Abstract
In Drosophila, dosage compensation of the single male X chromosome involves upregulation of expression of X linked genes. Dosage compensation complex or the male specific lethal (MSL) complex is intimately involved in this regulation. The MSL complex members decorate the male X chromosome by binding on hundreds of sites along the X chromosome. Recent genome wide analysis has brought new light into X chromosomal regulation. It is becoming increasingly clear that although the X chromosome achieves male specific regulation via the MSL complex members, a number of general factors also impinge on this regulation. Future studies integrating these aspects promise to shed more light into this epigenetic phenomenon.
Keywords: X chromosome, MSL, Drosophila, transcription, chromatin, roX RNA, acetylation
Introduction
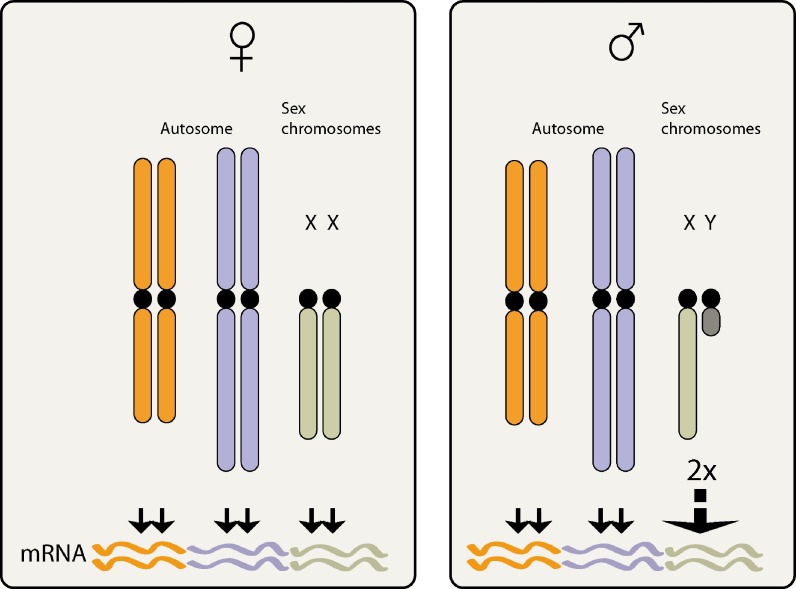
Dosage compensation is an exquisite example of a chromosome wide phenomenon for regulating gene expression (Payer and Lee 2008; Meyer 2005; Straub and Becker 2007; Mendjan and Akhtar 2007). In Drosophila this process involves upregulation of expression of X chromosomal genes in male flies by approximately two-fold in comparison to female flies which possess two X chromosomes (Fig. 1 ). In Drosophila melanogaster, dosage compensation is regulated by male specific lethal (MSL) factors whose product is essential for male survival. These factors are collectively called the MSL complex or the dosage compensation complex (DCC).
Fig. 1.
Dosage compensation in Drosophila melanogaster. Female cells have two X chromosomes, while males have one X and one degenerated Y chromosome. The male X produces the same dose of RNA as the female to compensate for the absence of homologue
Domain architecture
Who interacts with whom?
MSL1 and MSL2
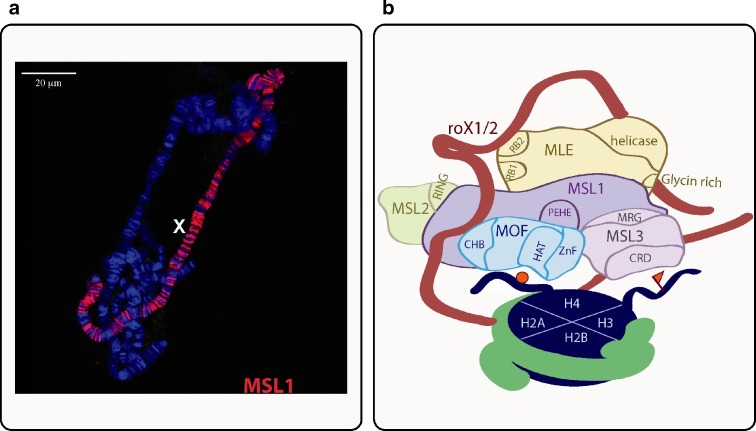
The MSL complex is a ribonucleoprotein complex that is composed of two long non-coding RNAs; roX1 or roX2 and at least five proteins namely MSL1, MSL2, MSL3, MOF and MLE (Fig. 2). All protein-coding genes are transcribed in both sexes (Kelley et al. 1995; Zhou et al. 1995; Gorman et al. 1995; Palmer et al. 1993; Kuroda et al. 1991; Hilfiker et al. 1997) however MSL2 mRNA translation is strictly inhibited by SXL, master sex regulator, in females (Beckmann et al. 2005). In the absence of MSL2, MSL1 protein is destabilized and presumably degraded because MSL1 protein is detected at very low level unless msl2 is expressed ectopically in females (Kelley et al. 1995). MSL1 can be regarded as an assembly platform of the complex because it interacts with all other protein members, except for MLE (Scott et al. 2000). Leucine zipper like motif at the amino (N) terminus interacts with MSL2 (Li et al. 2005) and carboxyl (C) terminus binds MOF and MSL3 (Scott et al. 2000). MSL3 and MOF contact occurs on different parts of MSL1, MSL3 being close to the C terminus and MOF with PEHE domain (Morales et al. 2004). MSL1 also contains a coiled coil domain however the importance of this domain has not been determined. MSL2 has a RING finger domain along with a cysteine rich motif at its C terminus (Zhou et al. 1995). The RING domain has two zinc finger clusters and mutations of polar residues chelating the first zinc ion have been shown to disrupt the interaction of MSL2 with MSL1 (Copps et al. 1998). Although the RING finger is conserved in many species, the novel combination of RING domain and cysteine cluster has been proposed to have an important contribution for the birth of ‘msl2 like’ genes and a driving force for the formation of compensasome (Marín 2003). MSL3 has an MRG domain located at the C-terminus that mediates the interaction with MSL1 (Morales et al. 2005). MRG domains are highly conserved in MRG gene family and they are thought to be interaction platforms in large complexes that are usually chromatin related (Bowman et al. 2006). Interestingly the MRG domain of MSL3 is interrupted by non-conserved sequences and the importance of these MSL3 specific linkers are yet to be determined (Morales et al. 2005).
Fig. 2.
MSL complex and known interactions. a) MSL complex binds specifically to X chromosome in male cells. This is clearly seen in polytene squashes of salivary glands from third instar male larvae. DNA is stained with Hoechst and MSL1 is detected by specific antibody. b) The MSL complex. MSL1 and MSL2 interact through RING domain of MSL2 and N terminus of MSL1. MOF chromobarrel (CHB) domain interacts with RNA. HAT (histone acetylase) domain acetylates H4K16 residue (represented by a red ball) and Zinc finger (ZnF) is important for the H4 specificity. MSL3 chromo- related domain (CRD) has been shown to bind to DNA and nucleosomes and been suggested to interact with tri-methylated H3 on K36 (H3K36me3 represented by red Flag). MSL3 and MOF bind MSL1 through ZnF and MRG domain, respectively. PEHE domain of MSL1 was shown to be crucial for MSL3 interaction. MLE has two RNA binding domains (RB1 and RB2) but only RB1 can bind RNA. Glycine rich region on the C terminus has a high affinity for RNAs. MLE could associate with the rest of the complex though RNA. The stoichiometry of the components and the mutual presence of roX RNAs are not known. The complex is not drawn to scale due to absence of any structural data therefore the figure must be seen as an artistic rendering of what is known
MOF and MSL3
The early observations of polytene squashes from the male larvae salivary glands revealed an interesting discovery that male X chromosome was enriched for a specific acetylation mark on the histone 4 lysine 16 (H4K16) (Turner et al. 1992). Observations of co-localization of this mark with MSL members and its absence in MSL mutants predicted a histone acetyltransferase (HAT) enzyme in the complex (Bone et al. 1994). Concordantly MOF, a member of MYST family of HATs, was shown to colocalize with MSL members and mark the male X chromosome on H4K16 (Gu et al. 1998, Hilfiker et al. 1997). MOF has a peculiar C2HC zinc finger motif, which mediates substrate recognition (Akhtar and Becker 2001), and its interaction with MSL1 (Morales et al. 2004). Although MOF binds to nucleosomes and its preferred substrate is H4 (Akhtar and Becker 2000; Smith et al. 2000), it can also acetylate MSL3 (Buscaino et al. 2003) and MSL1, nevertheless integration into the complex shifts the substrate specificity strongly to H4 (Morales et al. 2004). Both MOF and MSL3 have a chromo-related domain but accumulating evidence indicate that they may have different substrate specificities. Solution structure of MOF chromo-related domain revealed that this fold has five beta strands forming a barrel shape, therefore named as chromo-barrel domain (CBD), which is structurally similar to Tudor domains. This fold is quite different from chromodomains that bind lysine methylated histone tails through a hydrophobic cage formed by three conserved aromatic residues (Jacobs and Khorasanizadeh 2002; Nielsen et al. 2002). These residues are not found in MOF and in fact Arg387 of CBD may clash with superimposed methylated lysine H3 peptide (Nielsen et al. 2005). Instead MOF chromo-barrel domain in the context of the full-length protein is important for RNA binding activity in vivo and in vitro and mutation of a conserved tyrosine disrupts this interaction (Akhtar et al. 2000). Nucleic acid binding activity of a chromo domain is not unique to MOF but also well documented for dMi-2 protein of NuRD remodeling-HDAC complex in Drosophila (Bouazoune et al. 2002). Curiously, the CBD used for structure determination was not able to bind RNA by itself leading to the hypothesis that the fold may need additional surrounding residues for RNA binding activity (Nielsen et al. 2005). Supportive evidence for this idea came from the structural elucidation of yeast homologue of MOF, Esa1 chromo-domain (Shimojo et al. 2008). Computationally predicted Esa1 chromo-domain folds similar to MOF CBD and does not show nucleotide binding activity in vitro however structure of N terminally extended domain has an extra beta sheet which induces a loop in the barrel. This “knotted” barrel has a high affinity for RNA in vitro (Shimojo et al. 2008). In the same vein, MOF chromo-barrel domain could adopt a slightly different form in the context of full protein such that it has a high affinity for RNA.
MSL3 chromo related domain is predicted to fold similar to chromobarrel domain but unlike MOF, it contains the typical hydrophobic residues that forms the aromatic cage (Nielsen et al. 2005). MSL3 has been shown to bind nucleosomes that are methylated on H3K36 and mutation of hydrophobic residues causes the loss of this interaction (Sural et al. 2008, Larschan et al. 2007). Besides, the chromodomain by itself can bind to a nucleosomal template (Buscaino et al. 2006). Chromodomain structures of Eaf3 and MRG15, yeast and human homologue of MSL3, were solved and showed to bind methylated H3K36 (Zhang et al. 2006a; Sun et al. 2008). Nevertheless, Eaf3 interaction is a rather weak one and was suggested to take assistance from PhD finger for the optimal binding (Xu et al. 2008; Li et al. 2007). MRG15 binding to H3K36me3 is predicted to be different from canonical chromodomains because a beta strand in chromo-barrel preoccupies the histone peptide-binding groove (Zhang et al. 2006a). Interestingly, MSL3 chromo-related domain can bind DNA in vitro suggesting that nucleosome interaction may occur partly through DNA (Buscaino et al. 2006). Additionally, MSL3 localization to X-chromosome is lost upon RNAse treatment and MSL3 can bind roX RNA both in vivo and in vitro (Morales et al. 2005; Buscaino et al. 2003) therefore nucleic acid binding surfaces can be used to bind both RNA and DNA. Acetylated MSL3 cannot immunoprecipitate significant amounts of roX2 RNA; hence this posttranslational modification may be used as a regulatory switch for the substrate specificity of MSL3 (Buscaino et al. 2003). Structure determination of MSL3 will delineate the properties of this peculiar domain.
MLE
The second known enzyme associated with the MSL complex is MLE. MLE bears a modified DEAD box motif, DEIH, which is one of the key signatures for RNA helicases (Kuroda et al. 1991). Mutations in the DEAD box and the ATP binding pocket are lethal for flies (Richter et al. 1996; Lee et al. 1997). MLE fulfills single stranded nucleic acid binding, double stranded nucleic acid binding, ATPase activity and homo-hetero duplex unwinding activities in vitro (Lee et al. 1997). It has two RNA binding domains in the N terminal region but only RB2 domain was shown to bind RNA (Izzo et al. 2008). RNA binding and deletion of C terminal glycine rich region increases the ATPase activity (Izzo et al. 2008). Therefore MLE may undergo continuous self-regulation through its own domains. MLE X chromosome localization is RNAse sensitive and it has a very salt susceptible, weak interaction with the rest of the complex, suggesting the possibility that it may bind MSL proteins through an RNA intermediate, presumably the roX RNAs (Copps et al. 1998; Richter et al. 1996). However till today, direct MLE interaction with roX RNAs have not been shown.
roX RNAs
RoX RNAs were discovered in two different enhancer trap screens; one to look for sex specific expression in mushroom bodies of fly brains that causes dimorphic courtship behavior (Amrein and Axel 1997) and the other to look for differential expression of a reporter in mushroom bodies (Meller et al. 1997). These RNAs are male specific, confined to nucleus and expressed in all tissues of flies (Franke and Baker 1999; Amrein and Axel 1997; Meller et al. 1997). They both colocalize with MSL proteins along the X chromosome in males (Franke and Baker 1999). Although the big size and sequence difference, they are functionally redundant (Meller and Rattner 2002; Franke and Baker 1999). The only sequence similarity is a 30 bp sequence identity however deletion of this sequence has no phenotypic output (Kageyama et al. 2001). Many evidence suggest that roX RNAs exert their functions through a yet unpredictable tertiary structure or at least not in a strict sequence dependent manner. Additional to the inter-redundancy of roXs, there is also high intra-redundancy in each roX (Stuckenholz et al. 2003; Park et al. 2008). Successive ten percent deletions of roX1 and series of small deletions in roX2 do not change the male viability except for a region near 3′ end of roX1 that contains a predicted stem loop structure (Stuckenholz et al. 2003). Moreover, roX RNAs from other Drosophila species can be integrated into D.melonagester MSL complex in spite of the low sequence homology; 31% in the example of D.willistoni roX2 (Park et al. 2008). Recent findings suggest that there are evolutionary conserved “roX boxes” that may be the exchangeable functional units of rox RNAs (Park et al. 2008). Inarguably roX RNAs have physical contacts to the complex; they can be immunoprecipitated with MSL proteins and female expression of MSL2 causes stabilization of rox RNAs (Meller et al. 2000; Amrein and Axel 1997; Akhtar et al. 2000; Smith et al. 2000). Although, MLE, MOF and MSL3 have the capacity to bind roX RNAs, direct targets of roXs have not been determined but considering their size (roX1 3.7 kb and roX2 0.6 kb), it is plausible that they have several contact points. Interestingly, there is a time frame in the early hours of embryogenesis, where roX1 is transcribed in the absence of any detectable level of MSL components, but the rapid turnover of roX RNAs in the absence of the MSL complex led to the question how this stability is achieved. It was found that in the absence of maternal MLE, roX1 RNA is hardly detectable, therefore maternal stores of MLE can contribute to early stabilization of roX1 (Meller 2003).
Early events
What do females do?
The MSL complex formation is strictly inhibited in female cells, which is achieved by the master sex regulator, SXL protein. SXL is expressed only in females. In males, SXL pre-mRNA is spliced in a way that a premature stop codon is retained and the mRNA is degraded (Schütt and Nöthiger 2000). Many observations clearly showed MSL2 as the direct target of SXL. MSL2 mRNA has poly U stretches, binding sequences of SXL, in both UTRs and in males a 133 bp intron in 5′UTR, containing two of these binding sites are spliced out but retained in females (Bashaw and Baker 1995). Additionally transgenic constructs lacking poly U stretches enabled expression of MSL2 protein in females (Kelley et al. 1995). SXL modulates female specific intron retention by interfering with U2AF65 and U2AF35 snRNP interaction on the 3′ splice site (Merendino et al. 1999) and U1 recognition of the 5′ splice site (Förch et al. 2001). Msl2 mRNA translation inhibition occurs a by a dual mechanism, one conducted through 3′UTR binding and inhibition of 43S recruitment and the second one through 5′ UTR binding and prevention of 43S pre-initiation complex scanning (Beckmann et al. 2005; Grskovic et al. 2003; Gebauer et al. 2003; Kelley et al. 1997). 3′UTR control of SXL requires a co repressor, UNR, which is present in both male and female cytoplasm but is specifically recruited to the msl2 mRNA 3′UTR by SXL in females (Duncan et al. 2006; Abaza et al. 2006; Grskovic et al. 2003). Interestingly, UNR was recently found to have an important role in male dosage compensation (Patalano et al. 2009). Overexpression of UNR causes a preferential male lethality and loss of the MSL complex from the X chromosome. UNR also immnoprecipitates roX1 and roX2 however if this is a direct or indirect interaction has not been shown (Patalano et al. 2009).
Somatic versus germ line
Most of the available data on dosage compensation comes from observations of somatic cells, therefore much less is known how the male germ line deals with the dose problem. MLE had been known to function in spermatogenesis and consistently it can be detected in male germ line cells (Rastelli and Kuroda 1998). Interestingly MSL1, MSL2 and MSL3 are not observed in these cells and MLE, along with H4K16 acetylation, is not concentrated on X but rather scattered throughout the genome (Rastelli and Kuroda 1998; Rastelli et al. 1995). Nevertheless, expression microarray analysis showed that male germ line do compensate for the imbalance despite the absence of the MSL complex although the number of escaping genes are higher than those on the somatic cells (Gupta et al. 2006). The possibility of an MSL independent dosage compensation mechanism was pointed out before because msl mutant males can complete embryogenesis, survive up to third instar larvae—early pupae and additionally some genes are compensated in the absence of MSL, like runt (Baker et al. 1994). This mechanism could be the result of a complex buffering system inherent in genetic networks or another uncharacterized protein complex may function in the early dosage compensation (Zhang and Oliver 2007). Although the nature and the timing of an MSL independent mechanism is elusive, it appears that MSL mediated activation begins at blastoderm, coinciding with zygotic transcription start in embryos (Meller 2003; Franke et al. 1996; McDowell et al. 1996). In males with homozygous msl1, mle or msl3 mutant mothers, the onset of MSL detection on X chromosome is delayed; supporting the idea that maternal contribution may help balancing the low level of zygotic expression in initial stages of the MSL complex establishment (Franke et al. 1996; Rastelli et al. 1995). Initiation of dosage compensation relies on the expression of one of the roX RNAs (Meller 2003). In males, roX1 RNA transcription starts in the early stages of blastoderm (2 h After Egg Laying, AEL) and MSL2 localization to nuclei foci follows after. When roX1 is absent, MSL2 localization to the nuclear foci can only be seen after roX2 expression, which is nearly 6 h AEL. This may indicate that roX transcription may guide the MSL complex to the X chromosome.
Targeting the male X chromosome
Single gene versus global analyses
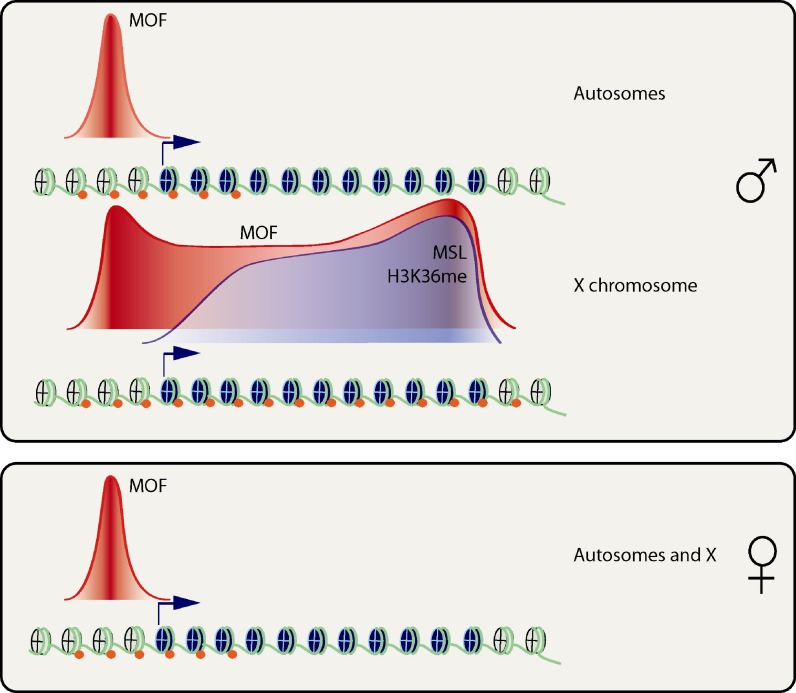
The advances of new technologies such as expression-arrays, high resolution tiling arrays and new generation sequencing technologies coupled with biochemical methods gave a totally new pace in understanding the mechanism of dosage compensation in Drosophila. MSL-chromatin interactions had often been carried out in polytene squashes of salivary glands by immunofluoresence but the resolution of this technique is low, therefore one big leap in the field occurred when MSL components were mapped in high resolution throughout the Drosophila genome by ChIP on chip method (Kind et al. 2008; Gilfillan et al. 2006; Legube et al. 2006; Alekseyenko et al. 2006; Alekseyenko et al. 2008; Straub et al. 2008). Although the immunoprecipitated proteins, cell type and embryonic stage are different in each case, common themes arose. First of all, not all genes on X are bound by the MSL complex and also there are a few autosomal sites that are clearly bound. The MSL complex members are mostly found on genes rather than intergenic sequences and when the binding profiles are averaged, a clear enrichment in the body and towards the end of the genes are observed (Gilfillan et al. 2006; Alekseyenko et al. 2006) (Fig. 3 ). Most target genes seem to be actively expressed however there is no significant correlation between the expression level and MSL abundance (Legube et al. 2006; Alekseyenko et al. 2006). Although most MSL target genes are actively transcribed, transcription per se is not sufficient to explain MSL binding because many genes that are bound by elongating form of RNAPII and canonical elongation factors are devoid of the MSL complex (Gilfillan et al. 2006; Legube et al. 2006). Also, MSL1 binding profiles of 4–6 embryos and third instar larvae salivary gland are fairly similar (Legube et al. 2006), supporting the notion that most compensated genes are selected early during development and bind irrespective of developmental changes (Kotlikova et al. 2006). Remarkably, MOF seems to have a unique status in the complex (Kind et al. 2008). Promoter binding of MOF is distributed throughout the whole genome in both males and females in an MSL independent manner whereas 3′ enrichment is restricted to the X chromosome in males and is MSL dependent (Kind et al. 2008). This led to the hypothesis that MOF plays an important role on the promoters of both sexes, and the MSL complex members binds this HAT to skew its location towards the end of the genes for specific acetylation and up-regulation of male X chromosome (Kind et al. 2008). Realization of 3′ enrichment of the MSL complex members stimulated investigation of associations to other well-known 3′ enriched epigenetic marks. Genome wide histone modifications from yeast and humans showed that Set2 dependent H3K36me3 is a conserved 3′ bias epigenetic mark associated with active genes (Barski et al. 2007; Pokholok et al. 2005). Mapping of H3K36me3 on X chromosome revealed that more than 90% of MSL targets are also enriched with this mark and there is a high correlation of MSL and H3K36me3 position on the gene (Larschan et al. 2007; Bell et al. 2008b). Interestingly, there seems to be a context dependent crosstalk between H3K36me3 and H4K16ac because reduction of Hypb, the enzyme required for final methylation state of H3K36 in Drosophila, causes a reduction of H4K16 acetylation mark on X-linked genes but not on autosomes (Bell et al. 2008b).
Fig. 3.
Global profiles of MSL components and associated histone marks averaged on a single transcriptional unit. In male cells, MSL components are enriched in the body of the genes peaking at the end along with the H3K36me3 mark. MOF shows a distribution peaking at promoters and at the 3′end of the genes. MOF peaks at the promoters are independent of MSL complex and also found in female cells and autosomal genes in males. H4K16 acetylation distribution tends to be broader in comparison to MOF distribution on target genes. Red balls indicate histone acetylation and transcriptional unit nucleosomes are depicted in blue
High affinity sites
One of the most obvious questions of dosage compensation is how the MSL complex recognizes the X chromosome specifically. An intriguing feature of the MSL complex is that partial complexes of MSL1 and MSL2 are able to bind 30–40 bands in polytene squashes in the absence of other MSL3, MOF or MLE, which have been named as Chromatin Entry Sites (CES) (Palmer et al. 1994; Lyman et al. 1997). MSL1 and MSL2 behave as the core of the complex because they are strictly dependent on each other for stability and they can localize to X without the other partners (Lyman et al. 1997; Kelley et al. 1995; Zhou et al. 1995). Surprisingly, roX1 and roX2 sites were the first CES to be mapped, due to their ability to recruit MSL components upon translocation to an autosomal site (Meller et al. 2000; Kelley et al. 1999). This feature of roX genes does not depend on their transcription but on a DNAse hypersensitive site (DHS) that can bind the MSL complex (Park et al. 2003; Kageyama et al. 2001). Initially, CES were thought to be the only sites for early MSL binding however, investigation of large X to autosome translocations showed that any segment of X was able to recruit MSL complexes even if they do not possess a previously mapped CES (Fagegaltier and Baker 2004; Oh et al. 2004). Moreover, translocated genomic segments from autosomes to X were devoid of MSL complexes (Oh et al. 2004). Identification of other MSL1 binding fragments by ChIP showed that only a subset of these fragments are able to recruit MSL1/2 when moved to autosomes, and the rest can do so only in the presence of over expressed MSL1/2 (Dahlsveen et al. 2006). Therefore, X chromosome seems to have a gradient of potential to recruit the MSL complex, named as the ‷“affinity model”(Fagegaltier and Baker 2004). Some sequences can recruit MSL independent of any apparent targeting determinant, called High Affinity Sites and other sequences, Low Affinity Sites, can only do so by the help of other mechanisms. The cipher of high affinity sites had remained a mystery due to absence of advance sequence algorithms and low number of mapped sites. But high throughput experiments discovered important clues about this mystery. High-resolution binding profiles of MSL1 and MSL2 in the absence of other MSL components revealed more than 130 Chromatin Entry sites (CES) or High Affinity sites (HAS) and a GA rich motif named as MRE motif (MSL Recognition) in these sites (Straub et al. 2008; Alekseyenko et al. 2008). Remarkably, this motif is slightly enriched on X chromosome and autosomal transposition of a minimal CES, containing as few as three MRE elements was able to recruit MSL, and up-regulate the upstream reporter gene (Alekseyenko et al. 2008). Since there are thousands of similar motifs scattered around the Drosophila genome, the choice of X chromosome still remains an unsolved issue. However, H3 depletion around the CES site indicates that accessibility could be an important player (Straub et al. 2008; Alekseyenko et al. 2008).
The importance of roX RNAs for the initial targeting have been shown in a number of cases (Ilik and Akhtar 2009). MSL protein complex per se have a weak affinity towards the chromatin but this is greatly enhanced with the presence and/or integration of roX RNAs (Li et al. 2008; Oh et al. 2003; Meller and Rattner 2002). In the absence of both roX RNAs, partial MSL complex can be located on a few X chromosome loci, autosomal loci and chromocenter (Li et al. 2008; Oh et al. 2003; Park et al. 2002; Meller and Rattner 2002). These sites have the intact complexes because all MSL proteins and H4K16 acetylation are seen coincidently (Meller and Rattner 2002; Oh et al. 2003). Moreover, overexpression of MSL1 and MSL2 can rescue male lethality to some extent (Oh et al. 2003). Nevertheless, there are opposing reports claiming that even though roX mutants are so severe that allows no detection by any means, they may still contribute to targeting and dosage compensation (Deng and Meller 2009; Deng et al. 2005). Therefore the role of roX RNAs in targeting is still an open issue.
Is the presence of entry sites on roX genes a coincidence? Compelling evidence suggest that the complex forms on the site of roX transcription in a co-transcriptional manner (Kelley et al. 2008; Oh et al. 2003). It is plausible that roX genes have acquired an entry site to establish the complex formation more efficiently. On the other hand, roX1 DHS was shown to play a role in roX1 transcription activation in males and MSL2 was shown to be important for this role as an independent task from the MSL complex (Bai et al. 2004; Rattner and Meller 2004). Similarly roX2 gene was found to have elements that bind MLE and regulates its transcription (Lee et al. 2004). Therefore, a complex regulatory network that contains components of the MSL complex may fine tune roX transcription and eventually formation of the MSL complex.
Beyond the high affinity sites
How is the complex located further from the high affinity sites? An intriguing observation upon roX gene translocation to autosomes was the spreading of the complex from the site of insertion in cis (Meller et al. 2000; Kelley et al. 1999). This spreading depends on the site of insertion, amount of the MSL complex, and presence of the competing roX transgene (Park et al. 2002). Because other large X to autosome translocations did not show any cis spreading, roX situation was pointed to be a unique feature of roX genes due to their function as the site of complex formation. (Fagegaltier and Baker 2004). MOF enzymatic activity is required for localization to low affinity sites but it is not known if this is due to its canonical histone acetylation activity or another protein acetylation event that may help maturation of the MSL complex (Buscaino et al. 2003; Gu et al. 2000). MLE helicase activity is also found to be important for LAS localization (Morra et al. 2008). Since MLE is required for the roX association into the complex (Meller et al. 2000), the phenotype can be a downstream result of an incomplete complex that is not able to spread further.
Enrichment of H3K36 methylation on actively transcribed genes prompted investigation of MSL3 as it contains a chromo related domain that could be a good candidate that can bind to this mark. By ChIP-chip analysis, it was seen that MSL3 chromodomain mutants, including the deletion mutant were enriched around the high affinity sites suggesting the requirement of intact chromo domain for spreading (Sural et al. 2008). In view of these results, Sural et al, proposes a two-step model, in which sequence dependent initial targeting to X chromosome is established on MRE containing chromatin entry sites and is followed by H3K36me3-chromodomain mediated spreading, analogous to heterochromatin spreading (Sural et al. 2008). Surprisingly, transgenic flies carrying the chromodomain mutants have a range of phenotypes ranging from fully viable to developmentally delayed to severely affecting male viability (Sural et al. 2008; Buscaino et al. 2006). This already suggests that chromodomain mediated spreading from high affinity sites cannot be the sole mechanism for the MSL binding pattern throughout the X chromosome. Transcription was noticed as a good candidate inferred from the high correlation between active transcriptional state and MSL binding. Indeed when MOF gene, a low affinity site, is translocated to an autosomal site, it can recruit MSL1 only in the presence of an endogenous or an exogenous promoter (Kind and Akhtar 2007). When the promoter is absent, blocked or reversed, MSL1 cannot bind. Effect of transcription extends beyond the MOF gene because blocking of RNA polymerase II by alpha amanitin decreases the occupancy MSL components on X-linked genes (Kind and Akhtar 2007). Binding of MSL1 after a strong activation of X linked genes by a Gal4 induced promoter had also been observed before (Sass et al. 2003). The passage of RNA Polymerase II may either expose targeting sequences that are normally hidden or it can change the chromatin marks such that the gene becomes a better target for the MSL complex. All these models are not mutually exclusive and genes may have evolved different strategies to recruit MSL depending on their need to compensate, their inherent affinity towards MSL or their plasticity during the development. One attractive possibility could be that transcription in combination with MSL proteins that recognize chromatin marks on active genes could facilitate spreading along the X chromosome.
Evolutionary considerations
Dosage compensation arises as an inevitable consequence of sex chromosome evolution. In Drosophila species, the dose problem begins with the evolution of Y chromosome (Carvalho 2002). Although the exact nature of Y chromosome formation is under debate (Carvalho 2002) the current model predicts the random acquisition of a male determining gene on an ancient autosome and prevention of recombination of that locus (Steinemann and Steinemann 2005). This leads to a strong tendency to accumulate degenerative mutations like transposition, duplication and finally the heterochromatinization of the whole chromosome (Steinemann and Steinemann 2005). Therefore the male cell nucleus is forced to balance this hemizygocity by formation of a novel complex acting on the X chromosome. There are plethora of evidence that once the MSL complex evolved, it was co-opted in other Drosophila species, which have a different sex chromosome history (Marín et al. 1996). In D. pseudoobscura, a fusion event between an autosomal chromosome (Muller D element) and original X chromosome (Muller A element) led to formation of a metacentric X chromosome and the similarization of both arms in terms of sequence identity (Gallach et al. 2007). Interestingly, the autosomal homolog of D element is lost in males and the newly translocated hemizygous arm is bound by MSL complex and acetylated on H4K16 (Bone and Kuroda 1996). On the contrary, in D. americana americana, a similar fusion event occurred but the males kept the autosomal homologue (Neo-Y) chromosome (Charlesworth et al. 1997). Since the two homologues can still recombine, there is no sign of degeneration on the neo-Y and no MSL binding on the X chromosome (Bone and Kuroda 1996).
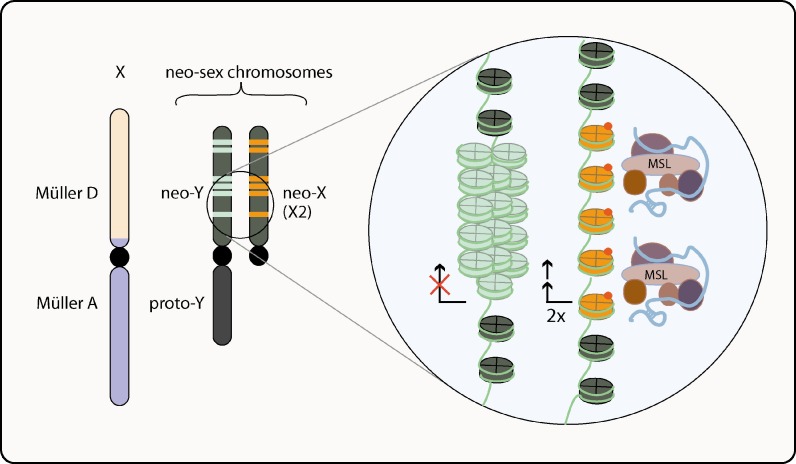
Perhaps the best tool to study the evolution of sex chromosomes and dosage compensation is the neo-sex chromosomes of D.miranda. D.miranda is closely related to D. Pseudoobscura and has the same metacentric X chromosome, which is fully dosage compensated. But in addition, a Robertsonian translocation of an autosome (Muller C element) to the Y chromosome generated a neo-Y chromosome (Macknight 1939). The fusion event is thought to occur about 2 million years ago and the homologue pair is kept in the male cells (neo-X chromosome) (Norman and Doane 1990). After the fusion event, neo-Y chromosome had undergone extensive random degeneration, including retrotransposition, duplication and nonsense mutations but most loci are still intact (Bachtrog 2006). Amazingly, the neo-X chromosome recruits MSL complex and acetylates H4K16 to the loci that are degenerating in the neo-Y homologue (Fig. 4 ) (Marín et al. 1996, Bone and Kuroda 1996). Interestingly, the core promoters of the lcp1-4 genes that are upregulated in the neo-X in response to degeneration in neo-Y show no apparent significant sequence alteration that could lead to two-fold upregulation (Steinemann and Steinemann 2007). Moreover, significant sequence variation of the neo-X chromosome from the old X shows that multiple selective sweeps of cis-acting regulatory regions did not occur (Yi et al. 2003). These results show that MSL complex recruitment may not require a strict gene-by-gene basis cis-acting sequence evolution.
Fig. 4.
Dosage compensation in Drosophila miranda. In male cells the proto X chromosome, (which is indicated by Muller A + Muller D) is compensated normally as in D.melanogaster. A translocation event of an autosomal arm onto the proto-Y chromosome created a neo-Y chromosome, which is in the process of degeneration. The degenerated loci are indicated as light colored bars. The autosomal homolog of neo-Y, also called neo-X or X2, shows upregulation at the loci in which there is degeneration on neo-Y. The upregulated loci are indicated in orange color and they correspond to similar loci as in the neo-Y. A hypothetical magnifier to one of these loci is shown. The degenerated loci on the neo-Y go under heterochromatinization due to retrotransposition and/or other means of molecular events leading to hemizygosity. In the homologous region on neo-X chromosome, the MSL complexes are recruited and upregulate the genes for dosage compensation
Since dosage compensation in Drosophila is an old problem and various subgroups use the same complex to cover up hemizygousity, MSL components are expected to be under stabilizing (purifying) selection. But recent experiments show that even two closely related Drosophila species, D. melanogaster and D. simulans that diverged 2.5 million years ago have highly asymmetric rapid evolution of MSL genes (Rodriguez et al. 2007). Interestingly the MOF acetylation site on MSL3 in D.melanogaster is unique in the Drosophila species (Kelley 2004). It is possible that there are other selective forces that are acting on the MSL complex of D.melanogaster like the male killing bacteria S.poulsonii (Veneti et al. 2005). MSL proteins could be evolving away from recognition by these bacteria. Nevertheless since the protein complexes tend to coevolve; the whole complex could be trying to fine tune to escape from selection while also trying to do its essential function. Curiously, MOF has been shown to bind LTR retrotransposons in D.melanogaster and inhibit their transposition (Matyunina et al. 2008). Inhibition of retrotransposition could also be a strong selective force on this subgroup. These recent findings can provide an explanation why it is difficult to find a consensus sequence for the MSL binding among other species.
MSL-like proteins in other organisms
The protein components of the MSL complex of D. melanogaster have clear homologues from yeast to mammals, except that MSL1 and MSL2 are not found in yeast (Marín 2003). Yeast NuA4 HAT complex contains Esa1 and Eaf3, homologues of MOF and MSL3 respectively (Eisen et al. 2001). However, stringent sequence analysis showed that yeast NuA4 complex is not the direct ancestor of compensasome in Drosophila, rather a novel complex arose with the concomitant evolution of MSL1 and MSL2 (Marín 2003). Although humans have entirely different strategy for the dosage compensation problem, the MSL complex is kept in mammals as well (Mendjan et al. 2006a; Smith et al. 2005; Taipale et al. 2005). However it seems that human MSL complex has evolved other functions in DNA damage response pathway and inhibition of tumor genesis (Gupta et al. 2005; Taipale et al. 2005; Smith et al. 2005). Recently it was shown that hMSL2 is responsible for the mono-ubiquitination of p53 and subsequent extra nuclear localization (Kruse and Gu 2008). Even though mammals shows X-inactivation in female nucleus, the active homologue of X is two-fold up regulated to reach the stoichiometry of autosomal gene expression level (Nguyen and Disteche 2006). The role of the MSL complex in this upregulation is a tempting hypothesis.
How general factors may impinge on the mechanism
One of the first issues addressed by high throughput experiments was the global regulation of expression on X by the MSL complex. A significant amount of earlier data proposed an alternative mechanism named inverse dose model for the X versus autosome balance (Bhadra et al. 2005; Bhadra et al. 2000; Bhadra et al. 1999). This model suggests that X chromosome inherently possesses sequences that recruit transcription factors for a roughly two fold up-regulation and the MSL complex functions to titrate MOF from autosomes to X to inhibit over-expression of autosomes by overriding the effects of hyper-upregulation of X (Birchler et al. 2003). RNAi against the components of the MSL complex and stringent normalization analysis of expression arrays showed that the MSL complex was indeed acting to up-regulate X-linked genes to the autosomal levels arguing against the inverse dosage model (Straub et al. 2005; Hamada et al. 2005). Moreover artificial recruitment of the MSL complex upstream of a reporter gene can cause its up regulation and roX autosomal transgenes can overcome silent heterochromatin (Henry et al. 2001; Kelley and Kuroda 2003).
Specific enrichment of X chromosome by H4K16 acetylation led to the belief that this canonical activation mark could be largely responsible for the up regulation. Indeed MOF can activate transcription in vivo and in vitro (Akhtar and Becker 2000). Moreover H4K16 acetylation can decondense 30 nm chromatin fiber in vitro (Shogren-Knaak et al. 2006). A simple prediction was that opening the chromatin might enable loading more polymerase RNA Polymerase II onto compensated genes. However, polymerase profiles show that there are not more polymerases on compensated genes than non-compensated ones (Gilfillan et al. 2006). Recent findings of new components related to dosage compensation tell us that the story may be much more complicated.
Identification of nuclear pore components, Nup153 and Mtor, in MSL purifications, and their effect on X linked gene expression suggest a link between dosage compensation and nuclear architecture (Mendjan et al. 2006b). Numerous findings indicate that position of a gene in the nuclear volume could affect its transcriptional status (Deniaud and Bickmore 2009; Branco and Pombo 2007; Akhtar and Gasser 2007). Although nuclear periphery was long known accepted as a repressive zone and a host for heterochromatin, nuclear pore complexes (NPC) can be a docking site for an induced gene (Köhler and Hurt 2007; Brown and Silver 2007). Close approximation of the X-linked genes to NPC may create a transcription competent domain/environment and may even provide up regulation of genes that are not bound by MSL but still dosage compensated. Interestingly, human interphase chromosomes are found to be associated with lamins in domains that are clearly demarcated by insulators showing that the genome can indeed be organized in discrete structures under the nuclear envelope (Guelen et al. 2008).
Another protein found to be associated with MSL is the JIL-1 kinase. JIL-1 can co-immunoprecipitate with MSL components and it is enriched on male X chromosome, although it is also distributed on other chromosomes (Jin et al. 2000). JIL-1 is the main kinase that is responsible for H3S10 phosphorylation (Wang et al. 2001). Although this mark was known to be a mitotic marker, it is also enriched in euchromatic regions and can antagonize heterochromatin spreading (Zhang et al. 2006b; Wang et al. 2001). Recently JIL-1 was shown to be an important activator in many genes in Drosophila and can relieve the promoter proximal pausing of RNAPII, which is thought to be a checkpoint after the initiation of transcription (Ivaldi et al. 2007; Saunders et al. 2006). Conceptually, selective recruitment of JIL-1 kinase by MSL to the X-linked genes may relieve this pausing more than autosomes and female X; helping twice as much transcription on male X. Albeit this attractive hypothesis, an opposing experiment demonstrated that RNA Polymerase II mediated transcription is independent of H3S10 phosphorylation and JIL-1 kinase affects transcription through maintaining the structural integrity of the chromosomes (Cai et al. 2008).
It seems that the male X chromosome is generally more sensitive to perturbations related to proteins that are responsible for general chromatin morphology. Two of these proteins are NURF, a chromatin remodeler, and Su(var)3–7, a protein responsible for heterochromatin formation by the help of HP1 (Spierer et al. 2005; Deuring et al. 2000). NURF is the founding member of ISWI family of remodelers and it contains ISWI protein as the catalytic subunit that enables sliding of nucleosomes (Bouazoune and Brehm 2006). Normally ISWI is not enriched on the male X chromosome or its mutations do not cause mislocalization of the MSL proteins, or the acetylation. Nevertheless, the male X chromosome looks much decondensed and broader in its absence and a functional MSL is required for this phenotype (Corona et al. 2002; Deuring et al. 2000). ISWI protein is also found in other complexes however this effect is related to NURF remodeler because aberrant phenotype of male X is repeated in NURF301 mutations, the main scaffold in NURF complex (Badenhorst et al. 2002). Recently it was found that roX null mutation could suppress the puffy appearance coming from the NURF mutations. Additionally, NURF can repress roX2 transcription in females (Bai et al. 2007). Similar to ISWI, Su(var)3–7 mutation causes male X chromosome decondensation, which can be suppressed by null MLE mutation (Spierer et al. 2008; Spierer et al. 2005). These antagonistic relations suggest that chromatin opening is not unchecked but is actually scrutinized by various complexes to maintain a sufficiently open—not more than necessary—state of chromatin. Actually, an analog system can be seen in a smaller scale on actively transcribing genes. Active genes have an increasing H3K36 di and trimethylation on the body of their genes and this mark is recognized by an HDAC complex, Rpd3S, which inhibits spurious transcription that may come from cryptic promoters (Carrozza et al. 2005). Interestingly, Rpd3S, the histone deacetylase and Set2, the enzyme required for H3K36 methylation play a role in dosage compensation (Buscaino et al. 2003; Bell et al. 2008a; Sural et al. 2008). Curiously, the components of the exosome, Dis3 and Rrp6, also copurify with MSL proteins (Mendjan et al. 2006b) suggesting that RNA degradation may be also be coupled to the system. In this sense exosome may degrade, antisense or cryptic transcripts that were generated uncontrolled due to open chromatin structure. Another fail-safe mechanism could be mediated by Supercoiling factor, of which genetic interaction with MSL has been shown (Furuhashi et al. 2006). Supercoilig Factor (SCF) was hypothesized to help decreasing the helical torsion that may have generated during chromatin remodeling however its role in dosage compensation is not determined yet (Furuhashi et al. 2006).
All these observations suggest that capabilities of MSL reach far beyond than expected before. Not only it behaves as a HAT complex but also acts as a mediator that fine tunes two fold upregulation by approaching to nuclear pore, cross talking with chromatin remodelers, heterochromatin proteins, and RNA degradation machines. Although the dazzling discoveries brought by powerful genetics, biochemistry and high throughput approaches, the new findings bring about their own mysteries the near future will be full of surprising discoveries.
Acknowledgements
We are very grateful to Boryana Petrova for preparation of figures and Thomas Conrad for providing the polytene squash picture. We thank Kent Duncan and members of the lab for critical reading of the manuscript and helpful discussions.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AEL
After Egg Laying
- CBD
Chromo barrel domain
- CES
Chromatin entry site
- DCC
Dosage compensation complex
- DHS
Dnase hypersensitive site
- HAS
High affinity sites
- HAT
Histone acetyltransferase
- HDAC
Histone deacetylase
- ISWI
Imitation switch
- LAS
Low affinity sites
- MLE
Maleless
- MRE
MSL recognition
- MOF
Males absent on the first
- MSL
Male specific lethal
- NPC
Nuclear pore complex
- NuRD
Nucleosome remodeling and deacetylase
- NURF
Nucleosome remodeling factor
- roX
RNA on X
- SCF
Supercoiling factor
- SXL
Sex lethal
- UTR
Untranslated region
References
- Abaza I, Coll O, Patalano S, Gebauer F. Drosophila UNR is required for translational repression of male-specific lethal 2 mRNA during regulation of X-chromosome dosage compensation. Genes Dev. 2006;20:380–9. doi: 10.1101/gad.371906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar A, Becker PB. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol Cell. 2000;5:367–75. doi: 10.1016/S1097-2765(00)80431-1. [DOI] [PubMed] [Google Scholar]
- Akhtar A, Becker PB. The histone H4 acetyltransferase MOF uses a C2HC zinc finger for substrate recognition. EMBO Rep. 2001;2:113–8. doi: 10.1093/embo-reports/kve022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar A, Gasser S. The nuclear envelope and transcriptional control. Nat Rev Genet. 2007;8:507–517. doi: 10.1038/nrg2122. [DOI] [PubMed] [Google Scholar]
- Akhtar A, Zink D, Becker PB. Chromodomains are protein-RNA interaction modules. Nature. 2000;407:405–9. doi: 10.1038/35030169. [DOI] [PubMed] [Google Scholar]
- Alekseyenko AA, Larschan E, Lai WR, Park PJ, Kuroda MI. High-resolution ChIP-chip analysis reveals that the Drosophila MSL complex selectively identifies active genes on the male X chromosome. Genes Dev. 2006;20:848–57. doi: 10.1101/gad.1400206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko AA, Peng S, Larschan E, Gorchakov AA, Lee OK, Kharchenko P, McGrath SD, Wang CI, Mardis ER, Park PJ, Kuroda MI. A sequence motif within chromatin entry sites directs MSL establishment on the Drosophila X chromosome. Cell. 2008;134:599–609. doi: 10.1016/j.cell.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein H, Axel R. Genes expressed in neurons of adult male Drosophila. Cell. 1997;88:459–69. doi: 10.1016/S0092-8674(00)81886-3. [DOI] [PubMed] [Google Scholar]
- Bachtrog D. Expression profile of a degenerating neo-y chromosome in Drosophila. Curr Biol. 2006;16:1694–9. doi: 10.1016/j.cub.2006.07.053. [DOI] [PubMed] [Google Scholar]
- Badenhorst P, Voas M, Rebay I, Wu C. Biological functions of the ISWI chromatin remodeling complex NURF. Genes Dev. 2002;16:3186–98. doi: 10.1101/gad.1032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Alekseyenko AA, Kuroda MI. Sequence-specific targeting of MSL complex regulates transcription of the roX RNA genes. EMBO J. 2004;23:2853–61. doi: 10.1038/sj.emboj.7600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Larschan E, Kwon SY, Badenhorst P, Kuroda MI. Regional control of chromatin organization by noncoding roX RNAs and the NURF remodeling complex in Drosophila melanogaster. Genetics. 2007;176:1491–9. doi: 10.1534/genetics.107.071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BS, Gorman M, Marín I. Dosage compensation in Drosophila. Annu Rev Genet. 1994;28:491–521. doi: 10.1146/annurev.ge.28.120194.002423. [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh T, Schones D, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bashaw GJ, Baker BS. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA-binding protein whose expression is sex specifically regulated by Sex-lethal. Development. 1995;121:3245–58. doi: 10.1242/dev.121.10.3245. [DOI] [PubMed] [Google Scholar]
- Beckmann K, Grskovic M, Gebauer F, Hentze MW. A dual inhibitory mechanism restricts msl-2 mRNA translation for dosage compensation in Drosophila. Cell. 2005;122:529–40. doi: 10.1016/j.cell.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Bell O, Conrad T, Kind J, Wirbelauer C, Akhtar A, Schubeler D. Transcription-coupled methylation of histone H3 at lysine 36 regulates dosage compensation by enhancing recruitment of the MSL complex in Drosophila melanogaster. Mol Cell Biol. 2008;28:3401–3409. doi: 10.1128/MCB.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell O, Conrad T, Kind J, Wirbelauer C, Akhtar A, Schübeler D. Transcription-coupled methylation of histone H3 at lysine 36 regulates dosage compensation by enhancing recruitment of the MSL complex in Drosophila melanogaster. Mol Cell Biol. 2008;28:3401–9. doi: 10.1128/MCB.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra MP, Bhadra U, Kundu J, Birchler JA. Gene expression analysis of the function of the male-specific lethal complex in Drosophila. Genetics. 2005;169:2061–74. doi: 10.1534/genetics.104.036020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra U, Pal-Bhadra M, Birchler JA. Role of the male specific lethal (msl) genes in modifying the effects of sex chromosomal dosage in Drosophila. Genetics. 1999;152:249–68. doi: 10.1093/genetics/152.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra U, Pal-Bhadra M, Birchler JA. Histone acetylation and gene expression analysis of sex lethal mutants in Drosophila. Genetics. 2000;155:753–63. doi: 10.1093/genetics/155.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler JA, Pal-Bhadra M, Bhadra U. Dosage dependent gene regulation and the compensation of the X chromosome in Drosophila males. Genetica. 2003;117:179–90. doi: 10.1023/A:1022935927763. [DOI] [PubMed] [Google Scholar]
- Bone JR, Kuroda MI. Dosage compensation regulatory proteins and the evolution of sex chromosomes in Drosophila. Genetics. 1996;144:705–13. doi: 10.1093/genetics/144.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone JR, Lavender J, Richman R, Palmer M, Turner BM, Kuroda MI. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes Dev. 1994;8:96–104. doi: 10.1101/gad.8.1.96. [DOI] [PubMed] [Google Scholar]
- Bouazoune K, Brehm A. ATP-dependent chromatin remodeling complexes in Drosophila. Chromosome Res. 2006;14:433–49. doi: 10.1007/s10577-006-1067-0. [DOI] [PubMed] [Google Scholar]
- Bouazoune K, Mitterweger A, Längst G, Imhof A, Akhtar A, Becker P, Brehm A. The dMi-2 chromodomains are DNA binding modules important for ATP-dependent nucleosome mobilization. EMBO J. 2002;21:2430–40. doi: 10.1093/emboj/21.10.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman BR, Moure CM, Kirtane BM, Welschhans RL, Tominaga K, Pereira-Smith OM, Quiocho FA. Multipurpose MRG domain involved in cell senescence and proliferation exhibits structural homology to a DNA-interacting domain. Structure. 2006;14:151–8. doi: 10.1016/j.str.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Branco MR, Pombo A. Chromosome organization: new facts, new models. Trends Cell Biol. 2007;17:127–34. doi: 10.1016/j.tcb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Brown C, Silver P. Transcriptional regulation at the nuclear pore complex. Curr Opin Genet Dev. 2007;17:100–106. doi: 10.1016/j.gde.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Buscaino A, Köcher T, Kind JH, Holz H, Taipale M, Wagner K, Wilm M, Akhtar A. MOF-regulated acetylation of MSL-3 in the Drosophila dosage compensation complex. Mol Cell. 2003;11:1265–77. doi: 10.1016/S1097-2765(03)00140-0. [DOI] [PubMed] [Google Scholar]
- Buscaino A, Legube G, Akhtar A. X-chromosome targeting and dosage compensation are mediated by distinct domains in MSL-3. EMBO Rep. 2006;7:531–8. doi: 10.1038/sj.embor.7400658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Bao X, Deng H, Jin Y, Girton J, Johansen J, Johansen KM. RNA polymerase II-mediated transcription at active loci does not require histone H3S10 phosphorylation in Drosophila. Development. 2008;135:2917–25. doi: 10.1242/dev.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozza MJ, Li B, Florens L, Suganuma T, Swanson S, Lee KK, Shia WJ, Anderson S, Yates J, Washburn M, Workman JL. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123:581–92. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Carvalho AB. Origin and evolution of the Drosophila Y chromosome. Curr Opin Genet Dev. 2002;12:664–8. doi: 10.1016/S0959-437X(02)00356-8. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D, Hnilicka J, Yu A, Guttman DS. Lack of degeneration of loci on the neo-Y chromosome of Drosophila americana americana. Genetics. 1997;145:989–1002. doi: 10.1093/genetics/145.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copps K, Richman R, Lyman LM, Chang KA, Rampersad-Ammons J, Kuroda MI. Complex formation by the Drosophila MSL proteins: role of the MSL2 RING finger in protein complex assembly. EMBO J. 1998;17:5409–17. doi: 10.1093/emboj/17.18.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona DF, Clapier CR, Becker P, Tamkun JW. Modulation of ISWI function by site-specific histone acetylation. EMBO Rep. 2002;3:242–7. doi: 10.1093/embo-reports/kvf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlsveen I, Gilfillan G, Shelest V, Lamm R, Becker P. Targeting determinants of dosage compensation in Drosophila. PLoS Genet. 2006;2:e5. doi: 10.1371/journal.pgen.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Meller V. Molecularly severe roX1 mutations contribute to dosage compensation in Drosophila. Genesis. 2009;47:49–54. doi: 10.1002/dvg.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Rattner BP, Souter S, Meller VH. The severity of roX1 mutations is predicted by MSL localization on the X chromosome. Mech Dev. 2005;122:1094–105. doi: 10.1016/j.mod.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Deniaud E, Bickmore WA. Transcription and the nuclear periphery: edge of darkness? Curr Opin Genet Dev. 2009;19:187–91. doi: 10.1016/j.gde.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Deuring R, Fanti L, Armstrong JA, Sarte M, Papoulas O, Prestel M, Daubresse G, Verardo M, Moseley SL, Berloco M, Tsukiyama T, Wu C, Pimpinelli S, Tamkun JW. The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol Cell. 2000;5:355–65. doi: 10.1016/S1097-2765(00)80430-X. [DOI] [PubMed] [Google Scholar]
- Duncan K, Grskovic M, Strein C, Beckmann K, Niggeweg R, Abaza I, Gebauer F, Wilm M, Hentze MW. Sex-lethal imparts a sex-specific function to UNR by recruiting it to the msl-2 mRNA 3′ UTR: translational repression for dosage compensation. Genes Dev. 2006;20:368–79. doi: 10.1101/gad.371406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A, Utley RT, Nourani A, Allard S, Schmidt P, Lane WS, Lucchesi JC, Cote J. The yeast NuA4 and Drosophila MSL complexes contain homologous subunits important for transcription regulation. J Biol Chem. 2001;276:3484–91. doi: 10.1074/jbc.M008159200. [DOI] [PubMed] [Google Scholar]
- Fagegaltier D, Baker BS. X chromosome sites autonomously recruit the dosage compensation complex in Drosophila males. Plos Biol. 2004;2:e341. doi: 10.1371/journal.pbio.0020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förch P, Merendino L, Martínez C, Valcárcel J. Modulation of msl-2 5′ splice site recognition by Sex-lethal. RNA. 2001;7:1185–91. doi: 10.1017/S1355838201010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, Baker BS. The rox1 and rox2 RNAs are essential components of the compensasome, which mediates dosage compensation in Drosophila. Mol Cell. 1999;4:117–22. doi: 10.1016/S1097-2765(00)80193-8. [DOI] [PubMed] [Google Scholar]
- Franke A, Dernburg A, Bashaw GJ, Baker BS. Evidence that MSL-mediated dosage compensation in Drosophila begins at blastoderm. Development. 1996;122:2751–60. doi: 10.1242/dev.122.9.2751. [DOI] [PubMed] [Google Scholar]
- Furuhashi H, Nakajima M, Hirose S. DNA supercoiling factor contributes to dosage compensation in Drosophila. Development. 2006;133:4475–83. doi: 10.1242/dev.02620. [DOI] [PubMed] [Google Scholar]
- Gallach M, Arnau V, Marín I. Global patterns of sequence evolution in Drosophila. BMC Genomics. 2007;8:408. doi: 10.1186/1471-2164-8-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F, Grskovic M, Hentze MW. Drosophila sex-lethal inhibits the stable association of the 40 S ribosomal subunit with msl-2 mRNA. Mol Cell. 2003;11:1397–404. doi: 10.1016/S1097-2765(03)00176-X. [DOI] [PubMed] [Google Scholar]
- Gilfillan GD, Straub T, De Wit E, Greil F, Lamm R, Van Steensel B, Becker P. Chromosome-wide gene-specific targeting of the Drosophila dosage compensation complex. Genes Dev. 2006;20:858–70. doi: 10.1101/gad.1399406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman M, Franke A, Baker BS. Molecular characterization of the male-specific lethal-3 gene and investigations of the regulation of dosage compensation in Drosophila. Development. 1995;121:463–75. doi: 10.1242/dev.121.2.463. [DOI] [PubMed] [Google Scholar]
- Grskovic M, Hentze MW, Gebauer F. A co-repressor assembly nucleated by Sex-lethal in the 3′UTR mediates translational control of Drosophila msl-2 mRNA. EMBO J. 2003;22:5571–81. doi: 10.1093/emboj/cdg539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Szauter P, Lucchesi JC. Targeting of MOF, a putative histone acetyl transferase, to the X chromosome of Drosophila melanogaster. Dev Genet. 1998;22:56–64. doi: 10.1002/(SICI)1520-6408(1998)22:1<56::AID-DVG6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Gu W, Wei X, Pannuti A, Lucchesi JC. Targeting the chromatin-remodeling MSL complex of Drosophila to its sites of action on the X chromosome requires both acetyl transferase and ATPase activities. EMBO J. 2000;19:5202–11. doi: 10.1093/emboj/19.19.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, De Klein A, Wessels L, De Laat W, Van Steensel B. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–51. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- Gupta A, Sharma GG, Young CS, Agarwal M, Smith ER, Paull TT, Lucchesi JC, Khanna KK, Ludwig T, Pandita TK. Involvement of human MOF in ATM function. Mol Cell Biol. 2005;25:5292–305. doi: 10.1128/MCB.25.12.5292-5305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Parisi M, Sturgill D, Nuttall R, Doctolero M, Dudko OK, Malley JD, Eastman P, Oliver B. Global analysis of X-chromosome dosage compensation. J Biol. 2006;5:3. doi: 10.1186/jbiol30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, Park PJ, Gordadze PR, Kuroda MI. Global regulation of X chromosomal genes by the MSL complex in Drosophila melanogaster. Genes Dev. 2005;19:2289–94. doi: 10.1101/gad.1343705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RA, Tews B, Li X, Scott MJ. Recruitment of the male-specific lethal (MSL) dosage compensation complex to an autosomally integrated roX chromatin entry site correlates with an increased expression of an adjacent reporter gene in male Drosophila. J Biol Chem. 2001;276:31953–8. doi: 10.1074/jbc.M103008200. [DOI] [PubMed] [Google Scholar]
- Hilfiker A, Hilfiker-Kleiner D, Pannuti A, Lucchesi JC. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 1997;16:2054–60. doi: 10.1093/emboj/16.8.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilik I and Akhtar A (2009) roX RNAs: Non-coding regulators of the male X chromosome in flies. RNA Biol, 6 [DOI] [PubMed]
- Ivaldi MS, Karam CS, Corces VG. Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila. Genes Dev. 2007;21:2818–31. doi: 10.1101/gad.1604007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A, Regnard C, Morales V, Kremmer E, Becker P. Structure-function analysis of the RNA helicase maleless. Nucleic Acids Res. 2008;36:950–62. doi: 10.1093/nar/gkm1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SA, Khorasanizadeh S. Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science. 2002;295:2080–3. doi: 10.1126/science.1069473. [DOI] [PubMed] [Google Scholar]
- Jin Y, Wang Y, Johansen J, Johansen KM. JIL-1, a chromosomal kinase implicated in regulation of chromatin structure, associates with the male specific lethal (MSL) dosage compensation complex. J Cell Biol. 2000;149:1005–10. doi: 10.1083/jcb.149.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y, Mengus G, Gilfillan G, Kennedy HG, Stuckenholz C, Kelley RL, Becker PB, Kuroda MI. Association and spreading of the Drosophila dosage compensation complex from a discrete roX1 chromatin entry site. EMBO J. 2001;20:2236–45. doi: 10.1093/emboj/20.9.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RL, Becker PB, Kuroda MI. Association and spreading of the Drosophila dosage compensation complex from a discrete roX1 chromatin entry site. EMBO J. 2001;20:2236–45. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RL, Kuroda MI. The Drosophila roX1 RNA gene can overcome silent chromatin by recruiting the male-specific lethal dosage compensation complex. Genetics. 2003;164:565–74. doi: 10.1093/genetics/164.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RL, Solovyeva I, Lyman LM, Richman R, Solovyev V, Kuroda MI. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell. 1995;81:867–77. doi: 10.1016/0092-8674(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Kelley RL, Wang J, Bell L, Kuroda MI. Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature. 1997;387:195–9. doi: 10.1038/387195a0. [DOI] [PubMed] [Google Scholar]
- Kelley RL, Meller VH, Gordadze PR, Roman G, Davis RL, Kuroda MI. Epigenetic spreading of the Drosophila dosage compensation complex from roX RNA genes into flanking chromatin. Cell. 1999;98:513–22. doi: 10.1016/S0092-8674(00)81979-0. [DOI] [PubMed] [Google Scholar]
- Kelley RL, Lee OK, Shim YK. Transcription rate of noncoding roX1 RNA controls local spreading of the Drosophila MSL chromatin remodeling complex. Mech Dev. 2008;125:1009–19. doi: 10.1016/j.mod.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind J, Akhtar A. Cotranscriptional recruitment of the dosage compensation complex to X-linked target genes. Genes Dev. 2007;21:2030–40. doi: 10.1101/gad.430807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind J, Vaquerizas JM, Gebhardt P, Gentzel M, Luscombe NM, Bertone P, Akhtar A. Genome-wide analysis reveals MOF as a key regulator of dosage compensation and gene expression in Drosophila. Cell. 2008;133:813–28. doi: 10.1016/j.cell.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Köhler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol. 2007;8:761–773. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- Kotlikova IV, Demakova OV, Semeshin VF, Shloma VV, Boldyreva LV, Kuroda MI, Zhimulev IF. The Drosophila dosage compensation complex binds to polytene chromosomes independently of developmental changes in transcription. Genetics. 2006;172:963–74. doi: 10.1534/genetics.105.045286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse J, Gu W. MSL2 promotes MDM2 independent cytoplasmic localization of p53. Journal of Biological Chemistry. 2008;284:3250–63. doi: 10.1074/jbc.M805658200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda MI, Kernan MJ, Kreber R, Ganetzky B, Baker BS. The maleless protein associates with the X chromosome to regulate dosage compensation in Drosophila. Cell. 1991;66:935–47. doi: 10.1016/0092-8674(91)90439-6. [DOI] [PubMed] [Google Scholar]
- Larschan E, Alekseyenko AA, Gortchakov AA, Peng S, Li B, Yang P, Workman JL, Park PJ, Kuroda MI. MSL complex is attracted to genes marked by H3K36 trimethylation using a sequence-independent mechanism. Mol Cell. 2007;28:121–33. doi: 10.1016/j.molcel.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Lee CG, Chang KA, Kuroda MI, Hurwitz J. The NTPase/helicase activities of Drosophila maleless, an essential factor in dosage compensation. EMBO J. 1997;16:2671–81. doi: 10.1093/emboj/16.10.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Reichman TW, Baik T, Mathews MB. MLE functions as a transcriptional regulator of the roX2 gene. J Biol Chem. 2004;279:47740–5. doi: 10.1074/jbc.M408207200. [DOI] [PubMed] [Google Scholar]
- Legube G, McWeeney SK, Lercher MJ, Akhtar A. X-chromosome-wide profiling of MSL-1 distribution and dosage compensation in Drosophila. Genes Dev. 2006;20:871–83. doi: 10.1101/gad.377506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Parry DA, Scott MJ. The amino-terminal region of Drosophila MSL1 contains basic, glycine-rich, and leucine zipper-like motifs that promote X chromosome binding, self-association, and MSL2 binding, respectively. Mol Cell Biol. 2005;25:8913–24. doi: 10.1128/MCB.25.20.8913-8924.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Gogol M, Carey M, Lee D, Seidel C, Workman JL. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science. 2007;316:1050–4. doi: 10.1126/science.1139004. [DOI] [PubMed] [Google Scholar]
- Li F, Schiemann AH, Scott MJ. Incorporation of the noncoding roX RNAs alters the chromatin-binding specificity of the Drosophila MSL1/MSL2 complex. Mol Cell Biol. 2008;28:1252–64. doi: 10.1128/MCB.00910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman LM, Copps K, Rastelli L, Kelley RL, Kuroda MI. Drosophila male-specific lethal-2 protein: structure/function analysis and dependence on MSL-1 for chromosome association. Genetics. 1997;147:1743–53. doi: 10.1093/genetics/147.4.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight RH. The Sex-Determining Mechanism of Drosophila Miranda. Genetics. 1939;24:180–201. doi: 10.1093/genetics/24.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín I. Evolution of chromatin-remodeling complexes: comparative genomics reveals the ancient origin of “novel” compensasome genes. J Mol Evol. 2003;56:527–39. doi: 10.1007/s00239-002-2422-1. [DOI] [PubMed] [Google Scholar]
- Marín I, Franke A, Bashaw GJ, Baker BS. The dosage compensation system of Drosophila is co-opted by newly evolved X chromosomes. Nature. 1996;383:160–3. doi: 10.1038/383160a0. [DOI] [PubMed] [Google Scholar]
- Matyunina L, Bowen N, McDonald J. LTR retrotransposons and the evolution of dosage compensation in Drosophila. BMC Mol Biol. 2008;9:55. doi: 10.1186/1471-2199-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell KA, Hilfiker A, Lucchesi JC. Dosage compensation in Drosophila: the X chromosome binding of MSL-1 and MSL-2 in female embryos is prevented by the early expression of the Sxl gene. Mech Dev. 1996;57:113–9. doi: 10.1016/0925-4773(96)00517-5. [DOI] [PubMed] [Google Scholar]
- Meller V. Initiation of dosage compensation in Drosophila embryos depends on expression of the roX RNAs. Mech Dev. 2003;120:759–67. doi: 10.1016/S0925-4773(03)00157-6. [DOI] [PubMed] [Google Scholar]
- Meller V, Rattner BP. The roX genes encode redundant male-specific lethal transcripts required for targeting of the MSL complex. EMBO J. 2002;21:1084–91. doi: 10.1093/emboj/21.5.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller VH, Wu KH, Roman G, Kuroda MI, Davis RL. roX1 RNA paints the X chromosome of male Drosophila and is regulated by the dosage compensation system. Cell. 1997;88:445–57. doi: 10.1016/S0092-8674(00)81885-1. [DOI] [PubMed] [Google Scholar]
- Meller VH, Gordadze PR, Park Y, Chu X, Stuckenholz C, Kelley RL, Kuroda MI. Ordered assembly of roX RNAs into MSL complexes on the dosage-compensated X chromosome in Drosophila. Curr Biol. 2000;10:136–43. doi: 10.1016/S0960-9822(00)00311-0. [DOI] [PubMed] [Google Scholar]
- Mendjan S, Akhtar A. The right dose for every sex. Chromosoma. 2007;116:95–106. doi: 10.1007/s00412-006-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendjan S, Taipale M, Kind J, Holz H, Gebhardt P, Schelder M, Vermeulen M, Buscaino A, Duncan K, Mueller J. Nuclear Pore Components Are Involved in the Transcriptional Regulation of Dosage Compensation in Drosophila. Mol Cell. 2006;21:811–823. doi: 10.1016/j.molcel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Mendjan S, Taipale M, Kind J, Holz H, Gebhardt P, Schelder M, Vermeulen M, Buscaino A, Duncan K, Mueller J, Wilm M, Stunnenberg HG, Saumweber H, Akhtar A. Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol Cell. 2006;21:811–23. doi: 10.1016/j.molcel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Merendino L, Guth S, Bilbao D, Martínez C, Valcárcel J. Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature. 1999;402:838–41. doi: 10.1038/45602. [DOI] [PubMed] [Google Scholar]
- Meyer BJ (2005) X-Chromosome dosage compensation. WormBook, 1–14 [DOI] [PMC free article] [PubMed]
- Morales V, Regnard C, Izzo A, Vetter I, Becker P. The MRG domain mediates the functional integration of MSL3 into the dosage compensation complex. Mol Cell Biol. 2005;25:5947–54. doi: 10.1128/MCB.25.14.5947-5954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales V, Straub T, Neumann MF, Mengus G, Akhtar A, Becker P. Functional integration of the histone acetyltransferase MOF into the dosage compensation complex. EMBO J. 2004;23:2258–68. doi: 10.1038/sj.emboj.7600235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra R, Smith ER, Yokoyama R, Lucchesi J. The MLE subunit of the Drosophila MSL complex uses its ATPase activity for dosage compensation and its helicase activity for targeting. Mol Cell Biol. 2008;28:958–66. doi: 10.1128/MCB.00995-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nat Genet. 2006;38:47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]
- Nielsen PR, Nietlispach D, Mott HR, Callaghan J, Bannister A, Kouzarides T, Murzin AG, Murzina NV, Laue ED. Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature. 2002;416:103–7. doi: 10.1038/nature722. [DOI] [PubMed] [Google Scholar]
- Nielsen PR, Nietlispach D, Buscaino A, Warner RJ, Akhtar A, Murzin AG, Murzina NV, Laue ED. Structure of the chromo barrel domain from the MOF acetyltransferase. J Biol Chem. 2005;280:32326–31. doi: 10.1074/jbc.M501347200. [DOI] [PubMed] [Google Scholar]
- Norman RA, Doane WW. Dosage compensation and dietary glucose repression of larval amylase activity in Drosophila miranda. Biochem Genet. 1990;28:601–13. doi: 10.1007/BF00553953. [DOI] [PubMed] [Google Scholar]
- Oh H, Park Y, Kuroda MI. Local spreading of MSL complexes from roX genes on the Drosophila X chromosome. Genes Development. 2003;17:1334–9. doi: 10.1101/gad.1082003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Bone JR, Kuroda MI. Multiple classes of MSL binding sites target dosage compensation to the X chromosome of Drosophila. Curr Biol. 2004;14:481–7. doi: 10.1016/j.cub.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Palmer M, Mergner VA, Richman R, Manning JE, Kuroda MI, Lucchesi JC. The male-specific lethal-one (msl-1) gene of Drosophila melanogaster encodes a novel protein that associates with the X chromosome in males. Genetics. 1993;134:545–57. doi: 10.1093/genetics/134.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer M, Richman R, Richter L, Kuroda MI. Sex-specific regulation of the male-specific lethal-1 dosage compensation gene in Drosophila. Genes Dev. 1994;8:698–706. doi: 10.1101/gad.8.6.698. [DOI] [PubMed] [Google Scholar]
- Park Y, Kelley RL, Oh H, Kuroda MI, Meller V. Extent of chromatin spreading determined by roX RNA recruitment of MSL proteins. Science. 2002;298:1620–3. doi: 10.1126/science.1076686. [DOI] [PubMed] [Google Scholar]
- Park Y, Mengus G, Bai X, Kageyama Y, Meller V, Becker P, Kuroda MI. Sequence-specific targeting of Drosophila roX genes by the MSL dosage compensation complex. Mol Cell. 2003;11:977–86. doi: 10.1016/S1097-2765(03)00147-3. [DOI] [PubMed] [Google Scholar]
- Park SW, Kuroda MI, Park Y. Regulation of histone H4 Lys16 acetylation by predicted alternative secondary structures in roX noncoding RNAs. Mol Cell Biol. 2008;28:4952–62. doi: 10.1128/MCB.00219-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patalano S, Mihailovich M, Belacortu Y, Paricio N, Gebauer F. Dual sex-specific functions of Drosophila Upstream of N-ras in the control of X chromosome dosage compensation. Development. 2009;136:689–98. doi: 10.1242/dev.027656. [DOI] [PubMed] [Google Scholar]
- Payer B, Lee JT. X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet. 2008;42:733–72. doi: 10.1146/annurev.genet.42.110807.091711. [DOI] [PubMed] [Google Scholar]
- Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, Bell GW, Walker K, Rolfe PA, Herbolsheimer E, Zeitlinger J, Lewitter F, Gifford DK, Young RA. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–27. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Rastelli L, Kuroda MI. An analysis of maleless and histone H4 acetylation in Drosophila melanogaster spermatogenesis. Mech Dev. 1998;71:107–17. doi: 10.1016/S0925-4773(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Rastelli L, Richman R, Kuroda MI. The dosage compensation regulators MLE, MSL-1 and MSL-2 are interdependent since early embryogenesis in Drosophila. Mech Dev. 1995;53:223–33. doi: 10.1016/0925-4773(95)00438-7. [DOI] [PubMed] [Google Scholar]
- Rattner BP, Meller V. Drosophila male-specific lethal 2 protein controls sex-specific expression of the roX genes. Genetics. 2004;166:1825–32. doi: 10.1534/genetics.166.4.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter L, Bone JR, Kuroda MI. RNA-dependent association of the Drosophila maleless protein with the male X chromosome. Genes Cells. 1996;1:325–36. doi: 10.1046/j.1365-2443.1996.26027.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez MA, Vermaak D, Bayes JJ, Malik HS. Species-specific positive selection of the male-specific lethal complex that participates in dosage compensation in Drosophila. Proc Natl Acad Sci USA. 2007;104:15412–7. doi: 10.1073/pnas.0707445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass GL, Pannuti A, Lucchesi J. Male-specific lethal complex of Drosophila targets activated regions of the X chromosome for chromatin remodeling. Proc Natl Acad Sci USA. 2003;100:8287–91. doi: 10.1073/pnas.1332749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Core LJ, Lis J. Breaking barriers to transcription elongation. Nat Rev Mol Cell Biol. 2006;7:557–67. doi: 10.1038/nrm1981. [DOI] [PubMed] [Google Scholar]
- Schütt C, Nöthiger R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development. 2000;127:667–77. doi: 10.1242/dev.127.4.667. [DOI] [PubMed] [Google Scholar]
- Scott MJ, Pan LL, Cleland SB, Knox AL, Heinrich J. MSL1 plays a central role in assembly of the MSL complex, essential for dosage compensation in Drosophila. EMBO J. 2000;19:144–55. doi: 10.1093/emboj/19.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H, Sano N, Moriwaki Y, Okuda M, Horikoshi M, Nishimura Y. Novel structural and functional mode of a knot essential for RNA binding activity of the Esa1 presumed chromodomain. J Mol Biol. 2008;378:987–1001. doi: 10.1016/j.jmb.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–7. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- Smith ER, Cayrou C, Huang R, Lane WS, Côté J, Lucchesi J. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol Cell Biol. 2005;25:9175–88. doi: 10.1128/MCB.25.21.9175-9188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Pannuti A, Gu W, Steurnagel A, Cook RG, Allis CD, Lucchesi JC. The drosophila MSL complex acetylates histone H4 at lysine 16, a chromatin modification linked to dosage compensation. Mol Cell Biol. 2000;20:312–8. doi: 10.1128/MCB.20.1.312-318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer A, Begeot F, Spierer P, Delattre M. SU(VAR)3–7 links heterochromatin and dosage compensation in Drosophila. PLoS Genet. 2008;4:e1000066. doi: 10.1371/journal.pgen.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer A, Seum C, Delattre M, Spierer P. Loss of the modifiers of variegation Su(var)3–7 or HP1 impacts male X polytene chromosome morphology and dosage compensation. J Cell Sci. 2005;118:5047–57. doi: 10.1242/jcs.02623. [DOI] [PubMed] [Google Scholar]
- Steinemann S, Steinemann M. Y chromosomes: born to be destroyed. Bioessays. 2005;27:1076–83. doi: 10.1002/bies.20288. [DOI] [PubMed] [Google Scholar]
- Steinemann S, Steinemann M. Evolution of sex chromosomes: dosage compensation of the Lcp1-4 gene cluster on the evolving neo-X chromosome in Drosophila miranda. Insect Mol Biol. 2007;16:167–74. doi: 10.1111/j.1365-2583.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- Straub T, Becker PB. Dosage compensation: the beginning and end of generalization. Nat Rev Genet. 2007;8:47–57. doi: 10.1038/nrg2013. [DOI] [PubMed] [Google Scholar]
- Straub T, Gilfillan G, Maier VK, Becker P. The Drosophila MSL complex activates the transcription of target genes. Genes Dev. 2005;19:2284–8. doi: 10.1101/gad.1343105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub T, Grimaud C, Gilfillan GD, Mitterweger A, Becker PB. The chromosomal high-affinity binding sites for the Drosophila dosage compensation complex. PLoS Genet. 2008;4:e1000302. doi: 10.1371/journal.pgen.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckenholz C, Meller V, Kuroda MI. Functional redundancy within roX1, a noncoding RNA involved in dosage compensation in Drosophila melanogaster. Genetics. 2003;164:1003–14. doi: 10.1093/genetics/164.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Hong J, Zhang P, Dong X, Shen X, Lin D, Ding J. Molecular basis of the interaction of Saccharomyces cerevisiae Eaf3 chromo domain with methylated H3K36. J Biol Chem. 2008;283:36504–12. doi: 10.1074/jbc.M806564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sural TH, Peng S, Li B, Workman JL, Park PJ, Kuroda MI. The MSL3 chromodomain directs a key targeting step for dosage compensation of the Drosophila melanogaster X chromosome. Nat Struct Mol Biol. 2008;15:1318–25. doi: 10.1038/nsmb.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M, Rea S, Richter K, Vilar A, Lichter P, Imhof A, Akhtar A. hMOF histone acetyltransferase is required for histone H4 lysine 16 acetylation in mammalian cells. Mol Cell Biol. 2005;25:6798–810. doi: 10.1128/MCB.25.15.6798-6810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM, Birley AJ, Lavender J. Histone H4 isoforms acetylated at specific lysine residues define individual chromosomes and chromatin domains in Drosophila polytene nuclei. Cell. 1992;69:375–84. doi: 10.1016/0092-8674(92)90417-B. [DOI] [PubMed] [Google Scholar]
- Veneti Z, Bentley JK, Koana T, Braig HR, Hurst GD. A functional dosage compensation complex required for male killing in Drosophila. Science. 2005;307:1461–3. doi: 10.1126/science.1107182. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang W, Jin Y, Johansen J, Johansen KM. The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell. 2001;105:433–43. doi: 10.1016/S0092-8674(01)00325-7. [DOI] [PubMed] [Google Scholar]
- Xu C, Cui G, Botuyan MV, Mer G. Structural basis for the recognition of methylated histone H3K36 by the Eaf3 subunit of histone deacetylase complex Rpd3S. Structure. 2008;16:1740–50. doi: 10.1016/j.str.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Bachtrog D, Charlesworth B. A survey of chromosomal and nucleotide sequence variation in Drosophila miranda. Genetics. 2003;164:1369–81. doi: 10.1093/genetics/164.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Oliver B. Dosage compensation goes global. Curr Opin Genet Dev. 2007;17:113–20. doi: 10.1016/j.gde.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang P, Du J, Sun B, Dong X, Xu G, Zhou J, Huang Q, Liu Q, Hao Q, Ding J. Structure of human MRG15 chromo domain and its binding to Lys36-methylated histone H3. Nucleic Acids Res. 2006;34:6621–8. doi: 10.1093/nar/gkl989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Deng H, Bao X, Lerach S, Girton J, Johansen J, Johansen KM. The JIL-1 histone H3S10 kinase regulates dimethyl H3K9 modifications and heterochromatic spreading in Drosophila. Development. 2006;133:229–35. doi: 10.1242/dev.02199. [DOI] [PubMed] [Google Scholar]
- Zhou S, Yang Y, Scott MJ, Pannuti A, Fehr KC, Eisen A, Koonin EV, Fouts DL, Wrightsman R, Manning JE. Male-specific lethal 2, a dosage compensation gene of Drosophila, undergoes sex-specific regulation and encodes a protein with a RING finger and a metallothionein-like cysteine cluster. EMBO J. 1995;14:2884–95. doi: 10.1002/j.1460-2075.1995.tb07288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]