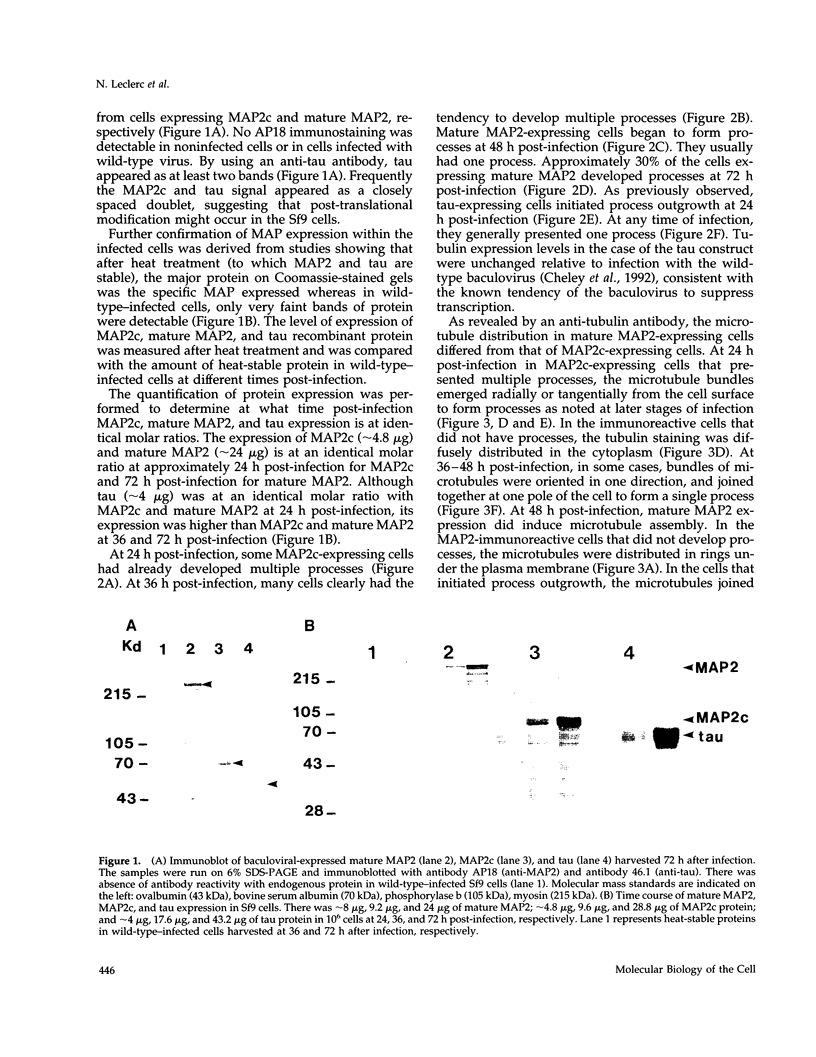
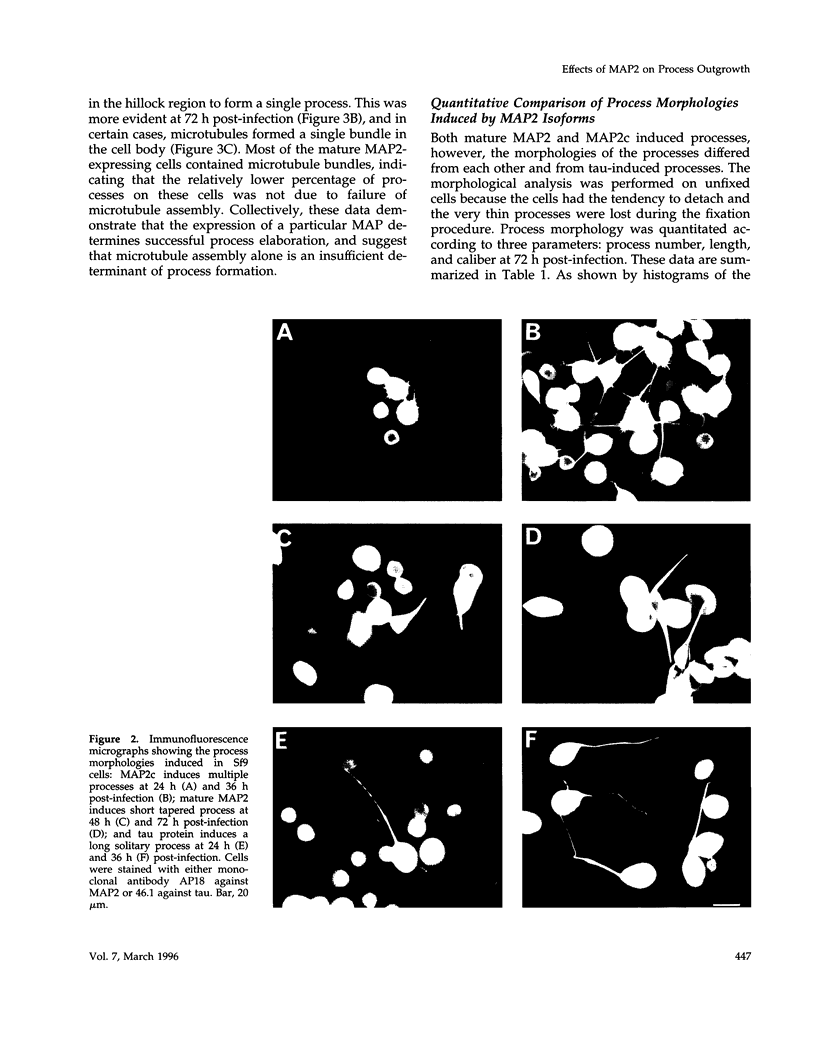
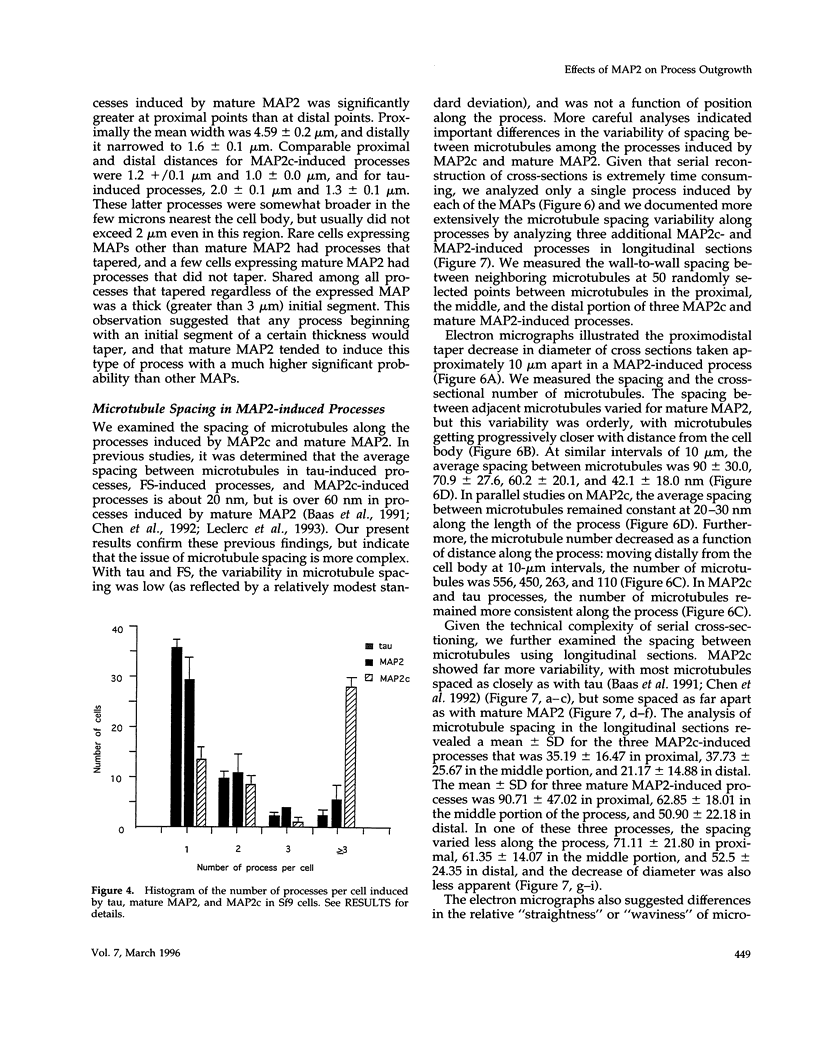
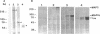Abstract
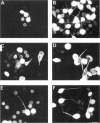
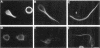
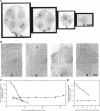
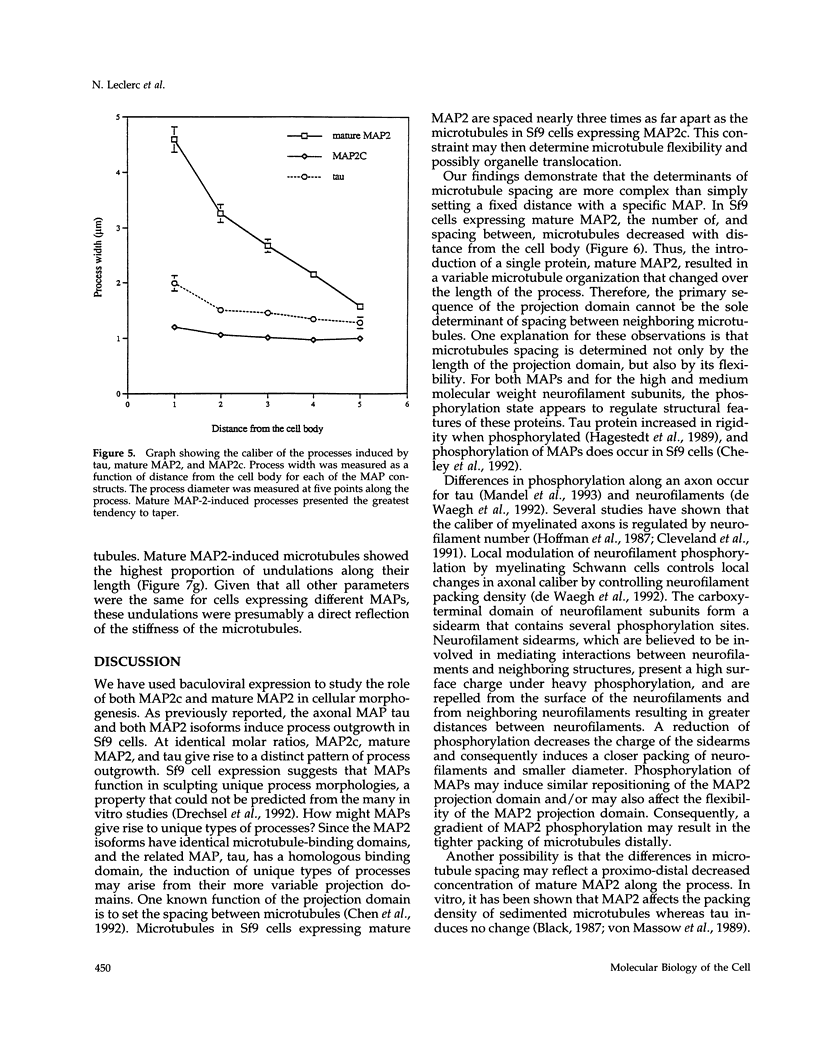
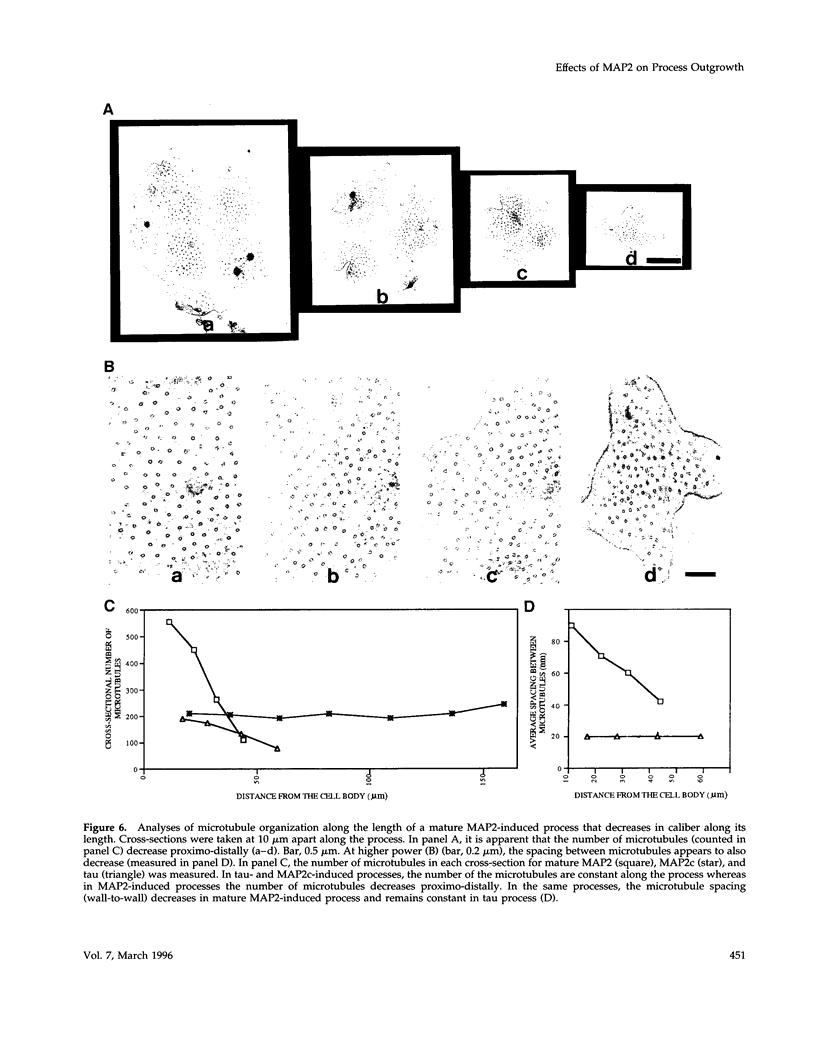
Microtubule-associated protein-2 (MAP2) is the most abundant MAP in neurons, where its distribution is restricted to the somatodendritic compartment. This molecule undergoes developmentally regulated alternative splicing, resulting in at least two isoforms, a juvenile isoform (termed MAP2c) and a mature isoform (MAP2), with greatly different molecular masses. Spodoptera frugiperda (Sf9) cell expression of the juvenile versus the mature MAP2 isoform generates two distinct patterns of process outgrowth. The smaller juvenile isoform induces multiple short thin processes. Mature MAP2 tends to induce single processes that are considerably thicker than those processes induced by juvenile MAP2. We found important differences in the variability of spacing between microtubules and the number of microtubules along the processes induced by MAP2c and mature MAP2. MAP2c showed variability with most microtubules spaced as closely as with tau, but some spaced as far apart as with mature MAP2. Over their length, the mature MAP2 processes demonstrate proximo-distal taper, which corresponds to a narrowing of the spacing between microtubules from 90 nm to 40 nm. Moreover, there is a decreased number of microtubules in mature MAP2-induced processes whereas in tau and MAP2-induced processes, the number of microtubules is constant along the length. Based on these observations, we conclude that MAP2 isoforms can serve as architectural elements by establishing specific morphological features of processes and specific arrangements of their microtubules.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P. W., Deitch J. S., Black M. M., Banker G. A. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Pienkowski T. P., Kosik K. S. Processes induced by tau expression in Sf9 cells have an axon-like microtubule organization. J Cell Biol. 1991 Dec;115(5):1333–1344. doi: 10.1083/jcb.115.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Sinclair G. I., Heidemann S. R. Role of microtubules in the cytoplasmic compartmentation of neurons. Brain Res. 1987 Sep 8;420(1):73–81. doi: 10.1016/0006-8993(87)90241-1. [DOI] [PubMed] [Google Scholar]
- Bartlett W. P., Banker G. A. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J Neurosci. 1984 Aug;4(8):1944–1953. doi: 10.1523/JNEUROSCI.04-08-01944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. M. Comparison of the effects of microtubule-associated protein 2 and tau on the packing density of in vitro assembled microtubules. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7783–7787. doi: 10.1073/pnas.84.21.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R., Lee G. Functional organization of microtubule-associated protein tau. Identification of regions which affect microtubule growth, nucleation, and bundle formation in vitro. J Biol Chem. 1993 Feb 15;268(5):3414–3419. [PubMed] [Google Scholar]
- Brandt R., Lee G. The balance between tau protein's microtubule growth and nucleation activities: implications for the formation of axonal microtubules. J Neurochem. 1993 Sep;61(3):997–1005. doi: 10.1111/j.1471-4159.1993.tb03613.x. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Paige J. L. Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc Natl Acad Sci U S A. 1981 May;78(5):3269–3273. doi: 10.1073/pnas.78.5.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A., Kosik K. S. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990 Feb 1;343(6257):461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- Caceres A., Mautino J., Kosik K. S. Suppression of MAP2 in cultured cerebellar macroneurons inhibits minor neurite formation. Neuron. 1992 Oct;9(4):607–618. doi: 10.1016/0896-6273(92)90025-9. [DOI] [PubMed] [Google Scholar]
- Caceres A., Potrebic S., Kosik K. S. The effect of tau antisense oligonucleotides on neurite formation of cultured cerebellar macroneurons. J Neurosci. 1991 Jun;11(6):1515–1523. doi: 10.1523/JNEUROSCI.11-06-01515.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheley S., Kosik K. S., Paskevich P., Bakalis S., Bayley H. Phosphorylated baculovirus p10 is a heat-stable microtubule-associated protein associated with process formation in Sf9 cells. J Cell Sci. 1992 Aug;102(Pt 4):739–752. doi: 10.1242/jcs.102.4.739. [DOI] [PubMed] [Google Scholar]
- Chen J., Kanai Y., Cowan N. J., Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992 Dec 17;360(6405):674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Monteiro M. J., Wong P. C., Gill S. R., Gearhart J. D., Hoffman P. N. Involvement of neurofilaments in the radial growth of axons. J Cell Sci Suppl. 1991;15:85–95. doi: 10.1242/jcs.1991.supplement_15.12. [DOI] [PubMed] [Google Scholar]
- Drechsel D. N., Hyman A. A., Cobb M. H., Kirschner M. W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992 Oct;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson K., Weisshaar B., Matus A. Actin depolymerisation induces process formation on MAP2-transfected non-neuronal cells. Development. 1993 Feb;117(2):689–700. doi: 10.1242/dev.117.2.689. [DOI] [PubMed] [Google Scholar]
- Fung M. C., Chiu K. Y., Weber T., Chang T. W., Chang N. T. Detection and purification of a recombinant human B lymphotropic virus (HHV-6) in the baculovirus expression system by limiting dilution and DNA dot-blot hybridization. J Virol Methods. 1988 Jan;19(1):33–42. doi: 10.1016/0166-0934(88)90005-5. [DOI] [PubMed] [Google Scholar]
- Garner C. C., Matus A. Different forms of microtubule-associated protein 2 are encoded by separate mRNA transcripts. J Cell Biol. 1988 Mar;106(3):779–783. doi: 10.1083/jcb.106.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagestedt T., Lichtenberg B., Wille H., Mandelkow E. M., Mandelkow E. Tau protein becomes long and stiff upon phosphorylation: correlation between paracrystalline structure and degree of phosphorylation. J Cell Biol. 1989 Oct;109(4 Pt 1):1643–1651. doi: 10.1083/jcb.109.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S. R., Landers J. M., Hamborg M. A. Polarity orientation of axonal microtubules. J Cell Biol. 1981 Dec;91(3 Pt 1):661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. N., Cleveland D. W., Griffin J. W., Landes P. W., Cowan N. J., Price D. L. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A. 1987 May;84(10):3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler S., Schulz B., Goedert M., Garner C. C. Molecular structure of microtubule-associated protein 2b and 2c from rat brain. J Biol Chem. 1990 Nov 15;265(32):19679–19684. [PubMed] [Google Scholar]
- Kitts P. A., Ayres M. D., Possee R. D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990 Oct 11;18(19):5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knops J., Kosik K. S., Lee G., Pardee J. D., Cohen-Gould L., McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991 Aug;114(4):725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R., LeClerc N., Kosik K. S. Organization of actin and microtubules during process formation in tau-expressing Sf9 cells. Cell Motil Cytoskeleton. 1994;28(3):256–264. doi: 10.1002/cm.970280308. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Caceres A. Tau protein and the establishment of an axonal morphology. J Cell Sci Suppl. 1991;15:69–74. doi: 10.1242/jcs.1991.supplement_15.10. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., McConlogue L. Microtubule-associated protein function: lessons from expression in Spodoptera frugiperda cells. Cell Motil Cytoskeleton. 1994;28(3):195–198. doi: 10.1002/cm.970280302. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- LeClerc N., Kosik K. S., Cowan N., Pienkowski T. P., Baas P. W. Process formation in Sf9 cells induced by the expression of a microtubule-associated protein 2C-like construct. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6223–6227. doi: 10.1073/pnas.90.13.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Wang D. H., Cowan N. J. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988 Nov 11;242(4880):936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- Ludin B., Matus A. The neuronal cytoskeleton and its role in axonal and dendritic plasticity. Hippocampus. 1993;3(Spec No):61–71. [PubMed] [Google Scholar]
- Matus A. Stiff microtubules and neuronal morphology. Trends Neurosci. 1994 Jan;17(1):19–22. doi: 10.1016/0166-2236(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Papandrikopoulou A., Doll T., Tucker R. P., Garner C. C., Matus A. Embryonic MAP2 lacks the cross-linking sidearm sequences and dendritic targeting signal of adult MAP2. Nature. 1989 Aug 24;340(6235):650–652. doi: 10.1038/340650a0. [DOI] [PubMed] [Google Scholar]
- Sharma N., Kress Y., Shafit-Zagardo B. Antisense MAP-2 oligonucleotides induce changes in microtubule assembly and neuritic elongation in pre-existing neurites of rat cortical neurons. Cell Motil Cytoskeleton. 1994;27(3):234–247. doi: 10.1002/cm.970270305. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P., Binder L. I., Viereck C., Hemmings B. A., Matus A. I. The sequential appearance of low- and high-molecular-weight forms of MAP2 in the developing cerebellum. J Neurosci. 1988 Dec;8(12):4503–4512. doi: 10.1523/JNEUROSCI.08-12-04503.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeyama T., Okabe S., Kanai Y., Hirokawa N. Dynamics of microtubules bundled by microtubule associated protein 2C (MAP2C). J Cell Biol. 1993 Jan;120(2):451–465. doi: 10.1083/jcb.120.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waegh S. M., Lee V. M., Brady S. T. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992 Feb 7;68(3):451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- von Massow A., Mandelkow E. M., Mandelkow E. Interaction between kinesin, microtubules, and microtubule-associated protein 2. Cell Motil Cytoskeleton. 1989;14(4):562–571. doi: 10.1002/cm.970140413. [DOI] [PubMed] [Google Scholar]