Abstract
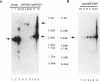
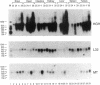
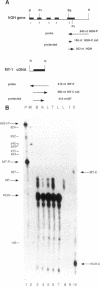
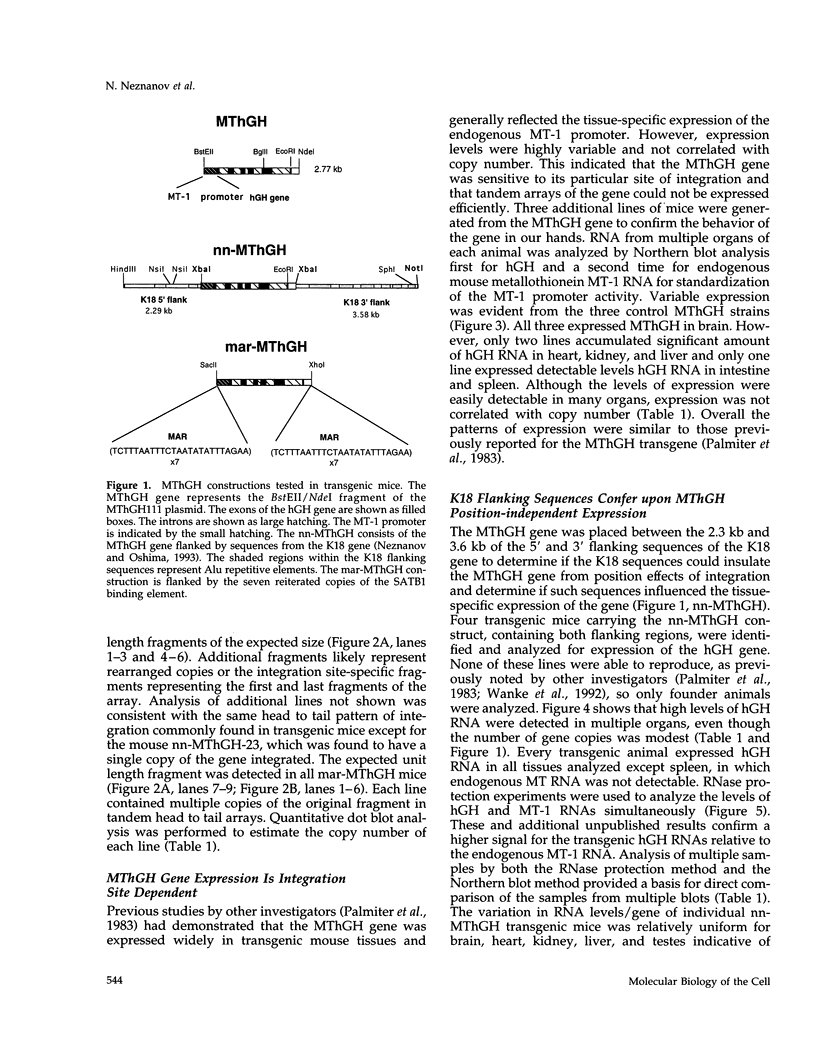
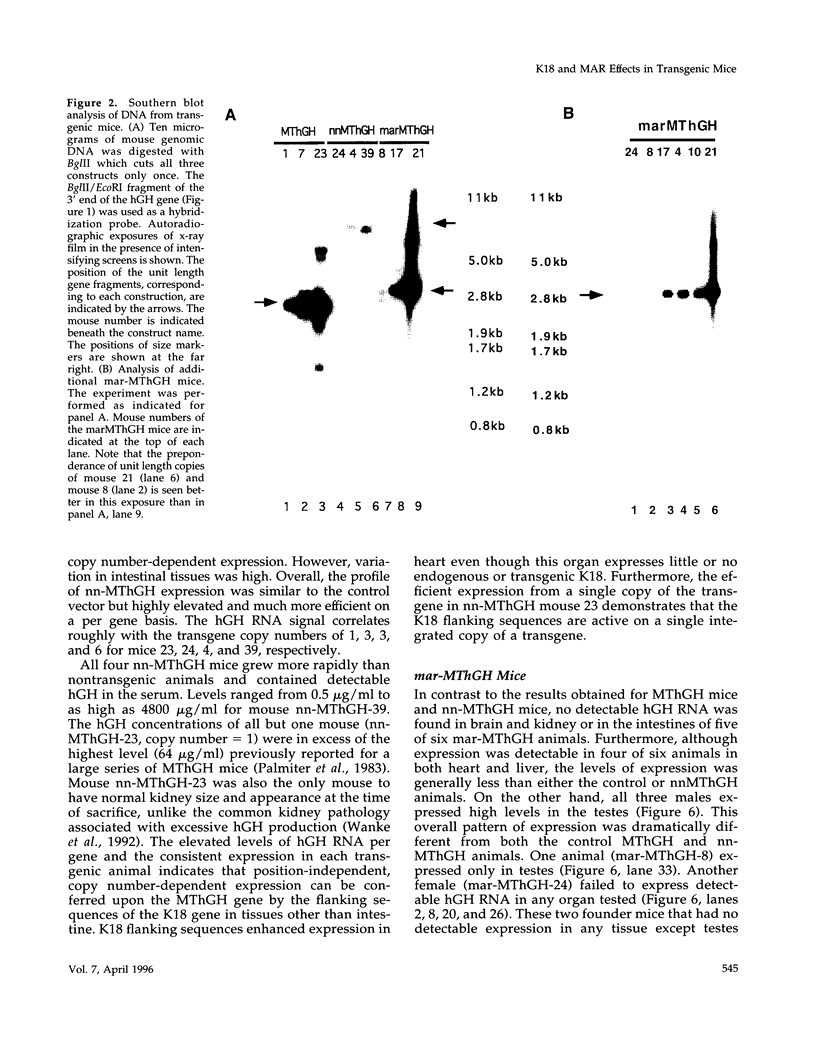
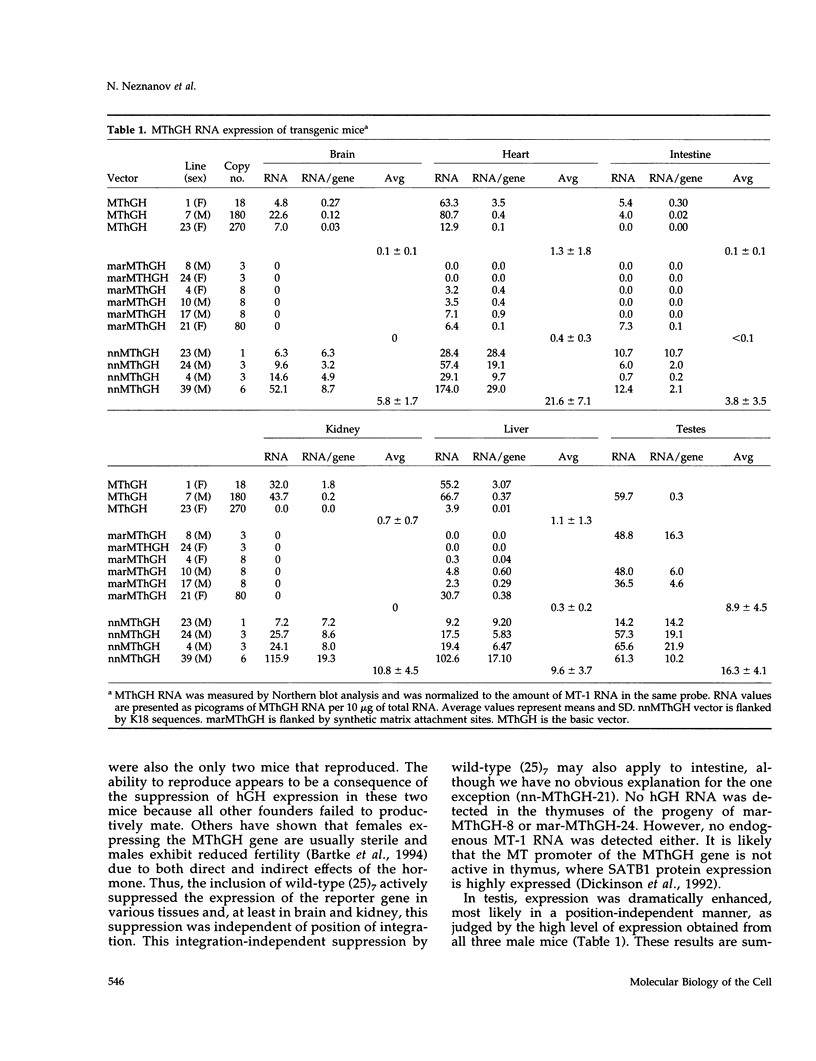
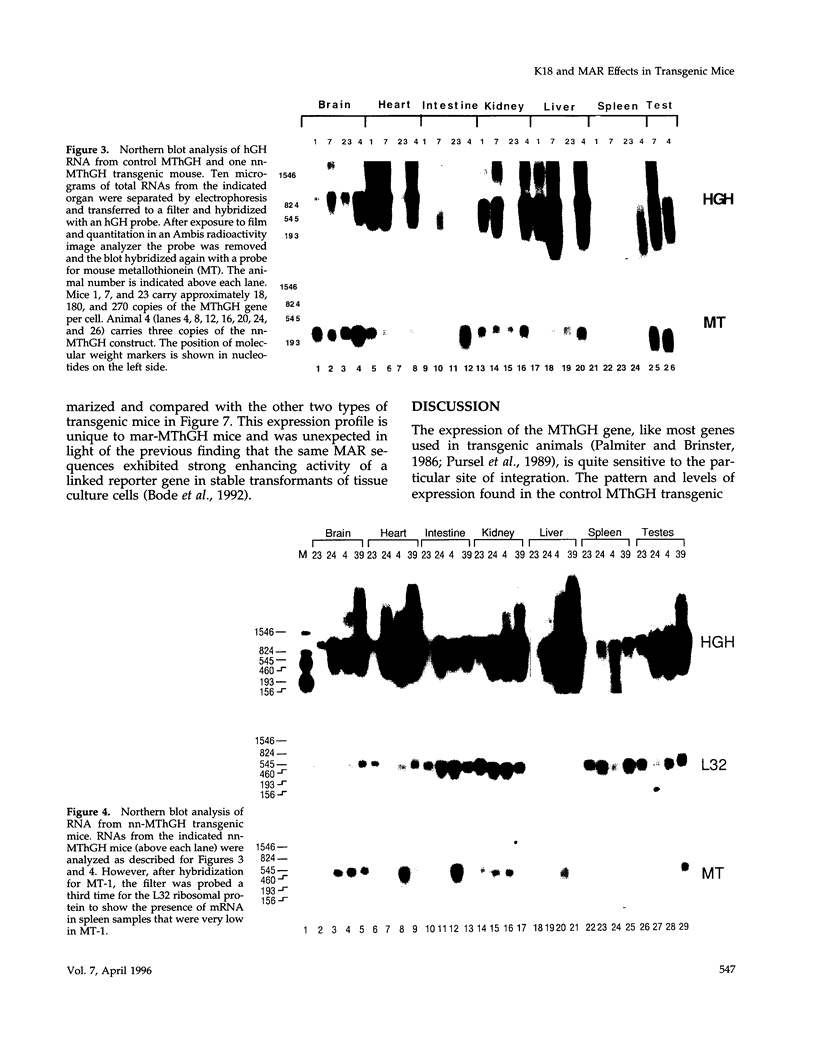
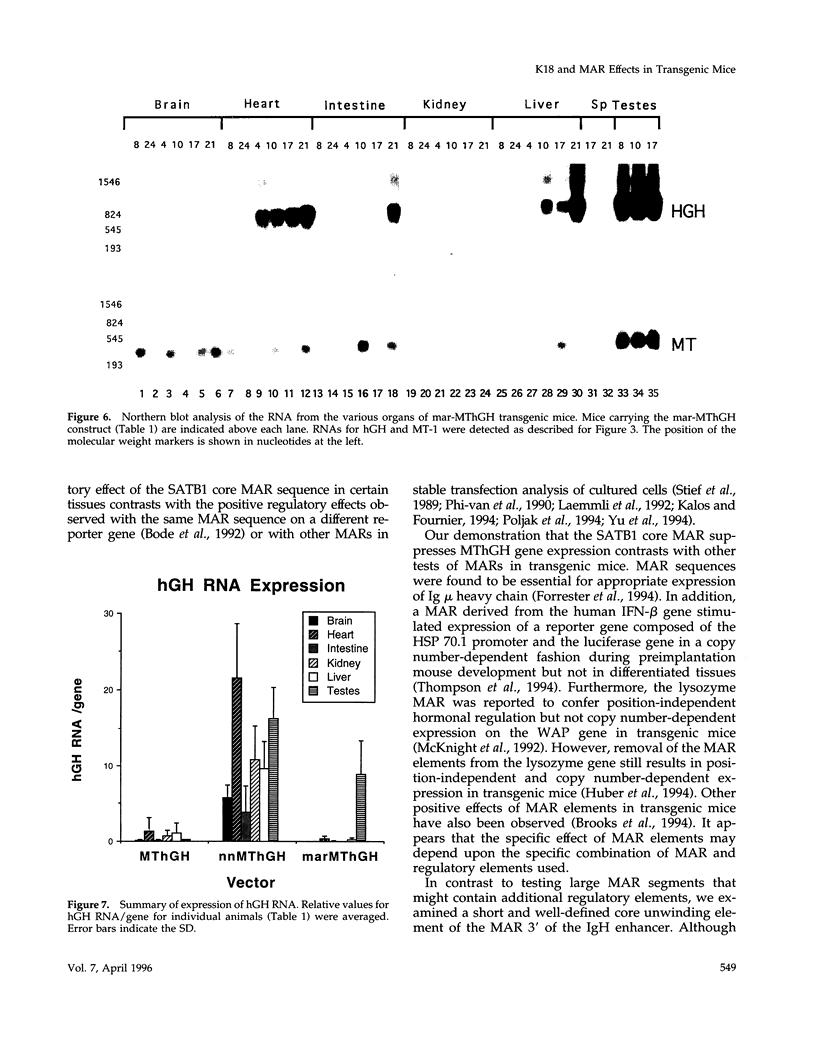
The 2.3 kb and 3.5 kb of DNA that flank the human keratin 18 (K18) gene and synthetic nuclear matrix attachment regions (MAR) composed of the binding sites for the SATB1 nuclear protein were fused to a reporter gene that utilizes the mouse metallothionein promoter and the human growth hormone gene (MThGH). Transgenic mice were generated from both constructions and the control MThGH gene to test K18 and SATB1 MAR sequences for the ability to insulate the reporter gene from integration site-specific position effects. The MThGH control gene was variably expressed in brain, heart, intestine, kidney, liver, and testes, confirming previous studies. In contrast, the MThGH gene insulated by the K18 flanking sequences was expressed in the same tissues of four independent transgenic animals at levels correlated with the copy number except for intestine. The average level of expression on a per gene basis of the K18 insulated gene was from 9- to 49-fold higher than the control. The MThGH gene linked to the SATB1 MAR sequences was completely repressed in the brains and kidneys of all six transgenic mice. However, expression was nearly as efficient in testes as the K18-insulated gene. Both the SATB1 MAR and the K18 flanking sequences confer position-independent transcriptional status on the reporter gene in some or many tissues. However, the effects are stimulatory for the K18 elements and generally suppressive for the SATB1 MAR elements.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Oshima R. G. A single human keratin 18 gene is expressed in diverse epithelial cells of transgenic mice. J Cell Biol. 1990 Sep;111(3):1197–1206. doi: 10.1083/jcb.111.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aladjem M. I., Groudine M., Brody L. L., Dieken E. S., Fournier R. E., Wahl G. M., Epner E. M. Participation of the human beta-globin locus control region in initiation of DNA replication. Science. 1995 Nov 3;270(5237):815–819. doi: 10.1126/science.270.5237.815. [DOI] [PubMed] [Google Scholar]
- Bartke A., Cecim M., Tang K., Steger R. W., Chandrashekar V., Turyn D. Neuroendocrine and reproductive consequences of overexpression of growth hormone in transgenic mice. Proc Soc Exp Biol Med. 1994 Sep;206(4):345–359. doi: 10.3181/00379727-206-43771. [DOI] [PubMed] [Google Scholar]
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Bode J., Kohwi Y., Dickinson L., Joh T., Klehr D., Mielke C., Kohwi-Shigematsu T. Biological significance of unwinding capability of nuclear matrix-associating DNAs. Science. 1992 Jan 10;255(5041):195–197. doi: 10.1126/science.1553545. [DOI] [PubMed] [Google Scholar]
- Brooks A. R., Nagy B. P., Taylor S., Simonet W. S., Taylor J. M., Levy-Wilson B. Sequences containing the second-intron enhancer are essential for transcription of the human apolipoprotein B gene in the livers of transgenic mice. Mol Cell Biol. 1994 Apr;14(4):2243–2256. doi: 10.1128/mcb.14.4.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chung J. H., Whiteley M., Felsenfeld G. A 5' element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993 Aug 13;74(3):505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Dickinson L. A., Kohwi-Shigematsu T. Nucleolin is a matrix attachment region DNA-binding protein that specifically recognizes a region with high base-unpairing potential. Mol Cell Biol. 1995 Jan;15(1):456–465. doi: 10.1128/mcb.15.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Ferns M. J., Hall Z. W. How many agrins does it take to make a synapse? Cell. 1992 Jul 10;70(1):1–3. doi: 10.1016/0092-8674(92)90525-h. [DOI] [PubMed] [Google Scholar]
- Fiering S., Epner E., Robinson K., Zhuang Y., Telling A., Hu M., Martin D. I., Enver T., Ley T. J., Groudine M. Targeted deletion of 5'HS2 of the murine beta-globin LCR reveals that it is not essential for proper regulation of the beta-globin locus. Genes Dev. 1995 Sep 15;9(18):2203–2213. doi: 10.1101/gad.9.18.2203. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., van Genderen C., Jenuwein T., Grosschedl R. Dependence of enhancer-mediated transcription of the immunoglobulin mu gene on nuclear matrix attachment regions. Science. 1994 Aug 26;265(5176):1221–1225. doi: 10.1126/science.8066460. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell. 1986 Aug 15;46(4):521–530. doi: 10.1016/0092-8674(86)90877-9. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Garrard W. T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Huber M. C., Bosch F. X., Sippel A. E., Bonifer C. Chromosomal position effects in chicken lysozyme gene transgenic mice are correlated with suppression of DNase I hypersensitive site formation. Nucleic Acids Res. 1994 Oct 11;22(20):4195–4201. doi: 10.1093/nar/22.20.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. K., Monks B. R., Liebhaber S. A., Cooke N. E. The human growth hormone gene is regulated by a multicomponent locus control region. Mol Cell Biol. 1995 Dec;15(12):7010–7021. doi: 10.1128/mcb.15.12.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M., Fournier R. E. Position-independent transgene expression mediated by boundary elements from the apolipoprotein B chromatin domain. Mol Cell Biol. 1995 Jan;15(1):198–207. doi: 10.1128/mcb.15.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R., Schedl P. A group of scs elements function as domain boundaries in an enhancer-blocking assay. Mol Cell Biol. 1992 May;12(5):2424–2431. doi: 10.1128/mcb.12.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R., Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991 Mar 8;64(5):941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Kohwi Y. Torsional stress stabilizes extended base unpairing in suppressor sites flanking immunoglobulin heavy chain enhancer. Biochemistry. 1990 Oct 16;29(41):9551–9560. doi: 10.1021/bi00493a009. [DOI] [PubMed] [Google Scholar]
- Kulesh D. A., Oshima R. G. Complete structure of the gene for human keratin 18. Genomics. 1989 Apr;4(3):339–347. doi: 10.1016/0888-7543(89)90340-6. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Käs E., Poljak L., Adachi Y. Scaffold-associated regions: cis-acting determinants of chromatin structural loops and functional domains. Curr Opin Genet Dev. 1992 Apr;2(2):275–285. doi: 10.1016/s0959-437x(05)80285-0. [DOI] [PubMed] [Google Scholar]
- McKnight R. A., Shamay A., Sankaran L., Wall R. J., Hennighausen L. Matrix-attachment regions can impart position-independent regulation of a tissue-specific gene in transgenic mice. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6943–6947. doi: 10.1073/pnas.89.15.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neznanov N. S., Oshima R. G. cis regulation of the keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Mar;13(3):1815–1823. doi: 10.1128/mcb.13.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neznanov N., Thorey I. S., Ceceña G., Oshima R. G. Transcriptional insulation of the human keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Apr;13(4):2214–2223. doi: 10.1128/mcb.13.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Norstedt G., Gelinas R. E., Hammer R. E., Brinster R. L. Metallothionein-human GH fusion genes stimulate growth of mice. Science. 1983 Nov 18;222(4625):809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Sandgren E. P., Koeller D. M., Brinster R. L. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol Cell Biol. 1993 Sep;13(9):5266–5275. doi: 10.1128/mcb.13.9.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phi-Van L., von Kries J. P., Ostertag W., Strätling W. H. The chicken lysozyme 5' matrix attachment region increases transcription from a heterologous promoter in heterologous cells and dampens position effects on the expression of transfected genes. Mol Cell Biol. 1990 May;10(5):2302–2307. doi: 10.1128/mcb.10.5.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak L., Seum C., Mattioni T., Laemmli U. K. SARs stimulate but do not confer position independent gene expression. Nucleic Acids Res. 1994 Oct 25;22(21):4386–4394. doi: 10.1093/nar/22.21.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Pursel V. G., Pinkert C. A., Miller K. F., Bolt D. J., Campbell R. G., Palmiter R. D., Brinster R. L., Hammer R. E. Genetic engineering of livestock. Science. 1989 Jun 16;244(4910):1281–1288. doi: 10.1126/science.2499927. [DOI] [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Martin N. C., Townes T. M., Palmiter R. D., Brinster R. L. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human beta-globin gene expression in transgenic mice. Genes Dev. 1989 Mar;3(3):314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- Stief A., Winter D. M., Strätling W. H., Sippel A. E. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature. 1989 Sep 28;341(6240):343–345. doi: 10.1038/341343a0. [DOI] [PubMed] [Google Scholar]
- Thompson E. M., Christians E., Stinnakre M. G., Renard J. P. Scaffold attachment regions stimulate HSP70.1 expression in mouse preimplantation embryos but not in differentiated tissues. Mol Cell Biol. 1994 Jul;14(7):4694–4703. doi: 10.1128/mcb.14.7.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorey I. S., Ceceña G., Reynolds W., Oshima R. G. Alu sequence involvement in transcriptional insulation of the keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Nov;13(11):6742–6751. doi: 10.1128/mcb.13.11.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke R., Wolf E., Hermanns W., Folger S., Buchmüller T., Brem G. The GH-transgenic mouse as an experimental model for growth research: clinical and pathological studies. Horm Res. 1992;37 (Suppl 3):74–87. doi: 10.1159/000182406. [DOI] [PubMed] [Google Scholar]
- Wu J., Grindlay G. J., Bushel P., Mendelsohn L., Allan M. Negative regulation of the human epsilon-globin gene by transcriptional interference: role of an Alu repetitive element. Mol Cell Biol. 1990 Mar;10(3):1209–1216. doi: 10.1128/mcb.10.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. H., Tsai W. H., Lee Y. M., Lei H. Y., Lai M. Y., Chen D. S., Yeh N. H., Lee S. C. Purification and characterization of nucleolin and its identification as a transcription repressor. Mol Cell Biol. 1994 Sep;14(9):6068–6074. doi: 10.1128/mcb.14.9.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Bock J. H., Slightom J. L., Villeponteau B. A 5' beta-globin matrix-attachment region and the polyoma enhancer together confer position-independent transcription. Gene. 1994 Feb 25;139(2):139–145. doi: 10.1016/0378-1119(94)90747-1. [DOI] [PubMed] [Google Scholar]
- Zhao K., Hart C. M., Laemmli U. K. Visualization of chromosomal domains with boundary element-associated factor BEAF-32. Cell. 1995 Jun 16;81(6):879–889. doi: 10.1016/0092-8674(95)90008-x. [DOI] [PubMed] [Google Scholar]
- al-Shawi R., Kinnaird J., Burke J., Bishop J. O. Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol. 1990 Mar;10(3):1192–1198. doi: 10.1128/mcb.10.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]