Abstract
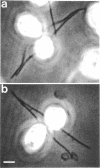
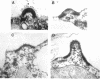
Sexual differentiation in the heterothallic alga Chlamydomonas reinhardtii is controlled by two mating-type loci, mt+ and mt-, which behave as a pair of alleles but contain different DNA sequences. A mutation in the mt minus-linked imp11 gene has been shown previously to convert a minus gamete into a pseudo-plus gamete that expresses all the plus gametic traits except the few encoded by the mt+ locus. Here we describe the iso1 mutation which is unlinked to the mt- locus but is expressed only in minus gametes (sex-limited expression). A population of minus gametes carrying the iso1 mutation behaves as a mixture of minus and pseudo-plus gametes: the gametes isoagglutinate but they do not fuse to form zygotes. Further analysis reveals that individual gametes express either plus or minus traits: a given cell displays one type of agglutinin (flagellar glycoprotein used for sexual adhesion) and one type of mating structure. The iso1 mutation identifies a gene unlinked to the mating-type locus that is involved in sex determination and the repression of plus-specific genes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair W. S. Characterization of Chlamydomonas sexual agglutinins. J Cell Sci Suppl. 1985;2:233–260. doi: 10.1242/jcs.1985.supplement_2.13. [DOI] [PubMed] [Google Scholar]
- Adair W. S., Monk B. C., Cohen R., Hwang C., Goodenough U. W. Sexual agglutinins from the Chlamydomonas flagellar membrane. Partial purification and characterization. J Biol Chem. 1982 Apr 25;257(8):4593–4602. [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Adair W. S., Mecham R. P., Heuser J. E. Chlamydomonas agglutinin is a hydroxyproline-rich glycoprotein. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5898–5901. doi: 10.1073/pnas.80.19.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debuchy R., Purton S., Rochaix J. D. The argininosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J. 1989 Oct;8(10):2803–2809. doi: 10.1002/j.1460-2075.1989.tb08426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersold W. T. Chlamydomonas reinhardi: heterozygous diploid strains. Science. 1967 Jul 28;157(3787):447–449. doi: 10.1126/science.157.3787.447. [DOI] [PubMed] [Google Scholar]
- Ferris P. J., Goodenough U. W. The mating-type locus of Chlamydomonas reinhardtii contains highly rearranged DNA sequences. Cell. 1994 Mar 25;76(6):1135–1145. doi: 10.1016/0092-8674(94)90389-1. [DOI] [PubMed] [Google Scholar]
- Ferris P. J., Goodenough U. W. Transcription of novel genes, including a gene linked to the mating-type locus, induced by Chlamydomonas fertilization. Mol Cell Biol. 1987 Jul;7(7):2360–2366. doi: 10.1128/mcb.7.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest C. L., Goodenough D. A., Goodenough U. W. Flagellar membrane agglutination and sexual signaling in the conditional GAM-1 mutant of Chlamydomonas. J Cell Biol. 1978 Oct;79(1):74–84. doi: 10.1083/jcb.79.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest C. L., Togasaki R. K. Selection for conditional gametogenesis in Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3652–3655. doi: 10.1073/pnas.72.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway R. E., Goodenough U. W. Genetic analysis of mating locus linked mutations in Chlamydomonas reinhardii. Genetics. 1985 Nov;111(3):447–461. doi: 10.1093/genetics/111.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W., Detmers P. A., Hwang C. Activation for cell fusion in Chlamydomonas: analysis of wild-type gametes and nonfusing mutants. J Cell Biol. 1982 Feb;92(2):378–386. doi: 10.1083/jcb.92.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W., Hwang C., Martin H. Isolation and genetic analysis of mutant strains of Chlamydomonas reinhardi defective in gametic differentiation. Genetics. 1976 Feb;82(2):169–186. doi: 10.1093/genetics/82.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W., Hwang C., Warren A. J. Sex-limited expression of gene Loci controlling flagellar membrane agglutination in the chlamydomonas mating reaction. Genetics. 1978 Jun;89(2):235–243. doi: 10.1093/genetics/89.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W. Tipping of flagellar agglutinins by gametes of Chlamydomonas reinhardtii. Cell Motil Cytoskeleton. 1993;25(2):179–189. doi: 10.1002/cm.970250207. [DOI] [PubMed] [Google Scholar]
- Goodenough U. W., Weiss R. L. Gametic differentiation in Chlamydomonas reinhardtii. III. Cell wall lysis and microfilament-associated mating structure activation in wild-type and mutant strains. J Cell Biol. 1975 Dec;67(3):623–637. doi: 10.1083/jcb.67.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupner G., Wills K. N., Tzukerman M., Zhang X. K., Pfahl M. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature. 1989 Aug 24;340(6235):653–656. doi: 10.1038/340653a0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Dec;52(4):536–553. doi: 10.1128/mr.52.4.536-553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J. A., Dutcher S. K. Cellular asymmetry in Chlamydomonas reinhardtii. J Cell Sci. 1989 Oct;94(Pt 2):273–285. doi: 10.1242/jcs.94.2.273. [DOI] [PubMed] [Google Scholar]
- Hunnicutt G. R., Snell W. J. Rapid and slow mechanisms for loss of cell adhesiveness during fertilization in Chlamydomonas. Dev Biol. 1991 Sep;147(1):216–224. doi: 10.1016/s0012-1606(05)80019-3. [DOI] [PubMed] [Google Scholar]
- Kindle K. L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Fukuzawa H., Shimada T., Saito T., Matsuda Y. Primary structure and expression of a gamete lytic enzyme in Chlamydomonas reinhardtii: similarity of functional domains to matrix metalloproteases. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4693–4697. doi: 10.1073/pnas.89.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Maheswaran S., Park S., Bernard A., Morris J. F., Rauscher F. J., 3rd, Hill D. E., Haber D. A. Physical and functional interaction between WT1 and p53 proteins. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5100–5104. doi: 10.1073/pnas.90.11.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N. C., Goodenough U. W. Gametic differentiation in Chlamydomonas reinhardtii. I. Production of gametes and their fine structure. J Cell Biol. 1975 Dec;67(3):587–605. doi: 10.1083/jcb.67.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. N., Yamamoto K. R. Regulatory crosstalk at composite response elements. Trends Biochem Sci. 1991 Nov;16(11):423–426. doi: 10.1016/0968-0004(91)90168-u. [DOI] [PubMed] [Google Scholar]
- Newman S. M., Boynton J. E., Gillham N. W., Randolph-Anderson B. L., Johnson A. M., Harris E. H. Transformation of chloroplast ribosomal RNA genes in Chlamydomonas: molecular and genetic characterization of integration events. Genetics. 1990 Dec;126(4):875–888. doi: 10.1093/genetics/126.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale S. M., Goodenough U. W. Cyclic AMP functions as a primary sexual signal in gametes of Chlamydomonas reinhardtii. J Cell Biol. 1987 Nov;105(5):2279–2292. doi: 10.1083/jcb.105.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional control of sexuality in Chlamydomonas reinhardi. J Gen Physiol. 1954 Jul 20;37(6):729–742. doi: 10.1085/jgp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Matsuda Y. Sexual agglutinin of mating-type minus gametes in Chlamydomonas reinhardii. I. Loss and recovery of agglutinability of gametes treated with EDTA. Exp Cell Res. 1984 Jun;152(2):322–330. doi: 10.1016/0014-4827(84)90634-7. [DOI] [PubMed] [Google Scholar]
- Snell W. J., Moore W. S. Aggregation-dependent turnover of flagellar adhesion molecules in Chlamydomonas gametes. J Cell Biol. 1980 Jan;84(1):203–210. doi: 10.1083/jcb.84.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlund A., Botchan M. R. The E2 trans-activator can act as a repressor by interfering with a cellular transcription factor. Genes Dev. 1990 Jan;4(1):123–136. doi: 10.1101/gad.4.1.123. [DOI] [PubMed] [Google Scholar]
- Tam L. W., Lefebvre P. A. Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics. 1993 Oct;135(2):375–384. doi: 10.1093/genetics/135.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waffenschmidt S., Woessner J. P., Beer K., Goodenough U. W. Isodityrosine cross-linking mediates insolubilization of cell walls in Chlamydomonas. Plant Cell. 1993 Jul;5(7):809–820. doi: 10.1105/tpc.5.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. P., Goodenough U. W. Molecular characterization of a zygote wall protein: an extensin-like molecule in Chlamydomonas reinhardtii. Plant Cell. 1989 Sep;1(9):901–911. doi: 10.1105/tpc.1.9.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gromoff E. D., Beck C. F. Genes expressed during sexual differentiation of Chlamydomonas reinhardtii. Mol Gen Genet. 1993 Nov;241(3-4):415–421. doi: 10.1007/BF00284695. [DOI] [PubMed] [Google Scholar]