Abstract
Up to date, the nature of the sepsis-induced vascular leakage is understood only partially, which limits pharmacological approaches for its management. Here we studied the protective effect of cAMP using endotoxin-induced hyperpermeability as a model for barrier dysfunction observed in gram-negative sepsis. We demonstrated that the alleviation of LPS-induced barrier compromise could be achieved by the specific activation of either protein kinase A (PKA) or Epac with cAMP analogues Bnz-cAMP or O-Me-cAMP, respectively. We next studied the involvement of PKA substrates VASP and filamin1 in barrier maintenance and LPS-induced barrier compromise. Depletion of both VASP and filamin1 with the specific siRNAs significantly exacerbated both the quiescent cells barrier and LPS-induced barrier dysfunction, suggesting barrier-protective role of these proteins. VASP depletion was associated with the more severe loss of ZO-1 peripheral staining in response to LPS, whereas filamin1-depleted cells reacted to LPS with more robust stress fiber induction and more profound changes in ZO-1 and VE-cadherin peripheral organization. Both VASP and filamin1 phosphorylation was significantly increased as a result of PKA activation. We next analyzed the effect of VASP and filamin1 depletion on the PKA-dependent alleviation of LPS-induced barrier compromise. We observed that Bnz-cAMP ability to counteract LPS-induced hyperpermeability was attenuated only by VASP, but not filamin1 depletion. Our data indicate that while PKA-dependent VASP phosphorylation contributes to the protective effect of cAMP elicited on LPS-compromised monolayers, filamin1 phosphorylation is unlikely to play a significant role in this process.
Keywords: Epac, PKA, VASP, filamin, LPS, barrier dysfunction
Introduction
Lipolysaccharide (LPS), or endotoxin, is an essential component of the cell wall of Gram-negative bacteria. The exposure to LPS results in the activation of a number of inflammatory pathways and therefore is often used as valuable model to study pathological processes associated with Gram-negative sepsis. Left untreated, these pathological processes can lead to the development of several morbid complications, including an acute lung injury (ALI) or its more severe form, adult respiratory distress syndrome (ARDS) (Jacobson and Garcia, 2007; Opal, 2007; Ware and Matthay, 2000). ALI and ARDS are characterized by the increased permeability of endothelial and epithelial barriers, leading to the accumulation of inflammatory cells and fluids in the lungs. The latter event is responsible for the inability of lung to maintain sufficient blood oxygenation and therefore could be considered a leading cause of ARDS-associated death.
Microvascular endothelial cells (EC) play a central role in the control of exchange of cells and fluids between blood vessel lumens and underlying lung tissue. Microvascular EC are known to respond to LPS with the loss of barrier function (Gong et al., 2008; Kolosova et al., 2008; Tiruppathi et al., 2008), aimed to facilitate the access of white blood cells to the infected tissue, but also responsible for the development of the lung edema. LPS-induced microvascular hyperpermeability was recently shown to be coupled to the activation of Src family kinases and phosphorylation of zonula adherence proteins (Gong et al., 2008). On the other hand, augmentation of LPS-induced barrier dysfunction by ATP was linked to the activation of protein kinase A (PKA), myosin phosphatase and Rac (Jacobson et al., 2006; Kolosova et al., 2005; Kolosova et al., 2008).
Activation of PKA by cAMP is generally known to be endothelial barrier protective. However, recent data show that depending on both the localization of the intracellular source of cAMP and the origin of endothelial cells used in study the effect could be either barrier protective or disruptive (Bindewald et al., 2004; Prasain et al., 2009; Sayner et al., 2006; Sayner et al., 2004). When different cAMP-elevating agents were employed to reduce LPS-induced vascular leakage in animal models (Irie et al., 2001; Lundblad et al., 2004; Miotla et al., 1998), the effect varied depending on the nature of the animal. In LPS-challenged mice, cAMP elevation significantly suppressed vascular leakage (Irie et al., 2001; Miotla et al., 1998), whereas treatment of LPS-challenged cats failed to demonstrate the protective effect of cAMP (Lundblad et al., 2004). The detailed analysis of the cAMP effect on LPS-challenged human pulmonary endothelium will help to better assess the potential therapeutic value of cAMP elevation.
The prospective mechanism by which cAMP opposes barrier dysfunction is likely to include the activation of both protein kinase A (PKA) and a novel cAMP effector Epac. Epac is a cAMP-activated guanine nucleotide exchange factor for Rap GTPases, which was recently shown to regulate integrity of endothelial junctions, state of actin cytoskeleton and stability of microtubules in EC (Kooistra et al., 2005; Sehrawat et al., 2008). Our knowledge about pivotal cAMP-dependent cellular processes depending on Epac had grown tremendously over the past decade. Here, we assessed the ability of Epac activation to attenuate LPS-induced barrier dysfunction in human pulmonary microvascular EC.
Vasodilator-stimulated protein VASP, a likely substrate for PKA, is known to associate with focal adhesions, tight and adherence junctions and regulate transendothelial permeability. The observation that VASP phosphorylation increases VASP interaction with junctional components (Comerford et al., 2002) let one hypothesize that VASP phosphorylation plays certain role in barrier regulation. However, lately this hypothesis was confronted by the results of several studies. Although it was clearly shown that VASP is required for the maintenance of barrier function under resting conditions, it does not appear to be essential for cAMP/cGMP-dependent enhancement of barrier function (Rentsendorj et al., 2008; Schlegel et al., 2008). Based on these data, the analysis of VASP and VASP phosphorylation involvement in barrier changes in response to LPS seems to be particularly interesting.
Another PKA substrate, filamin1 or filamin A, is known to associate with actin filaments and organize them into three-dimensional lattices (Popowicz et al., 2006). Filamin was shown to be essential for the mammalian cell locomotion, anchoring of transmembrane proteins, andgeneration of interfaces for protein-protein interaction. Filamin translocation from membrane to cytosol fraction precedes actin redistribution and increased gap formation, making this protein a plausible candidate for the key regulator of endothelial permeability (Hastie et al., 1997; Wang et al., 1997). The latest studies implicate filamin in the cellular signaling via its ability to recruit and inactivate certain membrane-associated regulatory proteins (p190RhoGAP) (Mammoto et al., 2007) or via the ability of the cleaved filamin fragment to migrate to the nuclei and bind certain nuclear receptors (Bedolla et al., 2009). Cleavage of filamin, switching it location and function from cytosol/cytoskeleton to nuclei, seems to be suppressed by filamin phosphorylation at Ser 2152 (Bedolla et al., 2009; Garcia et al., 2006). Several protein kinases, including PKA, are responsible for filamin phosphorylation at this residue (Jay et al., 2000). In light of these data, the analysis of the role of filamin and filamin phosphorylation in LPS-induced vascular hyperpermeability may provide valuable information pertinent to the management of this pathological condition.
In the current study, we evaluated the effect of cAMP elevation on LPS-induced endothelial hyperpermeability and analyzed downstream pathways linking cAMP elevation to the barrier enhancement. We found that cAMP elevation is capable of improving LPS-compromised barrier in human microvascular EC, and that the barrier-protective effect could be achieved by the specific activation of either PKA-dependent or Epac-dependent mechanisms. The analysis of the role of PKA substrates VASP and filamin1 demonstrated that whereas both VASP and filamin1 are critically involved in barrier maintenance, only VASP phosphorylation by PKA has the potential to impact barrier-protective effect of cAMP on LPS-compromised monolayer.
Materials and Methods
Reagents
LPS from E.coli (0127:B8, 900 000 u/mg), forskolin and isobutylmethylxantine (IBMX) were from Sigma. N6-Benzoyl-cAMP (bnz-cAMP) and 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (O-Me-cAMP) were obtained from Biolog Life Science Institute (Bremen, Germany). λ protein phosphatase and buffer for phosphatase reaction were purchased from New England Biolabs (Ipswich, MA).
Phospho-filamin and GAPDH antibodies were from Abcam (Cambridge, MA). VE-cadherin antibodies were from Cayman Chemical (Ann Arbor, MI). ZO-1 antibodies, as well as all reagents used for immunofluorescent staining, were obtained from Invitrogen (Carlsbad, CA). VASP antibody was from Calbiochem ((La Jolla, CA). Beta-actin antibody was from Sigma.
VASP-specific and filamin1-specific siRNA were from Santa Cruz Biotechnologies (Santa Cruz, CA). Non-specific control siRNA-1 was from Ambion (Austin, TX).
Cell culture
Human lung microvascular endothelial cells (HLMVEC) were purchased from Lonza (Walkersville, MD) and used at passages 6–7. They were cultured in media containing 5% FBS and maintained at 37°C in a humidified atmosphere of 5%CO2-95% air.
Measurement of transendothelial permeability
Transendothelial electrical resistance (TER) was measured using the highly sensitive biophysical assay with an electrical cell-substrate impedance sensor (Applied biophysics, Troy, NY) as described previously (Verin et al., 2001). Cells were grown to confluence on gold electrodes (resistance approximately 2000 Ohm). Media was changed to the fresh complete media 1 hour prior the experiment.
EC imaging
For immunofluorescence experiments, EC monolayers were plated on gelatin-covered coverslips. Media was changed to the fresh complete media 1 hour prior the experiment. Before immunostaining, cells were briefly washed with phosphate-buffered saline (PBS) and fixed in PBS solution of 4% formaldehyde. After permeabilization with 0.25% Triton X-100 and blocking, cells were stained with specific antibodies, fluorescent secondary antibodies and phalloidin conjugated with Alexa594 or Alexa350. After mounting in anti-fade mounting media, the coverslips were viewed and photographed with Zeiss Axio Observer video imaging system using Zeiss Axiovision software. To analyze the area of gaps, images taken with 40X objective (25–30 cells per microscope field) were processed with image J software (National Institute of Health, Bethesda, MD) to outline the borders of the cells and to compute the un-occupied area.
Western immunoblotting
Cells were grown in 12-well or 6-well plates; media was changed to the fresh complete media 1 hour prior the experiment. After stimulation, cells were rinsed with ice-cold PBS and lysed with PBS containing 1% SDS and 20mM NaF. After freezing-thawing and aspiration through 25g needle, samples were supplemented with Western blot loading buffer and boiled. Protein extracts were separated on 10% gel (for VASP and phospho-VASP separation) or 4–20% gels and transferred to nitrocellulose membrane. After staining with specific antibodies, membranes were developed and scanned using Kodak MI imaging system. Intensity of the specific bands was assessed in comparison to the intensity of GAPDH or beta-actin bands.
Depletion of endogenous VASP and filamin in EC
To reduce the expression of endogenous proteins, HLMVEC plated in 12-well plates (with or without coverslips) or in ECIS chambers were treated with specific siRNA or non-specific non-silencing siRNA. Transfection with 50 nM siRNA was performed using DharmaFECT1 transfection reagent (Dharmacon Research, Lafayette, CO) when cells reached 60% confluence. Cells were used for the described above experiments 48 h post-transfection.
Statistical analysis
Statistical analysis was performed on the data pooled from parallel experiments using one-way ANOVA test; results with p<0.05 were considered significantly different.
Results
LPS compromises the integrity of HLMVEC monolayer and affects barrier function
We have demonstrated earlier that 100 ng/ml LPS causes significant and sustained decrease in TER, indicative of the increased permeability of EC monolayer for water and ions (Kolosova et al., 2008). In this study, we challenged confluent HLMVEC monolayers with different doses of LPS to determine the range of LPS concentration leading to barrier dysfunction. The minimal LPS concentration used in this experiment (0.2ng/ml) was able to significantly decrease TER compared to the media control. The maximal TER decrease was seen at LPS concentrations equal and higher than 53 ng/ml (Fig. 1A). In parallel with TER measurements, we analyzed the formation of gaps in monolayers grown on the glass coverslips. Both methods revealed that the response to LPS develops gradually over the course of several hours and does not achieve its maximum until 6 hours post-LPS addition (Fig. 1).
Figure 1.
Immunocytochemical approach let us detect that observed changes in barrier integrity were simultaneous with the changes in junctional and contractile protein organization. Immunostaining with fluorescent phalloidin revealed that LPS induced initial moderate stress fiber formation, which was followed by the loss of the peripheral F-actin staining and the retraction of the cell mass toward the center. In quiescent cells, the staining for both adherence junction protein VE-cadherin and tight junction protein ZO-1 rendered strong cell border signal which appeared as a continous line of various thickness. LPS treatment initially led to the distortion of VE-cadherin/ZO-1 linear border pattern and eventually resulted in the emergence of areas with the visible reduction of VE-cadherin/ZO-1 periferal staining (Fig. 2). These areas of the deficient staining usually surrounded the gaps formed in the initially integral monolayer (indicated by arrows).
Figure 2.
cAMP elevation attenuates LPS-induced decrease in TER and gap formation
We had previously shown that LPS-induced barrier disruption can be attenuated by ATP, which is known to act, among other pathways, via the activation of PKA and myosin phosphatase (Kolosova et al., 2005; Kolosova et al., 2008). Here we determine if the elevation of PKA activator cAMP could affect LPS-induced hyperpermeability. As shown on Fig. 3A, pretreatment with either adenylate cyclase activator forskolin or phosphodyesterase inhibitor IBMX significantly decreased LPS-induced drop in TER. These results let us suggest that pathways depending on cAMP elevation do counteract barrier-disruptive pathways involved in the response of HLMVEC to LPS.
Figure 3.
We next attempted to identify these pathways. Since cellular cAMP sensors are known to include proteins other than PKA (Cheng et al., 2008), we employed cell-permeable cAMP analogues, specifically inducing either PKA or PKA-independent cAMP effector Epac (Stafford and Marnett, 2008). Fig. 3B, C shows that both PKA-specific activator Bnz-cAMP and Epac-specific activator O-Me-cAMP attenuate LPS-induced EC hyperepermeability and gap formation in EC monolayer. Combination of these compounds has additive effect on the improvement of barrier in LPS-treated EC (Fig. 3B). As was expected, only PKA activator Bnz-cAMP causes shift in VASP mobility due to the increase in PKA-dependent phosphorylation of VASP (Fig. 3D). These changes in mobility are similar to the changes induced by the adenylate cyclase activator forskolin. The band with the decreased mobility is specific to phospho-VASP, as incubation of forskolin-pretreated samples with λ protein phosphatase leads to the disappearance of this band (Fig 3E). Dissimilar to forskolin and Bnz-cAMP-induced changes, Epac-specific activator O-Me-cAMP does not cause VASP mobility shift (Fig 3D), indicative that this analogue elicits its effects via PKA-independent mechanism.
The role of VASP in forskolin-mediated suppression of LPS-induced hyperpermeability
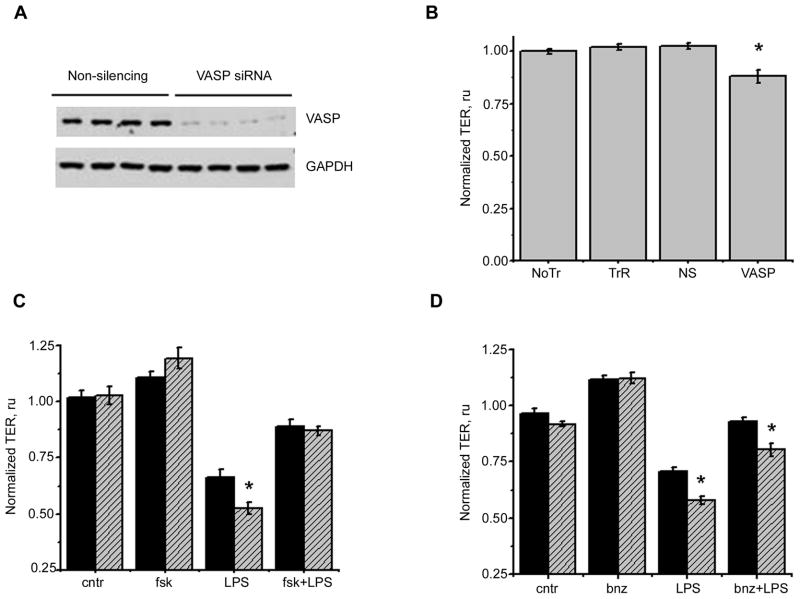
Continuing our search for the molecular pathway responsible for the protective effect of cAMP, we next analyzed the role of a known PKA substrate, vasodilator-stimulated phosphoprotein VASP, in HLMVEC barrier function. Pretreatment of HLMVEC with small interfering RNA (siRNA) resulted in the significant suppression of VASP expression (Fig. 4A). Analysis of the basal resistance in VASP-depleted cells revealed that these cells are characterized by a poorer barrier (Fig. 4B). Importantly, VASP-depleted cells demonstrated significantly exacerbated response to LPS (Fig. 4C, D). Analysis of the TER drop revealed that VASP depletion resulted in more rapid and robust TER decline in response to LPS, with the difference between VASP-depleted and control cells response being most obvious 6h post-LPS addition.
Figure 4.
To clarify the cytoskeletal events leading to HLMVEC hyperpermeability, we next analyzed the distribution of the tight junctional component ZO-1 in VASP-depleted cells responding to LPS. ZO-1 is known to interact with VASP (Comerford et al., 2002), and therefore seems to be a likely molecular link between the changes in VASP expression and increased endothelial permeability. Fig 5 shows that in control cells, LPS treatment leads to a partial loss of ZO-1 cell border signal concomitant with the transition of the border staining pattern from a straight to a zigzagged line. VASP depletion induces somewhat similar changes in ZO-1 distribution in the absence of LPS (note the appearance of areas with zigzagged pattern). In the presence of LPS, ZO-1 staining in VASP-depleted cells shows even more rigorous disturbance in the border pattern revealing sites with the total loss of ZO-1 border signal (indicated by arrows). Analysis of the F-actin rearrangement in VASP-depleted cells reveals greater loss of the peripheral actin, and, as a consequence, more prominent retraction of the cell mass toward the cell center. These findings let us speculate that VASP depletion distorts the ability of ZO-1 to associate with membrane structures, and, in particular, with peripheral actin, which, in turn, results in the more severe barrier compromise.
Figure 5.
To assess how VASP phosphorylation by PKA contributes to the cAMP-mediated attenuation of the response to LPS, we first analyzed how the level of VASP phosphorylation changes with cAMP elevation. We detected minimal phospho-VASP content in the quiescent cells; in contrast, both forskolin and bnz-cAMP pretreatment increased phospho-VASP level dramatically (Fig 6A). VASP/phospoVASP ratio did not change significantly upon LPS stimulation in either control or forskolin/bnz-cAMP-pretreated cells. We next analyzed intracellular redistribution of VASP in response to LPS. In quiescent cells, VASP signal co-localizes with the cell border, also showing parallel co-localization with some tubular intracellular structures (Fig 6B). LPS stimulation changes VASP localization dramatically, leading to the virtual disappearance of the cell border staining and appearance of the dotted staining reminiscent of the focal adhesion pattern. These changes coincide with the induction of stress fibers in LPS-treated cells. Pretreatment with forskolin and Bnz-cAMP blocks VASP redistribution, concomitant with the marked suppression of stress fiber induction.
Figure 6.
To ascertain that the blockade of VASP rearrangement by cAMP is causative to the improvement of the barrier in LPS-treated cells, we analyzed how the barrier protective effect of forskolin or Bnz-cAMP is affected by VASP depletion. To our surprise, forskolin ability to effectively prevent LPS-induced drop in TER was equal in VASP-depleted cells and in negative control-treated cells (Fig. 4C). In contrast, Bnz-cAMP ability to alleviate LPS-induced compromise was reduced in cells lacking VASP expression (Fig. 4D). Our data indicate that, although not being a major player, VASP phosphorylation by PKA certainly contributes to the barrier protective effect of cAMP. Another conclusion from the above results is that in order to reveal the causative role of VASP phosphorylation in barrier protection, one has to restrict activated pathways to the PKA cascade rather than analyze the events following activation of all cAMP-dependent effectors.
The role of filamin in forskolin-mediated suppression of LPS-induced hyperpermeability
We next analyzed the involvement of another PKA target, filamin1, in the regulation of HLMVEC barrier. Depletion of filamin1 expression by siRNA (Fig. 7A) resulted in the significant impairment of the basal resistance (Fig 7B). Similar to VASP-deficient cells, filamin1-deficient EC responded to LPS more readily than control siRNA-treated cells (Fig 7C, D).
Figure 7.
Filamin1, or filamin A, is an actin-cross-linking protein which contributes to the anchoring of actin cytoskeleton to the membrane proteins. To analyze how filamin1 depletion is linked to the barrier compromise, we assessed the distribution of actin, VE-cadherin and ZO-1 in control and filamin1 siRNA-treated cells (Fig. 8). We observed that in quiescent cells, filamin1 depletion causes changes in all three protein distribution. These changes are similar to the changes induced by LPS treatment, and include the distortion of VE-cadherin and ZO-1 border pattern and the induction of stress fiber formation. LPS-treated filamin1-deficient HLMVEC respond to LPS with significantly more robust stress fiber induction and somewhat increased thinning of VE-cadherin and ZO-1 peripheral staining. The distribution of F-actin inside these cells is shifted toward the formation of cell-crossing fibers versus membrane-underlying fibers. These findings let us speculate that LPS induction of filamin1-depleted cells results in unopposed polymerization of actin in non-membrane-associated areas, which, in turn, leads to the increased contractility and barrier compromise.
Figure 8.
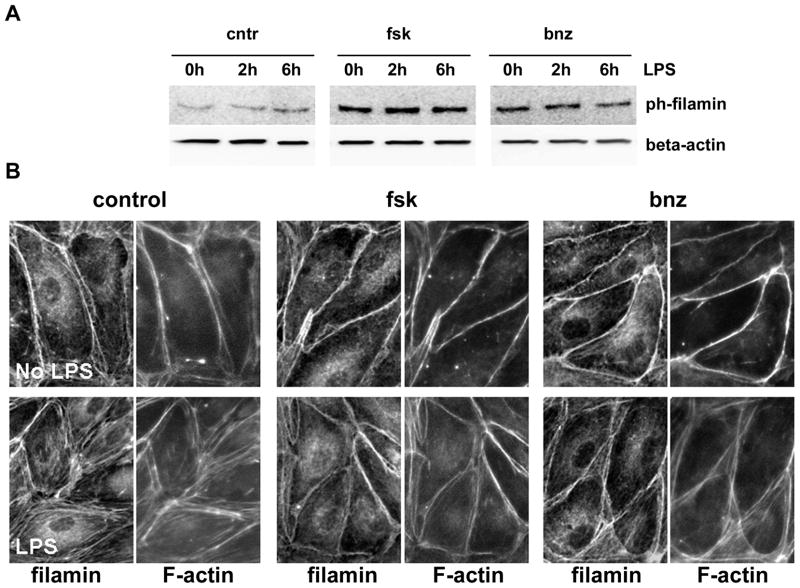
To evaluate the possible role of filamin1 phosphorylation in cAMP-mediated effects, we first assessed changes in the level of filamin phosphorylation in response to cAMP (Fig 9A). Filamin is constitutively phosphorylated at Ser 2152 in quiescent cells; however, the level of phosphorylation increases dramatically with forskolin/Bnz-cAMP pretreatment. Similar to VASP phosphorylation, we failed to observe significant changes in phospho-filamin level in response to LPS. We next analyzed filamin re-distribution in HLMVEC responding to LPS (Fig 9B). We observed that, aside from a portion which generates a diffuse cytoplasmic staining, filamin1 signal strongly colocalizes with F-actin in HLMVEC. In quiescent cells, filamin staining highlights the peripheral actin area, whereas in LPS-challenegd EC filamin signal underlines the induced stress fibers. Forskolin and Bnz-cAMP pretreatment blocks both stress fiber induction and redistribution of filamin from the peripheral area to the stress fibers.
Figure 9.
To assess whether the observed blockade of filamin redistribution contributes to the barrier protective effect of cAMP or simply is a consequence of the blocked stress fiber formation, we compared forskolin and Bnz-cAMP-induced barrier improvement in the control and filamin1-deficient cells. Analyzis of the whole time course of the response to LPS showed that, in contrast to the observed earlier effect of VASP depletion on barrier improvement, filamin1 depletion fails to affect forskolin or Bnz-cAMP-mediated rescue of the barrier (Fig 7C,D). These data show that, unlike PKA-dependent phosphorylation of VASP, filamin1 phosphorylation does not contribute significantly to the alleviation of barrier compromise by cAMP-elevating agents.
Discussion
During gram-negative sepsis, the normal physiological functions of endothelium are compromised, contributing to the sepsis-induced organ failure. Although there is a major debate about how much of the observed vascular dysfunction is due to the direct effect of LPS on endothelium and how much is induced by the release of proinflammatory mediators from macrophages and immune cells (Dauphinee and Karsan, 2006; Opal, 2007), the analysis of the direct effect of LPS is still necessary for the detailed understanding of the pathophysiology of sepsis. Up to date, the amount of studies analyzing mechanisms of LPS-induced endothelial barrier dysfunction is rather limited and offers only fragmented knowledge. Recently, it was shown that LPS in the range of 3–10000 ng/ml dose-dependently increases transendothelial albumin flux across monolayer (Gong et al., 2008). The measurement of TER undertaken here demonstrated that even lower LPS doses (hundreds pg/ml) induce HLMVEC hyperpermeability (Fig. 1). With latest studies placing LPS levels in septic patients in the range of 0.2 – 1 ng/ml (Buttenschoen et al., 2008; Marshall et al., 2002; Stief et al., 2007), our data suggest that hyperpermeability induced by the direct effect of LPS on endothelium definitely contributes to the barrier dysfunction seen in gram-negative sepsis.
Our experiments show that, similar to other edemagenic factors, LPS disrupts monolayer integrity causing the disturbance in the endothelial cytoskeletal organization. The stress fiber formation, induced by LPS, is rather moderate and is followed by the loss of peripheral actin signal, centripetal retraction of the cells and substantial gap formation in the monolayer (Fig. 2). This gap formation seems to correlate with the increasing deficit in the VE-cadherin/ZO-1 peripheral localization.
Analysis of the effect of forskolin and IBMX revealed that both activation of adenylate cycalse and inhibition of phosphodiesterases have a potential to alleviate LPS-induced hyperpermeability. The further employment of the specific cAMP analogues let us analyze the role of cAMP effectors PKA and Epac and show that the beneficial rescue of barrier could be achieved via either PKA-dependent or Epac-dependent pathways. Simultaneous activation of both cAMP effectors has an additive effect, suggesting that PKA-dependent and Epac-dependent pathways are not likely to share the majority of their downstream cytoskeletal targets (Fig. 3C).
We next studied the involvement of the known PKA substrate, VASP, into LPS-induced barrier dysfunction and alleviation of this dysfunction by cAMP elevators. Analysis of TER in monolayers challenged with LPS showed that VASP depletion exacerbates LPS-induced barrier compromise (Fig. 4C, D)). These results were consistent with our and other authors data, reporting increased endothelial permeability in response to VEGF (Furman et al., 2007), hydrogen peroxide (Rentsendorj et al., 2008) or bradykinin (Benz et al., 2008) and decreased improvement of barrier in response to ATP (Kolosova et al., 2005) in cells lacking VASP expression or activity. The analysis of LPS-induced redistribution of VASP partner protein, ZO-1, in VASP depleted cells showed that the mechanism of exacerbated barrier compromise likely includes the impairment of HLMVEC junctional organization via the distortion of ZO-1- F-actin contact (Fig. 5). Recent publication showed that decreased cytoskeletal anchorage of adhesion proteins VE-cadherin and β1-integrin can also contribute to barrier perturbance in VASP-deficient endothelium (Schlegel and Waschke, 2009).
As VASP association with partner proteins is believed to be regulated by PKA-dependent phosphorylation (Comerford et al., 2002), we analyzed how VASP localization and phosphorylation changes with LPS stimulation in forskolin and bnz-cAMP-pretreated cells. We showed that cAMP/PKA activation, increasing VASP phosphorylation dramatically, does not change VASP localization in quiescent cells. At the same time, LPS-induced relocation of VASP from the border region to the sites consistent in appearance with the focal adhesions is significantly suppressed by PKA activation (Fig. 6). These data let us suggest that VASP phosphorylation is not critical for the maintenance of VASP-border contacts in quiescent cells; however, it might be beneficiary for these contacts stabilization in the presence of contact-disrupting stimuli. We next assessed if the protective effect of cAMP on LPS-induced barrier dysfunction will be attenuated in the absence of VASP. We failed to reveal the effect of VASP depletion on forskolin-mediated improvement of LPS-compromised barrier (Fig. 4C). On contrary, when similar experiments were done with the specific PKA activator Bnz-cAMP, the protective effect of Bnz-cAMP was markedly reduced by the VASP knockdown (Fig. 4D). Based on these data, we conclude that VASP phosphorylation is likely represent only one of the many events initiated by forskolin treatment and therefore does not contribute significantly into forskolin barrier-protective effect. Nonetheless, the analysis of the experiment with the sole PKA activation clearly reveals the critical involvement of VASP phosphorylation in the barrier improvement.
We next assessed the possible involvement of yet another PKA substrate, filamin1 (filamin A), in barrier ma intenance in quiescent and LPS-challenged cells. We showed that filamin1 depletion dramatically compromised basal barrier and intensified barrier dysfunction in LPS-treated cells (Fig. 7). As filamin is known to be responsible for the orthogonal branching of F-actin and for the organization of F-actin in peri-membrane regions (Popowicz et al., 2006), we checked how F-actin and junctional proteins distributions are affected by the depletion of filamin1. We observed that filamin1 knockdown alone (in the absence of LPS) evokes F-actin rearrangement and VE-cadherin/ZO-1 junctional reorganization similar to those induced by LPS. In the presence of LPS, filamin1-depleted cells showed dramatically increased number of cell-crossing stress fibers (Fig. 8). We hypothesize that in filamin-depleted cells, where filamin-mediated organization of F-actin in the three-dimensional gels is no longer present, stress fibers induction occurs at a much higher rate. Our observations reveal important barrier-keeping role of filamin1 and link the depletion of filamin to the activation of stress fiber formation in pulmonary endothelium. On contrary, filamin degradation in spread human dermal microvascular EC was shown to be associated with the down-regulation of Rho activity, and Rho inactivation is known to suppress stress fiber formation (Mammoto et al., 2007). These data suggest that in confluent endothelial monolayers, the role of filamin may differ significantly from that in the spreading endothelial cells. Another possible explanation of ours and Mammoto (2007) disparate results may lie in the fact that degradation of filamin leads to the appearance of the shorter filamin products, and cleaved form of filamin is known to possess certain signaling properties (Bedolla et al., 2009). Future research will be needed to understand if the observed filamin contributions to cytoskeletal reorganization are cell-specific, cell state-specific or depend on whether filamin depletion or degradation was studied to assess the role of filamin.
The reports of the functional relevance of filamin1 phosphorylation are rather limited and suggest that filamin phosphorylation at Ser 2152 may affect the stability of this protein toward proteolysis (Bedolla et al., 2009; Garcia et al., 2006; Gorlin et al., 1990). Cleaved filamin1 is known to translocate from the cytosol to the nucleus, where it attains the new signaling role unrelated to the cytoskeleton regulation. Our data show that in quiescent cells at least a portion of filamin1 pool is constitutively phosphorylated at Ser2152, with cAMP elevation increasing this portion significantly (Fig. 9). Analysis of filamin1 localization showed that in HLMVEC filamin mostly co-localizes with F-catin structures, both peripheral actin and stress fibers, when the stress fiber induction takes place. Elevation of cAMP or specific activation of PKA prevents LPS-induced stress fiber formation, rendering filamin preferential co-localization with the cortical ring. This event could be facilitated by filamin phosphorylation or could simply be a consequence of the rearrangements underwent by the actin cytoskeleton. To assess if PKA-dependent filamin phosphorylation plays the role in the alleviation of LPS-induced hyperpermeability, we evaluated barrier protective effect of forskolin and Bnz-cAMP in control cells and cells deficient for filamin1. Our experiments failed to show the attenuation of the barrier-protective effect of cAMP/PKA in the cells lacking filamin expression (Fig. 7). Based on these data, we conclude that PKA-dependent phosphorylation of filamin1 is unlikely to play a significant role in the barrier improvement, elicited by cAMP-elevating agents.
In summary, here we demonstrated that LPS-induced barrier dysfunction could be significantly improved by the activation of both PKA-dependent and Epac-dependent pathways. The analysis of the involvement of PKA substrates VASP and filamin1 in the barrier regulation showed that whereas both proteins are critical for the barrier maintenance, only PKA-dependent phosphorylation of VASP contributes positively to the cAMP-mediated protection from LPS-induced barrier compromise.
Acknowledgments
We are grateful to Hong Fan for the skillful technical assistance. We thank Dr. E. Zemskov for the valuable help in preparation of this manuscript.
Grants. This work was supported by National Heart, Lung, and Blood Institute Grants HL-80675, HL-083327, HL-067307.
References
- Bedolla RG, Wang Y, Asuncion A, Chamie K, Siddiqui S, Mudryj MM, Prihoda TJ, Siddiqui J, Chinnaiyan AM, Mehra R, de Vere White RW, Ghosh PM. Nuclear versus cytoplasmic localization of filamin A in prostate cancer: immunohistochemical correlation with metastases. Clin Cancer Res. 2009;15(3):788–796. doi: 10.1158/1078-0432.CCR-08-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz PM, Blume C, Moebius J, Oschatz C, Schuh K, Sickmann A, Walter U, Feller SM, Renne T. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J Cell Biol. 2008;180(1):205–219. doi: 10.1083/jcb.200709181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindewald K, Gunduz D, Hartel F, Peters SC, Rodewald C, Nau S, Schafer M, Neumann J, Piper HM, Noll T. Opposite effect of cAMP signaling in endothelial barriers of different origin. Am J Physiol Cell Physiol. 2004;287(5):C1246–1255. doi: 10.1152/ajpcell.00132.2004. [DOI] [PubMed] [Google Scholar]
- Buttenschoen K, Kornmann M, Berger D, Leder G, Beger HG, Vasilescu C. Endotoxemia and endotoxin tolerance in patients with ARDS. Langenbecks Arch Surg. 2008;393(4):473–478. doi: 10.1007/s00423-008-0317-3. [DOI] [PubMed] [Google Scholar]
- Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40(7):651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. Faseb J. 2002;16(6):583–585. doi: 10.1096/fj.01-0739fje. [DOI] [PubMed] [Google Scholar]
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86(1):9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- Furman C, Sieminski AL, Kwiatkowski AV, Rubinson DA, Vasile E, Bronson RT, Fassler R, Gertler FB. Ena/VASP is required for endothelial barrier function in vivo. J Cell Biol. 2007;179(4):761–775. doi: 10.1083/jcb.200705002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E, Stracher A, Jay D. Calcineurin dephosphorylates the C-terminal region of filamin in an important regulatory site: a possible mechanism for filamin mobilization and cell signaling. Arch Biochem Biophys. 2006;446(2):140–150. doi: 10.1016/j.abb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Gong P, Angelini DJ, Yang S, Xia G, Cross AS, Mann D, Bannerman DD, Vogel SN, Goldblum SE. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem. 2008;283(19):13437–13449. doi: 10.1074/jbc.M707986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, Hartwig JH. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990;111(3):1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie LE, Patton WF, Hechtman HB, Shepro D. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. J Cell Physiol. 1997;172(3):373–381. doi: 10.1002/(SICI)1097-4652(199709)172:3<373::AID-JCP11>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Irie K, Fujii E, Ishida H, Wada K, Suganuma T, Nishikori T, Yoshioka T, Muraki T. Inhibitory effects of cyclic AMP elevating agents on lipopolysaccharide (LPS)-induced microvascular permeability change in mouse skin. Br J Pharmacol. 2001;133(2):237–242. doi: 10.1038/sj.bjp.0704073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol. 2006;291(2):L289–295. doi: 10.1152/ajplung.00343.2005. [DOI] [PubMed] [Google Scholar]
- Jacobson JR, Garcia JG. Novel therapies for microvascular permeability in sepsis. Curr Drug Targets. 2007;8(4):509–514. doi: 10.2174/138945007780362719. [DOI] [PubMed] [Google Scholar]
- Jay D, Garcia EJ, Lara JE, Medina MA, de la Luz Ibarra M. Determination of a cAMP-dependent protein kinase phosphorylation site in the C-terminal region of human endothelial actin-binding protein. Arch Biochem Biophys. 2000;377(1):80–84. doi: 10.1006/abbi.2000.1762. [DOI] [PubMed] [Google Scholar]
- Kolosova IA, Mirzapoiazova T, Adyshev D, Usatyuk P, Romer LH, Jacobson JR, Natarajan V, Pearse DB, Garcia JG, Verin AD. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circ Res. 2005;97(2):115–124. doi: 10.1161/01.RES.0000175561.55761.69. [DOI] [PubMed] [Google Scholar]
- Kolosova IA, Mirzapoiazova T, Moreno-Vinasco L, Sammani S, Garcia JG, Verin AD. Protective effect of purinergic agonist ATPgammaS against acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L319–324. doi: 10.1152/ajplung.00283.2007. [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579(22):4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- Lundblad C, Bentzer P, Grande PO. The permeability-reducing effects of prostacyclin and inhibition of Rho kinase do not counteract endotoxin-induced increase in permeability in cat skeletal muscle. Microvasc Res. 2004;68(3):286–294. doi: 10.1016/j.mvr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Huang S, Ingber DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J Cell Sci. 2007;120(Pt 3):456–467. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Walker PM, Foster DM, Harris D, Ribeiro M, Paice J, Romaschin AD, Derzko AN. Measurement of endotoxin activity in critically ill patients using whole blood neutrophil dependent chemiluminescence. Crit Care. 2002;6(4):342–348. doi: 10.1186/cc1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotla JM, Teixeira MM, Hellewell PG. Suppression of acute lung injury in mice by an inhibitor of phosphodiesterase type 4. Am J Respir Cell Mol Biol. 1998;18(3):411–420. doi: 10.1165/ajrcmb.18.3.2913. [DOI] [PubMed] [Google Scholar]
- Opal SM. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sépsis. Int J Med Microbiol. 2007;297(5):365–377. doi: 10.1016/j.ijmm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31(7):411–419. doi: 10.1016/j.tibs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol. 2009 doi: 10.1152/ajplung.90577.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsendorj O, Mirzapoiazova T, Adyshev D, Servinsky LE, Renne T, Verin AD, Pearse DB. Role of vasodilator-stimulated phosphoprotein in cGMP-mediated protection of human pulmonary artery endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2008;294(4):L686–697. doi: 10.1152/ajplung.00417.2007. [DOI] [PubMed] [Google Scholar]
- Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res. 2006;98(5):675–681. doi: 10.1161/01.RES.0000209516.84815.3e. [DOI] [PubMed] [Google Scholar]
- Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res. 2004;95(2):196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- Schlegel N, Burger S, Golenhofen N, Walter U, Drenckhahn D, Waschke J. The role of VASP in regulation of cAMP- and Rac 1-mediated endothelial barrier stabilization. Am J Physiol Cell Physiol. 2008;294(1):C178–188. doi: 10.1152/ajpcell.00273.2007. [DOI] [PubMed] [Google Scholar]
- Schlegel N, Waschke J. Impaired integrin-mediated adhesion contributes to reduced barrier properties in VASP-deficient microvascular endothelium. J Cell Physiol. 2009;220(2):357–366. doi: 10.1002/jcp.21772. [DOI] [PubMed] [Google Scholar]
- Sehrawat S, Cullere X, Patel S, Italiano J, Jr, Mayadas TN. Role of Epac1, an exchange factor for Rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell. 2008;19(3):1261–1270. doi: 10.1091/mbc.E06-10-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JB, Marnett LJ. Prostaglandin E2 inhibits tumor necrosis factor-alpha RNA through PKA type I. Biochem Biophys Res Commun. 2008;366(1):104–109. doi: 10.1016/j.bbrc.2007.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stief TW, Ijagha O, Weiste B, Herzum I, Renz H, Max M. Analysis of hemostasis alterations in sepsis. Blood Coagul Fibrinolysis. 2007;18(2):179–186. doi: 10.1097/MBC.0b013e328040bf9a. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Shimizu J, Miyawaki-Shimizu K, Vogel SM, Bair AM, Minshall RD, Predescu D, Malik AB. Role of NF-kappaB-dependent caveolin-1 expression in the mechanism of increased endothelial permeability induced by lipopolysaccharide. J Biol Chem. 2008;283(7):4210–4218. doi: 10.1074/jbc.M703153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L565–574. doi: 10.1152/ajplung.2001.281.3.L565. [DOI] [PubMed] [Google Scholar]
- Wang Q, Patton WF, Hechtman HB, Shepro D. A novel anti-inflammatory peptide inhibits endothelial cell cytoskeletal rearrangement, nitric oxide synthase translocation, and paracellular permeability increases. J Cell Physiol. 1997;172(2):171–182. doi: 10.1002/(SICI)1097-4652(199708)172:2<171::AID-JCP4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]