Abstract
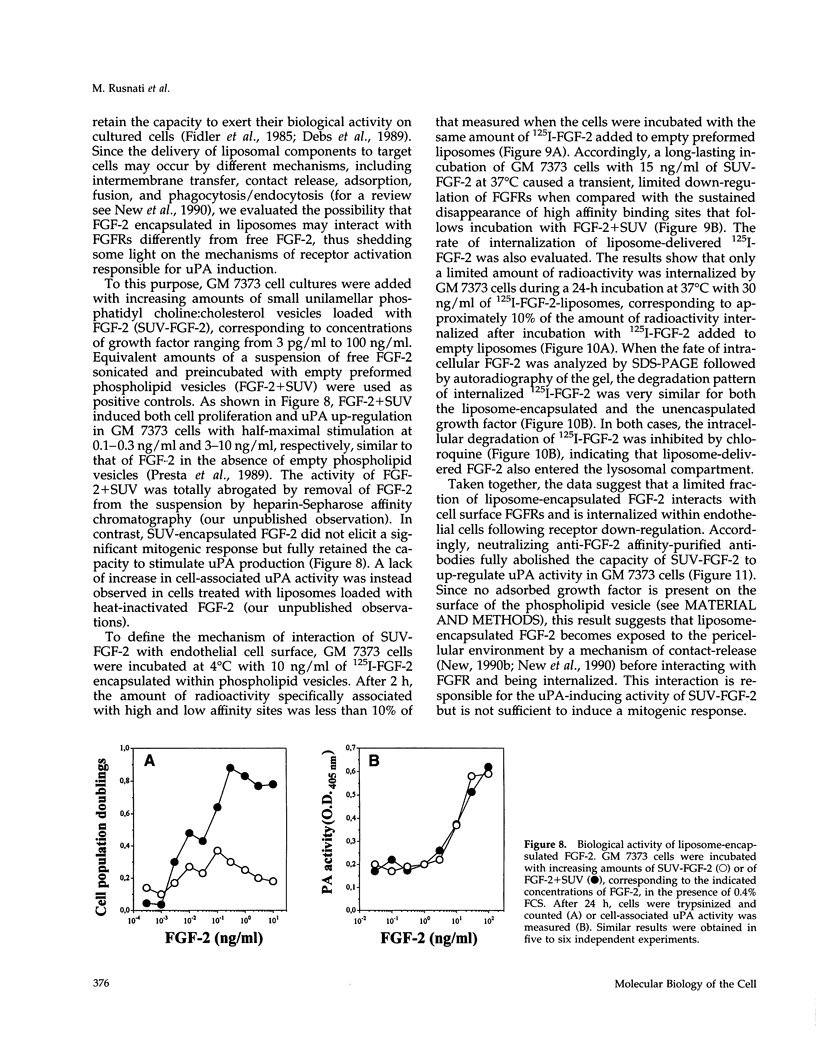
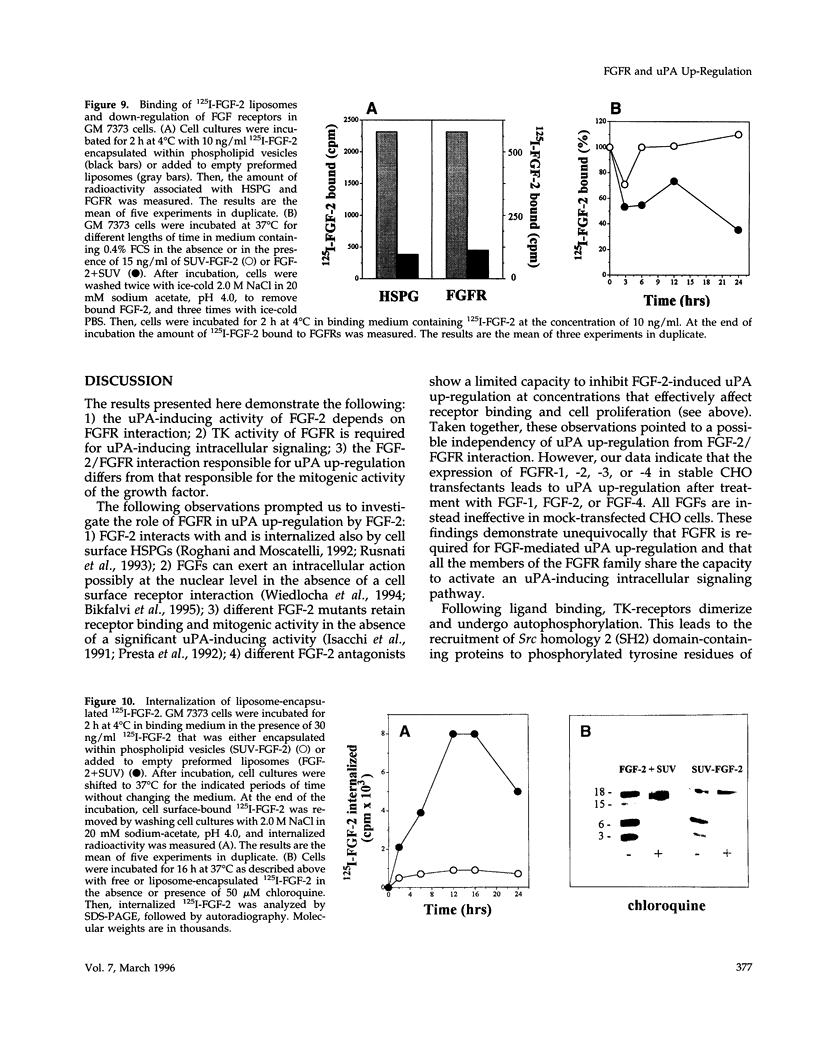
Basic fibroblast growth factor (FGF-2) induces cell proliferation and urokinase-type plasminogen activator (uPA) production in fetal bovine aortic endothelial GM 7373 cells. In the present paper we investigated the role of the interaction of FGF-2 with tyrosine-kinase (TK) FGF receptors (FGFRs) in mediating uPA up-regulation in these cells. The results show that FGF-2 antagonists suramin, protamine, heparin, the synthetic peptide FGF-2(112-155), and a soluble form of FGFR-1 do not inhibit FGF-2-mediated uPA up-regulation at concentrations that affect growth factor binding to cell surface receptors and mitogenic activity. In contrast, tyrosine phosphorylation inhibitors and overexpression of a dominant negative TK- mutant of FGFR-1 abolish the uPA-inducing activity of FGF-2, indicating that FGFR and its TK activity are essential in mediating uPA induction. Accordingly, FGF-2 induces uPA up-regulation in Chinese hamster ovary cells transfected with wild-type FGFR-1, -2, -3, or -4 but not with TK- FGFR-1 mutant. Small unilamellar phosphatidyl choline:cholesterol vesicles loaded with FGF-2 increased uPA production in GM 7373 cells in the absence of a mitogenic response. Liposome-encapsulated FGF-2 showed a limited but significant capacity, relative to free FGF-2, to interact with FGFR both at 4 degrees C and 37 degrees C and to be internalized within the cell. uPA up-regulation by liposome-encapsulated FGF-2 was quenched by neutralizing anti-FGF-2 antibodies, indicating that the activity of liposome-delivered FGF-2 is mediated by an extracellular action of the growth factor. Taken together, the data indicate that a distinct interaction of FGF-2 with FGFR, quantitatively and/or qualitatively different from the one that leads to mitogenicity, is responsible for the uPA-inducing activity of the growth factor.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Baird A., Schubert D., Ling N., Guillemin R. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2324–2328. doi: 10.1073/pnas.85.7.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Bergonzoni L., Caccia P., Cletini O., Sarmientos P., Isacchi A. Characterization of a biologically active extracellular domain of fibroblast growth factor receptor 1 expressed in Escherichia coli. Eur J Biochem. 1992 Dec 15;210(3):823–829. doi: 10.1111/j.1432-1033.1992.tb17485.x. [DOI] [PubMed] [Google Scholar]
- Besser D., Presta M., Nagamine Y. Elucidation of a signaling pathway induced by FGF-2 leading to uPA gene expression in NIH 3T3 fibroblasts. Cell Growth Differ. 1995 Aug;6(8):1009–1017. [PubMed] [Google Scholar]
- Bikfalvi A., Klein S., Pintucci G., Quarto N., Mignatti P., Rifkin D. B. Differential modulation of cell phenotype by different molecular weight forms of basic fibroblast growth factor: possible intracellular signaling by the high molecular weight forms. J Cell Biol. 1995 Apr;129(1):233–243. doi: 10.1083/jcb.129.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche G., Gas N., Prats H., Baldin V., Tauber J. P., Teissié J., Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer B., Thiery J. P. Cyclic AMP distinguishes between two functions of acidic FGF in a rat bladder carcinoma cell line. J Cell Biol. 1993 Feb;120(3):767–776. doi: 10.1083/jcb.120.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah A. T., McEwen D. G., Werner S., Xu J., Ornitz D. M. Fibroblast growth factor receptor (FGFR) 3. Alternative splicing in immunoglobulin-like domain III creates a receptor highly specific for acidic FGF/FGF-1. J Biol Chem. 1994 Apr 15;269(15):11620–11627. [PubMed] [Google Scholar]
- Chen P., Gupta K., Wells A. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J Cell Biol. 1994 Feb;124(4):547–555. doi: 10.1083/jcb.124.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L. Platelet-derived growth factor receptor signals. J Biol Chem. 1994 Dec 23;269(51):32023–32026. [PubMed] [Google Scholar]
- Coltrini D., Gualandris A., Nelli E. E., Parolini S., Molinari-Tosatti M. P., Quarto N., Ziche M., Giavazzi R., Presta M. Growth advantage and vascularization induced by basic fibroblast growth factor overexpression in endometrial HEC-1-B cells: an export-dependent mechanism of action. Cancer Res. 1995 Oct 15;55(20):4729–4738. [PubMed] [Google Scholar]
- Debs R. J., Düzgüneş N., Brunette E. N., Fendly B., Patton J., Philip R. Liposome-associated tumor necrosis factor retains bioactivity in the presence of neutralizing anti-tumor necrosis factor antibodies. J Immunol. 1989 Aug 15;143(4):1192–1197. [PubMed] [Google Scholar]
- Dell'Era P., Presta M., Ragnotti G. Nuclear localization of endogenous basic fibroblast growth factor in cultured endothelial cells. Exp Cell Res. 1991 Feb;192(2):505–510. doi: 10.1016/0014-4827(91)90070-b. [DOI] [PubMed] [Google Scholar]
- Dionne C. A., Crumley G., Bellot F., Kaplow J. M., Searfoss G., Ruta M., Burgess W. H., Jaye M., Schlessinger J. Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors. EMBO J. 1990 Sep;9(9):2685–2692. doi: 10.1002/j.1460-2075.1990.tb07454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J., Fogler W. E., Kleinerman E. S., Saiki I. Abrogation of species specificity for activation of tumoricidal properties in macrophages by recombinant mouse or human interferon-gamma encapsulated in liposomes. J Immunol. 1985 Dec;135(6):4289–4296. [PubMed] [Google Scholar]
- Fujimori A., Cheng S. L., Avioli L. V., Civitelli R. Dissociation of second messenger activation by parathyroid hormone fragments in osteosarcoma cells. Endocrinology. 1991 Jun;128(6):3032–3039. doi: 10.1210/endo-128-6-3032. [DOI] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Gherardi E., Stoker M. Hepatocyte growth factor--scatter factor: mitogen, motogen, and met. Cancer Cells. 1991 Jun;3(6):227–232. [PubMed] [Google Scholar]
- Grinspan J. B., Mueller S. N., Levine E. M. Bovine endothelial cells transformed in vitro by benzo(a)pyrene. J Cell Physiol. 1983 Mar;114(3):328–338. doi: 10.1002/jcp.1041140312. [DOI] [PubMed] [Google Scholar]
- Gualandris A., Presta M. Transcriptional and posttranscriptional regulation of urokinase-type plasminogen activator expression in endothelial cells by basic fibroblast growth factor. J Cell Physiol. 1995 Mar;162(3):400–409. doi: 10.1002/jcp.1041620312. [DOI] [PubMed] [Google Scholar]
- Isacchi A., Statuto M., Chiesa R., Bergonzoni L., Rusnati M., Sarmientos P., Ragnotti G., Presta M. A six-amino acid deletion in basic fibroblast growth factor dissociates its mitogenic activity from its plasminogen activator-inducing capacity. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2628–2632. doi: 10.1073/pnas.88.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Lu J., Chen H., Werner S., Williams L. T. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Mol Cell Biol. 1991 Sep;11(9):4627–4634. doi: 10.1128/mcb.11.9.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Williams L. T. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Katsuura M., Tanaka S. Topographic analysis of human epidermal growth factor by monospecific antibodies and synthetic peptides. J Biochem. 1989 Jul;106(1):87–92. doi: 10.1093/oxfordjournals.jbchem.a122826. [DOI] [PubMed] [Google Scholar]
- Keegan K., Johnson D. E., Williams L. T., Hayman M. J. Isolation of an additional member of the fibroblast growth factor receptor family, FGFR-3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1095–1099. doi: 10.1073/pnas.88.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F. G., Basilico C. Transcription from the polyoma late promoter in cells stably transformed by chimeric plasmids. Mol Cell Biol. 1985 Apr;5(4):797–807. doi: 10.1128/mcb.5.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Giancotti F. G., Presta M., Albelda S. M., Buck C. A., Rifkin D. B. Basic fibroblast growth factor modulates integrin expression in microvascular endothelial cells. Mol Biol Cell. 1993 Oct;4(10):973–982. doi: 10.1091/mbc.4.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Li Y., Basilico C., Mansukhani A. Cell transformation by fibroblast growth factors can be suppressed by truncated fibroblast growth factor receptors. Mol Cell Biol. 1994 Nov;14(11):7660–7669. doi: 10.1128/mcb.14.11.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokker N. A., Mark M. R., Luis E. A., Bennett G. L., Robbins K. A., Baker J. B., Godowski P. J. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 1992 Jul;11(7):2503–2510. doi: 10.1002/j.1460-2075.1992.tb05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P. A. Inhibition of the tyrosine kinase activity of the fibroblast growth factor receptor by the methyltransferase inhibitor 5'-methylthioadenosine. J Biol Chem. 1993 Feb 25;268(6):4244–4249. [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995 Jan 27;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. Basic fibroblast growth factor (bFGF) dissociates rapidly from heparan sulfates but slowly from receptors. Implications for mechanisms of bFGF release from pericellular matrix. J Biol Chem. 1992 Dec 25;267(36):25803–25809. [PubMed] [Google Scholar]
- Moscatelli D. Metabolism of receptor-bound and matrix-bound basic fibroblast growth factor by bovine capillary endothelial cells. J Cell Biol. 1988 Aug;107(2):753–759. doi: 10.1083/jcb.107.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Rifkin D. B. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y., Kihara K., Mizuno K., Masamune Y., Yoshitake Y., Nishikawa K. Direct effect of basic fibroblast growth factor on gene transcription in a cell-free system. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5216–5220. doi: 10.1073/pnas.89.12.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen J., Mäkelä T. P., Eerola E., Korhonen J., Hirvonen H., Claesson-Welsh L., Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991 Jun;10(6):1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Pepper M. S., Meda P. Basic fibroblast growth factor increases junctional communication and connexin 43 expression in microvascular endothelial cells. J Cell Physiol. 1992 Oct;153(1):196–205. doi: 10.1002/jcp.1041530124. [DOI] [PubMed] [Google Scholar]
- Presta M., Gualandris A., Urbinati C., Rusnati M., Coltrini D., Isacchi A., Caccia P., Bergonzoni L. Subcellular localization and biological activity of M(r) 18,000 basic fibroblast growth factor: site-directed mutagenesis of a putative nuclear translocation sequence. Growth Factors. 1993;9(4):269–278. doi: 10.3109/08977199308991587. [DOI] [PubMed] [Google Scholar]
- Presta M., Maier J. A., Ragnotti G. The mitogenic signaling pathway but not the plasminogen activator-inducing pathway of basic fibroblast growth factor is mediated through protein kinase C in fetal bovine aortic endothelial cells. J Cell Biol. 1989 Oct;109(4 Pt 1):1877–1884. doi: 10.1083/jcb.109.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta M., Maier J. A., Rusnati M., Moscatelli D., Ragnotti G. Modulation of plasminogen activator activity in human endometrial adenocarcinoma cells by basic fibroblast growth factor and transforming growth factor beta. Cancer Res. 1988 Nov 15;48(22):6384–6389. [PubMed] [Google Scholar]
- Presta M., Moscatelli D., Joseph-Silverstein J., Rifkin D. B. Purification from a human hepatoma cell line of a basic fibroblast growth factor-like molecule that stimulates capillary endothelial cell plasminogen activator production, DNA synthesis, and migration. Mol Cell Biol. 1986 Nov;6(11):4060–4066. doi: 10.1128/mcb.6.11.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta M., Statuto M., Isacchi A., Caccia P., Pozzi A., Gualandris A., Rusnati M., Bergonzoni L., Sarmientos P. Structure-function relationship of basic fibroblast growth factor: site-directed mutagenesis of a putative heparin-binding and receptor-binding region. Biochem Biophys Res Commun. 1992 Jun 30;185(3):1098–1107. doi: 10.1016/0006-291x(92)91739-d. [DOI] [PubMed] [Google Scholar]
- Presta M., Tiberio L., Rusnati M., Dell'Era P., Ragnotti G. Basic fibroblast growth factor requires a long-lasting activation of protein kinase C to induce cell proliferation in transformed fetal bovine aortic endothelial cells. Cell Regul. 1991 Sep;2(9):719–726. doi: 10.1091/mbc.2.9.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghani M., Moscatelli D. Basic fibroblast growth factor is internalized through both receptor-mediated and heparan sulfate-mediated mechanisms. J Biol Chem. 1992 Nov 5;267(31):22156–22162. [PubMed] [Google Scholar]
- Rusnati M., Urbinati C., Presta M. Internalization of basic fibroblast growth factor (bFGF) in cultured endothelial cells: role of the low affinity heparin-like bFGF receptors. J Cell Physiol. 1993 Jan;154(1):152–161. doi: 10.1002/jcp.1041540119. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994 Feb;4(1):25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Shanafelt A. B., Kastelein R. A. High affinity ligand binding is not essential for granulocyte-macrophage colony-stimulating factor receptor activation. J Biol Chem. 1992 Dec 15;267(35):25466–25472. [PubMed] [Google Scholar]
- Sherman L., Stocker K. M., Morrison R., Ciment G. Basic fibroblast growth factor (bFGF) acts intracellularly to cause the transdifferentiation of avian neural crest-derived Schwann cell precursors into melanocytes. Development. 1993 Aug;118(4):1313–1326. doi: 10.1242/dev.118.4.1313. [DOI] [PubMed] [Google Scholar]
- Springer B. A., Pantoliano M. W., Barbera F. A., Gunyuzlu P. L., Thompson L. D., Herblin W. F., Rosenfeld S. A., Book G. W. Identification and concerted function of two receptor binding surfaces on basic fibroblast growth factor required for mitogenesis. J Biol Chem. 1994 Oct 28;269(43):26879–26884. [PubMed] [Google Scholar]
- Strassmann G., Bertolini D. R., Eidelman O. Inhibition of immune reactions in vivo by liposome associated transforming growth factor (TGF) type beta 1. Clin Exp Immunol. 1991 Dec;86(3):532–536. doi: 10.1111/j.1365-2249.1991.tb02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Unson C. G., Macdonald D., Ray K., Durrah T. L., Merrifield R. B. Position 9 replacement analogs of glucagon uncouple biological activity and receptor binding. J Biol Chem. 1991 Feb 15;266(5):2763–2766. [PubMed] [Google Scholar]
- Wang J. K., Gao G., Goldfarb M. Fibroblast growth factor receptors have different signaling and mitogenic potentials. Mol Cell Biol. 1994 Jan;14(1):181–188. doi: 10.1128/mcb.14.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. H., Nuccitelli R. Inhibition of tyrosine phosphorylation prevents thrombin-induced mitogenesis, but not intracellular free calcium release, in vascular smooth muscle cells. J Biol Chem. 1992 Mar 15;267(8):5608–5613. [PubMed] [Google Scholar]
- Wiedłocha A., Falnes P. O., Madshus I. H., Sandvig K., Olsnes S. Dual mode of signal transduction by externally added acidic fibroblast growth factor. Cell. 1994 Mar 25;76(6):1039–1051. doi: 10.1016/0092-8674(94)90381-6. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]





