
EXPERIMENTAL PROCEDURES
Oocyte and cRNA Preparation
Xenopus oocytes were isolated as described previously (18) and incubated at 18 °C in a modified Liebovitz's L-15 medium (pH 7.5) with a mixture of antibiotics (gentamicin, penicillin, and streptomycin) and HEPES. The oocytes were injected with cRNAs encoding ClC-1, -2, -3, and -4; CFTR5; GABAC; Shaker B-IR; or KV1.2. Injection details may be found in the supplemental materials. The data were collected 2–5 days post injection. Methods for animal handling are in accordance with the National Institutes of Health guidelines, and the protocol was approved by the Animal Use and Care Committees of the Georgia Institute of Technology and Emory University.
Venom Preparation and Toxin Purification
Venom from L. quinquestriatus hebraeus was obtained from Latoxan (France) and prepared as described in the supplemental materials or Ref. 18. RP-HPLC was performed on partially fractionated scorpion venom (Lqh pf-venom) or on venom fractions. For toxin purification, venom was prepared at a concentration of 5 mg/ml in solution that contained 150 mm N-decanoyl-N-methylglucamine-Cl, 5 mm MgCl2, 10 mm TES, and 10 mm Tris·EGTA, pH 7.5. Subsequent fractions were resuspended in ND96 bath solution for the electrophysiological bio-assay (see the supplemental materials). All of the venom and fraction concentrations are stated as equivalent to the dried weight of whole venom.
Electrophysiology
Standard two-electrode voltage-clamp (TEVC) techniques were used to study whole cell ClC-2 currents expressed in Xenopus oocytes. The currents were acquired using a GeneClamp 500B amplifier and pClamp 8.4 software (Axon Instruments/Molecular Devices, Union City, CA). The data were acquired at 2 kHz and filtered at a corner frequency of 500 Hz. Bath solutions and voltage protocols for TEVC experiments with ClC channels were as described in the supplemental materials or Ref. 18. Inside-out multi-channel patch, inside-out and outside-out macropatch, and single-channel patch clamp experiments were performed as described previously (17, 18), using an Axopatch 200B amplifier (Axon). For all recordings the dashed line represents the zero current level. Further details of electrophysiology methods are provided in the supplemental materials.
To prevent loss of material, all of the solutions containing toxin were prepared immediately prior to experiments from a 20 μm stock. All of the solution lines and recording chambers were coated with bovine serum albumin to prevent loss of material because of sticking to plastic or glass.
Proteomic Analysis
The active toxin was analyzed via MALDI-MS at the Georgia Institute of Technology Bioanalytical Mass Spectrometry Facility. Edman degradation was performed at the Emory University Microchemical and Proteomics Facility. Sequence analysis of the natural and modified (reduced and alkylated) peptides was performed using Applied Biosystems model Procise-cLC automated protein sequencer (491cLC CLC capillary protein sequencing system; Applied Biosystems, Foster City, CA) using the manufacturer's cycles with slight modifications. Prior to sequencing, natural and modified samples were desalted and purified via RP-HPLC.
Homology Modeling
The homology model of the GaTx2 (GaTx2 is protected by a pending United States provisional patent, filed July 12, 2007, held by Georgia Tech Research Corporation) toxin was created using the Modeler 8v2 program, using neurotoxin P01 as the template structure (19). The GaTx2 homology model was then minimized via a 5-ps, 2500-step simulation with NAMDv2 utilizing the charmm22 force field. Prior to minimization, disulfide bridges were patched to ensure that the disulfide bonds remained intact during energy minimization.
GaTx2 Synthesis
Methods for peptide synthesis, purification, and folding have been described previously (20, 21). Solid phase synthesis was performed by Fmoc (N-(9-fluorenyl)methoxycarbonyl) chemistry, using the O-benzotriazole-N,N,N′,N′-tetramethyluronium hexafluorophosphate/hydroxybenzotriazole/N,N-diisopropylethylamine method on an Applied Biosystems 431A synthesizer. Oxidative cyclization of the crude linear peptide was performed under equilibrating conditions. The cyclized peptide was then purified from the reaction mixture via RP-HPLC and characterized by MALDI-MS and analytical HPLC.
Statistics
The data are expressed as the means ± S.E. for n observations. The differences were determined to be significant when p < 0.05, using paired and unpaired Student's t tests.
RESULTS
Isolation and Proteomic Characterization of the Active Toxin
We recently showed that a peptide component of scorpion venom is capable of specifically inhibiting the ClC-2 chloride channel (18). To isolate the active species from venom, we used RP-HPLC to separate the components of Lqh pf-venom, which contains only components smaller than 10 kDa. We tested each fraction for inhibition of ClC-2 channels expressed in Xenopus oocytes using TEVC. With initial separation performed using a C3 column, we observed that the fraction collected from 0 to 10 min (0–10% acetonitrile), Fraction A, retained activity similar to Lqh pf-venom (supplemental Fig. S1A, 45.7 ± 6.0% inhibition, fraction concentration of 0.1 mg/ml equivalent, n = 3, p = 0.01).
The brief retention time of the active component on the C3 column suggested that the toxin is very hydrophilic; therefore, further separation of components and isolation of the active toxin were accomplished by RP-HPLC using a C18 column, as summarized in supplemental Fig. S1B. The fractions were collected every 5 min and tested using the same protocol as in the prior round of fractionation. The activity was spread over a period of 15 min of elution covering a range of 7–15% acetonitrile. The active fractions showed activity similar to that of venom and fraction A (supplemental Fig. S1B, 45.2 ± 5.0% inhibition, fraction concentration of 0.1 mg/ml equivalent, n = 10, p = 0.01). These fractions were pooled and subjected to a final round of RP-HPLC using a C18 column (Fig. 1A).
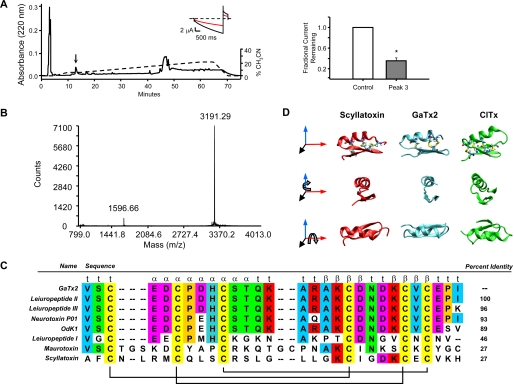
FIGURE 1.
Isolation and proteomic characterization of the active toxin. A, left panel, representative RP-HPLC chromatogram of the active venom subfraction, with the active peak indicated by the arrow. The elution gradient is represented by a dashed line. The right panel presents summary data for inhibition of ClC-2 by the active peak (n = 4). The inset shows representative traces from TEVC experiments in the absence (black trace) and presence (red trace) of the isolated peak at VM = −160 and +40 mV. B, MALDI-MS analysis of the active peak. C, sequence alignment of GaTx2 with other previously identified, highly related toxins. Disulfide bridge connectivity and predicted secondary structure are shown below and above the alignment, respectively. D, homology model of GaTx2 (middle column) shown with the NMR structures of Scyllatoxin (left column) and chlorotoxin (right column), in three orientations.
In the final stage of isolation, the active peak eluted at ∼5% acetonitrile (Fig. 1A). This peak fully recapitulated the activity of Lqh pf-venom (64.2 ± 5.3% inhibition at 0.1 mg/ml equivalent, n = 4, p = 0.025). A conservative estimate of toxin concentration was obtained from Edman degradation of the isolated peptide. These results suggested that this dilution of venom (0.1 mg/ml equivalent) contained 10 nm isolated toxin. A dose-response curve constructed using native toxin suggested a KD of ∼50 pm and a Hill coefficient of ∼1 (VM = −160 mV) (supplemental Fig. S1C). MALDI-MS analysis showed that the active peak had a mass of 3.2 kDa (Fig. 1B), well within the range expected for scorpion toxin inhibitors.
The primary structure of the peptide corresponding to the active peak was determined using N-terminal protein degradation and amino acid analysis (see supplemental materials). The toxin, which we named GaTx2 (gating modifier of anion channels 2), had a protein sequence of 1VSCEDCPDHCSTQKARAKCDNDKCVCEPI29 (Fig. 1C). MALDI-MS analysis of a reduced form of the toxin exhibited a mass shift of 348.2 Da (from 3191.29 to 3539.46 Da) upon reduction by dithiothreitol and modification of free cysteine residues by iodoacetimide, confirming that the six cysteine residues participate in three disulfide bonds in the folded toxin (data not shown). Comparison of this sequence to other known toxins revealed that GaTx2 bore 100% identity to a previously identified toxin called leiuropeptide II. This toxin was initially isolated in 1997, although no target was identified; it was initially postulated that this toxin was a K+ channel inhibitor based on disulfide bridge arrangement and spacing of critical lysine residues (22). Because it actually inhibits a Cl− channel, we have renamed this toxin to avoid confusion. Of the toxins shown in Fig. 1C, only four have been shown to be active against ion channels: neurotoxin P01, which inhibits apamin-sensitive K+ channels (23); scyllatoxin, which inhibits Ca2+-activated K+ channels (24); and maurotoxin and OdK1, both of which inhibit KV1.2 (25, 26).
The NMR structure of GaTx2 has been solved previously (22); however, the coordinates are not publicly available. Therefore, we created a homology model of GaTx2 based on the NMR structure of neurotoxin P01 (19), which bears 93% sequence identity to GaTx2, to make structural comparisons of GaTx2 to other known scorpion toxins (Fig. 1D, middle column). This model predicts that GaTx2 is composed of two β-strands and one α-helix, which are connected via three disulfide bonds. The basic fold and disulfide connectivity are consistent with the published NMR structure of leiuropeptide II (22). This basic fold is highly conserved among numerous toxins isolated from various scorpion species (27). We compared the tertiary structure of GaTx2 to both scyllatoxin, a K+ channel inhibitor, and chlorotoxin, a toxin isolated from a related scorpion that is thought to inhibit an unidentified Cl− channel from rat brain vesicles when reconstituted into lipid bilayers (24, 28). GaTx2 is noticeably more compact than both scyllatoxin (Fig. 1D, left column) and chlorotoxin (Fig. 1D, right column). Although the basic fold is similar between all three of these toxins, the tertiary structure itself is not enough to impart inhibitory activity against ClC-2, because chlorotoxin is unable to inhibit ClC-2 channels (18). The primary and secondary structures of GaTx2 are also very different from GaTx1, a recently isolated peptide inhibitor active against the CFTR Cl− channel, which also does not inhibit ClC-2 (17).
Inhibition of ClC-2 by Synthetic GaTx2
To determine whether the component that was isolated from venom is truly responsible for the observed inhibitory activity, we produced GaTx2 via solid phase chemical synthesis, as has been done for many other peptide inhibitors (20, 21). The folded synthetic toxin shows the same mass as the purified native toxin (supplemental Fig. S2A, right panel). Activity of the synthetic form of GaTx2 was tested initially via TEVC using the same voltage protocols as used during the isolation of the toxin. TEVC experiments showed that 10 nm synthetic GaTx2 inhibited steady state ClC-2-mediated currents by 61.4 ± 8.0% at VM = −160 mV (n = 3) (supplemental Fig. S2, B and C). This activity was almost exactly the same as that observed for the native toxin at the same concentration (64.2 ± 5.3, n = 4) (supplemental Fig. S2C).
Inhibition of ClC-2 by Synthetic GaTx2 Is Similar to That of Lqh pf-Venom
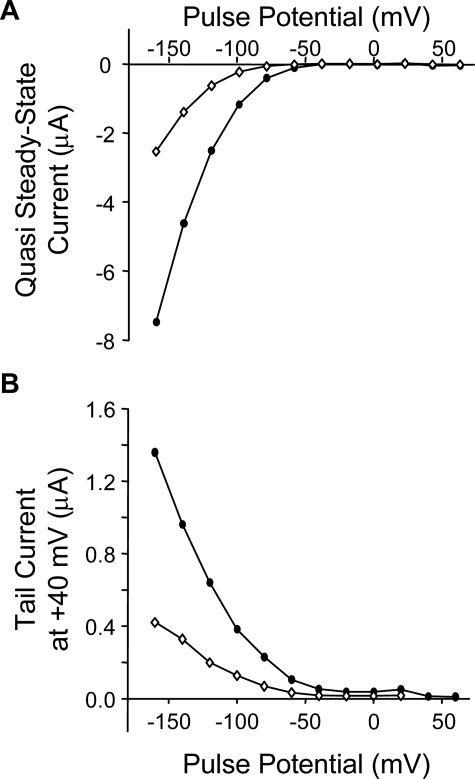
We previously showed that Lqh pf-venom inhibited ClC-2 currents across a range of voltages, for both the quasi-steady state activation currents and the tail currents elicited by a voltage step to +40 mV (18). To determine whether GaTx2 showed similar behavior, we tested the effect of 10 nm GaTx2 across a range of voltages from −160 to +60 mV. Similar to the activity of Lqh pf-venom, GaTx2 strongly inhibited inward ClC-2 currents and showed no effect at membrane potentials that do not activate the channel (Fig. 2A), drastically altering the current voltage relationship. There is also strong inhibition of tail currents at +40 mV from test potentials that strongly activate the channel (Fig. 2B), which could induce a shift in the V½ of channel activation to more hyperpolarizing potentials. However, because ClC-2 never reaches full activation, even at strongly hyperpolarizing potentials, it is not possible to determine the effect of GaTx2 on the conductance/voltage relationship for ClC-2 by calculating a true V½.
FIGURE 2.
Synthetic GaTx2 inhibits ClC-2 inward currents. Representative current voltage relationship for quasi steady state activation currents (A) and tail currents (B) in the absence (closed circles) and presence (open circles) of 10 nm synthetic GaTx2. The currents were elicited by a 3-s activation step ranging from −160 to +60 mV, with a tail pulse at +40 mV.
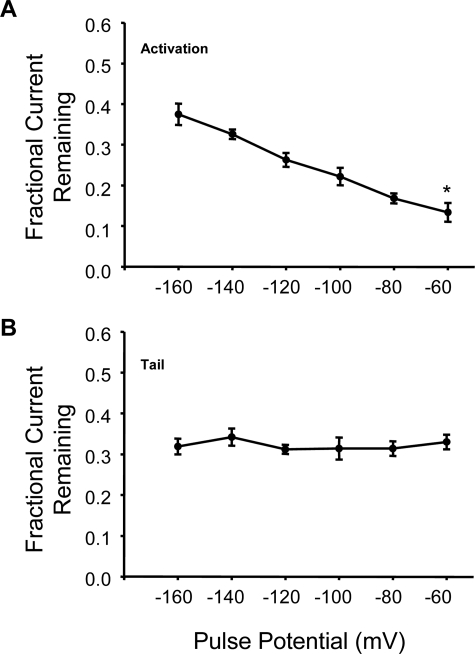
Lqh pf-venom also inhibited ClC-2 currents in a strongly voltage-dependent manner, with greater inhibition at more depolarizing potentials (18). To determine whether synthetic GaTx2 showed similar voltage dependence of inhibition, we examined the fractional current remaining across a range of membrane potentials from −160 to −60 mV. The currents were inhibited by 62.5 ± 2.6% at −160 mV (n = 3), but at −60 mV the currents were inhibited by 86.6 ± 2.3% (n = 3, p = 0.006) (Fig. 3A), consistent with the behavior observed for Lqh pf-venom (18). Also consistent with the activity of Lqh pf-venom is a lack of voltage-dependent inhibition of tail currents elicited at +40 mV (n = 3) (Fig. 3B). These results, therefore, suggest that GaTx2 is indeed the active component of Lqh pf-venom and inhibits via a similar mechanism.
FIGURE 3.
GaTx2 inhibits whole cell currents in a strongly voltage-dependent manner. Fractional current remaining of activation currents (A) and tail currents (B) in the presence of 10 nm synthetic GaTx2. The currents were compared with control currents at the same membrane potential. * indicates p < 0.01 compared with the value at −160 mV.
GaTx2 Is a Highly Selective ClC-2 Inhibitor
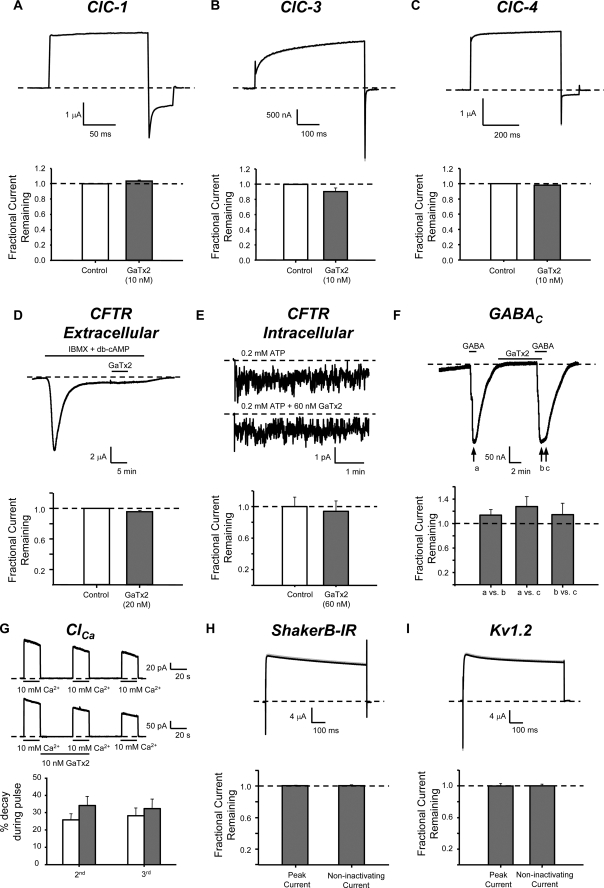
To determine whether GaTx2 could inhibit any other ion transport proteins, we tested the toxin on a number of related and unrelated ion channels and transporters, including members of the ClC family, other major chloride channels, and voltage-dependent K+ channels. We previously reported that Lqh pf-venom could not inhibit ClC-0 or ClC-1 (18). To confirm this we asked whether synthetic GaTx2 could inhibit ClC-1. We compared currents in the presence of toxin with those after washout of toxin to account for channel rundown. In the presence of 10 nm GaTx2, ClC-1 tail currents at VM = −120 mV from pulses to +60 mV were 1.03 ± 0.01-fold larger than currents after washout of toxin, implying no inhibition of ClC-1 (Fig. 4A). Application of 10 nm GaTx2 to ClC-3 likewise did not alter currents at VM = +80 mV (Fig. 4B; 0.90 ± 0.05-fold, n = 3, p = 0.18). GaTx2 also was not capable of inhibiting ClC-4-mediated Cl− transport measured at VM = +80 mV (10 nm GaTx2, 0.97 ± 0.02-fold, n = 3, p = 0.42) (Fig. 4C), indicating that GaTx2 does not inhibit a ClC protein that functions as a transporter. We also know from previous experiments that Lqh pf-venom does not inhibit ClC-0 (18). Thus, GaTx2 is not capable of inhibiting currents mediated by any other ClC channel or transporter on which we have tested it.
FIGURE 4.
GaTx2 specifically inhibits ClC-2. In TEVC experiments, GaTx2 was applied to oocytes expressing either ClC-1 (A), ClC-3 (B), or ClC-4 (C). Representative currents in the absence and presence of toxin are shown in the top panels (note that the lines overlap), whereas summary data are presented in the bottom panels. GaTx2 was unable to inhibit any other ClC protein (n = 3–4 for each type). D, representative TEVC experiment in which wild type CFTR channels were activated by a mixture of isobutylmethylxanthine and dibutyryl-cAMP. Channel activity increased rapidly, then decayed, and settled to steady state level. The channels were then exposed to 20 nm extracellular GaTx2 for 5 min. Summary data presented in the bottom panel show that GaTx2 cannot inhibit CFTR from the extracellular side (n = 2). E, inside-out multi-channel patch recording of FLAG-cut-ΔR-CFTR (VM = −80 mV). The channels were activated by application to the intracellular face of CFTR of either 0.2 mm ATP or 0.2 mm ATP + 60 nm GaTx2. Window currents for multiple 3-min windows were analyzed in the absence and presence of GaTx2, for multiple patches. Summary data in the bottom panel show that GaTx2 cannot inhibit CFTR from the intracellular side (n = 10 windows). F, TEVC recording of oocytes expressing GABAC channels (rho subunit) activated by 10 μm GABA (VM = −60 mV). The channels were exposed to 20 nm GaTx2 for 5 min and then activated by GABA again. GaTx2 was then removed, whereas the oocyte was still exposed to GABA. The bottom panel shows that GaTx2 did not inhibit GABAC currents (n = 3), and GABA-activated current did not increase when GaTx2 was removed. G, inside-out macropatch recording of endogenous Xenopus Ca2+-dependent Cl− channels (VM = −50 mV). The channels were activated by multiple applications of 10 mm Ca2+, and the amount of rundown was quantified. In some patches (lower traces), toxin was applied between the first and second application of Ca2+. The degree of rundown between the first and second application of Ca2+ did not differ between control patches and patches exposed to toxin (control, n = 5; GaTx2, n = 6). TEVC recording of Shaker ShB-IR K+ channels exposed to 20 nm GaTx2 (H) and KV1.2 channels exposed to 10 nm GaTx2 (I). The channels were activated by a voltage pulse to 0 mV from a holding potential of VM = −90 mV. The summary data show that GaTx2 was not capable of inhibiting Shaker ShB-IR K+ channels (n = 2) or KV1.2 channels (n = 4).
We recently identified and characterized GaTx1, a peptide inhibitor of currents mediated by CFTR chloride channels (17). CFTR was not inhibited by the venom fraction that contains GaTx2 (29). However, to corroborate these results we tested the effect of synthetic GaTx2 on CFTR. We first tested GaTx2 on CFTR currents by application of toxin to the extracellular face of the channels using TEVC. The channels were activated by 200 μm isobutylmethylxanthine plus 25 μm dibutyryl-cAMP. After steady state activation was achieved, 20 nm GaTx2 was applied for 5 min. No change in current was observed during the application of toxin (Fig. 4D; 0.95 ± 0.02-fold, n = 2), consistent with the notion that Lqh pf-venom could not inhibit CFTR currents from the extracellular face. Because GaTx1 only inhibited CFTR from the intracellular face (17), we applied 60 nm GaTx2 to the intracellular face of the channel using multi-channel patches. For these experiments we used FLAG-cut-ΔR-CFTR, a mutant CFTR lacking the R-domain, rendering it independent of phosphorylation by cAMP-dependent protein kinase and insensitive to dephosphorylation-mediated rundown. Channels were activated with 0.2 mm ATP and then reactivated with 0.2 mm ATP + 60 nm GaTx2. No inhibition of channel currents was observed (Fig. 4E; 0.91 ± 0.13-fold, n = 10, p = 0.74). Therefore, GaTx2 also is not capable of inhibiting CFTR from either the extracellular or intracellular face.
We next asked whether GaTx2 is capable of inhibiting currents from ligand-gated chloride channels formed by homomeric GABAC receptors. GABAC currents were measured by TEVC both in the absence and presence of GaTx2 (Fig. 4F). Comparison of current in the presence of 10 μm GABA to currents in the presence of 10 μm GABA plus 10 nm GaTx2 showed no change (13 ± 9.4% increase, n = 3, p = 0.32). GABA-induced Cl− currents also did not significantly increase upon washout of toxin (14 ± 19% increase, p = 0.74). This suggests that GaTx2 is not capable of inhibiting GABAC receptors.
Xenopus oocytes contain a large number of endogenous calcium-dependent chloride channels (ClCa). Application of 10 mm Ca2+ to the face of an excised inside-out patch rapidly activates these channels, which then run down over the course of the experiment. Therefore, control records and records in the presence of GaTx2 were obtained from different patches. To assess toxin activity, we measured current decay over the course of three pulsed exposures to high [Ca2+]; toxin was applied between the first and second Ca2+ pulses. If GaTx2 inhibited channel currents, we expected to observe a greater degree of apparent rundown in toxin-containing patches versus control patches. However, the decay between pulses was the same with and without 10 nm GaTx2 (Fig. 4G; 25.67 ± 3.65% (control, n = 5) versus 34.11 ± 5.26 (+ GaTx2, n = 6), p = 0.24), suggesting that GaTx2 is not capable of inhibiting these channels from the intracellular side. We were unable to test the effect of GaTx2 on ClCa when applied to the extracellular face because receptor-mediated activation of these channels exhibits irreversible desensitization after one exposure to receptor ligand (not shown).
Finally, because the toxin bearing the sequence of GaTx2 was originally predicted to be a K+ channel inhibitor, we sought to determine whether this toxin could inhibit the voltage-gated Drosophila Shaker K+ channel B variant with inactivation removed (ShB-IR), by exposing oocytes expressing ShB-IR to 20 nm GaTx2, a concentration that strongly inhibits ClC-2 currents (Fig. 4H). We observed no inhibition of ShB-IR currents, indicating that GaTx2 does not interact with this channel (1.00 ± 0.007-fold, n = 2). We similarly tested the effect of GaTx2 on the mammalian Shaker equivalent Kv1.2 and observed no change in K+ currents (Fig. 4I; 0.99 ± 0.03-fold, n = 4, p = 0.55). It is possible that GaTx2 may inhibit these channels at a much higher concentration, but it had no effect at a concentration that is saturating for inhibition of ClC-2-mediated currents (see below). Thus, we have shown that GaTx2 is a specific ClC-2 inhibitor, being unable to inhibit other members of the ClC family, CFTR, GABAC, ClCa, or major voltage-dependent K+ channels.
Kinetics of Inhibition of ClC-2 by GaTx2
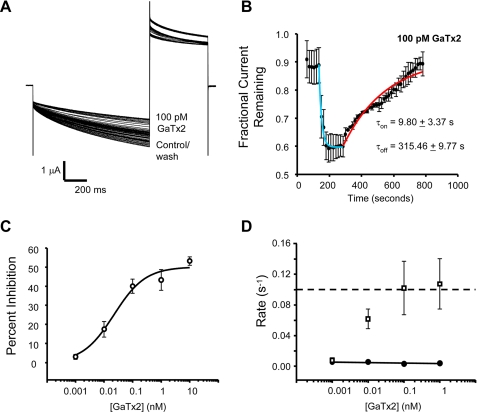
To gain a better understanding of the mechanism of inhibition of ClC-2 by GaTx2, we measured the rate of both onset of and recovery from inhibition of ClC-2 currents by direct application of GaTx2 to the extracellular face of the channel using TEVC; solution exchange in these experiments was complete within ∼10 s. To allow sufficient channel activation and maximal deactivation between pulses, the channels were activated every 15 s with a 1-s voltage pulse to VM = −100 mV, followed by a tail pulse to +40 mV. As a kinetic control, inhibition of ClC-2 currents by 1 mm Zn2+ showed that both inhibition and washout occurred very rapidly, with a time constant for recovery of ∼38 s (supplemental Fig. S3). The data obtained for both onset of inhibition and recovery from inhibition were fit with a single exponential function from the beginning of either onset or recovery from inhibition until the process had reached steady state. Time-dependent currents were inhibited by ∼95% by 1 mm Zn2+, as expected (30). These experiments were then repeated using GaTx2 over a broad concentration range. Data obtained in the presence of 100 pm GaTx2 showed a fast onset of inhibition and a very slow recovery from inhibition (Fig. 5, A and B). For low concentrations of toxin, steady state inhibition was achieved within 5 min. Using this protocol, we were able to construct a dose-response curve for steady state inhibition of ClC-2 time-dependent currents by synthetic GaTx2 at VM = −100 mV, which gave an apparent KD of 22 ± 10 pm and a Hill coefficient of ∼1 (Fig. 5C), consistent with the high affinity interaction observed for native GaTx2 (supplemental Figs. S1C and S2C). Maximal inhibition of ∼50% at this voltage likely arises from strong voltage dependence as well as protocol dependence of inhibition of ClC-2; this is also consistent with the behavior of Lqh pf-venom (18). The voltage dependence of inhibition likely reflects the voltage dependence of channel gating, although it is possible that the toxin may also bind in an intrinsically voltage-dependent manner. If GaTx2 does indeed function as a gating modifier, as suggested for Lqh pf-venom (18) and by results described below, its activity also should be state-dependent. Therefore, the incomplete inhibition observed using this voltage protocol may reflect incomplete inactivation of ClC-2 during the voltage protocol. We also observed that the pseudo-first order on-rate for GaTx2 increased as a function of [GaTx2], whereas the off-rate was independent of toxin concentration (Fig. 5D), as expected. The very slow off-rate (koff = 0.0034 s−1) is consistent with an intimate interaction between toxin and channel and is consistent with the off-rates of other very high affinity toxins (31). Although the on-rate is clearly concentration-dependent, we are unable to measure the forward rate constant accurately for rates faster than 0.1 s−1. This is due to a combination of the slow solution exchange rates and the slow voltage-induced activation and inactivation of ClC-2 channels requiring a voltage clamp protocol that pulses no more frequently than every 15 s. Thus, we cannot determine whether the onset of inhibition is a linear function of [GaTx2] and therefore represents a simple bimolecular interaction.
FIGURE 5.
GaTx2 inhibits ClC-2 with high affinity. The channels were activated every 15 s by a 1-s voltage pulse to −100 mV, followed by a tail pulse to +40 mV. GaTx2 was applied after channel activity had stabilized. A, experiment in the presence of 100 pm GaTx2. Note that time-dependent currents were inhibited by ∼40%. B, summary data for experiments in the presence of 100 pm GaTx2. Onset of inhibition occurred rapidly (n = 5 for this concentration), whereas recovery from inhibition was very slow, taking >7 min to return to base line. C, dose-response curve for inhibition of ClC-2 currents by synthetic GaTx2 at VM = −100 mV. Each point represents data from three to five TEVC experiments, fit with a three-parameter Hill equation. D, plot of first order rate constants for the onset of inhibition (open squares, n = 3–5), and recovery from inhibition (filled circles, n = 2–3). The dashed line represents an estimate of the detection limit of our experimental system, constrained by both the solution exchange rate and the low frequency of voltage pulses required to allow channels to return to the closed state. Note that some error bars are smaller than the symbols.
Inhibition of ClC-2 by GaTx2 Is Voltage-dependent
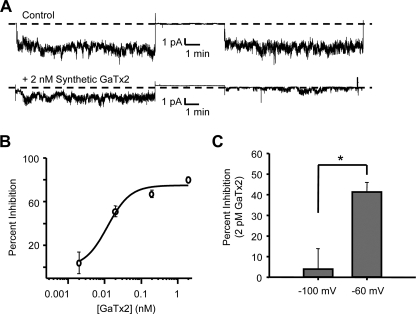
To confirm the activity of synthetic GaTx2, we recorded from inside-out multi-channel patches, where the pipette was backfilled with varying concentrations of synthetic toxin. The pipette was backfilled in such a way as to allow ∼10 min of control recording (at VM = −100 or −60 mV), followed by ∼10 min of recording in the presence of GaTx2. We then calculated the average window current from five separate 4-min windows in both control and experimental conditions (17). When no toxin was backfilled into the pipette, we observed no change in average window current, suggesting that there was no rundown over the course of the experiment (Fig. 6A). However, when 2 nm synthetic GaTx2 was backfilled into the pipette, the average window currents were drastically reduced at the end of the experiment (Fig. 6A, bottom panel; 80.4 ± 2.0% decrease). We repeated these experiments with varying concentrations of GaTx2 to obtain a dose-response curve, which provided KD = 12 ± 5 pm and a Hill coefficient of ∼1 for steady state inhibition at −100 mV using this multichannel patch protocol (Fig. 6B). This value is not significantly different from the KD measured using TEVC at the same membrane potential (Fig. 5C), suggesting that the pipette backfill technique was sufficient to accomplish steady state inhibition of the channel. The small difference in magnitude of maximal inhibition using the TEVC and multichannel patch protocols likely arises from differences in the Cl− gradient (no gradient for multichannel patches versus ∼3:1 out:in gradient for TEVC).
FIGURE 6.
Synthetic GaTx2 inhibits ClC-2 in a voltage-dependent manner. A, multi-channel inside-out patch recording of ClC-2 at VM = −100 mV in the presence of control extracellular solution (top panel) or extracellular solution containing 2 nm synthetic GaTx2 (bottom panel). The record at the left is from the beginning of the experiment, whereas the record at the right is from the end of the experiment, after the backfilled toxin had time to diffuse to the patch at the tip of the electrode. B, dose-response curve for inhibition of ClC-2 currents from multi-channel patches by GaTx2 at concentrations of 2 pm, 20 pm, 200 pm, and 2 nm (VM = −100 mV). All of the points contain data from 6 to 17 measurements of window current at each concentration. C, comparison of inhibition of ClC-2 by 2 pm GaTx2 when currents were measured at VM = −60 or −100 mV, in multichannel patches as shown in A. * indicates p < 0.001.
Our previous experiments with Lqh pf-venom indicated that inhibition of ClC-2 was voltage-dependent, with improved inhibitory efficacy at less hyperpolarizing potentials (18), suggesting that the affinity of GaTx2 is voltage-dependent. To further characterize this phenomenon, we compared the degree of inhibition of ClC-2 current by 2 pm synthetic GaTx2 at VM = −100 and −60 mV using multi-channel patches (Fig. 6C). At VM = −100 mV, 2 pm toxin did not inhibit ClC-2 significantly (4.0 ± 9.9% inhibition, n = 11 windows); however, at VM = −60 mV, ClC-2 currents were inhibited 41.4 ± 4.1% (n = 17 windows) by 2 pm toxin, confirming the notion that inhibition of ClC-2 by GaTx2 is voltage-dependent.
GaTx2 Slows ClC-2 Channel Opening
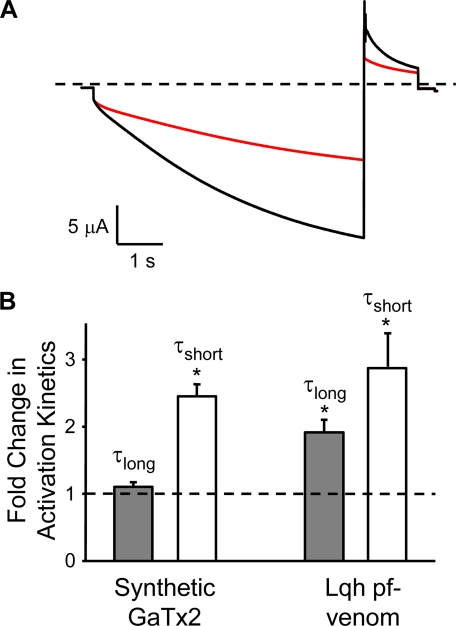
Toxins isolated from animal venoms may inhibit channel activity either by blocking the channel pore or by modifying channel gating via altering the voltage dependence of activation, thus causing a change in opening or closing rates at a given membrane potential (32). The activity of Lqh pf-venom suggested that the active component may act as a gating modifier caused by the significant slowing of the activation kinetics at −160 mV in the presence of venom (18). We tested the effect of 10 nm GaTx2 on the activation kinetics of ClC-2 to determine whether the activity of the isolated toxin was similar to that of Lqh pf-venom. Activation of ClC-2 macroscopic currents is best described by the sum of two exponential functions. In the presence of 10 nm GaTx2, we observed a 2.45 ± 0.18-fold (n = 3, p = 0.001) increase in the time constant for the fast component of channel activation, with no change in the slow time constant at low toxin concentrations (1.10 ± 0.06-fold increase, n = 3, p = 0.12) (Fig. 7). This activity is very similar to that observed for Lqh pf-venom, which showed a slowing of both the fast and slow components of channel activation at very high venom concentrations, although the change in the slow component of activation was less pronounced (18). This further suggests that GaTx2 inhibits ClC-2 channel activity via the same mechanism as Lqh pf-venom.
FIGURE 7.
GaTx2 alters the macroscopic opening rate. A, representative current trace in the absence (black trace) and presence (red trace) of 10 nm synthetic GaTx2. The currents were elicited by a 6-s pulse to −160 mV with a 1-s tail pulse to +40 mV. The dashed line represents the zero current level. B, effect of either synthetic GaTx2 or Lqh pf-venom on activation kinetics of ClC-2. The data are represented as fold change in τlong (gray bars) or τshort (white bars) compared with control values. The dashed line indicates no change. The data for Lqh pf-venom are from Ref. 18.
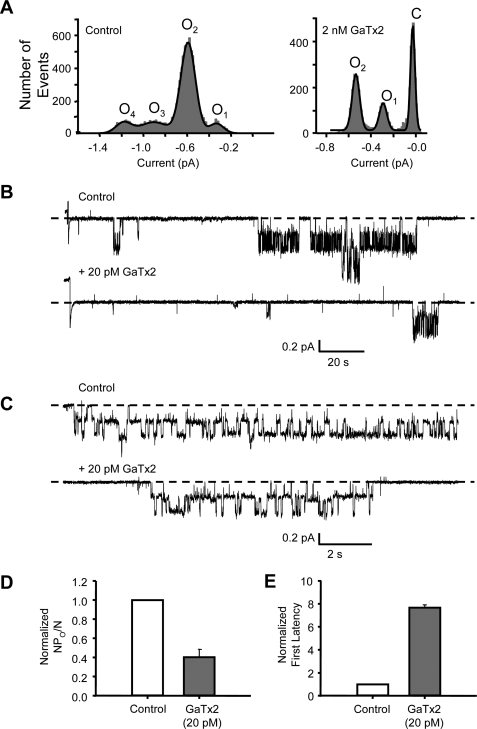
To examine the mechanism of inhibition in greater detail, we studied the effect of GaTx2 on single ClC-2 channels. To determine whether GaTx2 alters the single channel conductance of ClC-2, which may be indicative of pore block, we created all points amplitude histograms from segments of records with only one or two open channels, in the absence and presence of GaTx2 (records not shown). In the control portion of the experiment, two channels were active simultaneously, resulting in four conductance levels and no periods with zero channel activity (Fig. 8A, left panel). In the presence of 2 nm GaTx2, channel activity was drastically reduced such that only one active channel was observed, with the majority of the time spent in the closed state (Fig. 8A, right panel). Also, in the presence of a saturating concentration of GaTx2 (2 nm), the single protopore amplitude at −100 mV was unchanged from control (0.25 ± 0.01 pA versus 0.26 ± 0.01 pA, n = 3, p = 0.45) (Fig. 8A), as measured from the difference between open level 1 (O1) and open level 2 (O2) from the amplitude histograms created from 2-min sections of record in the absence and presence of toxin. This suggests that GaTx2 does not induce partial conductances, although this does not fully discount the possibility of inhibition via a pore block mechanism.
FIGURE 8.
Synthetic GaTx2 inhibits ClC-2 by slowing channel opening and does not affect single-channel conductance. A, all points amplitude histograms in the absence (left panel) and presence (right panel) of 2 nm GaTx2. B, representative single channel trace of ClC-2 with and without 20 pm synthetic toxin at VM = −100 mV. The traces shown begin with the step to −100 mV. C, expanded single channel trace of a ClC-2 burst in the absence and presence of 20 pm GaTx2, from the records shown in B. D, comparison of channel activity (as NPO/N) in the absence and presence of 20 pm GaTx2. E, comparison of latency to opening of the first double-barreled burst after stepping from 0 to −100 mV, from records such as those shown in B in the absence and presence of 20 pm GaTx2.
We next performed patch clamp experiments with either one or two channels in the patch and 20 pm GaTx2 backfilled into the pipette (Fig. 8, B–D). Measuring channel activity as NPO/N, we observed that NPO/N was reduced by 59.5 ± 8% (Fig. 8D, n = 2) at −100 mV, which is consistent with the reduction of window current observed in multichannel patches for this concentration of toxin (Fig. 6B). Some toxins act via a pore block mechanism by inducing long closed states within a channel burst (33). However, comparison of expanded recordings of bursts in the presence and absence of GaTx2 (Fig. 8C) showed no toxin-induced intraburst closures that would be consistent with a pore block mechanism, although it is of course impossible to know whether the toxin was bound to the channels from which these currents arose, because toxin does not alter conductance. This also suggests that once the channel opens in the presence of toxin, GaTx2 exerts no effect on fast gating, which controls the fast transitions between conductance levels observed within a channel burst for ClC channels.
Prior experiments with Lqh pf-venom led us to propose that ClC-2 is inhibited by this toxin via modification of channel gating; channel activation was slowed in the presence of venom (18). This was also found to be the case for synthetic GaTx2 (Fig. 7). The slow activation of ClC-2 channels evident in macroscopic recordings, such as shown in Fig. 7, reflects the slow voltage-induced activation of both the slow gates and the fast gates. The long interbust closures, as seen in Fig. 8B, are likely due to closure of the slow gate, which controls both protopores simultaneously. However, for ClC-2 both fast and slow gating appear to be coupled (15); thus, an inhibitor that affects either fast or slow gates could lead to prolongation of the apparent intraburst closed state. We reasoned that the effect of GaTx2 on single-channel gating may be apparent as a change in the latency to first opening of ClC-2 channels, measured as the time from the voltage step until the beginning of the first double-barreled open burst. For ClC channels. In the presence of 20 pm GaTx2, we observed a 7.67 ± 0.25-fold increase (from 13 ± 5 to 107 ± 40 s) (Fig. 8, B and E; n = 2) in the latency to first opening upon stepping from VM = 0 to VM = −100 mV, which is consistent with a modification of channel gating. The effect of GaTx2 on individual ClC-2 microscopic opening and closing rate constants under steady state conditions cannot be determined from these experiments but warrants further attention.
GaTx2 Is Not an Open Channel Blocker
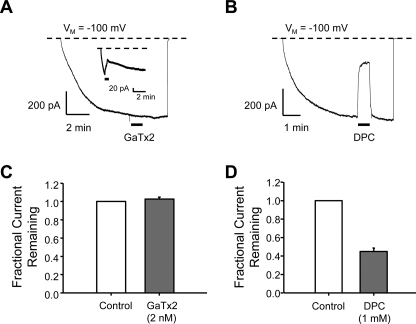
Thus far, our experiments have suggested that GaTx2 acts to slow channel opening via modification of channel gating. To provide further evidence for this mechanism, we tested the effect of GaTx2 on channels that had reached steady state activation using outside-out macropatches. We reasoned that if GaTx2 modifies channel gating, application of toxin to channels in the open state should not lead to a reduction in current amplitude, or current amplitude would be reduced very slowly. If the toxin inhibited via an open channel pore block mechanism, then currents should be reduced even if toxin was applied when channels are open. To test this notion, we activated ClC-2 channels in outside-out macropatches with a pulse to −100 mV and allowed current to reach steady state (Fig. 9). After steady state activation had been achieved, usually after several minutes, 2 nm GaTx2 was applied for at least 1 min using a fast perfusion system with a solution exchange time constant of ∼20 ms (18). Application of toxin did not inhibit open channel currents (1.02 ± 0.02-fold change, n = 5, p = 0.15) (Fig. 9, A and C), although application of toxin before steady state was reached led to substantial inhibition under these conditions (Fig. 9A, inset). In contrast, application of 1 mm .9 ± 3.0% inhibition (n = 9, p < 0.01) (Fig. 9, B and D) (34). These results suggest that DPC inhibited ClC-2 channels via an open channel pore block mechanism, whereas GaTx2 was not capable of inhibiting open channels. This also suggests that GaTx2 does not alter ClC-2 fast gating, because slowing of this process should resemble open channel block of macroscopic currents. Therefore, it seems likely that GaTx2 inhibits ClC-2 channel activation by inhibiting slow gating.
FIGURE 9.
GaTx2 cannot inhibit open ClC-2 channels. ClC-2 channels were studied using outside-out macropatches activated by a voltage step to VM = −100 mV. Channel activity was allowed to reach steady state. The patches were then exposed to either 2 nm GaTx2 (A) or 1 mm DPC (B). Summary data shown in C and D indicate that although GaTx2 was unable to inhibit ClC-2 steady state currents from open channels (n = 5) (C), DPC was able to inhibit very well (n = 9) (D). The inset in A, on the other hand, shows that if GaTx2 was applied to channels during the activation phase in this protocol, then currents were inhibited.
DISCUSSION
We showed previously that Lqh pf-venom contained a peptide component that inhibited the ClC-2 chloride channel by slowing channel opening (18). Here we report the successful isolation of the first peptide inhibitor of a ClC protein, GaTx2, and initial characterization of the mechanism of action. GaTx2 is a 3.2-kDa peptide with three disulfide bonds holding together a secondary structure composed of one α-helix and two β-strands. Although the sequence of GaTx2 has been described previously (22), this is the first identification of its molecular target. The target for this toxin originally was predicted to be a K+ channel based solely on sequence alignments with other known or putative K+ channel toxins. Therefore, this is also the first toxin that has been shown to be misclassified, raising the possibility that a number of other toxins capable of inhibiting anion-selective channels have already been sequenced.
The crystal structure of a bacterial ClC protein has been very useful for directing experiments aimed at gaining further understanding of the pore structures and fast gating mechanisms of ClC channel proteins (10, 35). Although the crystal structure has not been as useful for understanding slow gating, some progress has been made through mutagenesis studies (11, 13, 36, 37). The new crystal structures of the isolated C-terminal domain from ClC-0 will likely be very helpful in this regard (12). However, many of the conformational changes associated with channel gating are still unknown and are unlikely to be elucidated strictly by studying the available structures, which do not capture the dynamic nature of these proteins. The GaTx2 toxin will enable the biophysical study of these gating processes in greater detail.
The apparent dissociation constant of GaTx2 for ClC-2 at −100 mV, determined using multiple approaches including TEVC, which allowed direct application of toxin to the cell, and toxin application via a diffusion mechanism using pipette backfill, is ∼15 pm (Figs. 5C and 6B). Consistent with this high affinity interaction, recovery of ClC-2 currents from inhibition is very slow, with a time constant of >300 s. With an apparent Kd in the low picomolar range, GaTx2 inhibits ClC-2 with higher affinity than any other available drug and, in fact, is the best inhibitor of any chloride channel. The previously best available ClC inhibitor, a pentameric DIDS hydrolysis product, inhibits ClC-Ka with a K½ of 0.5 μm, whereas GaTx1 inhibits CFTR with a KD of 25 nm at −100 mV (17, 38).
Interestingly, inhibition of ClC-2 by GaTx2 seems to be protocol-dependent. Application of toxin to channels that have reached steady state activation did not reduce currents. However, significant inhibition was observed when channels were allowed to close in the presence of toxin, as observed for both TEVC recording and inside-out multi-channel patches. Therefore, GaTx2 does not mediate a simple open channel block mechanism such as that seen for some peptide toxins. Our TEVC and single channel data suggest that GaTx2 inhibits ClC-2 activity by slowing channel activation. Given that GaTx2 does not inhibit open ClC-2 channels, it is likely that the toxin preferentially binds to the closed state of the channel and prevents channel opening. The possibility that inhibition of ClC-2 currents by GaTx2 may not be mediated by a simple bimolecular interaction is very intriguing. The seemingly biphasic appearance of both the dose-response relationships (supplemental Fig. 1C; Figs. 5C and 6B) and recovery from inhibition (Fig. 5B) suggests that GaTx2 may bind to two or more sites that differ in affinity. ClC proteins are both structurally complex and regulated by very complicated gating mechanisms that are linked to permeation. In addition, fast and slow gating in ClC-2 are linked. Therefore, the idea that the interaction between GaTx2 and ClC-2 is not a simple one is not unreasonable and warrants further investigation.
Inhibition by GaTx2 is also voltage-dependent, with stronger inhibition at more physiological membrane potentials. This voltage-dependent inhibition also is consistent with a gating modification mechanism. Hanatoxin, a gating modifier of KV2.1 channels, inhibits with a KD of 42 nm at 0 mV. However, at +50 mV, hanatoxin inhibits the KV2.1 channels by only 40% at concentrations as high as 5 μm (39). This phenomenon is similar to the voltage-dependent inhibition reported here for GaTx2. It is at present mechanistically unclear why the maximal inhibition is ∼60% for TEVC, whereas 80% inhibition can be achieved using patch clamp techniques. It is possible that the differences in recording conditions, such as chloride concentrations, may affect the inhibitory activity of GaTx2. Such details regarding the mechanism of inhibition still need to be addressed. The voltage dependence and protocol dependence suggest that the mechanism by which GaTx2 inhibits ClC-2 is complex.
The physiological role of ClC-2 is still largely undefined. It is thought that ClC-2 may play a role in vascular smooth muscle cells and may be expressed on the apical membrane of epithelial cells along with CFTR, although this is still controversial (40). GaTx2 will be useful in determining the role of ClC-2 in these cells and may aid in determining the membrane localization of ClC-2 in specific cell types. Also, mutations in ClC-2 have been implicated in epilepsy (3), whereas under-activity of wild type ClC-2 has been implicated in constipation-associated inflammatory bowel disease (5). Therefore, GaTx2 may serve as a lead compound for peptidomimetic drugs that target ClC-2.
Supplementary Material
Acknowledgment
We thank M. C. Sullards for initial MALDI-MS experiments.
This work was supported, in whole or in part, by National Institutes of Health Grant DK066409. This work was also supported by the National Institutes of Health National Center for Research Resources, the Canadian Institutes of Health Research, the Cystic Fibrosis Foundation, and Children's Healthcare of Atlanta, Inc.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and supplemental text.
- CFTR
- cystic fibrosis transmembrane conductance regulator
- ShB-IR
- Shaker B K+ channel with inactivation removed
- TEVC
- two-electrode voltage clamp
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid
- Lqh
- L. quinquestriatus hebraeus
- RP-HPLC
- reversed phase high performance liquid chromatography
- GABAC
- γ-aminobutyric acid receptor, type C
- MALDI-MS
- matrix-assisted laser desorption ionization mass spectrometry
- pf
- partially fractionated
- DIDS
- 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid
- DPC
- diphenylamine-2-carboxylic acid.
REFERENCES
- 1.Jentsch T. J., Stein V., Weinreich F., Zdebik A. A. (2002) Physiol. Rev. 82, 503–568 [DOI] [PubMed] [Google Scholar]
- 2.Jentsch T. J., Neagoe I., Scheel O. (2005) Curr. Opin. Neurobiol. 15, 319–325 [DOI] [PubMed] [Google Scholar]
- 3.Haug K., Warnstedt M., Alekov A. K., Sander T., Ramírez A., Poser B., Maljevic S., Hebeisen S., Kubisch C., Rebstock J., Horvath S., Hallmann K., Dullinger J. S., Rau B., Haverkamp F., Beyenburg S., Schulz H., Janz D., Giese B., Müller-Newen G., Propping P., Elger C. E., Fahlke C., Lerche H., Heils A. (2003) Nat. Genet. 33, 527–532 [DOI] [PubMed] [Google Scholar]
- 4.Fahlke C. (2001) Am. J. Physiol. Renal. Physiol 280, F748–F757 [DOI] [PubMed] [Google Scholar]
- 5.Cuppoletti J., Malinowska D. H., Tewari K. P., Li Q. J., Sherry A. M., Patchen M. L., Ueno R. (2004) Am. J. Physiol. Cell Physiol 287, C1173–C1183 [DOI] [PubMed] [Google Scholar]
- 6.Cheng G., Kim M. J., Jia G., Agrawal D. K. (2007) Cardiovasc. Res 73, 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutzler R., Campbell E. B., Cadene M., Chait B. T., MacKinnon R. (2002) Nature 415, 287–294 [DOI] [PubMed] [Google Scholar]
- 8.Doyle D. A., Morais Cabral J., Pfuetzner R. A., Kuo A., Gulbis J. M., Cohen S. L., Chait B. T., MacKinnon R. (1998) Science 280, 69–77 [DOI] [PubMed] [Google Scholar]
- 9.Accardi A., Miller C. (2004) Nature 427, 803–807 [DOI] [PubMed] [Google Scholar]
- 10.Dutzler R., Campbell E. B., MacKinnon R. (2003) Science 300, 108–112 [DOI] [PubMed] [Google Scholar]
- 11.Duffield M., Rychkov G., Bretag A., Roberts M. (2003) J. Gen. Physiol 121, 149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer S., Dutzler R. (2006) Structure 14, 299–307 [DOI] [PubMed] [Google Scholar]
- 13.Hebeisen S., Biela A., Giese B., Müller-Newen G., Hidalgo P., Fahlke C. (2004) J. Biol. Chem. 279, 13140–13147 [DOI] [PubMed] [Google Scholar]
- 14.Saviane C., Conti F., Pusch M. (1999) J. Gen. Physiol. 113, 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Santiago J. A., Nehrke K., Arreola J. (2005) J. Gen. Physiol. 126, 591–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Possani L. D., Merino E., Corona M., Bolivar F., Becerril B. (2000) Biochimie 82, 861–868 [DOI] [PubMed] [Google Scholar]
- 17.Fuller M. D., Thompson C. H., Zhang Z. R., Freeman C. S., Schay E., Szakács G., Bakos E., Sarkadi B., McMaster D., French R. J., Pohl J., Kubanek J., McCarty N. A. (2007) J. Biol. Chem. 282, 37545–37555 [DOI] [PubMed] [Google Scholar]
- 18.Thompson C. H., Fields D. M., Olivetti P. R., Fuller M. D., Zhang Z. R., Kubanek J., McCarty N. A. (2005) J. Membr. Biol 208, 65–76 [DOI] [PubMed] [Google Scholar]
- 19.Blanc E., Fremont V., Sizun P., Meunier S., Van Rietschoten J., Thevand A., Bernassau J. M., Darbon H. (1996) Proteins 24, 359–369 [DOI] [PubMed] [Google Scholar]
- 20.Chang N. S., French R. J., Lipkind G. M., Fozzard H. A., Dudley S., Jr. (1998) Biochemistry 37, 4407–4419 [DOI] [PubMed] [Google Scholar]
- 21.Hui K., McIntyre D., French R. J. (2003) J. Gen. Physiol. 122, 63–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buisine E., Wieruszeski J. M., Lippens G., Wouters D., Tartar A., Sautiere P. (1997) J. Pept. Res. 49, 545–555 [DOI] [PubMed] [Google Scholar]
- 23.Zerrouk H., Laraba-Djebari F., Fremont V., Meki A., Darbon H., Mansuelle P., Oughideni R., van Rietschoten J., Rochat H., Martin-Eauclaire M. F. (1996) Int. J. Pept. Protein. Res 48, 514–521 [DOI] [PubMed] [Google Scholar]
- 24.Chicchi G. G., Gimenez-Gallego G., Ber E., Garcia M. L., Winquist R., Cascieri M. A. (1988) J. Biol. Chem. 263, 10192–10197 [PubMed] [Google Scholar]
- 25.Kharrat R., Mabrouk K., Crest M., Darbon H., Oughideni R., Martin-Eauclaire M. F., Jacquet G., el Ayeb M., Van Rietschoten J., Rochat H., Sabatier J. M. (1996) Eur. J. Biochem. 242, 491–498 [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Mottaleb Y., Clynen E., Jalali A., Bosmans F., Vatanpour H., Schoofs L., Tytgat J. (2006) FEBS. Lett. 580, 6254–6258 [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez de la Vega R. C., Possani L. D. (2004) Toxicon 43, 865–875 [DOI] [PubMed] [Google Scholar]
- 28.DeBin J. A., Maggio J. E., Strichartz G. R. (1993) Am. J. Physiol. Cell Physiol. 264, C361–C369 [DOI] [PubMed] [Google Scholar]
- 29.Fuller M. D., Zhang Z. R., Cui G., Kubanek J., McCarty N. A. (2004) Am. J. Physiol. Cell Physiol 287, C1328–C1341 [DOI] [PubMed] [Google Scholar]
- 30.Clark S., Jordt S. E., Jentsch T. J., Mathie A. (1998) J. Physiol. 506, 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein S. A., Miller C. (1993) Biophys. J 65, 1613–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonough S. (2003) in Calcium Channel Pharmacology ( McDonough S. ed.), Kluwer-Academic-Plenum Publishing, New York [Google Scholar]
- 33.Anderson C. S., MacKinnon R., Smith C., Miller C. (1988) J. Gen. Physiol. 91, 317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picollo A., Liantonio A., Babini E., Camerino D. C., Pusch M. (2007) J. Membr. Biol. 216, 73–82 [DOI] [PubMed] [Google Scholar]
- 35.Accardi A., Pusch M. (2003) J. Gen. Physiol. 122, 277–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estévez R., Pusch M., Ferrer-Costa C., Orozco M., Jentsch T. J. (2004) J. Physiol. 557, 363–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bykova E. A., Zhang X. D., Chen T. Y., Zheng J. (2006) Nat. Struct. Mol. Biol. 13, 1115–1119 [DOI] [PubMed] [Google Scholar]
- 38.Matulef K., Howery A. E., Tan L., Kobertz W. R., Du Bois J., Maduke M. (2008) ACS Chem. Biol. 3, 419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swartz K. J., MacKinnon R. (1997) Neuron 18, 665–673 [DOI] [PubMed] [Google Scholar]
- 40.Zdebik A. A., Cuffe J. E., Bertog M., Korbmacher C., Jentsch T. J. (2004) J. Biol. Chem. 279, 22276–22283 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.