Abstract
Background
The aim of this study was to determine whether DNA-associated micro-particles (MPs) in maternal plasma express fetal-derived human leukocyte antigen-G (HLA-G) or placental alkaline phosphatase (PLAP) and whether the levels differ between women with normotensive pregnancies and preeclampsia.
Methods
DNA-associated MPs expressing HLA-G or PLAP were examined in the plasma of normal pregnant women and preeclamptic patients using flow cytometric analysis.
Results
DNA-associated HLA-G+ MPs were significantly increased in maternal plasma compared to plasma from non-pregnant controls (p < 0.005), with highest levels found in first and second trimesters. DNA-associated PLAP+ MPs were also increased in maternal plasma compared to plasma from non-pregnant controls (p < 0.006), with highest levels in second and third trimesters. Term preeclamptic women had higher levels of DNA-associated MPs than control pregnant women. HLA-G+ MPs from the plasma of preeclamptic women had more DNA per MP than HLA-G+ MPs from the plasma of normal pregnant women (p < 0.03).
Conclusions
HLA-G and PLAP MPs increase in maternal circulation at different times during gestation. DNA amounts per HLA-G+ MP increase in preeclamptic women which might indicate dysfunctional extravillous cytotrophoblasts.
Keywords: Apoptotic micro-particles, fetal DNA, DNA-associated micro-particles, HLA-G, PLAP, preeclampsia, syncytiotrophoblast, extravillous cytotrophoblast
Introduction
Circulating placenta-derived micro-particles (MPs) in maternal plasma are likely released from apoptotic fetal cells, suggesting potential use as predictive biomarkers of pathogenic pregnancies and fetal development. Previous studies, using a sandwich ELISA that detects the fetal protein, placental alkaline phosphatase (PLAP), or its enzymatic activity, suggest an increase in syncytiotrophoblast MPs (STBMs) in plasma of women with early-onset preeclampsia compared to normal pregnant women. [1] [2] Although these studies indirectly found higher levels of STBMs in the second or third trimester of preeclamptic women compared to normal pregnant women,[1–3] the prediction of preeclampsia earlier in gestation might allow speedier medical intervention for improved clinical outcome.
As opposed to the later gestational detection of STBMs, fetal cell-free DNA (cfDNA) can be found as early as the 5th week of gestation.[4, 5] In addition, increased amounts of circulating fetal DNA correlate with pregnancy complications such as preterm labor,[6] polyhydramnios,[7] hyperemesis gravidarum,[8] aneuploidies,[9] and preeclampsia.[10] Several groups have also reported an increase in fetal cfDNA prior to the onset of preeclampsia,[10–12] suggesting that fetal cfDNA could be useful to monitor or predict preeclampsia.
Our lab reported a 10-fold enrichment of fetal cfDNA using a flow sorting technique based on forward light scatter and acridine orange fluorescence of MPs found in maternal plasma,[13] suggesting that plasma MPs contain DNA. An apoptotic trophoblastic cell line released PCR-amplifiable, DNase protected cfDNA in association with membranous apoptotic MPs, which we termed micro-particle DNA (mpDNA).[14] Because maternal plasma mpDNA generated in vivo displayed light scatter and DNA fluorescence similar to apoptotic mpDNA generated in vitro, mpDNA found in maternal circulation is likely of apoptotic origin. However, there are no studies addressing the potential physical association between circulating fetal MPs and fetal cfDNA. Therefore, the aims of this study were to investigate whether MPs from pregnant women expressed the placental cell markers HLA-G and PLAP over gestation, compare levels of MPs between normal pregnancies and those with late-onset preeclampsia and to test the hypothesis that quantitative abnormalities of HLA-G+ and PLAP+ MPs are reflective of preeclampsia.
Materials and Methods
Antibodies and reagents
Monoclonal antibody (mAb) MEM-G/1 (IgG1), which recognizes the α1 domain of HLA-G, isotype matched control mAb IgG1 mAb PLAP (H17E2, IgG1), which recognizes human placental alkaline phosphatase, and isotype matched control mAb were purchased from Serotec (Raleigh, NC). Phycoerythrin-labeled goat-anti-mouse immunoglobulin polyclonal Ab (PE-GAMIG) was purchased from BD Pharmingen (San Diego, CA). PicoGreen, which preferentially binds to dsDNA, was purchased from Invitrogen (Carlsbad, CA).
Isolation and Quantification of JEG-3 apoptotic MPs
JEG-3 apoptotic MPs were prepared as previously described[15] using double filtered (0.25 µm) PBS (dfPBS). MPs were centrifuged at room temperature, unless otherwise stated. MPs from 24 hour apoptotic supernatants were separated from detached cells by two centrifugation steps (300 × g, 5 minutes; 800 × g, 5 minutes). MP concentration was determined as previously described.[16] Briefly, 100 µL of fluorescent beads (counted at 500/µL with a hemocytometer) were added to 400 µL of each MP supernatant plus 300 µL of dfPBS for a total volume of 800 µL (1:2 dilution of MPs) and the bead count was stopped at 10,000. To rule out intrinsic “MP contamination” induced by the beads, a control bead count (100 µL beads + 700 µL of dfPBS = 800 µL) was determined prior to the MPs plus beads count (total MP count). After subtracting the number of background fluorescent beads (~ 1,000 beads), the specific MP count was obtained. The concentration of MPs in 800 µL was determined as follows: (MP concentration) = [(specific MP count) × (bead concentration)] / (bead count) and the appropriate number of MPs was centrifuged (25,000 × g, 1 hour at 4°C) in an SW 40 Ti Swinging-Bucket Rotor in a Beckman L8-M, Class H, ultracentrifuge (Beckman Coulter, Miami Lakes, FL) prior to flow cytometric staining.
Assessment of HLA-G expression on JEG-3 MPs by flow cytometry
Mild acid treatment of freshly prepared MPs was performed as previously described with slight modifications.[17] Briefly, 2 × 106 apoptotic MPs were centrifuged (10,000 rpm, 10 minutes) using an Eppendorf Centrifuge 5415c (Eppendorf, Westbury, NY, USA). The MP pellets were re-suspended in 70 µL of 0.2 M citrate-phosphate buffer, pH 3.0, supplemented with 0.1% BSA on ice. After one minute incubation, the sample was neutralized by adding a 15-fold excess (1 mL) of cold 0.1% BSA in PBS, pH 7.2, centrifuged and washed with PBS (pH 7.2). MPs were incubated on ice for 20 minutes with MEM-G/1 diluted in PBS pH 7.2 containing 1% BSA. An irrelevant mAb was used as IgG1 isotype control. After washing MPs once with 0.1% BSA in PBS (pH 7.2), MPs were labeled with PE-conjugated goat anti-mouse IgG for 20 minutes at 4 °C, countered-stained with PicoGreen (1:15,000 in PBS) and analyzed using a Beckman Coulter EPICS XL2 (Beckman Coulter). . Data was analyzed using EXPO 32 software (Beckman Coulter).
Plasma sample collection
Normal pregnancies
After obtaining Institutional Review Board approval from Baylor College of Medicine and written consent from the human subjects, 5 to 10 mL of peripheral blood was collected in vacutainer tubes containing 1.5 mL of ACD Solution A (trisodium citrate, 22.0 g/L; citric acid, 8.0 g/L; and dextrose 24.5 g/L) and processed within 24 hours. Plasma from pregnant women and non-pregnant controls was separated from whole blood by centrifugation at 800 × g for 10 minutes. Recovered plasma was centrifuged for an additional 10 minutes at 1,600 × g to remove residual cells. Finally, cell-free supernatant was removed and stored in −80°C freezer.
Preeclamptic and normotensive controls
Preeclampsia was defined as blood pressure 140/90 or greater plus proteinuria of 300mg or greater in 24 hours or 100 mg/dL or more in at least two random urine specimens collected 6 or more hours apart. Frozen (−80°C) plasma samples (3 minutes at 1,000 × g) from term preeclamptic and matched normal pregnancies were provide by Dr. Popek.
Labeling of plasma micro-particles
MP concentration was determined as described in Methods and Materials. Fluorescent beads (20,000) were added 15 µL plasma in double filtered (0.25 µm) PBS (dfPBS) to a total volume of 800 µL and a final concentration of 25 beads/µL. The number of beads counted by the flow cytometer was stopped at 1,000. All labeling procedures were performed at room temperature. One million MPs were re-suspended in dfPBS for a final volume of 66 µL, labeled with 3 µg MEM-G/1 or 1 µg PLAP and isotype control mAbs and mixed on a BD ADAMS Nutator (Aria Medical Equipment) for 6 minutes. Secondary PE-GAMIG (0.8 µg) was added directly to labeled MP samples and mixed for 5 minutes. MPs were then labeled with 2 µL PicoGreen for 10 minutes in dark. Unlabeled MPs were also used as negative controls. Both labeled and unlabeled MPs were re-suspended in 350 µL dfPBS and analyzed by an EPICS XL-2 flow cytometer. The number of events was stopped at 10,000. Data was analyzed using EXPO 32 software (Beckman Coulter).
Statistical analysis
Because the variables were highly skewed, comparisons between two groups (control pregnancy vs. preeclamptic pregnancy) were performed using a two-sample Wilcoxon Rank-Sum test. When more than two groups (non-pregnant controls, first, second, and third trimester plasma samples) were simultaneously considered, the Kruskal Wallis test was applied and two-sample Wilcoxon Rank-Sum tests adjusted using Bonferroni multiple comparisons tested inter-group differences. A two-sided p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using SAS® version 9.2.
Results
Concentration of total MPs during pregnancy
The number of total plasma MPs per mL from normal pregnant women over gestation and non-pregnant controls was quantitated by flow cytometry using a fluorescence bead assay.[16] Although the number of total MPs/mL increased from 9.3 × 106 MPs/mL during the first trimester, to 18.3 × 106 MPs/mL during second trimester and finally to 23.0 × 106 MPs/mL during the third trimester, no significant difference in the total number of MPs/mL for each trimester (or total pregnancy) was observed between normal pregnancies and non-pregnant controls (data not shown), indicating that the actual numbers of MPs are not reflective of in vivo status during normal pregnancy, and likely represent normal homeostasis of dying cells.
Characteristics of placenta-derived MPs in maternal plasma
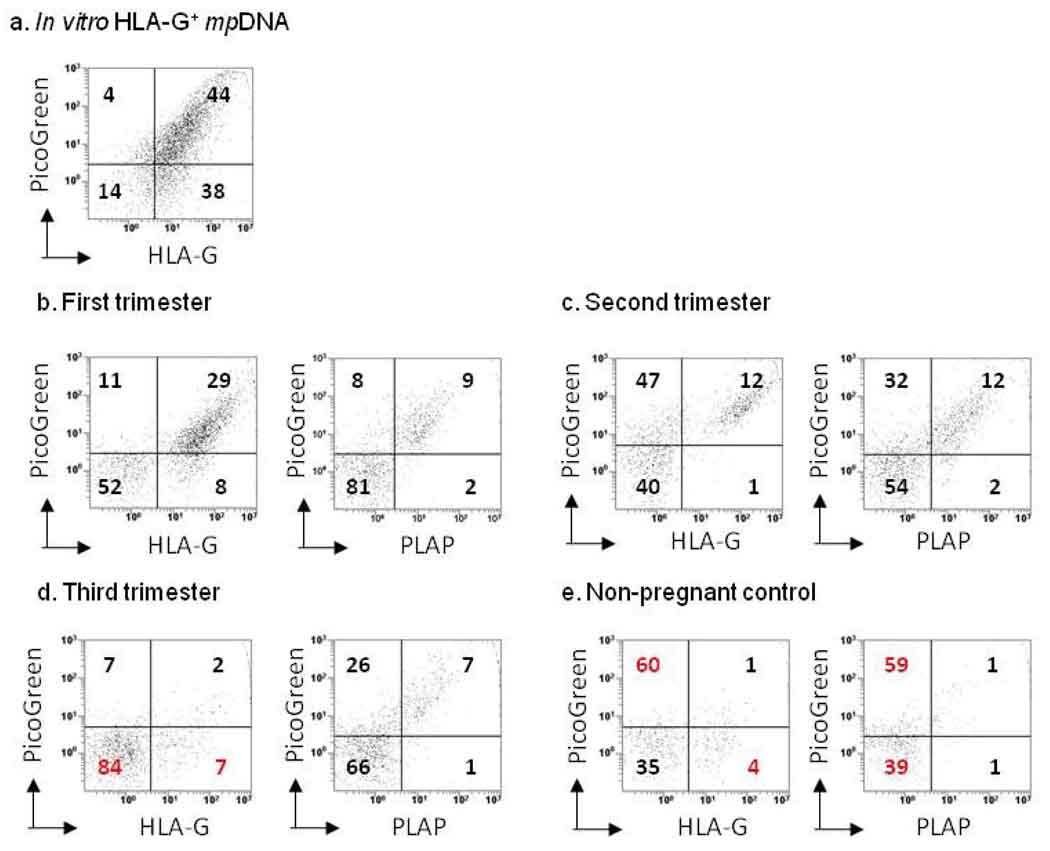
To determine the concentration of placenta-derived MPs in the plasma of normal pregnant women, plasma MPs were labeled with MEM-G/1 (anti-HLA-G mAb), anti-PLAP or appropriate isotype control mAbs and stained with PicoGreen for flow cytometry. Supernatants from isolated JEG-3 apoptotic MPs were used as a positive control for HLA-G+ MPs containing DNA (Figure 1a). Dot plots show the percentage of HLA-G+ MPs and PLAP+ MPs containing DNA in the plasma of normal pregnant women (Figure 1b–e), indicating that both types of fetal MPs contain DNA.
Figure 1. Flow cytometric characterization of maternal MPs in normotensive pregnancy.
MPs were quantitated by flow cytometry using a fluorescent bead assay described in Materials and Methods. For detection of HLA-G+ MPs, maternal (n = 31) and non-pregnant control (n = 8) plasma samples were labeled with MEM-G/1, or IgG1 isotype control mAbs. For detection of PLAP+ MPs, a subset of the original maternal (n = 16) and non-pregnant control (n = 5) plasma samples were labeled with anti-PLAP mAb, or IgG1 isotype control mAbs. After mAb labeling, MPs were counter-stained with PicoGreen (dsDNA dye) and analyzed by flow cytometry. JEG-3 derived MPs (n = 3) were used as a positive control for HLA-G+ mpDNA. A total of 10,000 events were taken. Dot plots show the percentage of HLA-G+ and PLAP+ mpDNA (x-axis, HLA-G or PLAP; y-axis, DNA). (a) Percentage of HLA-G+ mpDNA from in vitro JEG-3 MPs. (b) Percentage of first trimester HLA-G+ and PLAP+ mpDNA. (c) Percentage of second trimester HLA-G+ and PLAP+ mpDNA. (d) Percentage of third trimester HLA-G+ and PLAP+ mpDNA. (e) Percentage of HLA-G+ and PLAP+ mpDNA from non-pregnant control samples.
Number of HLA-G+ MPs in normal pregnancy
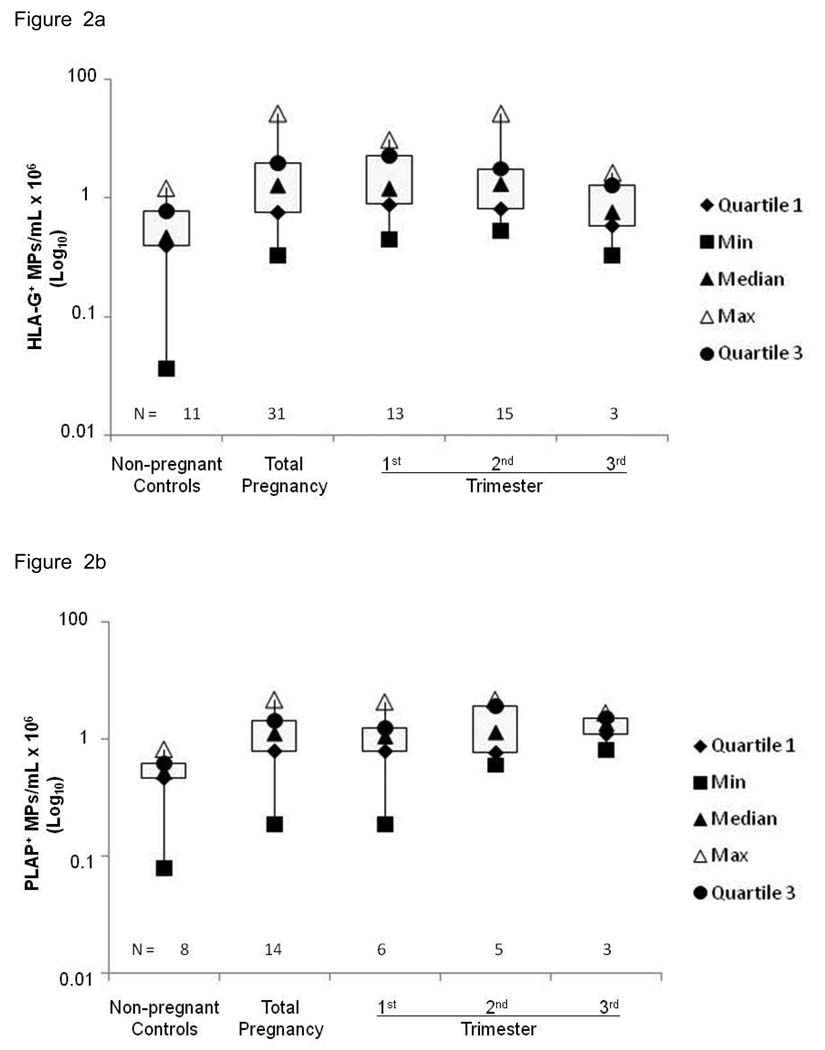
Concentrations of HLA-G+ MPs containing DNA in the plasma of the pregnant women are shown in Fig. 2a, which ranged from 0.2 – 27.1 × 106 MPs/mL, with a median of 1.6 × 106 MPs/mL in pregnant women and 0 – 1.5 × 106 MPs/mL, with a median of 0.5 × 106 MPs/mL in non-pregnant controls. The difference between the two groups was statistically significant (p < 0.005, Wilcoxon rank-sum test). A significant difference between the concentrations of HLA-G+ MPs containing DNA in the first trimester (1.4 × 106 MPs/mL, 0.2 – 9.7 × 106 MPs/mL) and non-pregnant controls (0.5 × 106 MPs/mL) and in the second trimester (1.7 × 106 MPs/mL, 0.3 – 27.1 × 106 MPs/mL) and non-pregnant controls (0.5 × 106 MPs/mL) was also observed (p < 0.02 and 0.01, respectively). However, no significant difference in concentrations of HLA-G+ MPs containing DNA between the third trimester and non-pregnant controls, or between trimesters was observed. Together, the data suggest that EVT-derived MPs are highest during the first and second trimesters of normal pregnancy.
Figure 2.
MPs were quantitated by flow cytometry using a fluorescent bead assay described in Materials and Methods. (a) First and second trimesters have the highest concentration of HLA-G MPs containing DNA. Maternal (n = 31) and non-pregnant control (n = 11) plasma samples were labeled with MEM-G/1, or IgG1 isotype control mAbs and stained with PicoGreen (dsDNA dye). A total of 10,000 events were taken by flow cytometry. The y-axis of the box plots represent, on a log scale, the concentration of HLA-G+ MPs containing DNA from non-pregnant controls and normal pregnancies at each trimester. Closed triangles = medians; diamonds = 1st quartile; circles = 3rd quartile; open triangles = max; squares = min. Asterisk (*) indicates significant difference (p < 0.05) compared to non-pregnant control samples. (b) Second and third trimesters have highest concentration of PLAP+ MPs containing DNA. Maternal (n = 16) and non-pregnant control (n = 8) plasma samples were labeled with anti-PLAP mAb or IgG1 isotype control mAbs and stained with PicoGreen (dsDNA dye). A total of 10,000 events were taken by flow cytometry. The y-axis of the box plots represent, on a log scale, the concentration of PLAP+ MPs containing DNA from non-pregnant controls and normal pregnancies at each trimester. Closed triangles = medians; diamonds = 1st quartile; circles = 3rd quartile; open triangles = max; squares = min. Asterisk (*) indicates significant difference (p < 0.01) compared to non-pregnant control samples.
Number of PLAP+ MPs in normal pregnancy
Concentrations of PLAP+ MPs containing DNA in the plasma of the pregnant women are shown in Fig. 2b, which ranged from 0 – 4.6 × 106 MPs/mL, with a median of 1.2 × 106 MPs/mL in pregnant women and 0 – 0.7 × 106 MPs/mL, with a median of 0.1 × 106 MPs/mL in non-pregnant controls. The difference between the two groups was statistically significant (p < 0.005, Wilcoxon rank-sum test). A significant difference between the concentrations of of PLAP+ MPs containing DNA of the second trimester (1.3 × 106 MPs/mL, 0.0 – 4.6 × 106 MPs/mL) and non-pregnant controls (0.1 × 106 MPs/mL) was also observed (p < 0.01). However, no significant difference in the concentrations of PLAP+ MPs containing DNA between the first trimester and non-pregnant controls, third trimester and non-pregnant controls, or between trimesters was observed. The data suggest that renewal of the syncytiotrophoblast (STB) layer by apoptosis occurs during the second trimester of gestation.
Number of HLA-G+ and PLAP+ MPs containing DNA in preeclampsia
Although elevated levels of STBMs in the plasma of preeclamptic women [2, 3] suggest specific tissue damage of the syncytiotrophoblast layer surrounding the placenta, it does not address whether there might be cellular death of extravillous cytotrophoblasts. Therefore, we measured the number of circulating HLA-G+ and PLAP+ MPs in the plasma of 10 preeclamptic and 8 normotensive women. Clinical characteristics of the patient groups are summarized in Table 1. Concentrations of MPs containing DNA in the plasma of the pregnant women are summarized in Table 2, which ranged from 39.0 – 256.6 × 106 MPs/mL, with a median of 119.5 × 106 MPs/mL in late-onset preeclamptic women and 29.1 – 160.5 × 106 MPs/mL with a median of 68.7 × 106 MPs/mL in normotensive plasma controls. The difference between the two groups was statistically significant (p < 0.04, Wilcoxon rank-sum test). A significant difference between the plasma concentrations of total MPs containing DNA in preeclamptic women (65.8 × 106 MPs/mL, 11.0 – 156.5 × 106 MPs/mL) and non-pregnant controls (21.1 × 106 MPs/mL, 8.3 – 49.0 × 106 MPs/mL) was also observed (p < 0.05) (Table 2). Together the data suggest that more cellular death of all types, both maternal and fetal, occurs during preeclampsia
Table 1.
Clinical characteristic of patient groups
| Characteristics | Normotensive Pregnancies (n = 8) |
Preeclamptic Pregnancies (n = 10) |
|---|---|---|
| Maternal age (years) | 24.0 (18.0 – 29.0) | 21.0 (19.0 – 40.0) |
| Parity (number) | 1.0 (1.0 – 3.0) | 0.5 (0.0 – 3.0) |
| Gestational age at delivery (weeks) | 39.9 (37.6 – 41.1) | *37.4 (33.7 – 41.1) |
| Birth weight (grams) | 3600.0 (1893.0 – 4005.0) | 3210.0.0 (1748.0 – 3725.0) |
| Diastolic blood pressure (mmHg) | 71.0 (58.0 – 86.0) | **90.0 (79.0 – 103.0) |
| Systolic blood pressure (mmHg) | 114.0 (106.0 – 127.0) | ***149.0 (131.0 – 160.0) |
| Protein | 0.0 (0.0 – 0.0) | 3.0 (1.0 – 4.0) |
Data are reported as median (min - max) of preeclamptic pregnancies compared to normotensive pregnant controls using the two-sample Wilcoxon Rank-Sum test.
p < 0.04,
p < 0.0001, and
p < 0.00002 compared to normotensive control group.
Table 2.
Characteristics of plasma MPs in preeclampsia
| Normotensive Pregnancies (n = 9) |
Preeclamptic Pregnancies (n = 10) |
|
|---|---|---|
|
Total MP/mL (maternal, EVT, STB) |
68.7 × 106 (29.1 – 60.5) | *119.5 × 106 (39.0 – 256.6) |
| Concentration (MPs/mL) | ||
|
TotalmpDNA/mL (maternal, EVT, STB) |
21.1 × 106 (8.3 – 49.0) | *65.8 × 106 (11.0 – 156.5) |
|
Neg. HLA-GmpDNA/mL (maternal and STB) |
15.1 × 106 (8.1 – 47.4) | **56.1 × 106 (9.8 – 148.8) |
|
Pos. HLA-G mpDNA/mL (EVT) |
3.5 × 106 (0.2 – 10.8) | 3.5 × 106 (0.8 – 45.2) |
| DNA MFI | ||
|
Total mpDNA MFI (maternal, EVT, STB) |
48.0 (24.0 – 104.0) | *94.5 (19.0 – 204.0) |
|
Neg. HLA-GmpDNA MFI (maternal and fetal) |
51.0 (24.0 – 110.0) | *98.0 (19.0 – 230.0) |
|
Pos. HLA-GmpDNA MFI (EVT) |
30.0 (15.0 – 78.0) | *55.5 (24.0 – 128.0) |
| Concentration (MPs/mL) | ||
|
Total mpDNA/mL (maternal, EVT, STB) |
21.5 × 106 (11.0 – 56.2) | **46.5 × 106 (9.8 – 200.1) |
|
Neg. PLAP mpDNA/mL (maternal and EVT) |
16.5 × 106 (8.7 – 52.2) | 21.1 × 106 (8.3 – 49.0) |
|
Pos. PLAPmpDNA/mL (STB) |
3.5 × 106 (1.2 – 10.8) | 5.0 × 106 (1.0 – 28.7) |
| DNA MFI | ||
|
Total mpDNA MFI (maternal, EVT, STB) |
70.0 (39.0 – 166.0) | 108.5 (24.0 – 213.0) |
|
Neg. PLAPmpDNA MFI (maternal and EVT) |
49.0 (28.0 – 119.0) | **82.5 (22.0 – 209.0) |
|
Pos. PLAP mpDNA MFI (STB) |
293.0 (220.0 – 421.0) | 280.0 (92.0 – 453.0) |
Data are reported as median (min - max) of preeclamptic pregnancies compared to normotensive pregnant controls using the two-sample Wilcoxon Rank-Sum test.
p < 0.05 and
p < 0.07 compared to normotensive pregnant control group
EVT: HLA-G+ extravillous cytotrophoblasts
STB: PLAP+ syncytiotrophoblast
HLA-G: Human leukocyte antigen-G
PLAP: Placental alkaline phosphatase
mpDNA: Micro-particles containing DNA
No significant difference in the concentration of DNA-associated HLA-G+ MPs and DNA-associated PLAP+ MPs was found in preeclamptic pregnancies compared to normotensive pregnancies (Table 2), suggesting that circulating levels of HLA-G+ MPs and PLAP+ MPs containing DNA per se might not reflect placental tissue damage in term preeclamptic women. No significant difference in the concentration of DNA-associated PLAP− MPs (maternal and HLA-G+ MPs) was observed, whereas a marginally significant difference in the concentration of DNA-associated MPs HLA-G− MPs (maternal and PLAP+ MPs) was observed in preeclamptic women (56.1 × 106 MPs/mL, 9.8 – 148.8 × 106 MPs/mL) compared to the MPs in normotensive pregnancies (15.1 × 106 MPs/mL, 8.1 – 47.4 × 106 MPs/mL, p < 0.07) (Table 2). Together, the data suggest an increase in maternal tissue damage and not placental damage.
Relationship between HLA-G+ MPs and PLAP+ MP DNA levels in preeclampsia
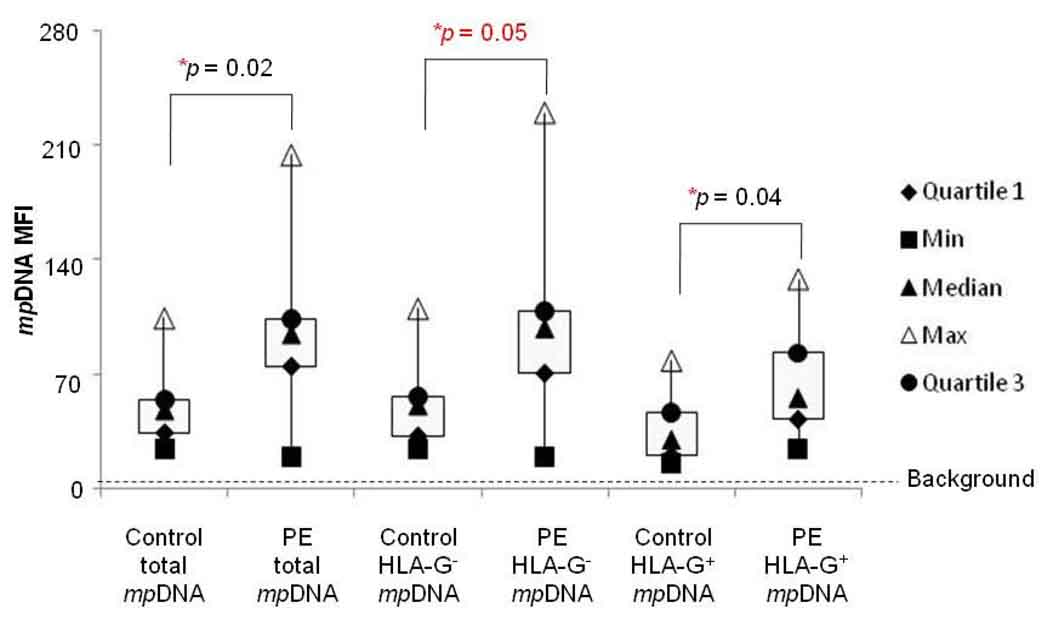
Previous studies have suggested that pregnancies associated with placental dysfunction, such as preeclampsia and fetal growth restriction, have higher concentrations of fetal cfDNA than normal pregnancies.[10, 18] However, significantly higher levels of HLA-G+ MPs or PLAP+ MPs containing DNA were not observed in preeclamptic women compared to normal pregnancies (Table 1). Therefore, we hypothesized that placenta-derived MPs in the plasma of preeclamptic women might have other characteristics. We therefore analyzed the levels of DNA per MP in maternal plasma using PicoGreen mean fluorescence intensity (MFI). As summarized in Table 2, a significant difference in DNA MFI for total plasma MPs containing DNA (maternal and fetal MPs) between preeclamptic women (94.5, 19.0 – 204.0) and normotensive pregnancies (48.0, 24.0 – 104.0; p < 0.02) was observed. The DNA MFI for HLA-G+ MPs was significantly higher in preeclamptic women (55.5, 24.0 – 128.0) compared to plasma from normotensive pregnancies (30.0, 15.0 – 78.0; p < 0.04), whereas the DNA MFI for PLAP+ MPs was slightly higher in preeclamptic women than in normal pregnant women, but did not reach statistical significance (Table 2). A significant difference in the DNA MFI of HLA-G− MPs containing DNA (maternal and PLAP+ MPs) between preeclamptic women (98.0, 19.0 – 230.0) and normotensive pregnancies (51.0, 24.0 – 110.0; p < 0.05) was observed. A marginally significant difference in DNA MFI of PLAP− MPs (maternal and HLA-G+ MPs) between preeclamptic women (82.5, 22.0 – 209.0) and normotensive pregnancies (49.0, 28.0 – 119.0; p < 0.07) was observed (Table 2).
Analysis of the ratio between the DNA MFI of PLAP+ MPs and that of HLA-G+ MPs in both preeclamptic pregnancies (5.4, 2.4 – 8.1) and normotensive pregnancies (12.2, 2.8 – 20.1), showed that the ratio was lower in preeclamptic women (p < 0.01) (Table 3). Together, the data suggest that although STBMs have more DNA per MP than EVT-MPs, EVT-MPs containing DNA most likely contribute to the elevated levels of circulating fetal cfDNA in term preeclamptic women. In addition, the relative amount of DNA per HLA-G+ MP, rather than the number of HLA-G+ MPs alone (Table 2) might indicate EVT tissue damage in term preeclamptic women. Earlier sampling and/or polychromatic flow cytometric assays are needed to confirm this observation.
Table 3.
Ratio of DNA MFI of PLAP+ MPs to HLA-G+ MPs is lower in term preeclamptic pregnancies.
| Normotensive Pregnancies |
Preeclamptic Pregnancies |
|
|---|---|---|
|
Amount of DNA per PLAP+mpDNA (fetal DNA MFI) |
293.0 (220.0 – 421.0) |
280.0 (92.0 – 453.0) |
|
Amount of DNA per HLA-G+mpDNA (fetal DNA MFI) |
30.0 (15.0 – 78.0) |
*55.5 (24.0 – 128.0) |
|
Ratio of HLA-G+ PLAP+mpDNA MFI to HLA-G+mpDNA MFI |
12.2 (2.8 – 20.1) |
*5.4 (2.4 – 8.1) |
Data are reported as median (min - max) of preeclamptic pregnancies compared to normotensive pregnant controls using the two-sample Wilcoxon Rank-Sum test.
p < 0.01 compared to normotensive pregnancies.
HLA-G (Human leukocyte antigen-G)
PLAP (Placental alkaline phosphatase)
mpDNA (Micro-particles containing DNA)
Discussion
This study investigated whether qualitative and quantitative differences in MPs over gestation and in comparison to preeclampsia were evident. We show, for the first time, that two types of trophoblast-derived MPs (HLA-G+ MPs and PLAP+ MPs) can be measured in the maternal circulation over gestation, that they contain DNA and that the type of MPs corresponds to fetal development. We next compared the number of total plasma MPs, HLA-G+ MPs and PLAP+ MPs between term preeclamptic women and normotensive pregnant women. The total number of MPs and DNA-associated MPs was significantly higher in women with preeclampsia than in normal controls, but this was not the case for HLA-G+ and PLAP+ MPs.
During placental development, human cytotrophoblast cells fuse together to form the STB layer or differentiate into EVTs, depending on levels of oxygen and timing. EVTs, which express HLA-G, are predominate during the first trimester when oxygen levels are low [19] and through the second trimester (14 –18 weeks of gestation) when oxygen levels slowly begin to rise.[20] By contrast, the majority of STBs, which express placental alkaline phosphatase (PLAP),[21] are found in the second trimester and third trimester when oxygen levels are high.[22] In keeping with these observations, our data showed that the majority of placenta-derived MPs containing DNA detected in the first and second trimesters of normal pregnancies originated from HLA-G+ cells, suggesting that the MPs are derived from invading EVTs undergoing apoptosis. [23] By contrast, the majority of PLAP+ MPs containing DNA were detected in the second trimester of normal pregnancies, suggesting that they are STBMs shed from the normal turnover and renewal of the syncytiotrophoblast layer. [24] Our data suggest that more EVT undergo apoptosis during the first and second trimester in normal pregnancies, whereas more STBs undergo apoptosis during the second trimester, consistent with what occurs during normal placental development.
Our system allows for the targeting of fetal antigens and avoids non-specific detection of maternal-derived MPs such as platelet MP, which constitutes 95% of the total MPs in maternal plasma.[25] A series of flow cytometric assays were performed to determine which form of HLA-G (native or denatured) was present on the plasma MPs. Monoclonal antibodies MEM-G/9, G233, and 87G recognize (different) conformational epitopes located on the heavy chain of HLA-G class I molecules in association with beta-2-microglobulin (β2m). By contrast, 4H84 and MEM-G/1 bind to (different) linear, non-conformational epitopes on the α1 domain of unfolded HLA-G heavy chains dissociated from β2m. MEM-G/1 bound best to the MPs in maternal plasma (p = 0.002, Supplemental Data, Figure 1). The linear epitope recognized by MEM-G/1 is likely already exposed on MPs from frozen maternal plasma samples, indicating that the material is already in a semi-denatured state. Support for our findings are data reported by Hunt et al.,[26] who showed that soluble HLA-G2, which lacks the α2 domain and does not associate with β2m, is the predominant isoform of HLA-G in the serum of normotensive pregnancies.
Although elevated levels of STBMs in the plasma of preeclamptic women [2, 3] suggest specific tissue damage of the syncytiotrophoblast layer surrounding the placenta, it does not address whether there might be cellular death of extravillous cytotrophoblasts. Our data show that the number of total MPs in maternal circulation is higher for women with preeclampsia than in matched normal pregnant women, which appears to contradict several other preeclamptic studies,[25, 27, 28] However, in previous studies, women with severe, early-onset preeclampsia (< 34 weeks of gestation) were studied, whereas our samples were from women with late-onset preeclampsia (> 34 weeks of gestation, suggesting different pathophysiologies.
We acknowledge that a limitation of this study is the difference in plasma MP preparation between the two aspects of the study. The total number of MPs from normal pregnancies and non-pregnant controls (Figure 2a, b) was lower than in the study comparing normotensive pregnancies to preeclamptic pregnancies (Table 2). Plasma samples from the first part of the study had two consecutive centrifugations (800 × g and 1600 × g), whereas the plasma samples from the second of the study were only centrifuged once (1000 × g). The second centrifugation step eliminates potential “contaminating” cells for the purpose of quantitative analysis of fetal DNA in maternal plasma via real-time PCR. Although a lower speed centrifugation (1000 × g) might contain “contaminating” cells, our flow cytometric analysis of plasma MPs indicates that the size of MPs is about 1 – 3 µm based on fluorescent beads of known sizes. Therefore, potentially “contaminating” cells from the single centrifugation step at 1000 × g are not in the electronic gate of the preeclamptic study. Furthermore, one of the primary aims of this study was to compare levels of MPs between normal pregnancies and those with late-onset preeclampsia. Because normal pregnancy and non-pregnant control samples as well as preeclamptic and normotensive control samples were collected the same way, each study has its own controls prepared in the same way and therefore it is unlikely that the differences in centrifugation speed changed the interpretation of the data. Uniformity of methods for isolation and quantification of plasma MPs in clinical practice will need to be addressed.
Consistent with previous reports of elevated levels of total cfDNA in preeclampsia compared to normotensive pregnancies,[10, 29] the total number of DNA-associated MPs (maternal + fetal MPs) in women with preeclampsia was higher compared to normal control pregnancies. Although we did not stain specifically for the many different types of maternal MPs containing DNA, it is likely that the HLA-G− MPs are derived from many different maternal cell types (endothelial cells, lymphocytes, and monocytes), all of which are elevated in preeclampsia.[30–32] Whether there is a difference in the amount of the total number of DNA-associated MPs before the development of preeclampsia remains to be tested.
Because preeclampsia is associated with abnormal EVT invasion, several studies have focused on protein levels of soluble HLA-G (sHLA-G) in maternal serum or maternal plasma as an early marker for preeclampsia. Studies by Yie et al., and Hackmon et al., reported that sHLA-G serum levels are significantly lower in early-onset preeclamptic women than normotensive controls.[33, 34] By contrast, we did not detect significant differences in the numbers of plasma DNA-associated HLA-G+ MPs between preeclamptic and normotensive pregnant women. Differences in severity and timing of preeclampsia might explain the differences in outcome. Both previous studies examined sHLA-G from women with severe early-onset preeclampsia, whereas we studied HLA-G+ MPs from women with late-onset preeclampsia. Based on the resistance of maternal blood flow in the uteroplacental circulation, preterm preeclampsia is associated with defective placentation, which is not the case for term preeclampsia.[35] This could explain why we did not detect a significant difference in the number of plasma DNA-associated HLA-G+ MPs between term preeclamptic patients and normal pregnant women.
We also confirmed the presence of STBMs in the plasma of women with late-onset preeclampsia using flow cytometric analysis. However, the levels of PLAP+ STBMs in the plasma of preeclamptic women were not significantly different from those observed in the plasma of normotensive pregnant women. Our results are supported by Goswami et al., who also did not observe a significant difference in the number of STBMs found in late-onset preeclamptic women compared to normal pregnant women. However, the authors did show a 2.5 increase in the number of STBMs found in early-onset preeclamptic women compared to normal pregnant women, perhaps because the placenta pathology is different.
Because there is no evidence of dysfunctional EVTs during late-onset preeclampsia, this might explain why Doppler sonography,[35] protein levels of sHLA-G[36] or numbers of HLA-G+ MPs are unlikely to detect significant differences in term preeclamptic women. However, a key factor in late-onset preeclampsia might be the increased amount of DNA per MP. Our data show that HLA-G+ MPs from the plasma of preeclamptic women have significantly more DNA per MP than HLA-G+ MPs from the plasma of normal pregnant women. By contrast, we did not detect a significant difference in the amount of DNA per between PLAP+ MP found in term preeclamptic women. If late-onset preeclampsia is associated with normal turnover of the STB layer, this could explain why there are similar amounts of DNA per PLAP+ MP found in the plasma of preeclamptic and normal pregnant women.
We hypothesize that the origin of HLA-G+ MPs containing high levels of DNA released into maternal plasma in term preeclamptic women might be due to destruction of a subset of extravillous cytotrophoblasts (polygonal extravillous trophoblasts) along the invasive pathway, rather than originating from the placenta itself. Polygonal extravillous trophoblasts are thought to be derived from small invasive extravillous cytotrophoblasts. They are large cells with big irregular shaped nuclei, express cytokeratin 7, which is a trophoblast intracellular marker, and their numbers increase with gestation. [37] Pregnancies complicated by preeclampsia may undergo late atherosclerotic changes in spiral arterioles, implying normal rearrangement of the spiral arteries. The fact that there is normal extravillous cytotrophoblast invasion in term preeclampsia would support the hypothesis that polygonal cells are the source of the increased DNA per HLA-G+ MP. However, few polygonal cells would be present in preterm preeclampsia because of shallow extravillous cytotrophoblast invasion. In addition, the increased nuclear material within polygonal cells supports the hypothesis that HLA-G+ MPs containing high amounts of DNA observed in late-onset preeclampsia could be released from apoptotic polygonal extravillous trophoblasts.
In conclusion, our data show that a flow cytometry-based approach holds promise for detection of circulating HLA-G+ or PLAP+ MPs containing DNA in normal and preeclamptic patients to allow prediction of preeclampsia. Polychromatic flow cytometry should be used to delineate the quantity and types of MPs over gestation.
Supplementary Material
Figure 3. Preeclamptic MPs contain more DNA.
MPs were quantitated by flow cytometry using a fluorescent bead assay described in Materials and Methods. Preeclamptic (n = 10) and normotensive (n = 9) plasma samples were labeled with MEM-G/1, or IgG1 isotype control mAbs, stained with PicoGreen (dsDNA dye) and analyzed by flow cytometry. A total of 10,000 events were taken. The y-axis of the box plots represents the mean fluorescent intensity of DNA per HLA-G+ MPs from preeclamptic and normal pregnancies. Closed triangles = medians; diamonds = 1st quartile; circles = 3rd quartile; open triangles = max; squares = min. Asterisk (*) indicates significant difference (p < 0.05) compared to non-pregnant control samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 2.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 3.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Birch L, English CA, O'Donoghue K, Barigye O, Fisk NM, Keer JT. Accurate and robust quantification of circulating fetal and total DNA in maternal plasma from 5 to 41 weeks of gestation. Clin Chem. 2005;51:312–320. doi: 10.1373/clinchem.2004.042713. [DOI] [PubMed] [Google Scholar]
- 5.Galbiati S, Smid M, Gambini D, Ferrari A, Restagno G, Viora E, et al. Fetal DNA detection in maternal plasma throughout gestation. Hum Genet. 2005;117:243–248. doi: 10.1007/s00439-005-1330-z. [DOI] [PubMed] [Google Scholar]
- 6.Leung TN, Zhang J, Lau TK, Hjelm NM, Lo YM. Maternal plasma fetal DNA as a marker for preterm labour. Lancet. 1998;352:1904–1905. doi: 10.1016/S0140-6736(05)60395-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhong S, Ng MC, Lo YM, Chan JC, Johnson PJ. Presence of mitochondrial tRNA(Leu(UUR)) A to G 3243 mutation in DNA extracted from serum and plasma of patients with type 2 diabetes mellitus. J Clin Pathol. 2000;53:466–469. doi: 10.1136/jcp.53.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekizawa A, Sugito Y, Iwasaki M, Watanabe A, Jimbo M, Hoshi S, et al. Cell-free fetal DNA is increased in plasma of women with hyperemesis gravidarum. Clin Chem. 2001;47:2164–2165. [PubMed] [Google Scholar]
- 9.Wataganara T, LeShane ES, Farina A, Messerlian GM, Lee T, Canick JA, et al. Maternal serum cell-free fetal DNA levels are increased in cases of trisomy 13 but not trisomy 18. Hum Genet. 2003;112:204–208. doi: 10.1007/s00439-002-0853-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhong XY, Holzgreve W, Hahn S. The levels of circulatory cell free fetal DNA in maternal plasma are elevated prior to the onset of preeclampsia. Hypertens Pregnancy. 2002;21:77–83. doi: 10.1081/PRG-120002911. [DOI] [PubMed] [Google Scholar]
- 11.Leung TN, Zhang J, Lau TK, Chan LY, Lo YM. Increased maternal plasma fetal DNA concentrations in women who eventually develop preeclampsia. Clin Chem. 2001;47:137–139. [PubMed] [Google Scholar]
- 12.Levine RJ, Qian C, Leshane ES, Yu KF, England LJ, Schisterman EF, et al. Two-stage elevation of cell-free fetal DNA in maternal sera before onset of preeclampsia. Am J Obstet Gynecol. 2004;190:707–713. doi: 10.1016/j.ajog.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff FZ, Lewis DE, Simpson JL. Cell-free fetal DNA in maternal blood: kinetics, source and structure. Hum Reprod Update. 2005;11:59–67. doi: 10.1093/humupd/dmh053. [DOI] [PubMed] [Google Scholar]
- 14.Orozco AF, Bischoff FZ, Horne C, Popek E, Simpson JL, Lewis DE. Hypoxia-induced membrane-bound apoptotic DNA particles: potential mechanism of fetal DNA in maternal plasma. Ann N Y Acad Sci. 2006;1075:57–62. doi: 10.1196/annals.1368.007. [DOI] [PubMed] [Google Scholar]
- 15.Orozco AF, Jorgez CJ, Horne C, Marquez-Do DA, Chapman MR, Rodgers JR, et al. Membrane protected apoptotic trophoblast microparticles contain nucleic acids: relevance to preeclampsia. Am J Pathol. 2008;173:1595–1608. doi: 10.2353/ajpath.2008.080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montes M, Jaensson EA, Orozco AF, Lewis DE, Corry DB. A general method for bead-enhanced quantitation by flow cytometry. J Immunol Methods. 2006;317:45–55. doi: 10.1016/j.jim.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polakova K, Bennink JR, Yewdell JW, Bystricka M, Bandzuchova E, Russ G. Mild acid treatment induces cross-reactivity of 4H84 monoclonal antibody specific to nonclassical HLA-G antigen with classical HLA class I molecules. Hum Immunol. 2003;64:256–264. doi: 10.1016/s0198-8859(02)00777-2. [DOI] [PubMed] [Google Scholar]
- 18.Caramelli E, Rizzo N, Concu M, Simonazzi G, Carinci P, Bondavalli C, et al. Cell-free fetal DNA concentration in plasma of patients with abnormal uterine artery Doppler waveform and intrauterine growth restriction--a pilot study. Prenat Diagn. 2003;23:367–371. doi: 10.1002/pd.596. [DOI] [PubMed] [Google Scholar]
- 19.Robins JC, Heizer A, Hardiman A, Hubert M, Handwerger S. Oxygen tension directs the differentiation pathway of human cytotrophoblast cells. Placenta. 2007;28:1141–1146. doi: 10.1016/j.placenta.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Lyall F. Priming and remodelling of human placental bed spiral arteries during pregnancy--a review. Placenta. 2005;26 Suppl A:S31–S36. doi: 10.1016/j.placenta.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Jones CJ, Fox H. An ultrahistochemical study of the distribution of acid and alkaline phosphatases in placentae from normal and complicated pregnancies. J Pathol. 1976;118:143–151. doi: 10.1002/path.1711180303. [DOI] [PubMed] [Google Scholar]
- 22.Matijevic R, Meekins JW, Walkinshaw SA, Neilson JP, McFadyen IR. Spiral artery blood flow in the central and peripheral areas of the placental bed in the second trimester. Obstet Gynecol. 1995;86:289–292. doi: 10.1016/0029-7844(95)00129-f. [DOI] [PubMed] [Google Scholar]
- 23.von Rango U, Krusche CA, Kertschanska S, Alfer J, Kaufmann P, Beier HM. Apoptosis of extravillous trophoblast cells limits the trophoblast invasion in uterine but not in tubal pregnancy during first trimester. Placenta. 2003;24:929–940. doi: 10.1016/s0143-4004(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 24.Redman CW, Sargent IL. Circulating Microparticles in Normal Pregnancy and Pre-Eclampsia. Placenta. 2008 doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Lok CA, Van Der Post JA, Sargent IL, Hau CM, Sturk A, Boer K, et al. Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertens Pregnancy. 2008;27:344–360. doi: 10.1080/10641950801955733. [DOI] [PubMed] [Google Scholar]
- 26.Hunt JS, Jadhav L, Chu W, Geraghty DE, Ober C. Soluble HLA-G circulates in maternal blood during pregnancy. Am J Obstet Gynecol. 2000;183:682–688. doi: 10.1067/mob.2000.106762. [DOI] [PubMed] [Google Scholar]
- 27.VanWijk MJ, Nieuwland R, Boer K, van der Post JA, VanBavel E, Sturk A. Microparticle subpopulations are increased in preeclampsia: possible involvement in vascular dysfunction? Am J Obstet Gynecol. 2002;187:450–456. doi: 10.1067/mob.2002.124279. [DOI] [PubMed] [Google Scholar]
- 28.Lok CA, Boing AN, Sargent IL, Sooranna SR, van der Post JA, Nieuwland R, et al. Circulating platelet-derived and placenta-derived microparticles expose Flt-1 in preeclampsia. Reprod Sci. 2008;15:1002–1010. doi: 10.1177/1933719108324133. [DOI] [PubMed] [Google Scholar]
- 29.Farina A, Sekizawa A, Iwasaki M, Matsuoka R, Ichizuka K, Okai T. Total cell-free DNA (beta-globin gene) distribution in maternal plasma at the second trimester: a new prospective for preeclampsia screening. Prenat Diagn. 2004;24:722–726. doi: 10.1002/pd.973. [DOI] [PubMed] [Google Scholar]
- 30.VanWijk MJ, Boer K, van der Meulen ET, Bleker OP, Spaan JA, VanBavel E. Resistance artery smooth muscle function in pregnancy and preeclampsia. Am J Obstet Gynecol. 2002;186:148–154. doi: 10.1067/mob.2002.119184. [DOI] [PubMed] [Google Scholar]
- 31.Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension. 2002;39:155–160. doi: 10.1161/hy0102.100778. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Quintero VH, Smarkusky LP, Jimenez JJ, Mauro LM, Jy W, Hortsman LL, et al. Elevated plasma endothelial microparticles: preeclampsia versus gestational hypertension. Am J Obstet Gynecol. 2004;191:1418–1424. doi: 10.1016/j.ajog.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 33.Yie SM, Li LH, Li YM, Librach C, et al. HLA-G protein concentrations in maternal serum and placental tissue are decreased in preeclampsia. Am J Obstet Gynecol. 2004;191:525–529. doi: 10.1016/j.ajog.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Hackmon R, Koifman A, Hyodo H, Glickman H, Sheiner E, Geraghty DE. Reduced third-trimester levels of soluble human leukocyte antigen G protein in severe preeclampsia. Am J Obstet Gynecol. 2007;197:255. doi: 10.1016/j.ajog.2007.06.033. e1–5. [DOI] [PubMed] [Google Scholar]
- 35.Melchiorre K, Wormald B, Leslie K, Bhide A, Thilaganathan B. First-trimester uterine artery Doppler indices in term and preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:133–137. doi: 10.1002/uog.5400. [DOI] [PubMed] [Google Scholar]
- 36.Yie SM, Taylor RN, Librach C. Low plasma HLA-G protein concentrations in early gestation indicate the development of preeclampsia later in pregnancy. Am J Obstet Gynecol. 2005;193:204–208. doi: 10.1016/j.ajog.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 37.Kemp B, Kertschanska S, Kadyrov M, Rath W, Kaufmann P, Huppertz B. Invasive depth of extravillous trophoblast correlates with cellular phenotype: a comparison of intra- and extrauterine implantation sites. Histochem Cell Biol. 2002;117:401–414. doi: 10.1007/s00418-002-0396-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.