Abstract
Context: Although acquired abnormalities of thyroid hormone metabolism are common, inherited defects in humans involving the synthesis of selenoproteins, including iodothyronine deiodinases, have been described in only one recent publication.
Objective: We report the study of a novel selenocysteine insertion sequence-binding protein 2 (SBP2) gene mutation (R128X) and its clinical and molecular characterization.
Subjects and Methods: A family of African origin was studied. The proband presented with growth retardation, low serum selenium level, and thyroid test abnormalities consisting of high serum total and free T4 concentrations associated with low T3, high rT3, and normal TSH. The entire coding region of the SBP2 gene was sequenced and minigenes constructed to explain the nature of the defect.
Results: The proband was homozygous for a nonsense gene mutation that produces an early stop codon (R128X). Both parents and a sister were heterozygous but showed no growth or thyroid test abnormalities. Despite the severity of the defect, the patient had a relatively mild phenotype, similar to that associated with partial SBP2 deficiency. In vitro analysis showed that the mutant minigene synthesized SBP2 from at least three downstream ATGs capable of generating molecules containing the essential functional domains. Treatment with l-T3 accelerated the growth velocity and advanced the bone age.
Conclusions: We identified a novel SBP2 gene mutation producing an early arrest in the synthesis of a full-length molecule. The demonstration that SBP2 isoforms containing all functional domains could be synthesized from three downstream ATGs explains the relatively mild phenotype caused by this defect.
The mild phenotype associated with a novel SBP2 gene mutation that produces an early stop codon is due to the synthesis of SBP2 isoforms from downstream ATGs that contain all of the protein’s functional domains.
A constant supply of thyroid hormone to tissues is regulated by a feedback system that involves the hypothalamus and the pituitary gland. Additional fine tuning of the amount of biologically active thyroid hormone available to target cells is provided by the local metabolism of the precursor T4 to produce the active hormone T3 and the inactive metabolite rT3 (1,2). The rate of their formation and subsequent degradation are dependent on three specific enzymes. These iodothyronine deiodinases, D1, D2, and D3, are selenoproteins present in different proportions in specific tissues and cells (3).
Physiological and pathological variations in the level of deiodinases are responsible for intracellular and extracellular changes in the concentration of iodothyronines. Most common are the consequences of calorie intake and illness (4). These acquired changes are not matched by inherited defects in iodothyronine metabolism. In the mouse, but not in humans, a reduction in D1 in the C3H strain is caused by a sequence difference reducing promoter activity (5,6). Gene manipulations resulting in deletions of each of the three deiodinases, alone and in combination, do produce characteristic phenotypes, none of which are lethal (7,8,9,10), although increased mortality in D3 knockout (D3KO) animals has been observed (9). Yet no genetic defects in any of the iodothyronine deiodinases has been so far identified in humans.
In 2005, we described in two families mutations in the selenocysteine insertion sequence-binding protein 2 (SECISBP2, MIM 607693; also called SBP2) gene, the probands of which presented with a transient growth retardation associated with abnormal thyroid function tests (11). The latter included high serum total and free T4 (FT4) concentrations, a modest reduction in serum T3, high rT3, and normal or slightly elevated TSH. Because SBP2 is a major determinant in the incorporation of selenocysteine during selenoprotein synthesis (12,13), the absence of lethality and mild phenotype were attributed, respectively, to the preservation of partial SBP2 activity and the hierarchy in the synthesis of selenoproteins (14,15). The latter allows the differential preservation of some selenoproteins in the presence of unfavorable conditions for their synthesis.
In this communication, we report a new family with SBP2 gene mutation producing an early stop codon. Evidence is provided that in this instance, the relatively mild phenotype is caused by alternative downstream translation start sites that produce smaller SBP2 molecules containing the intact functional domain.
Subjects and Methods
Case reports
The proband was born to nonconsanguineous parents from the same town in the eastern region of Ghana. Pregnancy, delivery at term (3.1 kg), and early development were thought to have been normal. He was 8.9 yr old when one of us (N.M.) was consulted because of short stature. He was proportionately short for age [height 113.5 cm, sd score (SDS) −3.36, centile <0.1] and for the height of his parents (target height 170.9 cm, SDS −1.06, target centile 14), and his bone age was delayed at 3.1 yr (height for bone age SDS +4.7). He was slim, weighing 18.5 kg (body mass index 14.4 kg.m2, weight for height 93%), whereas his head circumference was 51.0 cm (centile 2.4). No goiter or other somatic abnormalities were found, and he was clinically euthyroid.
Thyroid function tests revealed marked elevation of FT4 of 37.6 pmol/liter (normal range 12.0–25.0), low FT3 of 2.60 pmol/liter (normal range 3.6–8.5), and a normal TSH of 1.4 mU/liter. IGF-1 and IGF-binding protein-3 were normal at 11.1 nmol/liter and 2.2 mg/liter, respectively. Peak GH response to insulin hypoglycemia was 11.2 mU/liter and cortisol 668 nmol/liter. Blood selenium was low (see Fig. 1), but detailed assessment for malabsorption, including upper and lower gastrointestinal endoscopy and biopsy, were negative. Igs and CD 3:4:8 and inflammatory markers were negative. Karotype was 46XY and normal. Electrocardiography and echocardiography showed no evidence of cardiomyopathy.
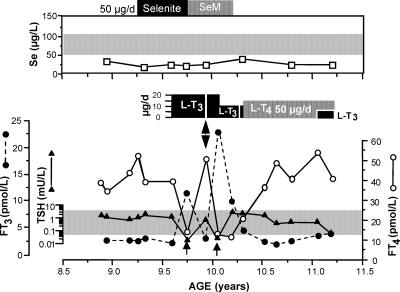
Figure 1.
Clinical course of the proband. Note the low serum Se concentration, which did not respond to Se treatment in the form of selenite or selenomethionine (SeM). Normal range is for children and adults living in England and Wales as provided by the Trace Elements Laboratory Department of Clinical Biochemistry, City Hospital, Birmingham, UK and is in agreement with values published in the Clinical and Analytical Handbook of the SAS Trace Element Center (32). The thyroid function test abnormalities (high serum T4 and low T3 concentrations in the presence of a normal serum TSH level), characteristic of SBP2 defects, were persistent during the initial 7-month period of observation. Treatment with 20 μg l-T3 reduced the serum T4 levels but also suppressed the serum TSH concentration (see arrows). Variations in serum FT3 concentrations are due to different intervals of time between blood sampling and the last dose of l-T3. The double-headed arrow indicates cessation of l-T3 treatment for 5 d, which promptly returned the baseline thyroid test abnormalities.
Thyroid function tests and measurements of selenium
Total T4 (TT4), TT3, and TSH were measured by chemiluminescence immunometric assays using the Elecsys Automated System (Roche Molecular Biochemicals GmbH and Hitachi, Ltd., both located in Indianapolis, IN). rT3 was measured using a commercial RIA kit (Adaltis Italia S.p.A, Bologna, Italy) and serum thyroglobulin by an in-house RIA (16). The serum FT4 index (FT4I) was calculated as the product of the serum TT4 concentration and the normalized resin T4 uptake ratio (17). Antibodies against thyroglobulin and thyroperoxidase were measured by passive hemagglutination (Fujirebio, Inc., Tokyo, Japan).
In the United Kingdom, FT4, FT3, and TSH were measured using a Siemens Immulite 2000 immunoassay analyzer.
Total serum selenium (Se) was measured by inductively coupled plasma mass spectrometry, using an Agilent 7500c (Agilent Technologies United Kingdom Ltd., Cheadle, UK). The instrument was operated in reaction cell mode, using hydrogen. Selenium was determined as 78Se, with germanium (72Ge) as an internal standard. Matrix-matched calibration standards were prepared in 4.5% human albumin solution. Before analysis, specimens and calibrators were diluted 11-fold with an aqueous solution containing 2% butanol, 1% NH4OH, and 0.05% each of EDTA and Triton X-100. Accuracy of batch analysis was monitored using commercial trace element reference sera (Centre de Toxicologie, INSP, Quebec, Canada). Quality of assay performance was confirmed by participation, with good results, in two international quality assessment schemes (UK NEQAS for Trace Elements, University of Surrey, UK; International Comparison Program, INSP, Quebec, Canada).
Measurement of glutathione peroxidase (GPx)
GPx was measured in leukocyte lysates and in serum using the Bioxytech GPx-340 kit (Oxis Research, Portland, OR) following the instructions of the manufacturer. Results are expressed as milliunits GPx per milligram protein and per milliliter for leukocytes and serum, respectively.
DNA sequencing
Studies were approved by the Institutional Review Boards of University of Chicago and Birmingham Children’s Hospital, and written informed consents were obtained. The entire coding region and intron exon junctions of the SBP2 gene were sequenced. Primer sequences and PCR conditions are provided in supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Isolation of RNA and real-time PCR
Lymphocytes were separated from whole blood using Ficoll-Paque PLUS (Amersham Biosciences, Piscataway, NJ), and RNA was extracted using phenol/guanidine isothiocyanate (TRIZOL; Invitrogen Life Technologies, Carlsbad, CA). The RNA was reverse transcribed with the Superscript II RNase H Reverse Transcriptase Kit (Invitrogen) using 2 μg total RNA and 100 ng random hexamers.
Reactions for the quantification of mRNAs [quantitative PCR (qPCR)] were performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), using SYBR Green I as detector dye. The reaction mixtures contained 12.5 μl QuantiTect SYBR Green PCR Kit (QIAGEN Inc., Valencia, CA), 0.3 μmol/liter of each primer, 50 ng template cDNA, and ribonuclease-free water to a final volume of 25 μl. The oligonucleotide primers for SBP2 were designed to cross exons and are shown in supplemental Table 2. The threshold of amplification in all measurements occurred at 23–28 PCR cycles. Values were corrected with the simultaneous determination of RNA polymerase II as internal control. Each sample was run in triplicate and expressed as percent change relative to the wild-type (WT) subject.
To quantify the relative proportion of normal and mutant SBP2 mRNA in lymphocyte of heterozygotes, mRNA was first reverse transcribed and the cDNAs were submitted to low cycle PCR to amplify the fragment containing the different SBP2 mRNA isoforms (oligonucleotide primers are shown in supplemental Table 2). They were then digested with Taq I, which cuts the WT but not mutant SBP2 sequence. Bands developed on agarose gel electrophoresis and stained with ethidium bromide were digitalized, and their density was measured using Image J, version 1.34 S (Rasband, W. S., Image J, U.S. National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997–2006) (18). Results are expressed as ratio of the mutant allele relative to the WT allele in heterozygous subjects and corrected with digestion of genomic DNA extracted from the same sample.
Analysis of the effects of the R128X mutation on SBP2 isoforms expression
The SBP2 minigenes, WT and exon 3a mutated (E3aΔT), were previously described (19). The latter has a deletion of a T that produces a stop (UGA) at amino acid 126 (K126X). The R128X minigene was created by introducing the C382T mutation into the SBP2 WT minigene using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and the following primers: 5′-CAAACAGTGAAGCATTGAAATGAGAA CACATGC-3′ and 5′-GCATGTGTTCTCATTTCAATGCTTCACTGTTTG-3′. Plasmid integrity was verified by sequencing. The minigenes were transiently transfected into human embryonic kidney HEK293T cells using 2 μg plasmid DNA per well of a six-well plate and the FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN). Cultures were maintained in a medium containing 25 nm sodium selenite. Cells were lysed 48 h after transfection and analyzed by Western blotting using antibodies to SBP2 as previously described (13). Bands were quantified using Image J, version 1.34 S (see above) and corrected for heat-shock protein 70.
Results
Clinical evaluation and course of the disease
Although it is unclear when the proband developed growth retardation, concern about his stature arose at the age of 8.9 yr, 2 months after his arrival to the United Kingdom. Assessment of his general health and nutrition were normal (see Case report above). The only positive findings were a low serum Se and abnormal thyroid function tests consisting of high FT4, low FT3, and normal TSH. Based on these results, the consulting general pediatrician (N.M.) suspected from the outset a defect in the conversion of T4 to T3 caused by deficiency in the Se-containing deiodinases. Thus, selenium replacement 50 μg/d in the form of selenite (6 months) and later selenomethionine (4 months) was given.
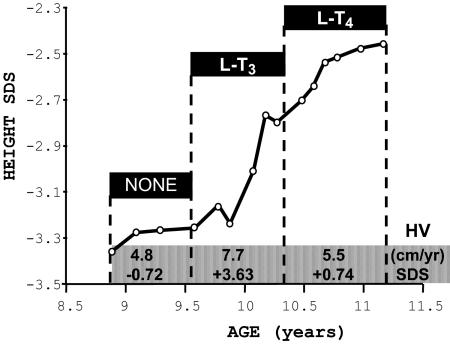
Over a period of 4 months, and despite treatment with sodium selenite, no changes in serum Se, thyroid function tests, or growth were observed (Figs. 1 and 2). Thus, a trial of treatment with l-T3 was initiated, first at a dose of 20 μg daily (given in two divided doses) and later 10 μg/d. During this period, in addition to an increase in height velocity (Fig. 2), there was advancement in bone age with narrowing of the gap between chronological and bone age (Table 1). Treatment with 20 μg l-T3 also reduced the serum FT4 levels and suppressed TSH below the lower limit of normal. No alterations in cardiac parameters were observed. Furthermore, the serum cholesterol concentration did not change, being 4.6 mmol/liter after 4 months treatment compared with 4.5 mmol/liter before initiation of treatment. Discontinuation of l-T3 administration for only 5 d resulted in a return of thyroid tests to pretreatment values (Fig. 1). It is at this point that further diagnostic and genetic testing was sought. Treatment with l-T4, which raised again the serum T4 levels, slowed down the increase in height velocity (Fig. 2), although the gap between chronological and bone age continued to narrow (Table 1). l-T3 at a lower dose was reinstated, but l-T4 was continued to maintain the basal serum T4 level.
Figure 2.
Effect of thyroid hormone treatment on linear growth of the proband (subject II-3, Fig. 3). Height is expressed as SDS in the ordinate and as a function of chronological age (33). Height velocities (HV) are changes in height over more than a 6-month interval and expressed in centimeters per year (34) and as SDS. In this plot, they are averaged for the three periods defined by the treatment given.
Table 1.
Bone age and linear growth
| Chronological age (yr) | Bone age (yr) | Difference (yr) | Height SDS | Treatment (months) |
|---|---|---|---|---|
| 8.9 | 3.1 | 5.8 | −3.36 | None |
| 10.2 | 5.2 | 5.0 | −2.77 | l-T3 (7) |
| 11.1 | 6.4 | 4.7 | −2.48 | l-T4 (9) |
Bone age is Tanner Whitehouse (TW20) (31), and difference is chronological minus bone age.
Genotyping and family study
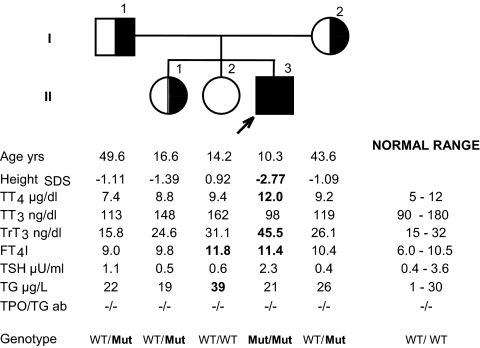
All members of the immediate family (family C) were tested. They were of normal stature, and tests of thyroid function were within the range of normal, except for the slight elevation of FT4I in one of the sisters (subject II-2, Fig. 3). Sequencing of the SBP2 gene revealed a novel mutation, a C to T transition in codon 128, located in exon 3, that results in the replacement of the normal arginine (CGA) with a stop (TGA). This early stop predicts an SBP2 defect in the proband and is in agreement with the observed thyroid phenotype. Both parents were heterozygous for the same mutation as was one of his sisters (subject II-1) but not the other (subject II-2, Fig. 3).
Figure 3.
Pedigree of the family (family C), age, height, and thyroid function data. Subjects are identified by generation (roman numerals on the left) and by a number on the right of each symbol. Results are aligned with the symbols in the pedigree. Values outside the normal range are in bold numbers. Height is given as SDS based on data from the British population obtained in 1990 (33). TG, Serum thyroglobulin; TGab, antibodies against thyroglobulin; TPOab, antibodies against thyroperoxidase. Normal ranges are for adults and adolescents except for the upper limit of normal for T3 of 210 ng/dl and rT3 of 38 ng/dl for adolescents. Genotyping results are also provided. Mut represents the SBP2 mutation R128X. Note that the blood sample from the proband was obtained 5 d after interruption of l-T3 treatment. Also by the age of 10.3 yr, the child had received 8 months l-T3 treatment, increasing his relative height compared with that at presentation (see case report).
Assessment of biochemical markers of selenoprotein synthesis
To assess the effect of this mutation on other selenoproteins, we measured the enzymatic activity of GPx in serum and in leukocytes. By virtue of their abundance, they represent predominantly GPx3 in serum and GPx1 in leukocytes. As shown on Table 2, the activity of the enzymes were markedly reduced in the proband but not in other members of the family, both heterozygous and carrying only the WT allele.
Table 2.
GPx activity
| Subject (see Fig. 3) | Relationship | Serum (mU/ml) | Leukocytes (mU/mg protein) |
|---|---|---|---|
| II-3 | Proband | 7 | 0.61 |
| I-2 | Mother | 70 | 65.5 |
| I-1 | Father | 67 | 33.8 |
| II-1 | Sister | 62 | NM |
| II-2 | Sister | 59 | 25.9 |
| Controls | Unrelated | 60 ± 11 | 18–70 |
Data are means of two separate determinations. NM, Not measured because whole blood was not available. Controls were from the United States: serum, nine subjects (mean ± sd); leukocytes, four subjects (range).
Gene transcription splicing and translation
Given the mild phenotype in the presence of a putative lack of SBP2, we explored the possibility that the mutation produced alternative splicing and the synthesis of a partial SBP2 molecule with possible functional activity. Studies along these lines were further prompted by the recent finding that SBP2 has several mRNA and protein isoforms (19).
Exon 3, where the mutation is located, is subject to alternative splicing normally producing three different SBP2 gene isoforms: 1) the intact isoforms containing the entire exon 3, 2) an isoform lacking 121 bp of the 5′-segment of exon 3, and 3) an isoform lacking the entire exon 3. Using qPCR and primer sequences crossing specific exons (see Subjects and Methods and supplemental Table 2), we tested for the relative proportion of the three isoforms of SBP2 mRNA described above in RNA extracted from leukocytes of all family members. qPCR of exons 4–5 was also performed to quantify all three isoforms in combination. Compared with the sister expressing only the WT alleles (100%), the expression of the intact exon 3, partial exon 3, absent exon 3, and exons 4–5 in the heterozygote parents were 74, 89, 62, and 76%, respectively, whereas the homozygote proband expressed 67, 79, 64, and 69%.
To determine whether there is a disproportionate abundance in mRNA harboring the mutant and WT sequence, fragments of cDNA derived from leukocytes of the heterozygote parents, which contain both WT and mutant nucleotides, were quantified after allele-specific digestion with TaqI (see Subjects and Methods and supplemental Table 2). The mean ratios of mutant relative to WT sequences were: 0.64/1 for intact exon 3 isoform, 0.62/1 for partial exon 3 isoform, and 0.60/1 for all isoforms combined. This suggests a modest allele-preferential expression due to nonsense-mediated decay in heterozygote subjects (20).
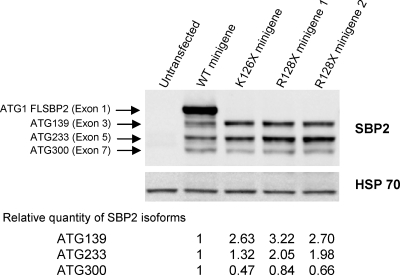
We then turned to the analysis of translation and transcription of the mutant and WT minigenes. Two colonies of plasmids containing the minigene with the mutation (R128X) were studied along with two controls, the minigene that produces a stop at the nearby codon 126 (E3aΔT or K126X) and the WT (19). As shown in Fig. 4, minigenes with stop codons at 126 and 128 lacked the full-length protein. However, they synthesized the smaller fragments from downstream ATGs at codons 139, 233, and 300, also produced by the WT minigene. Minigenes K126X and R128X synthesized 2- to 3-fold more SBP2 molecules from ATG 139 than the WT and those from ATG 233 were 2-fold more abundant in R128X but not K126X. Quantification of the smallest SBP2 (ATG 300) is less accurate.
Figure 4.
Western blot of SBP2 synthesized by minigenes expressing the mutant gene. Minigene constructs, identified above each lane, were transiently transfected into HEK293T cells, and products were electrophoresed, blotted, and developed with antibodies to SBP2. Bands generated from different ATGs are identified as described previously (19). Their relative proportion was quantified by density measurement using Image J and expressed relative to the corresponding band generated by the WT minigene and corrected for heat-shock protein (HSP) 70. FLSBP2, Full-length SBP2 protein.
Discussion
We previously reported the first occurrence of an inherited defect in thyroid hormone metabolism in humans (11). It was caused by mutations in the SBP2 gene. Three mutations were identified in two families. The missense mutation in the affected homozygous subjects of one family (family A) produced a nonsynonymous change in a conserved amino acid (R540Q), believed to be encoded by a hypomorphic allele. In the second family (family B), the affected compound heterozygous child had two mutations, one nonsense mutation (K438X) resulting in a truncation and another (IVS8ds+29G→A) producing a splicing defect. Given the expected devastating effect from complete impairment of selenoprotein synthesis (21), we hypothesized that the mild phenotype of the affected individuals of these families (limited to transient growth retardation and partial defect in thyroid hormone metabolism) must be due to preservation of sufficient SBP2 function in the affected subjects. Indeed, in family B, the splicing defect was partial, which allowed a 24% normal transcript (11). More recently, Bubenik and Driscoll (22) showed that the mutant SBP2 of family A had altered mRNA binding affinity so that it no longer stably interacted with a subset of SECIS elements including those of D2, GPx1, and D1 SECIS RNAs. Moreover, in vitro assays designed to mimic intracellular conditions, as well as other studies (23), showed that SBP2 exhibits preferential binding to some selenoprotein mRNAs compared with others. This seems to be the major determinant in dictating the hierarchy of selenoprotein synthesis and sensitivity to nonsense-mediated decay that occurs when the UGA decoding system is inefficient. It explained the preponderance of the deiodinase defect observed in family A we reported (11).
The reason for relatively mild phenotype of the proband of this newly identified family (family C), homozygous for an early stop codon in the SBP2 gene, was very surprising. An explanation was provided by our in vitro studies showing the synthesis of three SBP2 molecules from different downstream AUGs. All resulting isoforms contain the functional domains, including those required for selenocysteine insertion and RNA binding and ribosome interaction (residues present in exons 12–16) (24). Although quantification of SBP2 mRNA in leukocytes of family members and the relative proportion of SBP2 mRNA derived from the mutant compared with WT allele in heterozygotes suggest some nonsense-mediated decay, SBP2 isoforms ATG 130 and ATG 233 showed a 2- to 3-fold increase in truncated R128X minigene, indicating that a compensatory effect might exist.
There is no doubt that the thyroid phenotype in subjects deficient in SBP2 is secondary to the defect in iodothyronine metabolism due to a reduction in iodothyronine deiodinases. Yet, the serum thyroid abnormalities of high T4, low T3, and high rT3 do not perfectly match any of the mouse models of deiodinase deficiencies (5,7,8,9,10). All have increased serum T4 and normal T3 concentrations except for the D3KO mouse. The latter has low T4 and T3 concentrations. Serum rT3 is high in the D1KO and double D1/D2KO, and TSH is increased in the D2KO, C3H/D2KO, and D1/D2KO. As supported by our previous findings of reduced but not absent D2 activity in subjects with SBP2 mutations (11), it is logical to conclude that the phenotype in humans represents the combined but partial deficiency in all three deiodinases not fully reproduced in any of the available mouse models of total but selective deiodinase deficiencies.
Careful analysis of the phenotypes showed that the affected subject of the three families had no significant differences in the magnitude of thyroid function test abnormalities. Mean serum concentrations in affected subjects of families A, B, and C were, respectively, for T4 15.2, 14.9, and 14.0 μg/dl and for T3 83, 86, and 98 ng/dl. Similarly, no quantitative differences were observed in the reduction of the serum GPx activity, which in the subject of family C, presented herein, was 8.6-fold below the mean normal compared with 7.5-fold in families A and B (11). Unfortunately, no other selenoproteins, including deiodinases, could be measured in the subjects reported herein because of the limited amount of leukocytes and their low level of deiodinase expression. In addition, the family refused to provide skin samples for culture of fibroblasts as done in two other families with SBP2 gene mutations.
On the other hand, growth retardation was more significant in the affected boy presented herein. Heights of the probands for each of the three families (A, B, and C), at chronological ages of 11.5, 6, and 8.9 yr, were in the 5th, 97th and below the 1st centile, respectively, with bone ages of 7.5, 6, and 3.1 yr. Of note is that the proband of family B reached a normal stature by the age of 6 yr, when the first accurate measurement became available. Thus, in terms of linear and bone growth, the impairment was most severe in the subject homozygous for an early stop codon in the SBP2 gene (family C), followed by the child with the hypomorphic missense mutation (family A). The least and transient effect on growth was observed in the child expressing 24% of the WT SBP2 (family B).
The exact cause of growth retardation in individuals with SBP2 gene mutations remains unknown. Although the growth retardation observed in Se-deficient rodents (25,26) is associated with a reduction in GH and IGF-I (25), this was not found in the proband. Treatment with Se had no effect on the child reported herein or with higher Se doses given recently to subjects of family A (27). Based on the accelerated growth during l-T3 treatment, it appears that the growth retardation might be due to reduced thyroid hormone action on peripheral tissues. Yet, there was no correlation between the magnitude of serum T3 reduction and growth delay. In addition, in affected subjects of families A and B, catch-up growth was observed with the passage of time and without treatment.
The long-term effect of these SBP2 defects on the affected subjects is open to conjecture. Based on observation in selenoprotein-deficient animals, infertility and a propensity to develop cancer have been suggested (28,29). In a recent report, a 35-yr-old male with an SBP2 gene defect presented with azoospermia and bilateral high-frequency sensorineural hearing loss (30). These findings indicate that SBP2 defects may have as yet undetermined consequences.
Supplementary Material
Acknowledgments
We thank Dr. Neal H. Scherberg and his laboratory staff for performing the tests of thyroid function in sera and Dr. Ted Sheehan and his staff (City Hospital Birmingham, UK) for the blood selenium analyses. We thank all members of the family for their willingness to participate in this study.
Footnotes
This work was supported by Grants DK15070, DK20595, and RR04999 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 14, 2009
Abbreviations: FT4, Free T4; FT4I, FT4 index; Gpx, glutathione peroxidase; KO, knockout; qPCR, quantitative PCR; SBP2, selenocysteine insertion sequence-binding protein 2; SDS, sd score; TT4, total T4; WT, wild type.
References
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC 2008 Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Kester MH, Peeters RP, Visser TJ 2005 Biochemical mechanisms of thyroid hormone deiodination. Thyroid 15:787–798 [DOI] [PubMed] [Google Scholar]
- Bianco AC, Kim BW 2006 Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116:2571–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig RJ 2005 Regulation of type 1 iodothyronine deiodinase in health and disease. Thyroid 15:835–840 [DOI] [PubMed] [Google Scholar]
- Berry MJ, Grieco D, Taylor BA, Maia AL, Kieffer JD, Beamer W, Glover E, Poland A, Larsen PR 1993 Physiological and genetic analyses of inbred mouse strains with type I iodothyronine 5′ deiodinase deficiency. J Clin Invest 92:1517–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia AL, Berry MJ, Sabbag R, Harney JW, Larsen PR 1995 Structural and functional differences in the dio1 gene in mice with inherited type 1 deiodinase deficiency. Mol Endocrinol 9:969–980 [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Thai B, Wu SY, St Germain E, Parlow AF, St Germain DL, Galton VA 2006 Targeted disruption of the type 1 selenodeiodinase gene (dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 147:580–589 [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA 2001 Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 15:2137–2148 [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Liao XH, Van Sande J, Refetoff S, Galton VA, St Germain DL 2007 Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology 148:5680–5687 [DOI] [PubMed] [Google Scholar]
- Galton VA, Schneider MJ, Clark AS, St Germain DL 2009 Life without thyroxine to 3,5,3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology 150:2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S 2005 Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37:1247–1252 [DOI] [PubMed] [Google Scholar]
- Copeland PR 2003 Regulation of gene expression by stop codon recoding: selenocysteine. Gene 312:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp LV, Lu J, Striebel F, Kennedy D, Holmgren A, Khanna KK 2006 The redox state of SECIS binding protein 2 controls its localization and selenocysteine incorporation function. Mol Cell Biol 26:4895–4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BA, Xu XM, Gladyshev VN, Hatfield DL 2005 Selective rescue of selenoprotein expression in mice lacking a highly specialized methyl group in selenocysteine tRNA. J Biol Chem 280:5542–5548 [DOI] [PubMed] [Google Scholar]
- Köhrle J 2005 Selenium and the control of thyroid hormone metabolism. Thyroid 15:841–853 [DOI] [PubMed] [Google Scholar]
- Robin NI, Hagen SR, Collaço F, Refetoff S, Selenkow HA 1971 Serum tests for measurement of thyroid function. Hormones 2:266–279 [DOI] [PubMed] [Google Scholar]
- Barsano CP, Skosey C, DeGroot LJ, Refetoff S 1982 Serum thyroglobulin in the management of patients with thyroid cancer. Arch Intern Med 142:763–767 [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ 2004 Image processing with Image J. Biophoton Int 11:36–42 [Google Scholar]
- Papp LV, Wang J, Kennedy D, Boucher D, Zhang Y, Gladyshev VN, Singh RN, Khanna KK 2008 Functional characterization of alternatively spliced human SECISBP2 transcript variants. Nucleic Acids Res 36:7192–7206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartegni L, Chew SL, Krainer AR 2002 Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet 3:285–298 [DOI] [PubMed] [Google Scholar]
- Bosl MR, Takaku K, Oshima M, Nishimura S, Taketo MM 1997 Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp). Proc Natl Acad Sci USA 94:5531–5534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik JL, Driscoll DM 2007 Altered RNA-binding activity underlies abnormal thyroid hormone metabolism linked to a mutation in Sec insertion sequence binding protein 2. J Biol Chem 282:34653–34662 [DOI] [PubMed] [Google Scholar]
- Squires JE, Stoytchev I, Forry EP, Berry MJ 2007 SBP2 binding affinity is a major determinant in differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. Mol Cell Biol 27:7848–7855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland PR, Stepanik VA, Driscoll DM 2001 Insight into mammalian selenocysteine insertion: domain structure and ribosome binding properties of Sec insertion sequence binding protein 2. Mol Cell Biol 21:1491–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Reyes R, Egrise D, Nève J, Pasteels JL, Schoutens A 2001 Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J Bone Miner Res 16:1556–1563 [DOI] [PubMed] [Google Scholar]
- Schweizer U, Michaelis M, Köhrle J, Schomburg L 2004 Efficient selenium transfer from mother to offspring in selenoprotein-P-deficient mice enables dose-dependent rescue of phenotypes associated with selenium deficiency. Biochem J 378:21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg L, Dumitrescu AM, Liao XH, Bin-Abbas B, Hoeflich J, Köhrle J, Refetoff S 2009 Selenium supplementation fails to correct the selenoprotein synthesis defect in subjects with SBP2 gene mutations. Thyroid 19:277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP, Nagdas SK, Hill KE, Burk RF 2005 Selenoprotein P is required for mouse sperm development. Biol Reprod 73:201–211 [DOI] [PubMed] [Google Scholar]
- Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S, Doroshow JH 2004 Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res 64:962–968 [DOI] [PubMed] [Google Scholar]
- Mitchell C, Robertson V, Montano S, Lu J, Clemons N, Halsall D, Rajanayagam O, Castanet M, Baguely D, Fitzgerald R, Holmgren A, Coles A, Ogunko A, Chatterjee VK 2007 The adult phenotype of multiple selenoprotein deficiencies associated with a novel SECISBP2 gene defect. 8th International Workshop on Resistance to Thyroid Hormone and Action, Ponta Delgada, San Miguel, Azores, Portugal, p OP18 (Abstract) [Google Scholar]
- Tanner JM, Whitehouse RH, Cameron N, Marshall WA, Healy MJR, Goldstein H 1983 Assessment of skeletal maturity and prediction of adult height (TW2 method). 2nd ed. London: Academic Press [Google Scholar]
- Taylor A 2006 Clinical and analytical handbook of the SAS Trace Element Center. 4th ed. Guildford, UK: Supra-Regional Assay Service; 98–101, 161 [Google Scholar]
- Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA 1995 Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 73:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH, Takaishi M 1966 Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child 41:613–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.