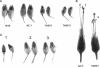Abstract
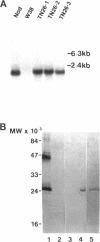
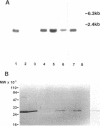
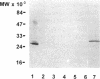
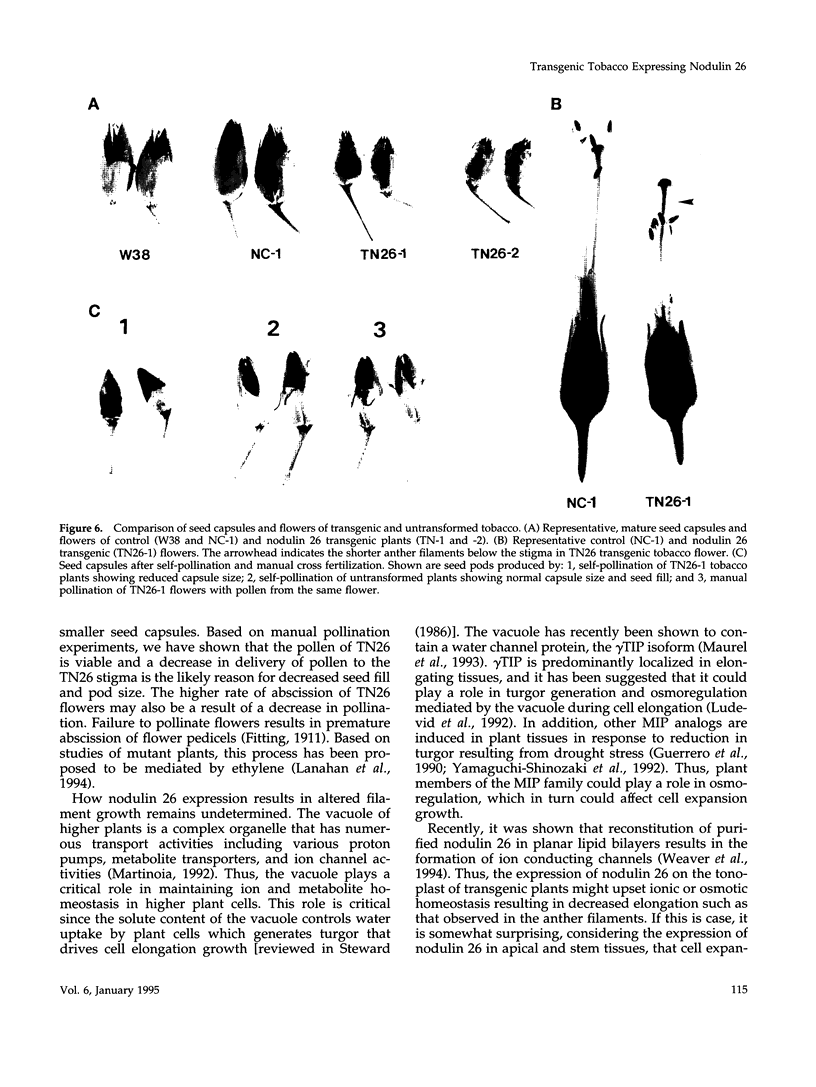
Nodulin 26 is an integral membrane protein of the symbiosome membrane of nitrogen-fixing soybean nodules. We expressed a nodulin 26 cDNA in transgenic tobacco (TN26 tobacco) under the control of the cauliflower mosaic virus 35S promoter to study subcellular targeting and the physiological effect(s) of its expression. Based on Northern and Western blots, the expression of nodulin 26 mRNA and protein in transgenic plants is high in apical shoot sections, flowers, and stems, low in mature leaves, and absent in roots. Western blot analysis revealed high levels of transgenic nodulin 26 protein in tonoplast membranes. In contrast, nodulin 26 protein was not found in isolated plasma membranes, the soluble fraction, nor in chloroplast and mitochondria-enriched membrane fractions. About 50-60% of the flowers and pods from TN26 tobacco plants abscised prematurely. Seed capsule size and seed fill per capsule from the remainder of surviving flowers were about 50% of that of control plants. Pollen viability was found to be normal, but flowers from TN26 tobacco plants showed shorter anther filaments compared with control plants. Normal seed production and capsule size was restored by manually crossing the stigmas from TN26 plants with isolated pollen from either transgenic or control plants. Thus, the aberrant filament growth could have resulted in the reproductive defects associated with the plants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Fortin M. G., Rea P. A., Verma D. P., Poole R. J. Presence of Host-Plasma Membrane Type H-ATPase in the Membrane Envelope Enclosing the Bacteroids in Soybean Root Nodules. Plant Physiol. 1985 Aug;78(4):665–672. doi: 10.1104/pp.78.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cheon C. I., Hong Z., Verma D. P. Nodulin-24 follows a novel pathway for integration into the peribacteroid membrane in soybean root nodules. J Biol Chem. 1994 Mar 4;269(9):6598–6602. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Raikhel N. V. Short peptide domains target proteins to plant vacuoles. Cell. 1992 Feb 21;68(4):613–616. doi: 10.1016/0092-8674(92)90134-x. [DOI] [PubMed] [Google Scholar]
- Ehring G. R., Zampighi G., Horwitz J., Bok D., Hall J. E. Properties of channels reconstituted from the major intrinsic protein of lens fiber membranes. J Gen Physiol. 1990 Sep;96(3):631–664. doi: 10.1085/jgp.96.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M. G., Morrison N. A., Verma D. P. Nodulin-26, a peribacteroid membrane nodulin is expressed independently of the development of the peribacteroid compartment. Nucleic Acids Res. 1987 Jan 26;15(2):813–824. doi: 10.1093/nar/15.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M. G., Zelechowska M., Verma D. P. Specific targeting of membrane nodulins to the bacteroid-enclosing compartment in soybean nodules. EMBO J. 1985 Dec 1;4(12):3041–3046. doi: 10.1002/j.1460-2075.1985.tb04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. M. The identification of subcellular fractions from mammalian cells. Methods Mol Biol. 1993;19:1–18. doi: 10.1385/0-89603-236-1:1. [DOI] [PubMed] [Google Scholar]
- Guerrero F. D., Jones J. T., Mullet J. E. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant Mol Biol. 1990 Jul;15(1):11–26. doi: 10.1007/BF00017720. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Chrispeels M. J. Protein sorting to the vacuolar membrane. Plant Cell. 1992 Aug;4(8):995–1004. doi: 10.1105/tpc.4.8.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Hubbard L., Reizer J., Ludevid D., Herman E. M., Chrispeels M. J. Vegetative and Seed-Specific Forms of Tonoplast Intrinsic Protein in the Vacuolar Membrane of Arabidopsis thaliana. Plant Physiol. 1992 Jun;99(2):561–570. doi: 10.1104/pp.99.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Herman E. M., Chrispeels M. J. An abundant, highly conserved tonoplast protein in seeds. Plant Physiol. 1989 Nov;91(3):1006–1013. doi: 10.1104/pp.91.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanahan M. B., Yen H. C., Giovannoni J. J., Klee H. J. The never ripe mutation blocks ethylene perception in tomato. Plant Cell. 1994 Apr;6(4):521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludevid D., Höfte H., Himelblau E., Chrispeels M. J. The Expression Pattern of the Tonoplast Intrinsic Protein gamma-TIP in Arabidopsis thaliana Is Correlated with Cell Enlargement. Plant Physiol. 1992 Dec;100(4):1633–1639. doi: 10.1104/pp.100.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C., Reizer J., Schroeder J. I., Chrispeels M. J., Saier M. H., Jr Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J Biol Chem. 1994 Apr 22;269(16):11869–11872. [PubMed] [Google Scholar]
- Maurel C., Reizer J., Schroeder J. I., Chrispeels M. J. The vacuolar membrane protein gamma-TIP creates water specific channels in Xenopus oocytes. EMBO J. 1993 Jun;12(6):2241–2247. doi: 10.1002/j.1460-2075.1993.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao G. H., Hong Z., Verma D. P. Topology and phosphorylation of soybean nodulin-26, an intrinsic protein of the peribacteroid membrane. J Cell Biol. 1992 Jul;118(2):481–490. doi: 10.1083/jcb.118.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Matsuoka K. Protein targeting to the vacuole in plant cells. Plant Physiol. 1993 Jan;101(1):1–5. doi: 10.1104/pp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Reizer J., Reizer A., Saier M. H., Jr The MIP family of integral membrane channel proteins: sequence comparisons, evolutionary relationships, reconstructed pathway of evolution, and proposed functional differentiation of the two repeated halves of the proteins. Crit Rev Biochem Mol Biol. 1993;28(3):235–257. doi: 10.3109/10409239309086796. [DOI] [PubMed] [Google Scholar]
- Roberts D. M., Besl L., Oh S. H., Masterson R. V., Schell J., Stacey G. Expression of a calmodulin methylation mutant affects the growth and development of transgenic tobacco plants. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8394–8398. doi: 10.1073/pnas.89.17.8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L. E., Stacey G. Bacterium release into host cells of nitrogen-fixing soybean nodules: the symbiosome membrane comes from three sources. Eur J Cell Biol. 1989 Jun;49(1):13–23. [PubMed] [Google Scholar]
- Sandal N. N., Marcker K. A. Soybean nodulin 26 is homologous to the major intrinsic protein of the bovine lens fiber membrane. Nucleic Acids Res. 1988 Oct 11;16(19):9347–9347. doi: 10.1093/nar/16.19.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A., Kent N. A., McHale M., Bangham J. A. Homology of MIP26 to Nod26. Nucleic Acids Res. 1988 Oct 11;16(19):9348–9348. doi: 10.1093/nar/16.19.9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer R., Matzeit V., Gronenborn B., Schell J., Steinbiss H. H. A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res. 1987 Jul 24;15(14):5890–5890. doi: 10.1093/nar/15.14.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi M. K., Day D. A. Electrogenic ATPase Activity on the Peribacteroid Membrane of Soybean (Glycine max L.) Root Nodules. Plant Physiol. 1989 Jul;90(3):982–987. doi: 10.1104/pp.90.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Reinders A., Hsu H. T., Sze H. Dissociation and Reassembly of the Vacuolar H-ATPase Complex from Oat Roots. Plant Physiol. 1992 May;99(1):161–169. doi: 10.1104/pp.99.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. D., Crombie B., Stacey G., Roberts D. M. Calcium-dependent phosphorylation of symbiosome membrane proteins from nitrogen-fixing soybean nodules : evidence for phosphorylation of nodulin-26. Plant Physiol. 1991 Jan;95(1):222–227. doi: 10.1104/pp.95.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. D., Shomer N. H., Louis C. F., Roberts D. M. Nodulin 26, a nodule-specific symbiosome membrane protein from soybean, is an ion channel. J Biol Chem. 1994 Jul 8;269(27):17858–17862. [PubMed] [Google Scholar]
- Williamson J. D., Hirsch-Wyncott M. E., Larkins B. A., Gelvin S. B. Differential Accumulation of a Transcript Driven by the CaMV 35S Promoter in Transgenic Tobacco. Plant Physiol. 1989 Aug;90(4):1570–1576. doi: 10.1104/pp.90.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoek A. N., Verkman A. S. Functional reconstitution of the isolated erythrocyte water channel CHIP28. J Biol Chem. 1992 Sep 15;267(26):18267–18269. [PubMed] [Google Scholar]