Abstract
Previously we showed that following hypoxia there is an increase in nuclear Ca++ -influx and Ca++/calmodulin-dependent protein kinase IV activity (CaMK IV) in the cerebral cortex of term guinea pig fetus. The present study tests the hypothesis that clonidine administration will prevent hypoxia-induced increased neuronal nuclear Ca++ -influx and increased CaMK IV activity, by blocking high affinity Ca++-ATPase. Studies were conducted in 18 pregnant guinea pigs at term, normoxia (Nx, n=6), hypoxia (Hx, n=6) and clonidine with Hx (Hx+Clo, n=6). The pregnant guinea pig was exposed to a decreased FiO2 of 0.07 for 60 min. Clonidine, an imidazoline inhibitor of high affinity Ca++-ATPase, was administered 12.5µg/kg IP 30 minutes prior to hypoxia. Hypoxia was determined biochemically by ATP and phosphocreatine (PCr) levels. Nuclei were isolated and ATP-dependent 45Ca++ -influx was determined. CaMK IV activity was determined by 33P-incorporation into syntide 2 for 2 min at 37oC in a medium containing 50 mM HEPES (pH 7.5), 2 mM DTT, 40 µM syntide 2, 0.2 mM 33P-ATP, 10 mM magnesium acetate, 5 µM PKI 5-24, 2 µM PKC 19-36 inhibitor peptides, 1 µM microcystine LR, 200 µM sodium orthovanadate and either 1 mM EGTA (for CaMK IV-independent activity) or 0.8 mM CaCl2 and 1 mM calmodulin (for total activity). ATP (µmoles/g brain) values were significantly different in the Nx (4.62±0.2), Hx (1.65±0.2, p<0.05 vs Nx), and Hx+Clo (1.92±0.6, p<0.05 vs. Nx). PCr (µmoles/g brain) values in the Nx (3.9±0.1), Hx (1.10±0.3, p<0.05 vs. Nx ), and Hx+Clo (1.14±0.3, p<0.05 vs. Nx). There was a significant difference between nuclear Ca++-influx (pmoles/mg protein/min) in Nx (3.98±0.4), Hx (10.38±0.7, p<0.05 vs. Nx), and Hx+Clo (7.35±0.9, p<0.05vs Nx, p<0.05 vs. Hx), and CaM KIV (pmoles/mg protein/min) in Nx (1314.00 ±195.4), Hx (2315.14±148.5, p<0.05 vs. Nx), and Hx+Clo (1686.75±154.3, p<0.05 vs. Nx, p <0.05 vs. Hx). We conclude that the mechanism of hypoxia-induced increased nuclear Ca++-influx is mediated by high affinity Ca++-ATPase and that CaMK IV activity is nuclear Ca++-influx dependent. We speculate that hypoxia-induced alteration of high affinity Ca++-ATPase is a key step that triggers nuclear Ca++-influx, leading to CREB protein-mediated increased expression of apoptotic proteins and hypoxic neuronal death.
Keywords: Hypoxia, Nuclear Ca++-influx, CaM K IV, Clonidine
INTRODUCTION
Calcium (Ca++) is a secondary messenger involved in a wide variety of cellular processes such as synaptic communication between neurons, muscle contraction, immune response, cell proliferation, and gene transcription [2, 29]. The frequency and magnitude of Ca++-flux elicited by various signaling ligands confer specificity to different signaling pathways by activating different Ca++-dependent enzymes [4,8]. One of the main target kinases of Ca++ is the multifunctional, multimeric Ca++/calmodulin-dependent kinase (CaMK) II, a family of serine/threonine kinases involved in many cellular processes [3, 17,31].
Calcium signals regulate numerous cell functions including motility, secretion, proliferation, cell survival, some forms of programmed cell death, and gene expression [29]. Until recently, Ca++ signaling was viewed essentially as a whole-cell process ubiquitously affecting all regions of the cytoplasm [7]. The concept that Ca++ signaling could be restricted to specific subcellular regions, with its corollary of independently regulated processes in individual cell compartments, was relatively alien in most studies. In addition to myosin light-chain kinase, CaM kinases II, III, and IV are also active in the nucleus [24, 27, 28, 30, 32, and 34]. CaM kinase isoforms are expressed in all tissues, and are targeted to the nucleus [23]. The end portion of the Ca++/calmodulin-activated chain of events that influences the transcription of genes is nuclear, but the initial Ca++-sensitive steps, may be cytoptasmic [15].
Hypoxic neuronal injury is a combination of processes including increased release of excitatory neurotransmitters, activation of excitatory amino acid neurotransmitter receptors, increased intracellular Ca++, production of free radicals, alteration of cellular membrane structure/function, and changes in nuclear Ca++-influx. An increase in intranuclear Ca++ concentration, acting through the Ca++ receptor calmodulin is a pervasive segment that regulates diverse cellular responses [31]. Ca++/calmodulin kinase IV (CaMK IV), is a key enzyme of the CaM kinase cascade and is enriched in the brain, predominantly localized in cell nuclei [36, 39]. Phosphorylation of Threonine196 in CaMK IV by an upstream protein kinase results in induction of Ca++/calmodulin-dependent activity (12). This regulatory mechanism allows to produce prolonged CaMK IV activation to regulate gene transcription, through cyclic AMP response element binding (CREB) protein [31]. CREB protein is phosphorylated by CaMK IV at serine133 which initiates transcription. CREB protein is a transcription factor that mediates responses to a number of physiological and pathological signals [12, 21].
Clonidine, an imidazoline, is a drug that acts as an α2-adrenoreceptor agonist. Clonidine is a lipid soluble drug which is able to cross the blood–brain barrier reducing the peripheral sympathetic effects of central α2-adrenoreceptors in hypertensive patients causing a hypotensive effect. This hypotensive effect is caused at lower doses, whereas at high doses no hypotensive effect is observed resulting from post-junctional α2-adrenoreceptor activation [9, 14]. Clonidine, has been shown to reduce intracellular Ca++ levels by modification of intracellular cAMP [33, 35]. It has also been shown to be a noncompetitive inhibitor of high affinity Ca++-ATPase [11]. Previously we have shown that clonidine inhibits high affinity Ca++-ATPase activity, and decreases intranuclear calcium influx in the neuronal nuclei of the cerebral cortex of newborn piglets. Thus, clonidine was selected to elucidate the importance of nuclear calcium influx in the apoptotic cascade.
In previous studies, we have shown that cerebral hypoxia results in increased nuclear Ca++-influx in neuronal nuclei of the cerebral cortex of guinea pig fetus and newborn piglets [5, 16, 21, and 37]. The nuclear Ca++-influx increased as a function of increase in cerebral tissue hypoxia, as measured by decrease in high-energy phosphates, ATP, and phosphocreatine (PCr). We have also demonstrated that cerebral hypoxia results in increased Ca++/calmodulin kinase (CaM kinase) IV activity and cyclic AMP response element binding (CREB) protein phosphorylation in neuronal nuclei of newborn piglets [38].
The present study tests the hypothesis that hypoxia results in increased nuclear Ca++-influx and increased CaM kinase IV activity, and the administration of Clonidine, an inhibitor of high affinity nuclear Ca++-ATPase, will prevent the hypoxia-induced increase in nuclear Ca++-influx and CaM kinase IV activity in neuronal nuclei of the cerebral cortex of term guinea pig fetus. The pharmacologic agent clonidine was selected to test this hypothesis secondary to its ability to decrease high affinity Ca++-ATPase activity [11].
MATERIAL AND METHODS
The experimental protocol for all the studies conducted was approved by the Institutional Animal Care and Use Committees of Drexel University College of Medicine. Guidelines for animal experimentation were observed. Pregnant guinea pigs (Hilltop Laboratory animals, Scottsdale, PA, USA) of 60 days (term = 63 days) gestation were used. The term pregnant guinea pigs (n = 18) were divided into normoxic, hypoxic and hypoxic-treated with clonidine, an inhibitor of high affinity nuclear Ca++-ATPase. In the hypoxic group (n=6), the pregnant animals were individually allowed to breathe 7% oxygen for 60 min in a custom-designed chamber fitted with a probe to monitor oxygen tension. In the normoxic group (n=6), the pregnant animals were exposed to 21% oxygen under the same conditions. In the hypoxic treated with clonidine (Hx+Clo, n=6) group guinea pigs were administered clonidine (12.5µg/kg, I.P.) over 30 min before the induction of hypoxia. Following hypoxic or normoxic exposure, pregnant animals were anesthetized with intraperitoneal administration of pentobarbital (50 mg/kg) and a cesarean delivery was performed. Of a litter of four to six fetuses from each mother, the cerebral cortex of one fetus was removed and frozen within 4–10 seconds in liquid nitrogen for biochemical analysis and the remaining cerebral cortical tissue from fetuses of the litter was used for isolation of neuronal nuclei.
Determination of Brain tissue ATP and PCr
Tissue hypoxia was confirmed biochemically by determining the levels of high energy phosphates ATP and phosphocreatine (PCr). The concentrations of ATP and PCr in the cerebral cortex were determined by an enzyme-coupled assay. Deproteinized cortical homogenate from 500 mg of frozen cortex was ground to a powder in 7% (v/v) perchloric acid (1 ml/100 mg brain) under liquid nitrogen, allowed to thaw on ice, then centrifuged at 4000 g for 5 min. Aliquots of supernatant were neutralized with KOH–K2CO3 and centrifuged at 2000 g for 5 min. ATP and PCr concentrations were determined in a 1-ml volume containing buffer (50 mM triethanolamine, 5 mM MgCl2, 1 mM EDTA, 2 mM glucose), 400 µl of the neutralized 2000 g supernatant, and 20 µl NADP. Readings were taken every 5 min after the addition of 10 µl hexokinase until a steady state was reached. The ATP concentration was calculated from the increase in absorbance at 340 nm during the 20 min after the addition of hexokinase. A 20-µl volume of ADP and 20 µl of creatine kinase were then added and readings taken at 5-min intervals until a second steady state was reached. PCr concentration was calculated from the increase in absorbance at 340 nm after the addition of creatine kinase.
Isolation of cerebral cortical neuronal nuclei
Cerebral cortical nuclei were isolated by homogenizing 1-g of brain tissue in 15 volumes of a medium containing 0.32 M sucrose, 10 mM Tris–HCl and 1 mM MgCl2 (pH 6.8). The homogenate was filtered through a nylon bolting mesh (size 110 µm) and subsequently centrifuged at 850 g for 10 min. The nuclei were recovered through a discontinuous gradient with a final sucrose concentration of 2.1 M, which increases the yield of large neuronal nuclei. The nuclei were purified by centrifugation for 60 min at 70, 000 g. The nuclear pellet was collected, re-homogenized and used as the nuclear preparation. Purity of neuronal nuclei was assessed by phase contrast microscope. Neuronal nuclei were characterized by the presence of one nucleolus per nucleus, whereas, others have multiple nucleoli per nucleus. The final nuclear preparation was examined for purity by specific markers of subcellular fractions using western blot analysis and was found to be devoid of any microsomal, mitochondrial or plasma membrane contaminant with a purity of neuronal nuclei of 90%. The protein concentration was determined.
Determination of nuclear Ca2+-influx
Ca2+ influx was determined in 300 µl medium composed of 50 mM Tris buffer (pH 7.4) containing the neuronal nuclei (150 µg), 1 µM 45Ca2+, and 1 mM ATP. A separate set of reactions was run without ATP to determine the baseline and thus provide for the ATP-dependent Ca2+ influx. The assay was carried out at 37 °C for 2 min. The rate of nuclear Ca2+-influx is linear for 4 min, under our experimental conditions. After incubation, the samples were filtered on a glass fiber filter and washed three times in 20 mM Tris (pH 7.2), 100 mM potassium chloride buffer. The radioactivity was counted in a Rackbeta scintillation counter (Pharmacia, Gaithersburg, MD, USA). Results are expressed as pmol/mg protein/min.
Determination of CaM kinase IV activity in neuronal nuclei
CaM kinase IV activity was determined according to Park and Soderling [25] by phosphorus 33-labeled P-incorporation into syntide-2 (synthetic peptide substrate for CaM kinase) for 15 min at 37 °C in a 38 µl medium containing 50 mM HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid) (pH 7.0), 2 mM dithiothreitol (DTT), 40 µM syntide-2, 0.2 mM γ33P ATP, 10 mM magnesium acetate, 5 µM protein kinase inhibitor 5–24, 2 µM protein kinase C inhibitor peptides, 1 µM microcystine, 200 µM sodium orthovanadate, and either 1 mM EGTA (ethyleneglycol-bis-[β-aminoethylether]-N,N,N′,N′-tetra-acetic acid) (for Ca2+/CaM-independent activity) or 0.8 mM calcium chloride and 1 mM Calmodulin (for total activity). CaM kinase IV activity was expressed as pmol/mg protein/min.
Statistical analysis
Statistical analysis of biochemical measurements was performed using a one-way analysis of variance (ANOVA) and Tukey-test for comparison among groups. A P value <0.05 was considered significant. All values shown are mean ± standard deviation (S.D.).
RESULTS
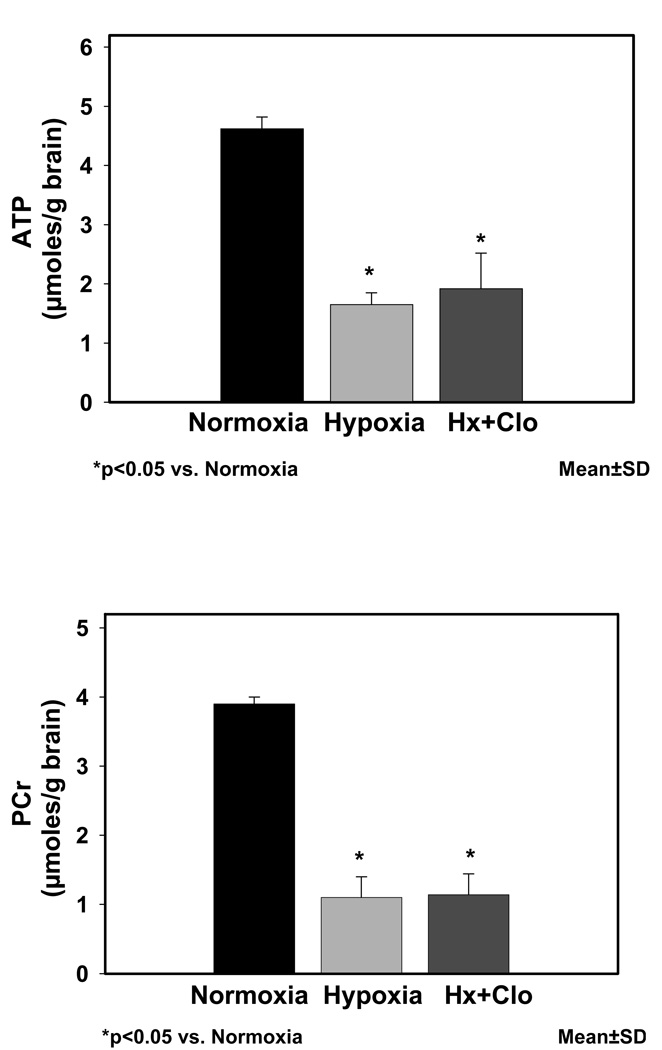
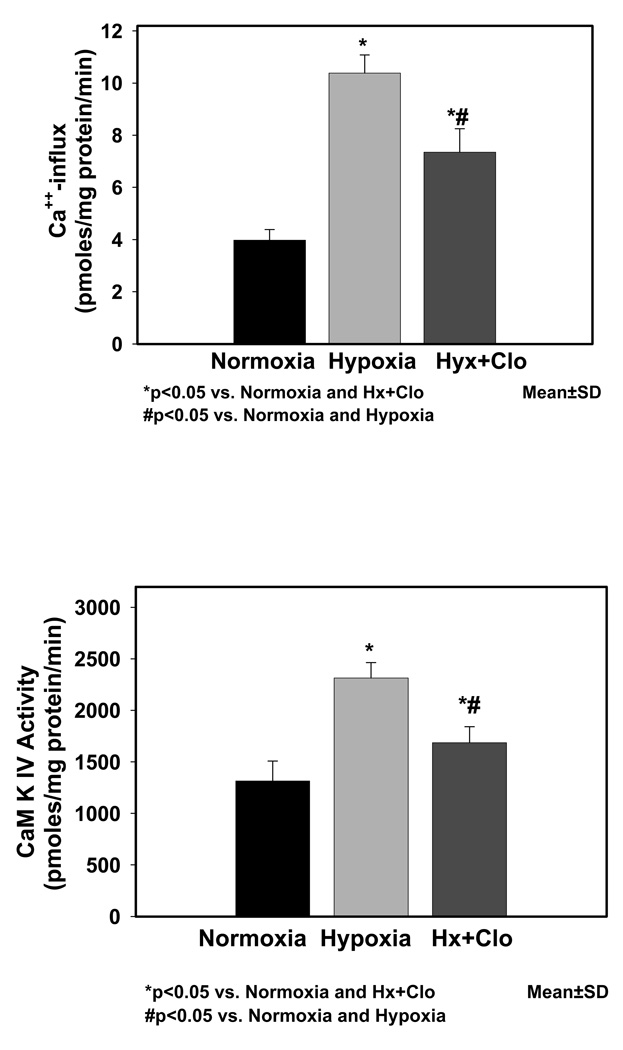
Brain tissue hypoxia in guinea pig fetuses was documented by determining the ATP and PCr levels (Fig. 1) in the cerebral cortical tissue. ATP (µmoles/g brain) values were significantly different in the Nx (4.62±0.2), Hx (1.65±0.2, p<0.05 vs. Nx), and Hx+Clo (1.92±0.6, p<0.05 vs. Nx). PCr (µmoles/g brain) values were significantly different in the Nx (3.9±0.1), Hx (1.10±0.3, p<0.05 vs. Nx), and Hx+Clo (1.14±0.3, p<0.05 vs. Nx). Results of Ca++-influx in neuronal nuclei of Nx, Hx, Hx+Clo groups are shown in Fig. 2. There was a significant difference between nuclear Ca++ influx (pmoles/mg protein/min) in Nx (3.98±0.4), Hx (10.38±0.7, p<0.05 vs. Nx), and Hx+Clo 7.35±0.9, (p<0.05vs. Nx, p<0.05 vs. Hx). CaM kinase IV activity in neuronal nuclei of Nx, Hx, Hx + Clo groups are shown in Fig. 3. Neuronal nuclear CaM kinase IV activity (pmoles/mg protein/min) was 1314.00±195.4 in Nx, 2315.14±148.5 in Hx group (p<0.05 vs. Nx), and 1686.75±154.3 in Hx+Clo group (p<0.05 vs. Nx, p <0.05 vs. Hx). In addition, we performed experiments on normoxic animals treated with clonidine and both the Ca++-influx and the CaM kinase IV activity was comparable with the normoxic control group.
Figure 1.
High energy phosphates ATP and PCr levels during normoxic, hypoxic and hypoxic pretreated with clonidine. The concentration of ATP and phosphocreatine is expressed as µmoles/g brain and shown on the Y-axis
Figure 2.
Figure 2a: Results of Ca++-influx (pmoles/mg protein/min) in neuronal nuclei of normoxic, hypoxic and hypoxic pretreated with clonidine are shown in Fig. 2a. Data is presented as mean±standard deviation.
Figure 2b: CaM kinase IV activity (pmoles/mg protein/min) in neuronal nuclei of normoxic, hypoxic and hypoxic pretreated with clonidine are shown in Fig. 2b. Data is presented as mean±standard deviation.
DISCUSSION
In previous studies we have shown that cerebral hypoxia results in increased activity of high affinity Ca++-ATPase in neuronal nuclei of the cerebral cortex of guinea pig fetuses and newborn piglets [37]. The activity of high affinity Ca++-ATPase in neuronal nuclei increased as a function increase in cerebral tissue hypoxia as measured by high energy phosphates, ATP and phosphocreatine [19]. In addition, we demonstrated that the density of IP4 and IP3 receptor also increased in neuronal nuclei following hypoxic exposure [20, 37]. Furthermore, we showed that hypoxia results in increased intranuclear Ca++-influx and the activity of nuclear CaM kinase IV as a function of cerebral tissue hypoxia [5, 13and 40]. The present study specifically focuses on testing the hypothesis that the increased nuclear Ca++-influx and the subsequent increase in CaM kinase IV activity in neuronal nuclei is due to increased high affinity Ca++-ATPase, a mechanism of nuclear Ca++-influx and therefore inhibition of high affinity Ca++-ATPase with clonidine, an inhibitor of the enzyme, will prevent the hypoxia-induced increase in nuclear Ca++ and the activity of CaM kinase IV in neuronal nuclei.
The results of the present study demonstrate that cerebral hypoxia resulted in increased Ca++-influx in neuronal nuclei of the cerebral cortex of the term guinea pig fetus. The administration clonidine decreased the hypoxia-induced increase in nuclear Ca++-influx demonstrating that high affinity Ca++-ATPase is the mechanism of increased nuclear Ca++-influx during hypoxia. The results of the present study also demonstrate that the hypoxia-induced increase in the activity of CaM kinase IV in neuronal nuclei of the cerebral cortex of the guinea pig fetus is attenuated by the administration of clonidine suggesting that the increase in CaM kinase IV activity during hypoxia is mediated by high affinity Ca++-ATPase-dependent nuclear Ca++-influx.
Previously, we have shown that hypoxia results in increased expression of pro-apoptotic proteins Bax and Bad in the nuclear, mitochondrial and the cytosolic fractions of the cerebral cortex of the guinea pigs and newborn piglets [1, 6 and 26]. However, the expression of the anti-apoptotic proteins Bcl-2 and Bcl-xl did not increase resulting in an increased ratio of proapototic/antiapoptotic proteins. The results of the present study demonstrate that the increased nuclear Ca++-influx is a key step that leads to activation of CaM kinase IV which subsequently results in activation of CREB protein and triggers the expression of apoptotic proteins. Thus the high-affinity Ca++-ATPase –dependent nuclear Ca++-influx is a potential mechanism that may regulate the expression of proapoptotic proteins in the cerebral tissue of hypoxic fetus.
In previous studies we have also shown that cerebral hypoxia results in increased activity of high affinity Ca++-ATPase, the mechanism responsible for increased nuclear Ca++-influx. We also showed that administration of a nitric oxide synthase (NOS) inhibitor, N-Nitro-L-arginine (NNLA) prior to hypoxia attenuated the hypoxia-induced increase in high affinity Ca++-ATPase activity indicating that the increase in Ca++-ATPase activity during hypoxia is mediated by nitric oxide [10]. We also showed that administration of NOS inhibitors prevent the hypoxia-induced increase in nuclear Ca++-influx [22]. In addition, NO donors increased the nuclear Ca++-influx. These studies demonstrated that the increase in nuclear Ca++-influx during hypoxia is NO-mediated [22]. We propose that the NO-mediated alteration in high affinity Ca++-ATPase is the mechanism of hypoxia-induced increase in nuclear Ca++-influx in neuronal nuclei of the cerebral cortex of the guinea pig fetus at term.
We have investigated the effect of hypoxia in-utero on Ca++-influx in neuronal nuclei of the preterm and term guinea pig fetuses [16]. The data showed that hypoxia results in increased nuclear Ca++-influx at both the gestational ages, however, the effect of hypoxia on nuclear Ca++ -influx was higher in term fetus indicating that the mechanisms of Ca++-influx are more susceptible to hypoxia in the guinea pig fetus at term [16]. We have also demonstrated that hypoxia in utero results in increased activity of high affinity Ca++-ATPase in neuronal nuclei of the guinea pig fetus [37] and increased Ca++-influx measured fluorometrically with Fura-2 [38]. Therefore, a pretreatment with a high affinity Ca++-ATPase inhibitor, such as clonidine, could be a potential strategy for preventing hypoxia-induced cascade of cell death in the term fetus. We have also shown that cerebral hypoxia in-utero resulted in increased activity of CaM kinase IV in neuronal nuclei of the cerebral cortex of preterm as well as term guinea pig fetuses. Furthermore, we have shown that cerebral hypoxia in- utero results in increased expression of proapoptotic proteins Bax and Bad at both the gestational ages, however, the effect of hypoxia on the expression of pro-apoptotic proteins was higher in the term fetus [1].
These above mentioned studies along with our earlier work investigating the effect of cerebral hypoxia in-utero on lipid peroxidation, the activity of Na+, K+-ATPase, modification of N-methyl-D-aspartate (NMDA) receptor ion channel and its modulatory sites in the developing brain during gestation [18], demonstrate increased susceptibility of the fetus brain at term. Since cerebral hypoxia in utero results in increased nuclear Ca++-influx accompanied with increased CaM kinase IV activity and increased expression of proapoptotic proteins Bax and Bad, blockade of nuclear Ca++-influx would be a potential strategy for preventing transcription-dependent mechanism of neuronal death in the guinea pig fetus.
In summary, the results of the present study demonstrated that cerebral hypoxia resulted in increased Ca++-influx and increased CaM kinase IV activity in neuronal nuclei of the cerebral cortex of the guinea pig fetus at term and the administration of clonidine, an inhibitor of high affinity Ca++-ATPase, prevented the hypoxia-induced increase in nuclear Ca++-influx and CaM kinase IV activity. We conclude that the increased nuclear Ca++-influx and increased CaM kinase IV activity in neuronal nuclei of the cerebral cortex are high affinity Ca++-ATPase –dependent. We propose that the increased CaM kinase IV activity would lead to increased activation of CREB protein during hypoxia and blockade by clonidine would prevent the subsequent increased expression of proapototic proteins that result in activation of caspase-cascade of programmed cell death in the brain of the hypoxic fetus at term.
Acknowledgement
This study was supported by grant from the National Institutes of Health, NIH-HD20337. The authors express their thanks to Ms. Anli Zhu for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abedin N, Ashraf Q, Mishra OP, Delivoria-Papadopoulos M. Effect of hypoxia on the expression of pro- and anti-apoptotic proteins in neuronal nuclei of the guinea pig fetus during gestation. Brain Res Dev Brain Res. 2005;156:32–37. doi: 10.1016/j.devbrainres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling–an overview. Semin Cell Dev Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- 3.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 4.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca++ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 5.Delivoria-Papadopoulos M, Akhter W, Mishra OP. Hypoxia-induced Ca2+-influx in cerebral cortical neuronal nuclei of newborn piglets. Neurosci Lett. 2003;342:119–123. doi: 10.1016/s0304-3940(03)00256-8. [DOI] [PubMed] [Google Scholar]
- 6.Delivoria-Papadopoulos M, Ashraf QM, Mishra OP. Effect of Hypoxia on Expression of Apoptotic Proteins in Nuclear, Mitochondrial and Cytosolic Fractions of the Cerebral Cortex of Newborn Piglets: The Role of Nuclear Ca++-influx. Neurochem Res. 2008 doi: 10.1007/s11064-007-9568-6. [DOI] [PubMed] [Google Scholar]
- 7.Denton RM, McCormack JG. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980;119:1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- 8.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 9.Galeotti N, Bartolini A, Ghelardini C. Alpha-2 agonists induce amnesia through activation of the Gi-protein signalling pathway. Neuroscience. 2004;126:451–460. doi: 10.1016/j.neuroscience.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Gavini G, Zanelli SA, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. Effect of nitric oxide synthase inhibition on high affinity Ca++-ATPase during hypoxia in cerebral cortical neuronal nuclei of newborn piglets. Brain Res. 2000;887:385–390. doi: 10.1016/s0006-8993(00)03069-9. [DOI] [PubMed] [Google Scholar]
- 11.Gorini A, Villa RF. Effect of in vivo treatment of clonidine on ATP-ase's enzyme systems of synaptic plasma membranes from rat cerebral cortex. Neurochem Res. 2001;26:821–827. doi: 10.1023/a:1011616219687. [DOI] [PubMed] [Google Scholar]
- 12.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 13.Hornick K, Chang E, Zubrow AB, Mishra OP, Delivoria-Papadopoulos M. Mechanism of Ca++/calmodulin-dependent protein kinase IV activation and of cyclic AMP response element binding protein phosphorylation during hypoxia in the cerebral cortex of newborn piglets. Brain Res. 2007;1150:40–45. doi: 10.1016/j.brainres.2007.02.079. [DOI] [PubMed] [Google Scholar]
- 14.Huwyler J, Fricker G, Torok M, Schneider M, Drewe J. Transport of clonidine across cultured brain microvessel endothelial cells. J Pharmacol Exp Ther. 1997;282:81–85. [PubMed] [Google Scholar]
- 15.Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maulik D, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. Effect of hypoxia in calcium influx and calcium/calmodulin-dependent kinase activity in neuronal nuclei of the guinea pig during development. Am J Obst. Gynecol. 2002;186:658–662. doi: 10.1067/mob.2002.122392. [DOI] [PubMed] [Google Scholar]
- 17.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332(Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra OP, Ashraf QM, Delivoria-Papadopoulos M. Modification of modulatory sites of NMDA receptor in the fetal guinea pig brain during development. Neurochem. Res. 1992;17:1223–1228. doi: 10.1007/BF00968404. [DOI] [PubMed] [Google Scholar]
- 19.Mishra OP, Delivoria-Papadopoulos M. Effect of graded hypoxia on high-affinity Ca Ca++-ATPase activity in cortical neuronal nuclei of newborn piglets. Neurochem Res. 2001;26:1335–1341. doi: 10.1023/a:1014205702905. [DOI] [PubMed] [Google Scholar]
- 20.Mishra OP, Delivoria-Papadopoulos M. Inositol tetrakisphosphate (IP4)- and inositol triphosphate (IP3)-dependent Ca++-influx in cortical neuronal nuclei of newborn piglets following graded hypoxia. Neurochem Res. 2004;29:391–396. doi: 10.1023/b:nere.0000013742.19074.7e. [DOI] [PubMed] [Google Scholar]
- 21.Mishra OP, Delivoria-Papadopoulos M. Nitric oxide-mediated Ca++-influx in neuronal nuclei and cortical synaptosomes of normoxic and hypoxic newborn piglets. Neurosci Lett. 2002;318:93–97. doi: 10.1016/s0304-3940(01)02484-3. [DOI] [PubMed] [Google Scholar]
- 22.Mishra OP, Zubrow AB, Ashraf QM, Delivoria-Papadopoulos M. Nuclear Ca++-influx, Ca++/calmodulin-dependent protein kinase IV activity and CREB protein phosphorylation during post-hypoxic reoxygenation in neuronal nuclei of newborn piglets: the role of nitric oxide. Neurochem Res. 2006;31:1463–1471. doi: 10.1007/s11064-006-9204-x. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y, Okuno S, Sato F, Fujisawa H. An immunohistochemical study of Ca++/calmodulin-dependent protein kinase IV in the rat central nervous system: light and electron microscopic observations. Neuroscience. 1995;68:181–194. doi: 10.1016/0306-4522(95)00092-w. [DOI] [PubMed] [Google Scholar]
- 24.Ohta Y, Ohba T, Miyamoto E. Ca++/calmodulin-dependent protein kinase II: localization in the interphase nucleus and the mitotic apparatus of mammalian cells. Proc Natl Acad Sci U S A. 1990;87:5341–5345. doi: 10.1073/pnas.87.14.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park IK, Soderling TR. Activation of Ca++/calmodulin-dependent protein kinase (CaM-kinase) IV by CaM-kinase kinase in Jurkat T lymphocytes. J Biol Chem. 1995;270:30464–30469. doi: 10.1074/jbc.270.51.30464. [DOI] [PubMed] [Google Scholar]
- 26.Ravishankar S, Ashraf QM, Fritz K, Mishra OP, Delivoria-Papadopoulos M. Expression of Bax and Bcl-2 proteins during hypoxia in cerebral cortical neuronal nuclei of newborn piglets: effect of administration of magnesium sulfate. Brain Res. 2001;901:23–29. doi: 10.1016/s0006-8993(01)02109-6. [DOI] [PubMed] [Google Scholar]
- 27.Sahyoun N, LeVine H, 3rd, Bronson D, Cuatrecasas P. Ca++-calmodulin-dependent protein kinase in neuronal nuclei. J Biol Chem. 1984;259:9341–9344. [PubMed] [Google Scholar]
- 28.Sahyoun N, LeVine H., 3rd Cuatrecasas, Ca++/calmodulin-dependent protein kinases from the neuronal nuclear matrix and post-synaptic density are structurally related. Proc Natl Acad Sci U S A. 1984;81:4311–4315. doi: 10.1073/pnas.81.14.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santella L, Carafoli E. Calcium signaling in the cell nucleus. FASEB J. 1997;11:1091–1109. [PubMed] [Google Scholar]
- 30.Sikorska M, MacManus JP, Walker PR, Whitfield JF. The protein kinases of rat liver nuclei. Biochem Biophys Res Commun. 1980;93:1196–1203. doi: 10.1016/0006-291x(80)90616-6. [DOI] [PubMed] [Google Scholar]
- 31.Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasan M, Edman CF, Schulman H. Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J Cell Biol. 1994;126:839–852. doi: 10.1083/jcb.126.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmons SD, Geisert E, Stewart AE, Lorenzon NM, Foehring RC. alpha2-Adrenergic receptor-mediated modulation of calcium current in neocortical pyramidal neurons. Brain Res. 2004;1014:184–196. doi: 10.1016/j.brainres.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Wakim BT, Picken MM, DeLange RJ. Identification and partial purification of a chromatin bound calmodulin activated histone 3 kinase from calf thymus. Biochem Biophys Res Commun. 1990;171:84–90. doi: 10.1016/0006-291x(90)91359-z. [DOI] [PubMed] [Google Scholar]
- 35.Wang WZ, Yuan WJ, Pan YX, Tang CS, Su DF. Interaction between clonidine and N-methyl-D-aspartate receptors in the caudal ventrolateral medulla of rats. Exp Brain Res. 2004;158:259–264. doi: 10.1007/s00221-004-1902-5. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe S, Okuno S, Kitani T, Fujisawa H. Inactivation of calmodulin-dependent protein kinase IV by autophosphorylation of serine 332 within the putative calmodulin-binding domain. J Biol Chem. 1996;271:6903–6910. doi: 10.1074/jbc.271.12.6903. [DOI] [PubMed] [Google Scholar]
- 37.Zanelli SA, Spandou E, Mishra OP, Delivoria-Papadopoulos M. Hypoxia modified nuclear calcium uptake pathways in the cerebral cortex of the guinea-pig fetus. Neurosci. 2005;130:949–955. doi: 10.1016/j.neuroscience.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Zanelli SA, Numagami Y, McGowan JE, Mishra OP, Delivoria-Papadopoulos M. NMDA receptor-mediated calcium influx in cerebral cortical synaptosomes of the hypoxic guinea pig fetus. Neurochem Res. 1999;24:437–446. doi: 10.1023/a:1020950019986. [DOI] [PubMed] [Google Scholar]
- 39.Zubrow AB, Delivoria-Papadopoulos M, Ashraf QM, Fritz KI, Mishra OP. Nitric oxide-mediated Ca++/calmodulin-dependent protein kinase IV activity during hypoxia in neuronal nuclei from newborn piglets. Neurosci Lett. 2002;335:5–8. doi: 10.1016/s0304-3940(02)01138-2. [DOI] [PubMed] [Google Scholar]
- 40.Zubrow AB, Delivoria-Papadopoulos M, Fritz KI, Mishra OP. Effect of neuronal nitric oxide synthase inhibition on Ca++/calmodulin kinase kinase and Ca++/calmodulin kinase IV activity during hypoxia in cortical nuclei of newborn piglets. Neuroscience. 2004;125:937–945. doi: 10.1016/j.neuroscience.2004.02.027. [DOI] [PubMed] [Google Scholar]